Abstract
The association of transmembrane (TM) helices underlies membrane protein structure and folding. Structural studies of TM complexes are limited by complex stability and the often time-consuming selection of suitable membrane mimics. Here, methodology for the efficient, preparative scale construction of covalent TM complexes and the concomitant high-throughput selection of membrane mimics is introduced. For the employed integrin αIIbβ3 model system, the methodology identified phospholipid bicelles, including their specific composition, as the best membrane mimic. The method facilitates structure determination by NMR spectroscopy as exemplified by the measurement of previously inaccessible residual dipolar couplings and 15N relaxation parameters.
The structure determination of membrane proteins, which account for 20–30% of genes in typical genomes,1 represents an area of active research. A central theme of membrane protein structure and folding is the association of transmembrane (TM) helices.2,3 For example, in the ubiquitous family of integrin cell-adhesion receptors the destabilization of the complex formed between the α and β TM helices leads to receptor activation.4,5 NMR spectroscopy is highly suitable to contribute to the structural and dynamic characterization of TM complexes if a membrane mimic that supports native TM interactions can be identified.6–12 The direct NMR screening of membrane mimics, ranging from organic solvents, detergent micelles, lipid nanodiscs, amphiphols to phospholipid bicelles,6–12 is revealing, but slow and material intensive. In addition, structural studies may be limited by TM complex stability. For example, commonly used membrane protein aligning media such as negatively charged polyacrylamide gels13,14 and G-tetrad DNA liquid crystals15 can dissociate TM complexes as observed for integrin αIIbβ3.4 Monomerdimer exchange kinetics also affect NMR parameter adversely by contributing to transverse relaxation rates, even in the slow exchange limit.16 This hampers NMR studies directly, but also indirectly since sample temperature may need to be chosen to optimize exchange kinetics rather than rotational correlation time. For the bicelle-embedded αIIbβ3 TM complex, the population of dimer resonances in slow exchange with monomer is maximal at 28° C,4 whereas the monomeric subunits can be studied at temperatures of up to 40° C.10,17 Here, methodology for the study of TM complexes is introduced that accelerates the selection of membrane mimics and enhances complex stability.
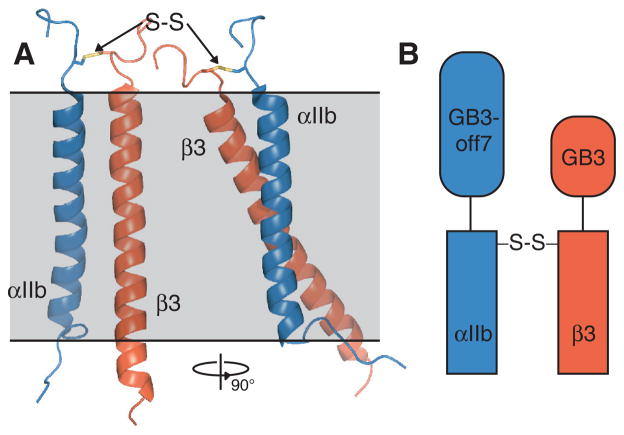
The heterodimeric integrin αIIbβ3 TM complex (Figure 1A) is used as model system for the association of TM helices. Based on the premise that physiological interactions lead to αIIb-β3 association, we hypothesized that the relative suitability of a membrane mimic can be judged by the amount of heterodimer obtainable in its presence. Accordingly, we have developed a high-throughput approach to quantify this parameter as a function of membrane mimic in parallel using microgram protein quantities within one day. To permit simple and rapid detection of αβ dimer quantities, SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) was employed. For this purpose but also to stabilize the complex for NMR studies, αIIb(Ala963Cys) and β3(Gly690Cys) substitutions were introduced that are suitable to covalently cross-link the heterodimer (Figure 1A). In the general case of an unknown TM complex structure, short, flexible linkers can decouple the disulfide linkage from the dimer interface,18 or disulfide linkage sites can be screened for the most efficient cross-linking pattern,5,18 which already provides useful structural information.5 To separate αβ dimer from other possible species (α, β, αα and ββ) according to mass, non-interacting fusion proteins of different sizes were initially used for the 42-residue αIIb and 43-residue β3 peptides (Figure 1B).
Figure 1.
Integrin αIIbβ3 model system. (A) Illustration of αIIb(A963C)-β3(G690C) disulfide linkage in the αIIbβ3 heterodimer. The linkage is modeled on the αIIbβ3 TM complex structure (PDB ID 2K9J)4 using the program Modeller.19 (B) Illustration of fusion proteins used for αIIb and β3 TM peptides.
In vitro, the αIIbβ3 TM complex had been studied in organic solvent,20 detergent micelles,21 and isotropic bicelles.4 Representatives from these systems were evaluated (Figure 2), but any additional membrane mimic may be included in the assay. As outlined in detail in Supporting Information, subsequent to reducing spontaneously formed cystines, disulfide-linked dimers were accumulated for 1 hr in the presence of the hydrophobic oxidant Cu2+· [phenanthroline]2 at protein concentrations of 10 μM and a molar ratio of αIIb:β3:short-chain lipid/detergent:long-chain lipid of 1:1:2000:600 before detection by SDS-PAGE (Figure S1–S2). Under this scheme, disulfide-linked dimer ratios will differ from equilibrium ratios and dimerization in general may be susceptible to unspecific disulfide formation arising from random intermolecular collisions.
Figure 2.
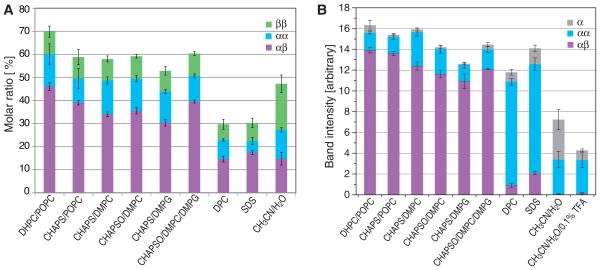
Relative quantities of accumulated αIIbβ3 TM species as a function of membrane mimic. (A) Dimeric species obtained with GB3-off7-αIIb(Ala963Cys) and GB3-β3(Gly690Cys) proteins. The size of each color-coded bar denotes its molar ratio among α, β, αα, ββ and αβ species. For visual clarity, explicit bars were omitted for α and β. (B) Species obtained with GB3-αIIb(Ala963Cys) and β3(Gly690Cys) proteins. The size of each color-coded bar approximates the mass distribution of GB3-tagged αIIb among α, αα and αβ species. Figure S4B depicts the corresponding distribution for β3. In all experiments the molar ratio of αIIb:β3:short-chain lipid/detergent:long-chain lipid was 1:1:2000:600 with protein concentrations of 10 μM. Error bars denote the standard error of the mean of three experiments. Abbreviations are DHPC (1,2-dihexanoyl-sn-glycero-3-phosphocholine), POPC (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol), CHAPS (3-[(cholamidopropyl) dimethyl-ammonio]-1-propane sulfonate), DMPC (1,2-dimyristoyl-sn-glycero-3-phosphocholine), CHAPSO (3-([3-Cholamidopropyl]dimethylammonio)-2-hydroxy-1-propanesulfonate), DMPG (1,2-dimyristoyl-sn-glycero-3-phosphoglycerol), DPC (dodecylphosphocholine), SDS (sodium dodecyl sulfate), and TFA (trifluoroacetic acid).
Quantification of the obtained species from integrated gel band intensities revealed large differences among membrane mimics (Figure 2A). In organic solvent and micelles little preferences between disulfide-linked dimeric species were observed. Bicelles produced the highest molar ratio of covalent αIIbβ3 heterodimer and identified DHPC/POPC as the most suitable membrane mimic. In each reaction, not all monomeric peptide was consumed, which may have arisen from the exhaustion of oxidant, the terminal oxidation of sulfhydryls or hindered sulfhydryl accessibility. Oxidant is not meaningfully exhausted during the reaction (Figure S2B). Terminal sulfhydryl oxidation was determined to occur for 8.6% of e.g. β3 peptides (Figure S3), which left accessibility as an important factor. Membrane proteins often aggregate unspecifically in the presence of unsuitable membrane mimics9 and the inability to consume monomeric peptides is therefore viewed as negative for the suitability of a membrane mimic. However, in the present case, the fusion proteins may also interfere with accessibility. Reactions were therefore repeated with only one fusion protein (GB3) on either the αIIb or β3 subunit. For bicelles, this increased dimerization, especially heterodimerization, to a level of unused monomer that is fully explained by terminal sulfhydryl oxidation (Figure 2B and S4B). For micelles, less monomer also remained; however for αIIb mostly homodimerization was increased, which may explain the direct observation of αIIb homodimers in micelles.22 In organic solvent, heterodimerization remained low. If fusion proteins were changed to the interacting off7-MBP pair,23 no changes compared to the original off7-GB3 combination was observed (Figures S6–S7). This showed that Cys accessibility played an important role in dimerization reactions, making the use of only one fusion protein preferable. Nevertheless, relative heterodimerization levels were overall similar and consistently identified bicelles, specifically DHPC/POPC-based bicelles, as the best membrane mimic for αIIbβ3.
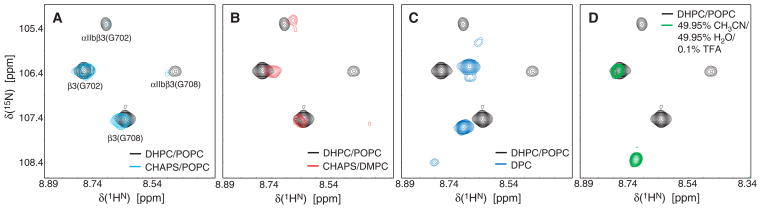
If our initial hypothesis is valid, NMR spectral properties must correlate with the detected heterodimerization efficiencies. Backbone H-N correlation spectra of non-covalently associated 1H/14N-labeled αIIb/2H/15N-labeled β3 TM peptides (without fusion proteins) were thus compared. Spectral quality decreased significantly from DHPC/POPC bicelles to CHAPS/POPC to CHAPS/DMPC bicelles (Figures 3A–B) in correlation with lowered amounts of observed heterodimer (Figures 2 and S4B). For CHAPS/DMPG bicelles, which produced the least heterodimer among bicelles, extremely broad lines were observed (data not shown). These observations are explained by increasing TM complex off-rates, resulting in a shift to intermediate exchange kinetics for the monomer-heterodimer equilibrium. Complex lifetime was therefore a decisive factor in the accumulation of disulfide-linked dimer. The stronger resonance intensities of monomeric relative to dimeric signals (Figure 3A–B) indicated that, at the time of initiating disulfide formation, most peptides had been monomeric (KD > peptide concentration of 100 μM). Heterodimer therefore accumulated over time to be the dominant species in competition to side reactions. In the presence of DPC and SDS micelles, aside from a dominant set of resonances, presumed to correspond to monomeric β3 peptide (Figure S4B), additional relatively broad resonances were observed (Figure 3C and Figure S5). In SDS micelles, the close chemical shift congruence between both sets of resonances suggested an unspecific interaction rather than defined dimerization. In DPC micelles, chemical shifts were better resolved but many peaks exhibited two sets of weak resonances (Figure 3C), which also indicated structural inhomogeneity. For the 50% CH3CN/50% H2O organic solvent mixture, peptide solubility at pH 7.4 was too low to prepare a NMR sample. Spectra of αIIbβ3 TM peptides were reported in this solvent system in the presence of 0.1% trifluoroacetic acid (TFA; see Figure S1 of Yang et al.20).
Figure 3.
H-N correlation spectra of non-covalently associated integrin 1H/14N-labeled αIIb and 2H/15N-labeled β3 TM domains as a function of membrane mimic. (A) Comparison of DHPC/POPC with CHAPS/POPC bicelles at relative contour levels of 1:0.70. In the slow exchange regime, monomeric and heterodimeric resonances are observable4,10 and were observed here for the 15N-labeled β3 subunit as indicated. (B) Comparison of DHPC/POPC with CHAPS/DMPC bicelles at relative contour levels of 1:0.49. (C) Comparison of DHPC/POPC bicelles with DPC micelles at relative contour levels of 1:0.84. (D) Comparison of DHPC/POPC bicelles with 49.95% CH3CN/49.95% H2O/0.1% TFA at relative contour levels of 1:2.52. Peptide concentrations of 0.1 mM were used in all samples with a molar ratio of αIIb:β3:short-chain lipid/detergent:long-chain lipid of 1:1:2000:600. Spectra were recorded using 2H/15N-labeled peptides in 25 mM HEPES·NaOH, pH 7.4 solution at a 1H frequency of 700 MHz at 28 °C.
However, this decreased the pH to 1.8 and consequently will make it unlikely for carboxylate groups such as β3(Asp723), which stabilizes the αIIbβ3 complex,24 to remain deprotonated. One set of relatively intense resonances was observed in this solvent system (Figure 3D). In sum, the relative quantities of obtained covalent αIIbβ3 heterodimer successfully guided the selection of membrane mimics for NMR studies.
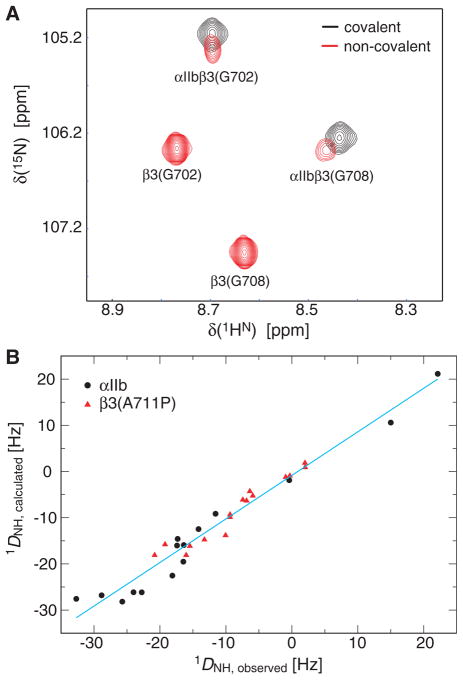
To verify that the introduced disulfide bond did not alter the αIIbβ3 TM complex structure, spectra of covalent and non-covalent dimers were compared in DHPC/POPC bicelles. Close chemical shift congruence was obtained for heterodimeric resonances (Figure 4A), indicating that dimer packing was virtually unchanged. As expected, the resonance lineshapes of covalent dimer surpassed those of non-covalent dimer (Figure 4A). The absence of exchange broadening permitted straightforward 15N relaxation analysis. Specifically, rotational correlation times of monomeric and covalently-linked αIIbβ3 (Table 1) confirmed their oligomeric states in bicelles,10,17 further illustrating that disulfide-linked homooligomers arose from random intermolecular collision in bicelles. To test whether the TM complex could be aligned relative to the magnetic field, the covalently-linked αIIbβ3 TM complex incorporating the recently identified β3(A711P) substitution,25 was immersed in a stretched, negatively charged polyacrylamide gel.13,14 The complex remained associated in the gel matrix at a temperature of 40° C as evidenced by the invariance of chemical shifts (data not shown). The αIIbβ3(A711P) complex structure is unknown at present, but structures of the monomeric subunits are known and conformed well to the measured 1DHN RDC (Figure 4B). This confirmed the absence of significant backbone changes upon heterodimerization.4
Figure 4.
Structural properties of the covalently associated integrin αIIbβ3 TM complex. (A) Comparison of H-N correlation spectra of covalently and non-covalently associated αIIbβ3 TM domains. 1H/14N-labeled αIIb and 2H/15N-β3 TM peptides or 2H/15N-labeled, covalently linked αIIb(Ala963Cys)-β3(Gly690Cys) peptides were reconstituted at concentrations of 0.1 mM each in 200 mM DHPC, 60 mM POPC, 25 mM HEPES·NaOH, pH 7.4. Spectra were recorded at 700 MHz and 28 °C. (B) Residual dipolar coupling (RDC) of the αIIb(A963C)-β3(G690C) cross-linked integrin αIIbβ3(A711P) TM complex at 40°C. Measured 1DNH couplings were fitted to the monomeric αIIb and β3(A711P/K716A) TM structures (PDB ID 2K1A and 2L91),17,25 respectively, to back-calculate 1DNH (R=0.980).
Table 1.
Rotational correlation times, τc, of bicelle-embedded integrin αIIbβ3 TM domains at 35 °C
Isotropic models were adequate for interpreting 15N relaxation rates.26
Measurements of covalently linked αIIbβ3 in bicelles consisting of 350 mM DHPC and b105 mM POPC or c105 mM DMPC. Protein was present at 0.5 mM in 25 mM HEPES·NaOH, pH 7.4.
In conclusion, the present study established a comprehensive, high-throughput approach for membrane mimic selection but also for the optimal preparative production of TM protein complexes. Based on the studied heterodimer formation efficiencies (Figure 2), the αIIbβ3 complex is best reconstituted in DHPC/POPC bicelles. To minimize lipid cost during the production of covalently linked αIIbβ3 on a preparative scale, CHAPS/DMPC bicelles offer an attractive compromise between αIIbβ3 yield and cost. The covalently linked αIIbβ3 complex permitted the acquisition of previously inaccessible dynamic parameter and structural constraints, which significantly improves the study of TM protein complexes. The presented approach will be suited to study larger complexes of membrane proteins if a suitable disulfide bond location can be identified. Finally, we note that the preference of the integrin αIIbβ3 TM complex for phospholipid bicelles will certainly not be universal. For the seven TM helix bundle, G protein-coupled receptor opsin, CHAPS-based bicelles provided higher protein stability than DHPC-based bicelles.27 In addition, micelles are useful membrane mimics for a range of transmembrane systems28,29 as well as diverse membrane-surface associated proteins.30,31
Supplementary Material
Acknowledgments
We thank Ansgar Siemer for critically reading the manuscript. This work is supported by National Institutes of Health (HL089726).
Footnotes
The authors declare no competing financial interest.
Experimental details and supporting figures. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Krogh A, Larsson B, von Heijne G, Sonnhammer ELL. J Mol Biol. 2001;305:567. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 2.Bowie JU. Nature. 2005;438:581. doi: 10.1038/nature04395. [DOI] [PubMed] [Google Scholar]
- 3.White SH, Wimley WC. Annu Rev Biophys Biomolec Struct. 1999;28:319. doi: 10.1146/annurev.biophys.28.1.319. [DOI] [PubMed] [Google Scholar]
- 4.Lau T-L, Kim C, Ginsberg MH, Ulmer TS. EMBO J. 2009;28:1351. doi: 10.1038/emboj.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu J, Luo BH, Barth P, Schonbrun J, Baker D, Springer TA. Mol Cell. 2009;34:234. doi: 10.1016/j.molcel.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raschle T, Hiller S, Etzkorn M, Wagner G. Curr Opin Struct Biol. 2010;20:471. doi: 10.1016/j.sbi.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Popot JL. Annual Review Of Biochemistry, Vol 79; Annual Reviews: Palo Alto. 2010;79:737. doi: 10.1146/annurev.biochem.052208.114057. [DOI] [PubMed] [Google Scholar]
- 8.Shenkarev ZO, Lyukmanova EN, Paramonov AS, Shingarova LN, Chupin VV, Kirpichnikov MP, Blommers MJJ, Arseniev ASJ. Am Chem Soc. 2010;132:5628. doi: 10.1021/ja9097498. [DOI] [PubMed] [Google Scholar]
- 9.Krueger-Koplin RD, Sorgen PL, Krueger-Koplin ST, Rivera-Torres AO, Cahill SM, Hicks DB, Grinius L, Krulwich TA, Girvin MEJ. Biomol NMR. 2004;28:43. doi: 10.1023/B:JNMR.0000012875.80898.8f. [DOI] [PubMed] [Google Scholar]
- 10.Lau T-L, Partridge AP, Ginsberg MH, Ulmer TS. Biochemistry. 2008;47:4008. doi: 10.1021/bi800107a. [DOI] [PubMed] [Google Scholar]
- 11.Kim HJ, Howell SC, Van Horn WD, Jeon YH, Sanders CR. Prog Nucl Magn Reson Spectrosc. 2009;55:335. doi: 10.1016/j.pnmrs.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warschawski DE, Arnold AA, Beaugrand M, Gravel A, Chartrand E, Marcotte I. Biochim Biophys Acta-Biomembr. 2011;1808:1957. doi: 10.1016/j.bbamem.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Ulmer TS, Ramirez BE, Delaglio F, Bax A. J Am Chem Soc. 2003;125:9179. doi: 10.1021/ja0350684. [DOI] [PubMed] [Google Scholar]
- 14.Meier S, Haussinger D, Grzesiek S. J Biomol NMR. 2002;24:351. doi: 10.1023/a:1021609207024. [DOI] [PubMed] [Google Scholar]
- 15.Lorieau J, Yao LS, Bax A. J Am Chem Soc. 2008;130:7536. doi: 10.1021/ja801729f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McConnell HM. J Chem Phys. 1958;28:430. [Google Scholar]
- 17.Lau T-L, Dua V, Ulmer TS. J Biol Chem. 2008;283:16162. doi: 10.1074/jbc.M801748200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ulmer TS, Yaspan B, Ginsberg MH, Campbell ID. Biochemistry. 2001;40:7498. doi: 10.1021/bi010338l. [DOI] [PubMed] [Google Scholar]
- 19.Sali A, Blundell TL. J Mol Biol. 1993;234:779. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 20.Yang J, Ma YQ, Page RC, Misra S, Plow EF, Qin J. Proc Natl Acad Sci U S A. 2009;106:17729. doi: 10.1073/pnas.0909589106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li RH, Babu CR, Lear JD, Wand AJ, Bennett JS, DeGrado WF. Proc Natl Acad Sci U S A. 2001;98:12462. doi: 10.1073/pnas.221463098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Remorino A, Korendovych IV, Wu YB, DeGrado WF, Hochstrasser RM. Science. 2011;332:1206. doi: 10.1126/science.1202997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Binz HK, Amstutz P, Kohl A, Stumpp MT, Briand C, Forrer P, Grutter MG, Pluckthun A. Nat Biotechnol. 2004;22:575. doi: 10.1038/nbt962. [DOI] [PubMed] [Google Scholar]
- 24.Kim C, Lau T-L, Ulmer TS, Ginsberg MH. Blood. 2009;113:4747. doi: 10.1182/blood-2008-10-186551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim C, Schmidt T, Cho E-G, Ye F, Ulmer TS, Gins-berg MH. Nature. 2012;481:209. doi: 10.1038/nature10697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dosset P, Hus JC, Blackledge M, Marion D. J Biomol NMR. 2000;16:23. doi: 10.1023/a:1008305808620. [DOI] [PubMed] [Google Scholar]
- 27.McKibbin C, Farmer NA, Jeans C, Reeves PJ, Khorana HG, Wallace BA, Edwards PC, Villa C, Booth PJ. J Mol Biol. 2007;374:1319. doi: 10.1016/j.jmb.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 28.MacKenzie KR, Prestegard JH, Engelman DM. Science. 1997;276:131. doi: 10.1126/science.276.5309.131. [DOI] [PubMed] [Google Scholar]
- 29.Chill JH, Louis JM, Miller C, Bax A. Protein Sci. 2006;15:684. doi: 10.1110/ps.051954706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao JN, Warren GZ, Estolt-Povedano S, Zammit VA, Ulmer TS. J Biol Chem. 2011;286:42545. doi: 10.1074/jbc.M111.306951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lokappa SB, Ulmer TS. J Biol Chem. 2011;286:21450. doi: 10.1074/jbc.M111.224055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.