Abstract
The Borrelia burgdorferi BpaB proteins of the spirochete's ubiquitous cp32 prophages are DNA-binding proteins, required both for maintenance of the bacteriophage episomes and for transcriptional regulation of the cp32 erp operons. Through use of DNase I footprinting, we demonstrate that BpaB binds the erp operator initially at the sequence 5′-TTATA-3′. Electrophoretic mobility shift assays indicated that BpaB also binds with high affinity to sites located in the 5′ noncoding regions of two additional cp32 genes. Characterization of the proteins encoded by those genes indicated that they are a single-stranded DNA-binding protein and a nuclease, which we named SsbP and NucP, respectively. Chromatin immunoprecipitation indicated that BpaB binds erp, ssbP, and nucP in live B. burgdorferi. A mutant bacterium that overexpressed BpaB produced significantly higher levels of ssbP and nucP transcript than did the wild-type parent.
INTRODUCTION
The cp32 prophages are ubiquitous throughout the genus Borrelia, replicating autonomously as circular episomes in the Lyme disease-associated species and as circular and/or linear elements in the relapsing fever borreliae (12, 14, 16, 21, 50, 51, 52, 53, 64). Individual bacteria are naturally infected by numerous distinct cp32 variants; the B. burgdorferi type strain B31 contains 12 different cp32 variants (14, 15, 40). The cp32 elements of Lyme disease spirochetes, such as B. burgdorferi, are of particular interest, since they encode Erp, RevA, and Mlp lipoproteins. These bacterial surface proteins are produced by the bacterium during mammalian infection, and those with known functions serve as adhesins for host components, such as plasmin(ogen), laminin, and complement factor H (1, 7, 8, 9, 10, 13, 22, 25, 26, 32, 33, 38, 42, 43, 49, 63). Sequences of the erp, revA, and mlp loci generally vary between different cp32s (Fig. 1).
Fig 1.
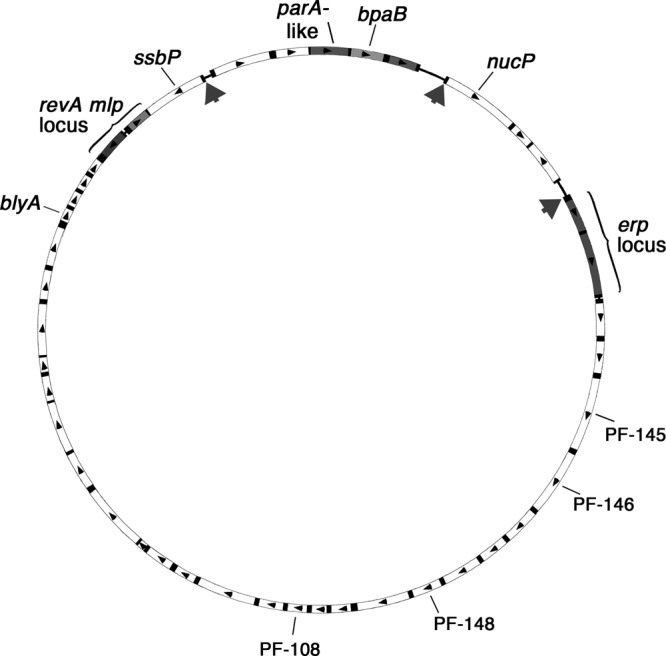
Schematic of cp32-1 from B. burgdorferi type strain B31, a representative member of the prophage family. The boxes represent open reading frames, with arrows indicating directions of transcription. The sequences of ORFs shown in white are >90% identical between all known cp32s, while the shaded ORFs generally exhibit significant variation between replicons. The genes described in this report are identified. The large arrows indicate the locations of mapped BpaB-binding sites. A ChIP survey indicated additional in vivo BpaB-binding sites in the vicinities of blyA and the undefined ORFs PF-145, PF-146, PF-148, and PF-108.
A third region of variable sequence encodes replication/segregation proteins (14, 15, 53) (Fig. 1). These genes encode a protein similar to the ParA proteins of other bacterial plasmids and a protein that appears to be functionally similar to ParB proteins, named BpaB (borrelial ParB analog) (20, 48, 53, 55, 67). Previous studies in our laboratory demonstrated that cp32-encoded BpaB proteins bind to erp operator DNA and repress the transcription of erp operons (11, 30). Although the BpaB allele of each cp32 differs in overall sequence from the alleles of other cp32s, they all possess a conserved domain that facilitates binding to erp operator DNA (11). Thus, all tested cp32-encoded BpaB proteins bind the same site in all tested erp operators (11). Following up on those results, the ability of BpaB to bind elsewhere on cp32 elements was investigated. BpaB was found to bind DNA sequences immediately 5′ of two previously undefined cp32 genes and to positively affect their transcription levels. Biochemical activities were identified for the protein products of those two genes, which we named nucP (nuclease of the prophage) and ssbP (single-stranded DNA-binding protein of the prophage).
MATERIALS AND METHODS
Bacterial strains and culture conditions.
All studies used derivatives of the B. burgdorferi type strain, B31, cultured at 34°C in Barbour-Stoenner-Kelly II medium (66). Chromatin immunoprecipitation (ChIP) was performed using the infectious clone B31-MI-16, which contains all of the genetic elements present in the sequenced, nonclonal B31-MI culture (14, 24, 41).
Studies on the cellular effects of BpaB overproduction used B. burgdorferi strains KS50 and KS52, which are derivatives of B31 that carry pSZW53-4 or pBLS705, respectively (29, 30). pBLS705 contains the lp56 bpaB (allele bpaB56) under the control of a TetR-repressible promoter, post (30, 60). Transcription from the post promoter was induced in early-exponential-phase cultures (approximately 105 bacteria/ml) by addition of anhydrotetracycline (ATc) at a final concentration of 0.5 μg/ml. After further cultivation to final densities of approximately 107 bacteria/ml, bacteria were harvested by centrifugation.
Recombinant BpaB, NucP, and SsbP.
Polyhistidine-tagged recombinant BpaB proteins were produced as previously described (11). The allele of the BpaB gene carried by the strain B31 lp56 plasmid (BpaB56) was used for electrophoretic mobility shift assays (EMSAs) (11, 30). Unless otherwise specified, purified BpaB56 was dialyzed against EMSA buffer (100 nM dithiothreitol, 50 mM Tris [pH 7.5], 50 mM KCl, 10% [vol/vol] glycerol, 0.01% [vol/vol] Tween 20, 0.1% [vol/vol] phenylmethanesulfonyl fluoride). The BpaB allele carried by the strain B31 cp32-1 (BpaB321) was purified for use in the production of specific antibodies (described below).
Recombinant NucP was produced from a clone of the strain B31 cp32-1 nucP gene (open reading frame [ORF] BBP29) (14, 67) in pET101 (Invitrogen, Carlsbad, CA). Purified NucP was dialyzed against 10 mM Tris (pH 7.5), 50 mM KCl, 100 nM dithiothreitol, 10% (vol/vol) glycerol. Recombinant SsbP was produced from the cp32-1 ssbP gene (ORF BBP35) (14) cloned into pET200 (Invitrogen). Purified SsbP was dialyzed against EMSA buffer.
Proteins were concentrated using 10 kDa Amicon centrifugal units (Millipore, Billerica, MA). Final concentrations were measured spectrophotometrically as a ε280 value of 2.64 × 104 M−1 cm−1 (44) or by Bradford assay (Bio-Rad, Hercules, CA). Purities were determined by SDS-PAGE and staining with Coomassie brilliant blue. Aliquots were stored at −80°C.
DNase I footprinting.
Synthetic DNAs were produced by IDT (Coralville, IA). Oligonucleotide P50F (Table 1) was 32P labeled at the 5′ terminus using T4 polynucleotide kinase (New England BioLabs, Beverly, MA) and [γ-32P]ATP (ICN Radiochemicals, Irvine, CA) (44). Unincorporated [γ-32P]ATP was removed with Sephadex G-10 centrifuge columns (GE Healthcare, Little Chalfont, United Kingdom) equilibrated with TE buffer (100 mM Tris-HCl, pH 8.0, 10 mM EDTA). Duplex DNA was prepared by annealing with complementary oligonucleotide P50R (Table 1), as described previously (44), to produce labeled double-stranded probe P50. DNA concentrations were measured spectrophotometrically as a ε260 value of 1.31 × 104 M−1 cm−1 (per base).
Table 1.
Oligonucleotides used in this work
| Oligonucleotide designation | Sequence (5′ to 3′) | Purpose |
|---|---|---|
| P50F | TGTAACAGCTGAATGTAACAAAATTATATATTTAAATCTTTGAAATATTG | DNase footprinting |
| P50R | CAATATTTCAAAGATTTAAATATATAATTTTGTTACATTCAGCTGTTACA | DNase footprinting |
| Bio-LHB3F | biotin-TAAAAAAGTTGGCAAAAATAGT | Labeled probe B-3.1 |
| P3-1R | CAATTATTTCACAACCAGTAAAACTT | Labeled probe B-3.1 |
| IRcomp1F | AAAATAGTTTTTGCTATATACTTATTTTTA | Competitor cB-1 |
| IRcomp1R | TAAAAATAAGTATATAGCAAAAACTATTTT | Competitor cB-1 |
| IRcomp2F | TAAATAACCATAGGAGTAAAAAGATGGAAA | Competitor cB-2 |
| IRcomp2R | TTTCCATCTTTTTACTCCTATGGTTATTTA | Competitor cB-2 |
| IRcomp3F | ATCTTTCAAACAATAATAATCCACAAGAAA | Competitor cB-3 |
| IRcomp3R | TTTCTTGTGGATTATTATTGTTTGAAAGAT | Competitor cB-3 |
| Bio-4-6 | biotin-TAATAGAGAATTTAGCTAAGCCCTATTTTTTTG | Labeled probe B-4 |
| 4-7 | CGGAAGCTTATTTTTGCTTGAATATTATTTTGT | Labeled probe B-4 |
| Fla3 | GGGTCTCAAGCGTCTTGG | flaB Q-RT-PCR |
| Fla4 | GAACCGGTGCAGCCTGAG | flaB Q-RT-PCR |
| ssbP-QF | GGTATGCTTTAGTTGCAGCTCTTGG | ssbP Q-RT-PCR |
| ssbP-QR | GTTGTAGGGCTATTTACCCAGTCTT | ssbP Q-RT-PCR |
| nucP-QF | GTAGCGACTCTAATTATATGAGTAG | nucP Q-RT-PCR |
| ERP-1 | ATGTAACAGCTGAATG | cp32-1 Q-PCR |
| ERP-108 | CTTAAATTATGTCTAGTATCACTC | cp32-1 Q-PCR |
| ERP-1 | ATGTAACAGCTGAATG | cp32-3 Q-PCR |
| ERP-58 | AGTCTAATCATATCCTCAGACAGG | cp32-3 Q-PCR |
| ERP-401 | AATATTGCAATTATTAGCTGTTG | cp32-4 Q-PCR |
| ERP-404 | ATTCATTCTTAGGGTTTTCATATC | cp32--4 Q-PCR |
| nucP-QR | CACTTTTGATATTAAGGCCTCATCT | nucP Q-RT-PCR |
| erpA-141F | AGAATAATAGTAATAACTGGG | erpA Q-RT-PCR |
| erpA-142R | CTAGTGATATTGCATATTCAG | erpA Q-RT-PCR |
| Bio104F | TGTTAAAATGTAACAGCTGAAT | SsbP function |
| 104R | ATTCAGCTGTTACATTTTATCT | SsbP function |
| A43R | GCAATATTTCAAAGATTTAAA | SsbP function |
| A15R | TCATACCACAATACATAATAA | SsbP function |
| A21R | TAATAAATGCATATTTCAAA | SsbP function |
| M13-R | GGAAACAGCTATGACCATG | Amplify linear DNA used in NucP functional assays |
| BSV19 | TAAGATAGGTAAGAAATTACCCGG | Amplify linear DNA used in NucP functional assays |
Footprinting reaction conditions were essentially as described by Hudson and Fried (27). Protein samples were dialyzed against 10 mM Tris, 50 mM KCl, 1 mM dithiothreitol (DTT), 1 mM MgCl2, 1 mM CaCl2, pH 7.5. BpaB protein sample concentrations ranged from 4 to 23 μM (0.084 to 0.5 mg/ml). Each reaction mixture contained 5 ng labeled P50 DNA (50 bp). In addition, Maxam-Gilbert limited cleavage sequencing of untreated labeled DNA was performed and analyzed on the same gels (37).
EMSA.
EMSAs were performed as previously described (11, 45). The oligonucleotides used to make labeled probes and unlabeled competitors are listed in Table 1. Some oligonucleotides were synthesized with a covalently attached biotin on the 5′ end (IDT). Some labeled dsDNA probes were produced by PCR and purified as described previously (2). Other labeled and unlabeled dsDNAs were produced by annealing pairs of complementary oligonucleotides as follows: equal molar concentrations of the two oligonucleotides were mixed, heated to 94°C for 5 min, and then cooled gradually to room temperature over a period of >30 min.
Mapping of additional BpaB-binding sites.
Probe B-3.1, which encompasses the 5′ terminus of nucP and upstream DNAs, was produced by PCR, using oligonucleotides Bio-LHB3F and P3-1R, from template pOMB65 (15, 67). Probe B-4, which encompasses the 5′ end of ssbP and upstream DNAs, was produced using primers Bio-4-6 and -4-7 and a plasmid clone of the strain B31 cp32-1 ssbP locus. Labeled amplicons were purified as previously described (11). The unlabeled competitor DNAs cB-1, cB-2, and cB-3 were produced by annealing the oligonucleotide pairs IRcomp1F-IRcomp1R, IRcomp2F-IRcomp2R, and IRcomp3F-IRcomp3R, respectively (Table 1). EMSA competitors were used at concentrations giving 100-fold molar excess over the probes.
ChIP.
Monospecific murine antibodies were raised against the BpaB321 allele, which is encoded by cp32-1 and is present in all of the subcultures of strain B31 used in these studies. Prior to immunization, samples of blood were collected, pooled, and processed into serum (preimmune serum). BALB/c mice were injected subcutaneously with 10 μg recombinant BpaB321 in 80 μl 60% AlOH (mass/vol), followed by 2 additional injections 2 weeks apart. One week after the final boost, the mice were euthanized, and their blood was pooled and processed into serum. Preserum and antiserum were assessed for anti-BpaB antibodies by immunoblotting and enzyme-linked immunosorbent assay (ELISA) with purified recombinant BpaB321 and with B. burgdorferi whole-cell lysate. The preserum did not contain any antibodies reactive with BpaB321 or any other borrelial protein.
ChIP was performed as previously described (29, 30, 58, 59). Following cross-linking and cell lysis, BpaB-containing complexes were immunoprecipitated using antibodies and protein A magnetic Dynabeads (Invitrogen, Carlsbad, CA). Donkey anti-mouse IgG (Santa Cruz Technologies, Santa Cruz, CA) was used as a control for nonspecific antibody binding. Resin beads without added antibody were used as additional controls.
Borrelial supernatants (800 μl) were incubated with antibody-bead complexes, or control beads alone, for 20 min at room temperature. The supernatants were poured off, and then the beads were incubated a second time with a fresh aliquot of lysate. The bead complexes were washed 3 times with immunoprecipitation (IP) wash buffer (Invitrogen Protein A Dynabead kit) and then resuspended in IP buffer. After transfer to clean microcentrifuge tubes, bead complexes were washed 4 times with IP buffer supplemented with 500 mM NaCl, followed by 2 washes with TE buffer (10 mM Tris, 1 mM EDTA). The beads were resuspended in TE and incubated at 65°C for 18 h. The eluted DNA was purified using DNeasy Blood and Tissue kits (Qiagen, Valencia, CA), and antigens were eluted from antibody using elution buffer (Immunoprecipitation Kit; Invitrogen). Immunoprecipitation was verified by Western blot analysis following antigen elution.
The eluted DNA was digested with Tsp509I, cloned into the compatible EcoRI site of pUC19, and then transformed into Escherichia coli. Colonies were screened directly by PCR (61). The inserts of 20 randomly selected plasmid clones were sequenced.
Quantitative PCR (Q-PCR).
Relative copy number comparisons of cp32s in different B. burgdorferi strains were assessed by PCR using identical masses of total bacterial DNA as templates. Total DNA was extracted from ATc-treated KS50 and KS52 (described above) using DNeasy Blood and Tissue kits (Qiagen). Limited PCR was performed using previously described oligonucleotide primer pairs that specifically amplify the cp32-1 erpAB, cp32-3 erpG, and cp32-4 erpHY loci (15) (Table 1). PCRs were performed for 15, 18, or 21 cycles; the products were subjected to agarose gel electrophoresis and ethidium bromide staining; and band intensities were compared densitometrically.
Q-RT-PCR.
B. burgdorferi strains KS50 and KS52 were cultured in 10 ml BSK-II plus 0.5 μg/ml ATc. Cultures were collected at midexponential phase (approximately 107 bacteria/ml). Total RNA was extracted, and transcript levels were determined by quantitative reverse transcription-PCR (Q-RT-PCR), as described by Miller (39). The oligonucleotides are listed in Table 1. The results were compared by a two-tailed Student's t test.
Functional analyses of SsbP.
The affinities of SsbP for single-stranded DNA (ssDNA) and dsDNA were determined by EMSA, using precast 20% nondenaturing polyacrylamide gels (Invitrogen). The labeled probes were either biotinylated oligonucleotide Bio104F alone (ssDNA) or Bio104F annealed with the complementary oligonucleotide 104R (dsDNA). For specificity experiments, the unlabeled competitor ssDNAs A43R, A15R, and A21R were included in EMSA reaction mixtures at concentrations giving 100-fold molar excess over the labeled probes. The binding affinity (KD) was determined using previously described methods (45).
Functional analyses of NucP.
Recombinant NucP was purified from E. coli. Since E. coli may produce endogenous DNases, the same parental E. coli strain was transformed with a pET200-based plasmid that contains nucP in the inverse orientation (control E. coli). Both the NucP-producing and control strains were subjected to simultaneous, identical IPTG (isopropyl-β-d-thiogalactopyranoside) induction and protein purification treatments. Equal volumes of each purified protein/bacterial extract were incubated with linear DNA in 1× New England BioLabs restriction endonuclease buffer 1, 2, 3, or 4. Buffer 1 (1×) contained 10 mM Bis Tris-propane-HCl (pH 7.0), 10 mM MgCl2, 1 mM dithiothreitol. Buffer 2 (1×) contained 10 mM Tris-HCl (pH 7.9), 50 mM NaCl, 10 mM MgCl2, 1 mM dithiothreitol. Buffer 3 (1×) contained 50 mM Tris-HCl (pH 7.9), 100 mM NaCl, 10 mM MgCl2, 1 mM dithiothreitol. Buffer 4 (1×) contains 20 mM Tris-acetate (pH 7.9), 50 mM sodium acetate, 10 mM magnesium acetate, 1 mM dithiothreitol. For the illustrated experiments, the substrate DNA was an approximately 1.2-kb linear fragment produced from pBLS590 (3) using oligonucleotide primers BSV-19 and M13-F (Table 1). The reaction mixtures were incubated at 37°C for various times and then terminated by heating to 99°C for 10 min. Samples were electrophoresed in 1.8% agarose gels, and DNA was visualized with ethidium bromide.
RESULTS
Identification of a high-affinity BpaB-binding site by DNase footprinting.
EMSA-based analyses indicated that BpaB binds with high affinity to a sequence within a 20-bp region of the cp32 erp operator DNA (11). To more precisely define the high-affinity BpaB-binding sequence, DNase I footprinting experiments were undertaken, using 32P-labeled erp operator DNA. Protein was added at concentrations ranging up to 23 μM, well beyond the KD of BpaB binding to the DNA (11). DNase I binds to the minor groove of the DNA and cuts the phosphodiester backbones of both strands independently (56). Its size (∼40 Å) helps to prevent it from cutting the DNA under and adjacent to the bound protein (34). At the lowest concentrations of BpaB that afforded any discernible protection, the 5-nucleotide sequence 5′-TTATA-3′ was protected from DNase I digestion (Fig. 2, lanes 2 to 8). As BpaB concentrations were increased, additional nucleotides flanking the initial binding site were protected from DNase I activity, consistent with previous EMSA data (30, 45).
Fig 2.
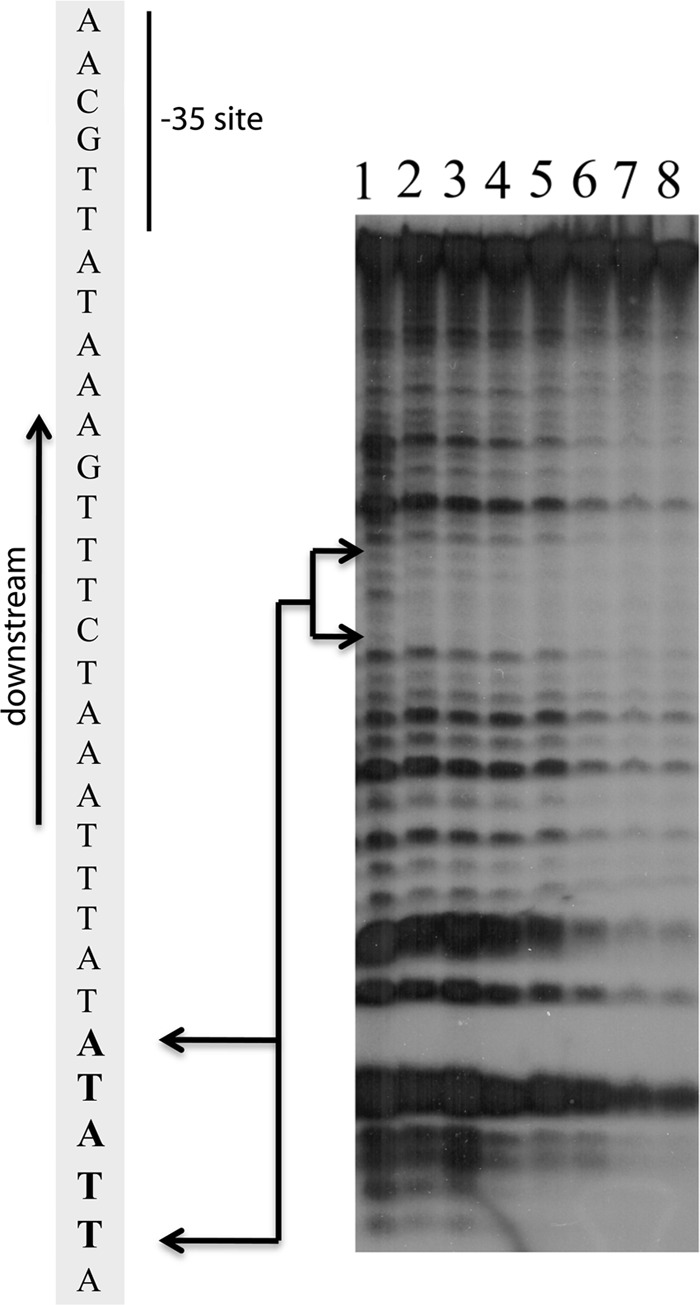
Identification of a high-affinity BpaB-binding site by DNase I footprinting. The illustrated representative experiment was conducted with the addition of increasing concentrations of BpaB to samples containing 5 ng 32P-labeled P50 erp operator noncoding dsDNA. Lanes 1 to 8 contained BpaB at final concentrations of 0, 4, 8, 10, 12, 15, 20, and 23 μM, respectively. The lower concentrations of BpaB protected the nucleotides TTATA from DNase1. Increasing protein concentrations led to expansion of protected nucleotides, consistent with EMSA data indicating the binding of additional BpaB molecules to DNA as the protein concentration increases (11, 30).
BpaB binds to other cp32 sequences.
The ability of cp32-encoded BpaB proteins to bind with high affinity to the erp operator led us to query whether the protein binds to other cp32 loci. To that end, EMSAs were performed using purified recombinant BpaB and various cp32 fragments. Protein binding was observed when examining labeled probe B-3.1, a segment of plasmid cp32-1 that includes 5′ noncoding DNA and the initial codons of the gene previously referred to as ORF-8/7, ORF-8, paralog family 165, and bppA (Fig. 3 and 4) (14, 18, 23, 52). Biochemical analyses of the gene's product demonstrated that it is a nuclease, so the gene was renamed nucP (see below). BpaB bound to the nucP promoter-containing DNA with approximately 1.4-fold-greater affinity than to the erpAB operator probe (Fig. 4A). Binding to a control DNA fragment derived from the promoter region of the B. burgdorferi flaB gene, which lacks a BpaB-binding sequence, did not occur (Fig. 4A) (11). The binding region of probe B3.1 was refined through the use of unlabeled competitor DNAs that consisted of DNA sequences within B3.1. DNA fragment cB-1 competed BpaB away from probe B3.1, whereas fragments cB-2 and cB-3 did not detectably compete for BpaB binding (Fig. 4B). Thus, the BpaB-binding site 5′ of nucP is within the region spanned by competitor cB-1 (Fig. 3).
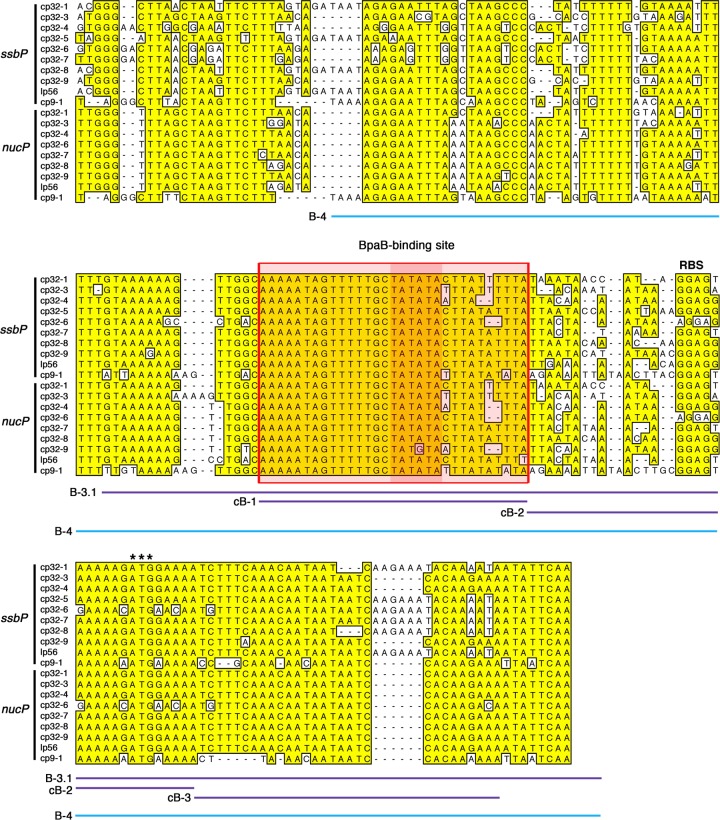
Fig 3.
Alignment of the 5′ noncoding regions and the first 15 to 17 codons of the ssbP and nucP genes found on the native cp32 family members of B. burgdorferi type strain B31. Each gene's ribosome binding site (RBS) is indicated above the alignment, and initiation codons are indicated by three asterisks. Sequences found in the majority of loci are boxed and shaded. Sequences encompassed by labeled DNA probes and unlabeled competitors are indicated by colored lines below the alignment.
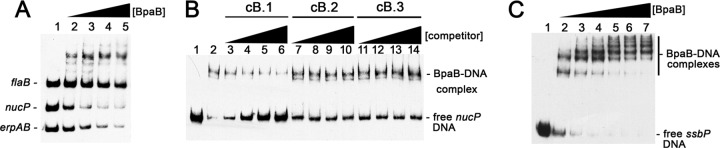
Fig 4.
BpaB binds cp32 DNA 5′ of nucP and ssbP. (A) EMSA of BpaB with labeled DNA probes consisting of sequence 5′ of the cp32-1 nucP ORF (probe B-3.1), the cp32-1 erpAB operator, and the chromosomal flaB ORF. Lane 1 contained only DNAs. Lanes 2 to 5 also contained recombinant BpaB321 at concentrations of 0.03, 0.06, 0.09, 0.12, and 0.15 μM. Densitometric analyses indicated that BpaB binds the site 5′ of nucP with an approximately 1.4-fold-greater affinity than the site in the erpAB operator. (B) EMSA of BpaB, labeled nucP 5′ noncoding DNA (probe B-3.1), and unlabeled competitor DNAs. Lane 1 contained probe B-3.1 only, while the other lanes also included 0.1 μM BpaB321. Lanes 3 to 6, 7 to 10, and 11 to 14 additionally contained unlabeled probes at molar concentrations that were 63-, 125-, 250-, and 500-fold greater than that of the labeled probe. (C) EMSA indicating binding of BpaB to DNA 5′ of ssbP. Lane 1 contained only labeled ssbP DNA (probe B-4), while lanes 2 to 7 contained BpaB321 at concentrations of 0.3, 0.6, 0.9, 1.2, 1.4, and 1.5 μM.
All cp32 elements contain a second region that is nearly identical to the initial 15 to 17 codons of nucP and the approximately 140 bp immediately 5′ of nucP, forming the promoter region and initial codons of a second gene (Fig. 1 and 3) (14, 15, 18, 67). The extensive similarities between these two regions have led to their being described as “inverted repeats,” although several kilobases separates the two sequences (14, 15, 18, 67). We subsequently demonstrated that this second locus, previously referred to as ORF-4 or paralog family 161 (14, 18), encodes a single-stranded DNA-binding protein that we named SsbP (see below). Other than their initial 15 to 17 codons, the nucP and ssbP open reading frames do not share any significant homology with each other. EMSA using purified BpaB indicated that the protein also binds the promoter region of ssbP (Fig. 4C).
Together, these data indicate that BpaB binds with high affinity to the region 5′ of the nucP and ssbP genes that is outlined in Fig. 3. The nucP and ssbP BpaB-binding sites contain a sequence, 5′-TATATA-3′, that is similar to the erp operator high-affinity site (Fig. 3).
ChIP was then used to examine whether BpaB binds to the nucP and ssbP loci in live B. burgdorferi cells. A simple survey approach was taken. Following in vivo cross-linking, BpaB-containing complexes were immunoprecipitated, DNA digested with a restriction endonuclease, then cloned into an E. coli plasmid. The inserts of 20 random clones were sequenced. Control ChIP using irrelevant antibodies or no antibodies did not yield any cloned borrelial DNAs, confirming the specificity of these analyses.
One of the clones contained inserts derived from a nucP locus; nucleotide polymorphisms indicated that it came from either cp32-3, cp32-6, cp32-9, or lp56. Two clones contained fragments of ssbP loci, one from either cp32-1 or cp32-7 and the other from cp32-7, cp32-8, or lp56. Two other clones contained erp operator DNAs, consistent with our previous results indicating that BpaB binds that locus in vivo (11, 30). These data demonstrated that live B. burgdorferi contains BpaB protein bound to the 5′ noncoding regions of the cp32 nucP and ssbP operons, as well as to the erp operator.
Seven ChIP clones contained other fragments of cp32 DNAs: three contained DNA from the region that includes genes for putative bacteriophage terminase and portal proteins (PF-145 and PF-146, respectively), three clones contained DNA near the holin-encoding blyA, and the other two contained loci with unknown functions (PF-148 and PF-109) (Fig. 1). The remaining 6 clones contained fragments of other B. burgdorferi replicons, including the main chromosome. Further studies are ongoing to precisely map those additional BpaB-binding sites and to determine if BpaB affects the expression of genes at those loci.
BpaB modulates transcription of nucP and ssbP.
As noted above, all strains of B. burgdorferi naturally contain numerous distinct cp32 replicons, each of which produces a BpaB protein (11, 14, 30). BpaB is required for cp32 maintenance, so deletion of a bpaB gene results in elimination of that replicon from the cell (20, 55). Thus, it is impossible to delete all copies of bpaB from a cell and still examine the effects of BpaB on cp32 gene expression in a native context. We therefore examined the effects of BpaB on cp32 genes by using B. burgdorferi that overexpress that protein (30). B. burgdorferi KS52 contains an inducible bpaB gene, while KS50 carries the empty vector. Addition of the inducing compound ATc to KS52 increases production of BpaB to levels well above those of uninduced cultures but does not have any effect on the production of BpaB or any other protein in the control strain KS50 (29, 30).
We previously demonstrated that BpaB represses erp expression (30), a result also observed in the present studies (Fig. 5B). In contrast, overproduction of BpaB by B. burgdorferi significantly enhanced the transcript levels of both ssbP and nucP (Fig. 5B).
Fig 5.
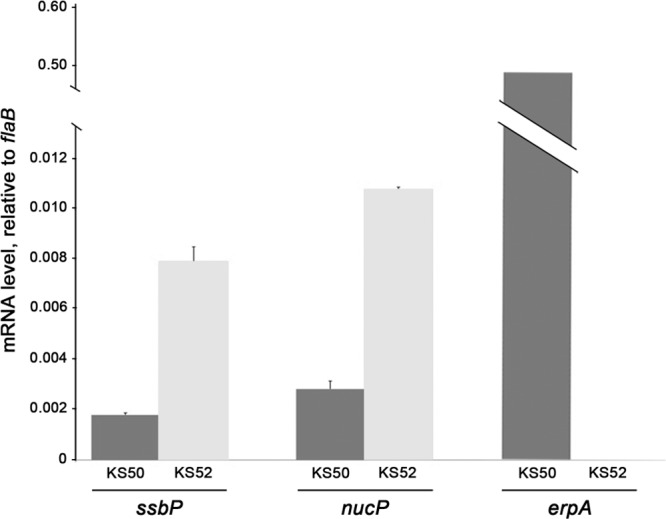
Cellular levels of BpaB affect expression of ssbP and nucP. B. burgdorferi strains KS52 (which contains bpaB on a plasmid under the control of the ATc-inducible ostp promoter) and KS50 (which carries the parental, empty ostp vector) were incubated with ATc, and total RNAs were purified. Q-RT-PCR was used to determine the levels of ssbP, nucP, and erpA relative to the constitutively expressed housekeeping flaB gene. The illustrated results are representative of two independent analyses, each performed in triplicate using separately prepared batches of cDNA. Standard errors are indicated. Differences in expression levels for each gene with and without enhanced levels of BpaB were statistically significant (P < 0.05) by two-tailed Student's t tests.
Replication of each cp32 element requires a specific cis-encoded BpaB allele (20, 55). The BpaB56 allele utilized in the above-described studies was derived from the strain B31 lp56 replicon, while the strain examined contains only cp32-1, cp32-3, and cp32-4 and lacks lp56 (15). Although BpaB56 is not predicted to affect replication of the cp32s present in the analyzed bacteria, we nonetheless addressed the possibility that the altered expression levels of ssbP and nucP might have been due to changes in cp32 copy numbers. Q-PCR of cp32-1, cp32-3, and cp32-4 target loci indicated that enhanced cellular levels of BpaB56 did not cause significant differences in copy numbers (Fig. 6).
Fig 6.
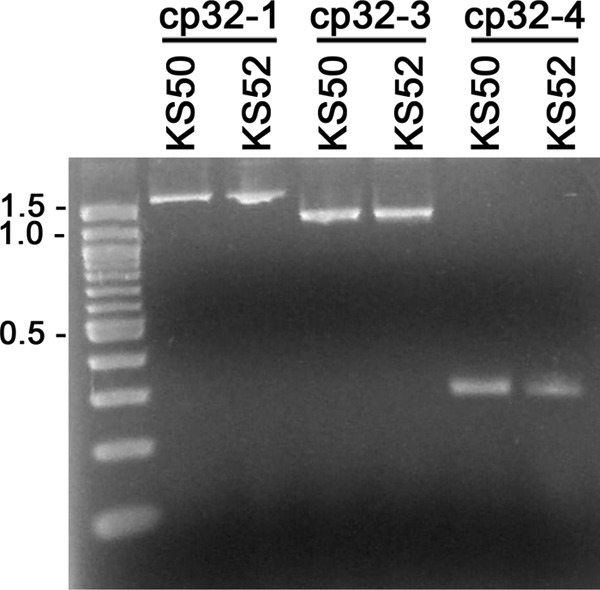
Enhanced production of BpaB56 does not affect the relative copy numbers of cp32-1, cp32-3, and cp32-4. Oligonucleotide primer pairs specific for the resident cp32s of sibling strains KS50 and KS52 were utilized for limited PCR, followed by agarose gel electrophoresis and staining with ethidium bromide. Data for 15 PCR cycles are shown. Similar results were obtained after 18 or 21 cycles.
SsbP is a nonspecific single-stranded DNA-binding protein.
The ssbP genes are highly conserved among all cp32 elements (14, 51). Blast-P analyses of the previously unannotated gene products revealed similarities (E values ranging up to 1013) with proteins of other bacterial species that interact with ssDNA, such as RecT and ERF (http://www.ncbi.nlm.nih.gov/BLAST).
The predicted function of SsbP was examined by EMSA using labeled ssDNA and dsDNA. Recombinant SsbP bound to the randomly selected ssDNA 5′-TGTTAAAATGTAACAGCTGAAT-3′ in a dose-dependent manner, with a KD of approximately 2 nM (Fig. 7A). In contrast, almost no binding occurred with dsDNA with the same sequence (Fig. 7A).
Fig 7.
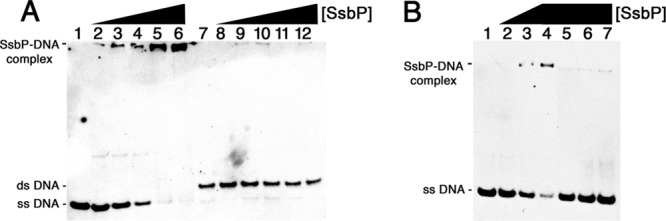
SsbP is a nonspecific ssDNA-binding protein. (A) SsbP binds ssDNA, but not dsDNA. Lanes 1 to 6 contained the labeled ssDNA probe ssBio104. Lanes 7 to 12 contained the labeled dsDNA probe dsBio104. Lanes 1 and 7 did not contain any added protein. SsbP was added to the DNAs at the following final concentrations: lanes 2 and 8, 0.083 ng/ml; lanes 3 and 9, 0.17 ng/ml; lanes 4 and 10, 0.25 ng/ml; lanes 5 and 11, 8.3 ng/ml; and lanes 6 and 12, 2.1 ng/ml. (B) SsbP binds nonspecifically. Lanes 1 to 7 contained the labeled ssDNA probe ssBio104. Lane 1 contained labeled probe only. Lanes 2 to 4 included SsbP added to final concentrations of 0.083 ng/ml, 0.83 ng/ml, or 1.7 ng/ml, respectively. Lanes 5 to 7 contained probe, 1.7 ng/ml SsbP, and 100× excesses of unlabeled ssDNA probes A42-R, A15-R, and A21-R, respectively.
The question of whether SsbP binding to ssDNA is sequence specific was addressed through addition of unlabeled ssDNAs to EMSAs. Three randomly selected ssDNA sequences competed for SsbP against the above-described labeled ssDNA probe, indicating that SsbP binding is not dependent upon the DNA sequence (Fig. 7B).
NucP is a DNase.
The nucP gene product likewise had not previously been functionally characterized. An earlier study by our laboratory gave this gene the placeholder name bppA (Borrelia plasmid protein A) (23). Subsequent Blast-P analyses of the predicted gene product found modest similarities with some bacteriophage-encoded nucleases (e.g., an E value of 105 similarity to LHK-Exo exonuclease of Laribacter hongkongensis) (62). That possible function was addressed by incubating recombinant NucP protein with linear dsDNA, as it would be a substrate of either exonuclease or endonuclease. Since the recombinant protein was produced by E. coli and therefore might contain E. coli-encoded DNases, extracts that do not encode NucP were also produced from E. coli. Cultures were processed simultaneously, in identical manners. Incubation of the NucP-producing E. coli extract with linear DNA resulted in almost complete digestion within 8 h (Fig. 8). In contrast, the control E. coli extract yielded appreciably less degradation of the DNA, even after 24 h of incubation. DNA digestion by NucP was similarly observed when using a variety of buffers. While additional studies are required to determine the optimal conditions for DNA digestion by NucP, as well as to define the mechanism of degradation, these data indicate that NucP is a nuclease.
Fig 8.
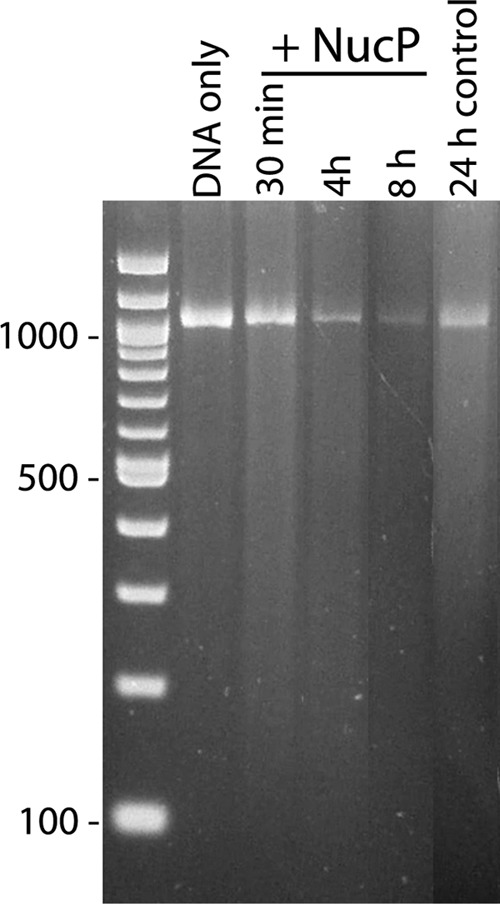
NucP is a DNase. E. coli that produces polyhistidine-tagged NucP from an inducible lac promoter or that carries the expression vector with nucP inserted in the inverse orientation (E. coli control) was subjected to identical treatments for protein purification using immobilized nickel beads. A linearized DNA was incubated with purified NucP for 30 min or 4 or 8 h or with E. coli control extract for 24 h and then subjected to agarose gel electrophoresis and ethidium bromide staining. Rapid DNA degradation was repeatedly observed with NucP. E. coli control extracts possessed appreciably less nuclease activity, presumably due to residual native E. coli DNase(s).
DISCUSSION
Previous studies indicated roles for BpaB in maintenance of small borrelial replicons, including the cp32s (5, 20, 54, 55). Almost all replicons encode two unique proteins that have been associated with maintenance: a ParA/SopA homolog and BpaB, leading to speculation that BpaB may function in a manner analogous to the ParB/SopB proteins of other bacterial replicons (5, 11, 20, 30, 54, 55). In addition to their roles in plasmid maintenance, some ParB/SopB proteins directly regulate the expression of plasmid genes (4, 19, 31, 46, 47). This and previous studies indicate that cp32-encoded BpaB proteins are also regulatory factors (30). Despite their overall differences in amino acid sequence, the BpaB proteins encoded by members of the cp32 replicon family all contain a conserved amino acid motif that participates in binding to the 5′-TTATA-3′ sequence of erp operator sites (11, 30). The present studies indicate that cp32-encoded BpaB proteins also bind to a sequence that is located adjacent to the transcriptional promoters of two additional cp32 genes, ssbP and nucP. Recombinant BpaB56 bound with high affinity to both sites in vitro, and ChIP indicated that BpaB321 was bound in live bacteria. Intriguingly, BpaB repressed the transcription of erp operons (30) yet enhanced the expression of both ssbP and nucP. Studies are ongoing to define the mechanism(s) by which BpaB affects the expression of ssbP and nucP.
These studies also determined that ssbP encodes a single-stranded DNA-binding protein and that nucP encodes a nuclease. The predicted sequence of SsbP bears strong similarity to those of other single-stranded DNA-binding proteins, many of which are of bacteriophage origin (6, 28). Intriguingly, a large number of such bacteriophage-encoded proteins are coexpressed with a DNase, and the paired proteins function in homologous recombination (17, 28, 36, 57, 62). As emphasized in Fig. 3, the 5′ noncoding regions of all ssbP and nucP genes are nearly identical, suggesting that these unlinked genes are probably regulated through the same mechanism and thereby coexpressed. The effect of BpaB on both nucP and ssbP transcript levels supports that hypothesis. Thus, SsbP-NucP may play roles in the gene shuffling that is known to occur between cp32 prophages (48, 50). Since cp32s are ubiquitous in Lyme disease spirochetes and have coevolved with their borrelial host, it is also possible that cp32-encoded SsbP and NucP are involved with the RecA-independent recombination events that occur at the bacterium's vlsE antigenic-variation locus (35, 65).
In conclusion, cp32-encoded BpaB proteins bind to multiple sites on the cp32 prophages. Both stimulatory (ssbP and nucP) and repressive (erp) effects of BpaB have been observed (this work and reference 30). Almost all of the other small replicons that naturally occur in B. burgdorferi encode BpaB-like proteins but do not bind the same DNA sequence as do the cp32-encoded BpaBs (11, 14). The effects of cp32 BpaB proteins on cp32 gene expression suggest that other plasmids' paralogs may also participate in the regulation of genes on those replicons.
ACKNOWLEDGMENTS
This work was funded by National Institutes of Health grant R01-AI044254 to Brian Stevenson.
We thank Catherine Brissette, Sherwood Casjens, and Michael Fried for their insightful comments on this work and the manuscript.
Footnotes
Published ahead of print 22 June 2012
REFERENCES
- 1. Alitalo A, et al. 2002. Complement inhibitor factor H binding to Lyme disease spirochetes is mediated by inducible expression of multiple plasmid-encoded outer surface protein E paralogs. J. Immunol. 169:3847–3853 [DOI] [PubMed] [Google Scholar]
- 2. Babb K, et al. 2006. Borrelia burgdorferi EbfC, a novel, chromosomally-encoded protein, binds specific DNA sequences adjacent to erp loci on the spirochete's resident cp32 prophages. J. Bacteriol. 188:4331–4339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Babb K, McAlister JD, Miller JC, Stevenson B. 2004. Molecular characterization of Borrelia burgdorferi erp promoter/operator elements. J. Bacteriol. 186:2745–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bartosik AA, Lasocki K, Mierzejewska J, Thomas CM, Jagura-Burdzy G. 2004. ParB of Pseudomonas aeruginosa: interactions with its partner ParA and its target parS and specific effects on bacterial growth. J. Bacteriol. 186:6983–6998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beaurepaire C, Chaconas G. 2005. Mapping of essential replication functions of the linear plasmid lp17 of B. burgdorferi by targeted deletion walking. Mol. Microbiol. 57:132–142 [DOI] [PubMed] [Google Scholar]
- 6. Botstein D, Matz MJ. 1970. A recombination function essential to the growth of bacteriophage P22. J. Mol. Biol. 54:417–440 [DOI] [PubMed] [Google Scholar]
- 7. Brissette CA, Bykowski T, Cooley AE, Bowman A, Stevenson B. 2009. Borrelia burgdorferi RevA antigen binds host fibronectin. Infect. Immun. 77:2802–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brissette CA, et al. 2009. Borrelia burgdorferi infection-associated surface proteins ErpP, ErpA, and ErpC bind human plasminogen. Infect. Immun. 77:300–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brissette CA, et al. 2010. The borrelial fibronectin-binding protein RevA is an early antigen of human Lyme disease. Clin. Vac. Immunol. 17:274–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brissette CA, Verma A, Bowman A, Cooley AE, Stevenson B. 2009. The Borrelia burgdorferi outer-surface protein ErpX binds mammalian laminin. Microbiology 155:863–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burns LH, et al. 2010. BpaB, a novel protein encoded by the Lyme disease spirochete's cp32 prophages, binds to erp Operator 2 DNA. Nucleic Acids Res. 38:5443–5455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carlyon JA, Marconi RT. 1998. Cloning and molecular characterization of a multicopy, linear plasmid-carried, repeat motif-containing gene from Borrelia turicatae, a causative agent of relapsing fever. J. Bacteriol. 180:4974–4981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carroll JA, El-Hage N, Miller JC, Babb K, Stevenson B. 2001. Borrelia burgdorferi RevA antigen is a surface-exposed outer membrane protein whose expression is regulated in response to environmental temperature and pH. Infect. Immun. 69:5286–5293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Casjens S, et al. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs of an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:490–516 [DOI] [PubMed] [Google Scholar]
- 15. Casjens S, van Vugt R, Tilly K, Rosa PA, Stevenson B. 1997. Homology throughout the multiple 32-kilobase circular plasmids present in Lyme disease spirochetes. J. Bacteriol. 179:217–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Casjens SR, Eggers CH, Schwartz I. 2010. Borrelia genomics: chromosome, plasmids, bacteriophages and genetic variation, p 27–54 In Samuels DS, Radolf JD. (ed), Borrelia: molecular biology, host interaction and pathogenesis. Calister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 17. Chen WY, Ho JW, Huang JD, Watt RM. 2011. Functional characterization of an alkaline exonuclease and single strand annealing protein from the SXT genetic element of Vibrio cholerae. BMC Mol. Biol. 12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dunn JJ, et al. 1994. Complete nucleotide sequence of a circular plasmid from the Lyme disease spirochete, Borrelia burgdorferi. J. Bacteriol. 176:2706–2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ebersbach G, Gerdes K. 2005. Plasmid segregation mechanisms. Annu. Rev. Genet. 39:453–479 [DOI] [PubMed] [Google Scholar]
- 20. Eggers CH, et al. 2002. Identification of loci critical for replication and compatibility of a Borrelia burgdorferi cp32 plasmid and use of a cp32-based shuttle vector for the expression of fluorescent reporters in the Lyme disease spirochaete. Mol. Microbiol. 43:281–295 [DOI] [PubMed] [Google Scholar]
- 21. Eggers CH, Samuels DS. 1999. Molecular evidence for a new bacteriophage of Borrelia burgdorferi. J. Bacteriol. 181:7308–7313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. El-Hage N, et al. 2001. Surface exposure and protease insensitivity of Borrelia burgdorferi Erp (OspEF-related) lipoproteins. Microbiology 147:821–830 [DOI] [PubMed] [Google Scholar]
- 23. El-Hage N, Stevenson B. 2002. Simultaneous coexpression of Borrelia burgdorferi Erp proteins occurs through a specific, erp locus-directed regulatory mechanism. J. Bacteriol. 184:4536–4543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fraser CM, et al. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580–586 [DOI] [PubMed] [Google Scholar]
- 25. Hellwage J, et al. 2001. The complement regulatory factor H binds to the surface protein OspE of Borrelia burgdorferi. J. Biol. Chem. 276:8427–8435 [DOI] [PubMed] [Google Scholar]
- 26. Hovis KM, et al. 2006. Selective binding of Borrelia burgdorferi OspE paralogs to factor H and serum proteins from diverse animals: possible expansion of the role of OspE in Lyme disease pathogenesis. Infect. Immun. 74:1967–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hudson JM, Fried MG. 1989. A streamlined, low ionic strength DNase1 footprinting reaction. Biotechniques 7:812–814 [PubMed] [Google Scholar]
- 28. Iyer LM, Koonin EV, Aravind L. 2002. Classification and evolutionary history of the single-strand annealing proteins, RecT, Redbeta, ERF and RAD52. BMC Genomics 3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jutras BL, et al. 2012. EbfC (YbaB) is a new type of bacterial nucleoid-associated protein, and a global regulator of gene expression in the Lyme disease spirochete. J. Bacteriol. 194:3395–3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jutras BL, et al. 2012. BpaB and EbfC DNA-binding proteins regulate production of the Lyme disease spirochete's infection-associated Erp surface proteins. J. Bacteriol. 194:778–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalnin K, Stegalkina S, Yarmolinsky M. 2000. pTAR-encoded proteins in plasmid partitioning. J. Bacteriol. 182:1889–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kenedy MR, Akins DR. 2011. The OspE-related proteins inhibit complement deposition and enhance serum resistance of Borrelia burgdorferi, the Lyme disease agent. Infect. Immun. 79:1451–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kraiczy P, Skerka C, Brade V, Zipfel PF. 2001. Further characterization of complement regulator-acquiring surface proteins of Borrelia burgdorferi. Infect. Immun. 69:7800–7809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leblanc B, Moss T. 2009. DNase I footprinting. Methods Mol. Biol. 543:37–47 [DOI] [PubMed] [Google Scholar]
- 35. Liveris D, Mulay V, Sandigursky S, Schwartz I. 2008. Borrelia burgdorferi vlsE antigenic variation is not mediated by RecA. Infect. Immun. 76:4009–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lopes A, Amarir-Bouhram J, Faure G, Petit MA, Guerois R. 2010. Detection of novel recombinases in bacteriophage genomes unveils Rad52, Rad51 and Gp2.5 remote homologs. Nucleic Acids Res. 38:3952–3962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maxam A, Gilbert WS. 1977. A new method for sequencing DNA. Proc. Natl. Acad. Sci. U. S. A. 74:560–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Metts MS, McDowell JV, Theisen M, Hansen PR, Marconi RT. 2003. Analysis of the OspE determinants involved in binding of factor H and OspE-targeting antibodies elicited during Borrelia burgdorferi infection. Infect. Immun. 71:3587–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miller JC. 2005. Example of real-time quantitative reverse transcription-PCR (Q-RT-PCR) analysis of bacterial gene expression during mammalian infection: Borrelia burgdorferi in mouse tissues. Curr. Protoc. Microbiol. 1D.3. [DOI] [PubMed] [Google Scholar]
- 40. Miller JC, et al. 2000. A second allele of eppA in Borrelia burgdorferi strain B31 is located on the previously undetected circular plasmid cp9-2. J. Bacteriol. 182:6254–6258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miller JC, von Lackum K, Babb K, McAlister JD, Stevenson B. 2003. Temporal analysis of Borrelia burgdorferi Erp protein expression throughout the mammal-tick infectious cycle. Infect. Immun. 71:6943–6952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Porcella SF, Fitzpatrick CA, Bono JL. 2000. Expression and immunological analysis of the plasmid-borne mlp genes of Borrelia burgdorferi strain B31. Infect. Immun. 68:4992–5001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Radolf JD, Caimano MJ, Stevenson B, Hu LT. 2012. Of ticks, mice, and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat. Rev. Microbiol. 10:87–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rasimas J, Kar SR, Pegg AE, Fried MG. 2003. Effects of protein and DNA alkylation on complex stability. J. Biol. Chem. 278:7973–7980 [DOI] [PubMed] [Google Scholar]
- 45. Riley SP, et al. 2009. Borrelia burgdorferi EbfC defines a newly-identified, widespread family of bacterial DNA-binding proteins. Nucleic Acids Res. 37:1973–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rodionov O, Lobocka M, Yarmolinsky M. 1999. Silencing of genes flanking the P1 plasmid centromere. Science 283:546–549 [DOI] [PubMed] [Google Scholar]
- 47. Sawitzke JA, et al. 2002. Transcriptional interference by a complex formed at the centromere-like partition site of plasmid P1. J. Bacteriol. 184:2447–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stevenson B, Casjens S, Rosa P. 1998. Evidence of past recombination events among the genes encoding the Erp antigens of Borrelia burgdorferi. Microbiology 144:1869–1879 [DOI] [PubMed] [Google Scholar]
- 49. Stevenson B, El-Hage N, Hines MA, Miller JC, Babb K. 2002. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect. Immun. 70:491–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stevenson B, Miller JC. 2003. Intra- and interbacterial genetic exchange of Lyme disease spirochete erp genes generates sequence identity amidst diversity. J. Mol. Evol. 57:309–324 [DOI] [PubMed] [Google Scholar]
- 51. Stevenson B, et al. 2000. The relapsing fever spirochete Borrelia hermsii contains multiple, antigen-encoding circular plasmids that are homologous to the cp32 plasmids of Lyme disease spirochetes. Infect. Immun. 68:3900–3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stevenson B, Tilly K, Rosa PA. 1996. A family of genes located on four separate 32-kilobase circular plasmids in Borrelia burgdorferi B31. J. Bacteriol. 178:3508–3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stevenson B, Zückert WR, Akins DR. 2001. Repetition, conservation, and variation: the multiple cp32 plasmids of Borrelia species, p 87–100 In Saier MH, García-Lara J. The spirochetes: molecular and cellular biology. Horizon Press, Oxford, United Kingdom [Google Scholar]
- 54. Stewart PE, Chaconas G, Rosa P. 2003. Conservation of plasmid maintenance functions between linear and circular plasmids in Borrelia burgdorferi. J. Bacteriol. 185:3202–3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stewart PE, Thalken R, Bono JL, Rosa P. 2001. Isolation of a circular plasmid region sufficient for autonomous replication and transformation of infectious Borrelia burgdorferi. Mol. Microbiol. 39:714–721 [DOI] [PubMed] [Google Scholar]
- 56. Suck D, Lahm A, Oefner C. 1988. Structure refined to 2 Å of a nicked DNA octanucleotide complex with DNase1. Nature 332:464–468 [DOI] [PubMed] [Google Scholar]
- 57. Vellani TS, Myers RS. 2003. Bacteriophage SPP1 Chu is an alkaline exonuclease in the SynExo family of viral two-component recombinases. J. Bacteriol. 185:2465–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wade JT, Reppas NB, Church GM, Struhl K. 2005. Genomic analysis of LexA binding reveals the permissive nature of the Escherichia coli genome and identifies unconventional target sites. Genes Dev. 19:2619–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wade JT, Struhl K. 2004. Association of RNA polymerase with transcribed regions in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 101:17777–17782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Whetstine CR, Slusser JG, Zückert WR. 2009. Development of a single-plasmid-based regulatable gene expression system for Borrelia burgdorferi. Appl. Environ. Microbiol. 75:6553–6558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Woodman ME. 2008. Direct PCR of intact bacteria (colony PCR). Curr. Protoc. Microbiol. Appendix 3D. [DOI] [PubMed] [Google Scholar]
- 62. Yang W, et al. 2011. Structural and functional insight into the mechanism of an alkaline exonuclease from Laribacter hongkongensis. Nucleic Acids Res. 39:9803–9819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yang X, et al. 1999. Identification, characterization, and expression of three new members of the Borrelia burgdorferi Mlp (2.9) lipoprotein gene family. Infect. Immun. 67:6008–6018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang H, Marconi RT. 2005. Demonstration of cotranscription and 1-methyl-3-nitroso-nitroguanidine induction of a 30-gene operon of Borrelia burgdorferi: evidence that the 32-kilobase circular plasmids are prophages. J. Bacteriol. 187:7985–7995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhang J-R, Hardham JM, Barbour AG, Norris SJ. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89:275–285 [DOI] [PubMed] [Google Scholar]
- 66. Zückert WR. 2007. Laboratory maintenance of Borrelia burgdorferi. Curr. Protoc. Microbiol. 12C:1–10 [DOI] [PubMed] [Google Scholar]
- 67. Zückert WR, Meyer J. 1996. Circular and linear plasmids of Lyme disease spirochetes have extensive homology: characterization of a repeated DNA element. J. Bacteriol. 178:2287–2298 [DOI] [PMC free article] [PubMed] [Google Scholar]