Abstract
Interleukin-1β (IL-1β) is an inflammatory cytokine that exerts marked effects on neuronal function and survival. Here we examined the effects of IL-1β on synapses between rat hippocampal neurons in culture using an imaging-based assay to quantify clusters of the scaffolding protein postsynaptic density 95 fused to green fluorescent protein. Treatment with IL-1β for 24 h induced a 23±3 % loss in the number of synaptic sites. Pharmacological studies indicated that synapse loss was mediated by the IL-1 receptor with subsequent activation of two pathways. COX2-mediated prostaglandin production and postsynaptic activation of a Src family tyrosine kinase were required. Presynaptic release of glutamate with subsequent activation of NMDA receptors was necessary for IL-1β-induced synapse loss. Neither Src activation nor prostaglandin E2 (PGE2) application alone was sufficient to reduce the number of synapses. However, in cells expressing constitutively active or pharmacologically activated Src, PGE2 induced synapse loss. Thus, IL-1β reduces the number of synaptic connections by simultaneously activating multiple pathways that require both pre- and post-synaptic activity. These results highlight targets that may prove important for pharmacotherapy of neuroinflammatory disease.
Keywords: Interleukin-1, excitotoxicity, NMDA receptor, prostaglandin E2, Src tyrosine kinase, glutamate release
Introduction
Interleukin-1β (IL-1β) is a pro-inflammatory cytokine (Allan et al., 2005) that contributes to neuronal injury associated with HIV encephalitis (Persidsky et al., 1997), Alzheimer’s disease (McGeer and McGeer, 2011), ischemia (Boutin et al., 2001) and epilepsy (Vezzani et al., 2011). Determining the mechanism of the deleterious in vivo effects of IL-1β has proven difficult because it exerts complex actions that depend on cell type, concentration and duration of exposure (Pinteaux et al., 2009).
IL-1 receptors are located on virtually all CNS cell types, although the neurotoxic actions of IL-1β result primarily from activating receptors on neurons and astrocytes (Allan et al., 2005). The type 1 IL-1 receptor (IL-1R1) is a Toll-like type receptor that couples to several signaling pathways including those mediated by Src family kinases and p38 MAP kinases (Weber et al., 2010). These kinase cascades affect many aspects of neurophysiology including gene expression and the release of both survival promoting and toxic factors (Allan et al., 2005; Fogal and Hewett, 2008). A considerable body of work relates the actions of IL-1β on cell signaling pathways to changes in neuronal survival (Rothwell and Luheshi, 2000; Allan et al., 2005; Fogal and Hewett, 2008). However, the mechanisms leading to neuronal death are not necessarily the same as those that produce dendritic changes and synapse loss (Kim et al., 2008; Waataja et al., 2008). Thus, how activation of pathways implicated in cell death processes contribute to the effects of IL-1β on the integrity of synaptic networks is not known.
IL-1β produces changes in dendrite development (Gilmore et al., 2004), dendritic structure (Li et al., 2003) and synaptic plasticity (Katsuki et al., 1990; Kelly et al., 2003) that are thought to impair network function. For example, IL-1 activation of Src family kinases phosphorylates the NMDA receptor increasing its sensitivity to glutamate (Viviani et al., 2006; Salter et al., 2009). This action likely accounts for IL-1β-induced exacerbation of excitotoxic insults (Rothwell and Luheshi, 2000; Allan et al., 2005; Fogal and Hewett, 2008). IL-1β receptor activation is also known to increase prostaglandin E2 (PGE2) production with effects on glutamate release (Sang et al., 2005; Marty et al., 2008).
Here we examined the effects of IL-1β on the synapses that form between rat hippocampal neurons in culture using an imaging-based assay to quantify clusters of the scaffolding protein postsynaptic density 95 fused to green fluorescent protein (PSD95-GFP). IL-1β produced a marked loss of synaptic connections. The mechanism of loss required both presynaptic glutamate release and postsynaptic NMDA receptor asctivation. The activation of multiple pathways by IL-1β highlights targets that may prove important for pharmacotherapy of neuroinflammatory disease.
Materials and Methods
Materials
Materials were obtained from the following sources: the PSD95-GFP expression vector (pGW1-CMV-PSD95-EGFP) was kindly provided by Donald B. Arnold; constitutively active Src (CA-Src) with the Y527F mutation in pLNCX expression vector was kindly provided by Joan Brugge (Harvard University; Addgene plasmid 13660); the Syn-GFP expression vector (pSynaptophysin-EGFPN1) was kindly provided by Jane Sullivan (University of Washington, Seattle, WA); the expression vector for DsRed2 (pDsRed2-N1; CMV promoter) from Clontech (Mountain View, CA); Dulbecco’s modified Eagle medium (DMEM), fetal bovine serum and horse serum from Invitrogen (Carlsbad, CA); recombinant rat IL-1β and IL1-ra from R&D Systems (Minneapolis, MN); pyrazolopyrimidine 2 (PP2) and PP3 from Calbiochem-Novabiochem Corp. (La Jolla, CA); ω-conotoxin MVIIC (ω-CgTx) from Bachem (Torrence, CA); 6-Isopropoxy-9-oxoxanthene-2-carboxylic acid (AH6809), (4Z)-7-[(rel-1S,2S,5R)-5-((1,1′-Biphenyl-4-yl)methoxy)-2-(4-morpholinyl)-3-oxocyclopentyl]-4-heptenoic acid hemicalcium salt hydrate (AH23848), C2-ceramide, N-[2-(Cyclohexyloxy)-4-nitrophenyl]methanesulfonamide (NS398), 4-(4-Fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole (SB203580), DL-2-amino-5-phosphonopentanoic acid (AP5), dizocilpine (MK801); tetanus toxin (TetTx) and all other reagents from Sigma (St. Louis, MO).
Cell culture
Rat hippocampal neurons were grown in primary culture as described previously (Kim et al., 2008) with minor modifications. Fetuses were removed on embryonic day 17 from maternal rats, anesthetized with CO2, and sacrificed by decapitation. Hippocampi were dissected and placed in Ca2+ and Mg2+-free HEPES-buffered Hanks salt solution (HHSS), pH 7.45. HHSS was composed of the following (in mM): HEPES 20, NaCl 137, CaCl2 1.3, MgSO4 0.4, MgCl2 0.5, KCl 5.0, KH2PO4 0.4, Na2HPO4 0.6, NaHCO3 3.0, and glucose 5.6. Cells were dissociated by trituration through a 5 ml pipette and a flame-narrowed Pasteur pipette, pelleted and re-suspended in DMEM without glutamine, supplemented with 10% fetal bovine serum and penicillin/streptomycin (100 U/ml and 100 μg/ml, respectively). Dissociated cells were then plated at a density of 100,000–200,000 cells/dish onto a 25-mm-round cover glass (#1) glued to cover a 19 mm diameter opening drilled through the bottom of a 35 mm Petri dish. The coverglass was precoated with matrigel (200 μL, 0.2mg/mL). Neurons were grown in a humidified atmosphere of 10% CO2 and 90% air (pH 7.4) at 37 °C, and fed on days 1 and 6 by exchange of 75% of the media with DMEM, supplemented with 10% horse serum and penicillin/streptomycin. Cells used in these experiments were cultured without mitotic inhibitors for at least 12 days, resulting in a mixed glial-neuronal culture. Immunocytochemistry experiments demonstrated that these cultures were composed of 18±2 % neurons, 70 ± 3 % astrocytes and 9 ± 3 % microglia (Kim et al., 2011).
Transfection
Rat hippocampal neurons were transfected between 10 and 13 days in vitro using a modification of a protocol described previously (Kim et al., 2008). Briefly, hippocampal cultures were incubated for at least 20 min in DMEM supplemented with 1 mM kynurenic acid, 10 mM MgCl2, and 5 mM HEPES, to reduce neurotoxicity. A DNA/calcium phosphate precipitate containing 1 μg plasmid DNA per well was prepared, allowed to form for 30 min at room temperature and added to the culture. After a 90 min incubation, cells were washed once with DMEM supplemented with MgCl2 and HEPES and then returned to conditioned media, saved at the beginning of the procedure. The transfection efficiency ranged from 2 to 11%.
Confocal imaging
Transfected neurons were transferred to the stage of a Yokogawa spinning disk confocal microscope (Nikon TE2000-S) and viewed through a 60X oil-immersion objective (NA. 1.40). Images were acquired with a Photometrics Cascade cooled CCD camera (1004 × 1002 pixels) controlled with MetaMorph software (version 7.6.1). Because of the low transfection efficiency, only one cell in the field expressed fluorescent proteins. For experiments in which the same neuron was imaged before and after a 24-h interval, the location of the cell was recorded using the coordinates of a motorized stage on the microscope. Eight optical sections spanning 7 μm in the z-dimension were collected (1 μm steps) and combined through the z-axis into a compressed z stack. A Krypton-Argon laser was used to excite GFP at 488 nm and emission collected at 520 nm (35 nm bandpass). The excitation and emission wavelengths for DsRed2 were 568 nm and 628 nm (40 nm band-pass), respectively. A laser scanning confocal microscope was used to image cells expressing Syn-GFP and DsRed2 as previously described (Kim and Thayer, 2009).
Image processing
To count and label PSD95-GFP and Syn-GFP puncta an automated algorithm was created using MetaMorph 6.2 image processing software described previously (Waataja et al., 2008). Briefly, maximum z-projection images were created from the DsRed2 and GFP image stacks. Next, a threshold was applied to the DsRed2 image to optimize visualization of fine processes. Then a circular top-hat filter (50 pixels) was applied to minimize bloom surrounding the soma. This created a 1-bit image that was used as a mask via a logical AND function with the GFP maximum z-projection. A top-hat filter (80 pixels) was applied to the masked GFP image. A threshold set 1.5 s.d. above the mean intensity inside the mask was then applied to the contrast-enhanced image. Structures between 8 and 80 pixels (approximately 0.37 to 3.12 μm in diameter) were counted as synaptic sites. The structures were then dilated and superimposed on the DsRed2 maximum z-projection for visualization. Fluorescent puncta counts were presented as mean ± s.e.m. where n is the number of cells, each from a separate cover glass over multiple cultures. We used Student’s t-test for single or ANOVA with Bonferroni post-test for multiple statistical comparisons.
Results
IL-1β induced synapse loss requires glutamate release and activation of NMDA receptors
IL-1β is elevated during neuroinflammatory conditions and influences neuronal survival and synaptic transmission. Here we examined the effects of IL-1β on the number of synaptic connections between hippocampal neurons in culture. Changes in the number of synapses were monitored by imaging cells expressing PSD95-GFP and DsRed2 as previously described (Waataja et al., 2008). In Fig. 1A, we show representative images of neurons 48 hrs after transfection with expression plasmids for PSD95-GFP and DsRed2. PSD95-GFP expressed as discrete puncta that contrasted well from diffuse green fluorescence found throughout the cell. Expressed DsRed2 filled the soma and dendrites and was used to track morphological changes, as a mask for image processing and to determine cell viability based on cytoplasmic retention of the fluorescent protein. Image processing identified and counted puncta by locating intensity peaks of the appropriate size (mean diameter = 0.52 μm) in contact with the DsRed2 mask. We demonstrated previously that PSD95-GFP puncta represent functional synapses (Waataja et al., 2008). The PSD95-GFP puncta co-localize with functional presynaptic sites for neurotransmitter release and they co-localize with synaptically driven postsynaptic Ca2+ increases (Waataja et al., 2008). PSD95-GFP puncta co-localize with NMDA receptor immunoreactivity and after treatments that increase the number of puncta the new puncta also co-localize with NMDA receptors (Kim and Thayer, 2009). NMDA-induced loss of PSD95-GFP puncta correlates with loss of evoked EPSC amplitude and proteasome inhibitors protect the number of puncta and EPSC amplitude (Waataja et al., 2008).
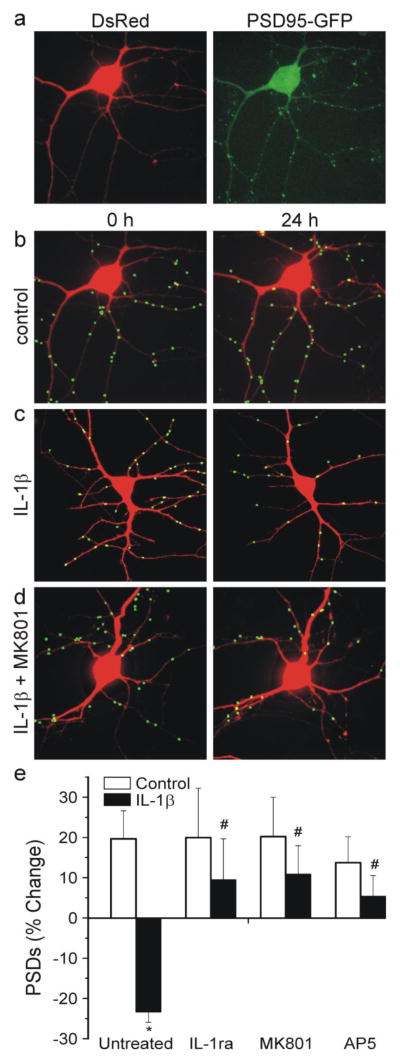
Fig. 1.
Activation of IL-1β receptors induces synapse loss via NMDA receptors. a Confocal fluorescent images display maximum z-projections of neurons expressing PSD95-GFP and DsRed2. Processing of PSD95-GFP images identified PSDs as fluorescent puncta meeting intensity and size criteria and in contact with a mask derived from the DsRed2 image. The images in (a) were processed to produce the 0 h image in (b). b–d, labeled PSDs were dilated and overlaid on the DsRed2 image for visualization purposes. Processed images show cells before (0 h) and 24 h after no treatment (b control), treatment for 24 h with 3 ng/ml IL-1β c, and treatment with 10 μM MK801 15 min prior to and during 24 h treatment with IL-1β d. e Bar graph summarizes the effects of IL-1β on changes in PSD95-GFP puncta after 24 h treatment under control conditions (control, open bars) or following treatment with 3 ng/ml IL-1β (solid bars). Cells were untreated (n=45) or treated with 1 μg/ml IL-1ra (n=12), 10 μM MK801 (n=13) or 300 μM AP5 (n=6) as indicated. Data are mean ± SEM. *p<0.05 relative to control (Student’s t test); #, p<0.05 relative to IL-1β untreated (ANOVA with Bonferroni post-test).
Treatment with 3 ng/ml IL-1β for 24 hr decreased the number of fluorescent puncta by 23±3 %, (n=45) (Fig. 1C). IL-1β treated cells had significantly fewer PSDs than control cells (Fig. 1E). Application of the IL-1β receptor antagonist IL-1ra (1 μg/ml) 15 min prior to and during exposure to IL-1β, completely prevented synapse loss (Fig. 1E). Synapse loss induced by IL-1β was mediated by NMDA receptors because treatment with NMDA receptor antagonists completely blocked synapse loss. As shown in Fig 1D and E, MK801 (10 μM) prevented IL-1β-induced synapse loss. Interestingly, the competitive antagonist AP5 (300 μM) also protected from synapse loss when applied prior to and during IL-1β exposure. The protection afforded by the competitive antagonist AP5 suggests that IL-1β evoked-synapse loss requires glutamate mediated activation of NMDA receptors.
We next examined the role of presynaptic glutamate release in IL-1β induced synapse loss using inhibitors of presynaptic voltage-gated Ca2+ channels (VGCCs) and vesicular release. ω-conotoxin MVIIC inhibits presynaptic VGCCs required for glutamatergic synaptic transmission. In cultures pretreated for 15 min with 1 μM ω-conotoxin MVIIC, IL-1β failed to induce synapse loss (Fig. 2B). Tetanus toxin (TetTx) inhibits glutamate release by enzymatic cleavage of vesicle associated membrane proteins to prevent vesicle fusion. We treated cultures for 24 h with 0.3 μg/ml TetTx and then examined the effects of IL-1β on synaptic number. In cultures treated with TetTx, IL-1β failed to induce synapse loss. Thus, excitatory synaptic transmission is required for IL-1β-induced NMDA receptor activation.
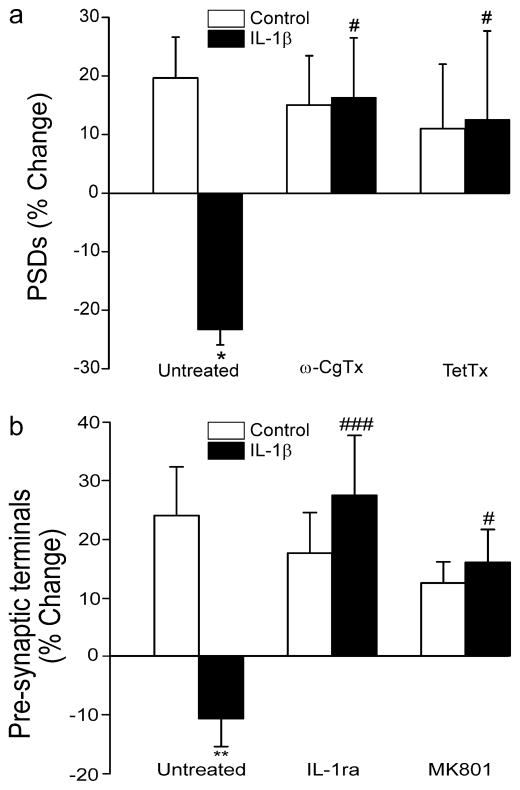
Fig. 2.
IL-1β requires glutamate release to evoke synapse loss. a–b Bar graphs summarize the effects of IL-1β on changes in PSD95-GFP (a) or Syn-GFP (b) puncta after 24 h treatment under control conditions (control, open bars) or following treatment with 3 ng/ml IL-1β (solid bars). a Cells expressing PSD95-GFP were untreated (n=45) or pretreated with 1 μM ω-conotoxin MVIIC (ω-CgTx; n=9) for 15 min or 0.3 μg/ml tetanus toxin (TetTx; n=5) for 24 h as indicated. b Cells expressing Syn-GFP were untreated (n=21) or treated with 1 μg/ml IL-1ra (n=10) or 10 μM MK801 (n=11) as indicated. Data are mean ± SEM. *p<0.05, **p<0.01 relative to control (Student’s t test); #, p<0.05, ### p<0.001 relative to IL-1β untreated (ANOVA with Bonferroni post-test).
The loss of PSDs following treatment with IL-1β suggested that presynaptic terminals might also be lost. We counted presynaptic terminals using an approach similar to that described for quantifying PSDs. Hippocampal cultures were transfected with a vector to express the presynaptic marker synaptophysin fused to GFP (Syn-GFP) as previously described (Kim and Thayer, 2009). Treatment with 3 ng/ml IL-1β for 24 hr decreased the number of Syn-GFP puncta by 11±5 %, (n=21; p<0.01) (Fig. 2B). Application of the IL-1β receptor antagonist IL-1ra (1 μg/ml) 15 min prior to and during exposure to IL-1β, completely prevented synapse loss (Fig. 2B). Consistent with the idea that IL-1β produces parallel pre- and post-synaptic loss by over activation of NMDA receptors, treatment with the NMDA receptor antagonist MK801 (10 μM) completely blocked the loss of Syn-GFP fluorescent puncta.
IL-1β-induced synapse loss requires activation of the Src and p38 MAP kinase pathways
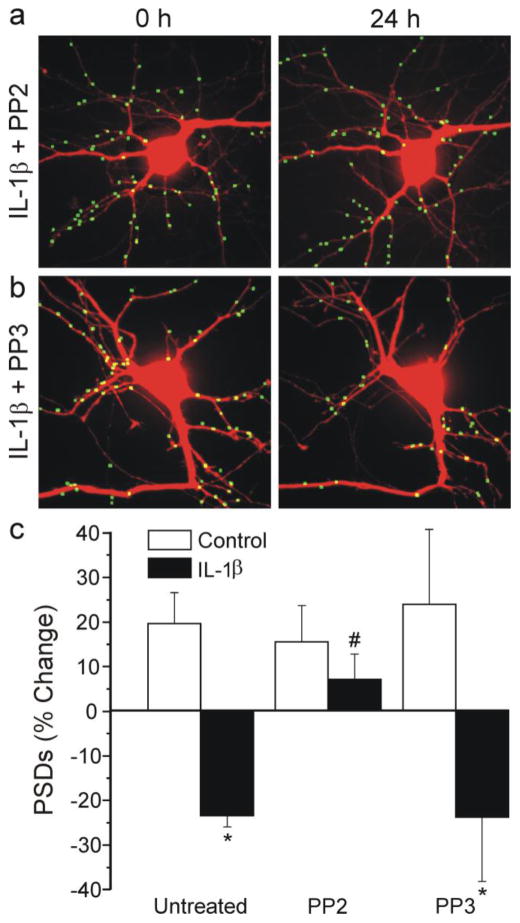
The IL-1β receptor activates Src family kinases in hypothalamic neurons via N-sphingomyelinase-mediated ceramide production (Sanchez-Alavez et al., 2006). NMDA receptors are sensitized to glutamate following phosphorylation by Src family kinases (Viviani et al., 2006; Salter et al., 2009). Thus, we hypothesized that the activity of a Src family kinase might be required for IL-1β-induced synapse loss. We treated hippocampal cultures with the Src family kinase inhibitor PP2 or its inactive analog PP3. As shown in Fig 3A, 10 μM PP2 completely blocked IL-1β-induced synapse loss. In contrast, PP3 had no effect (Fig. 3B). Thus, PP2 afforded significant protection relative to its inactive analog (Fig. 3C). These data are consistent with the hypothesis that sensitization of the NMDA receptor is required for IL-1β-induced synapse loss.
Fig. 3.
IL-1β evokes synapse loss via activation of a Src family kinase. a–b Images displaying PSDs were processed as described in Methods. Images show PSDs before (0 h) and 24 h after treatment with 3 ng/ml IL-1β in the presence of 10 μM PP2 (a) or PP3 (b). c Bar graph summarizes the effects of IL-1β on changes in PSD95-GFP puncta after 24 h treatment under control conditions (control, open bars) or following treatment with 3 ng/ml IL-1β (solid bars). Cells were untreated (n=45) or treated with 10 μM PP2 (n=14) or 10 μM PP3 (n=6) as indicated. Data are mean ± SEM. *p<0.05 relative to control (Student’s t test); #, p<0.05 relative to IL-1β untreated (ANOVA with Bonferroni post-test).
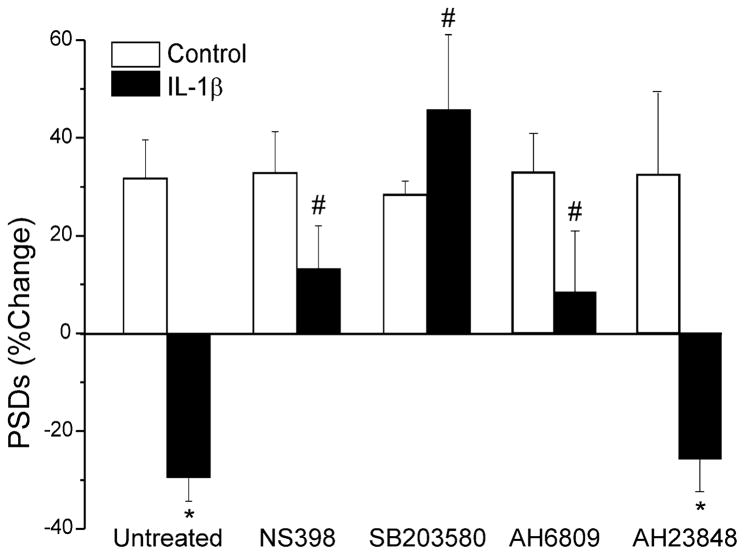
Activation of the IL-1β receptor is also known to increase COX-2 expression via p38 MAPK (Weber et al., 2010). Pretreating the culture with the COX-2 inhibitor NS398 (20 μM) 15 min prior to and during exposure to IL-1β completely prevented synapse loss (Fig. 4). Treating with the MAPK inhibitor, SB203580 (50 μM) also completely prevented IL-1β induced synapse loss (Fig. 4). These data suggested that prostaglandins might play a role in synapse loss. IL-1β has been shown to increase prostaglandin E2 (PGE2) in hippocampal cultures via both direct actions on neurons (Sang et al., 2005; Marty et al., 2008) and by acting on astrocytes (Hartung et al., 1989). Indeed, the EP1–2 receptor antagonist AH6809 (10 μM) prevented IL-1β-induced synapse loss (Fig. 4). In contrast, the EP4 antagonist, AH23848 (10 μM), did not affect the IL-1β response.
Fig. 4.
IL-1β induced synapse loss requires prostaglandin production. Bar graph summarizes the effects of IL-1β on changes in PSD95-GFP puncta after 24 h treatment under control conditions (control, open bars) or following treatment with 3 ng/ml IL-1β (solid bars). Cells were untreated (n=45) or treated with 20 μM NS398 (n=9), 50 μM SB203580 (n=7), 10 μM AH6809 (n=10) or 10 μM AH23848 (n=11) as indicated. Data are mean ± SEM. *p<0.05 relative to control (Student’s t test); #, p<0.05 relative to IL-1β untreated (ANOVA with Bonferroni post-test).
Simultaneous activation of Src and PGE2 pathways is required for IL-1β-induced synapse loss
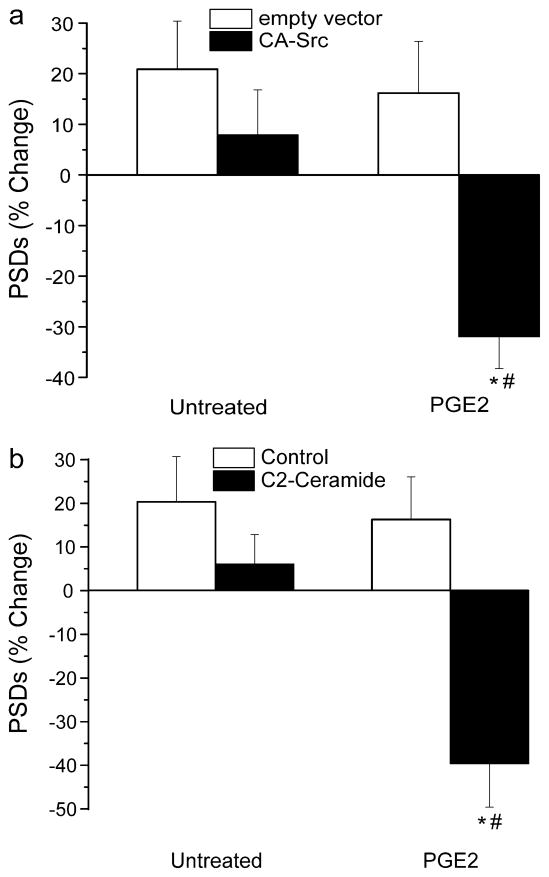
We next determined whether PGE2 induced synapse loss. Treatment with PGE2 (10 μM) did not affect synaptic number when applied alone (Fig. 5A). However, in cells expressing constitutively active Src (CA-Src), PGE2 induced synapse loss comparable to that evoked by IL-1β (Fig. 5A). Cells expressing CA-Src for 48 h exhibited reduced survival and displayed morphological changes that included swelling of the soma and increased dendritic arborization. Thus, these experiments were performed 24 h after transfection. We also employed a pharmacological approach that did not require prolonged exposure to elevated Src activity prior to IL-1β application. The sphingomyelin pathway is known to participate in IL-1β signaling (Sanchez-Alavez et al., 2006) and C2-ceramide activates Src. As shown in Figure 5B, C2-ceramide had no effect on synapse number when applied alone. However, when applied in combination with PGE2 marked synapse loss occurred. Thus, the COX-2 product PGE2 evoked synapse loss only with coincident Src kinase activation.
Fig. 5.
Synapse loss requires coincident activation of the EP2 receptor and Src kinase. a Bar graph summarizes the changes in synapse number in cells transfected with empty vector (open bars) or a CA-Src expression plasmid (solid bars) following 24 h exposure to no treatment (untreated, n=9) or treatment with 10 μM PGE2 (n=11). *p<0.05 relative to empty vector (Student’s t test); #, p<0.05 relative to untreated CA-Src (ANOVA with Bonferroni post-test). b Bar graph summarizes the changes in synapse number in cells under control conditions (control, open bars) or following treatment with 10 μM C2-ceramide (solid bars) following 24 h exposure to no additional treatment (untreated, n=4) or treatment with 10 μM PGE2 (n=13). *p<0.05 relative to control (Student’s t test); #, p<0.05 relative to C2-ceramide untreated (ANOVA with Bonferroni post-test).
Discussion
IL-1β significantly reduced the number of synaptic connections between hippocampal neurons. Loss of synapses required the activation of two signaling pathways downstream from the IL-1 receptor (Fig. 6). IL-1β activated a COX-2 pathway previously established in both neurons (Sang et al., 2005; Marty et al., 2008) and astrocytes (Hartung et al., 1989) and a Src tyrosine kinase pathway (Viviani et al., 2006;(Weber et al., 2010). The Src and COX-2 pathways were both necessary for IL-1β-induced synapse loss, but activation of either pathway alone was not sufficient to induce loss. Pre- and post-synaptic activity were required to produce sufficient stimulation of NMDA receptors to induce the network to adapt to the presence of inflammatory cytokine. We speculate that activation of the COX2 pathway increases glutamate release (Sang et al., 2005) and that Src activation sensitizes NMDA receptors to glutamate (Salter et al., 2009). Figure 6 displays a model that incorporates the results from this study with published studies.
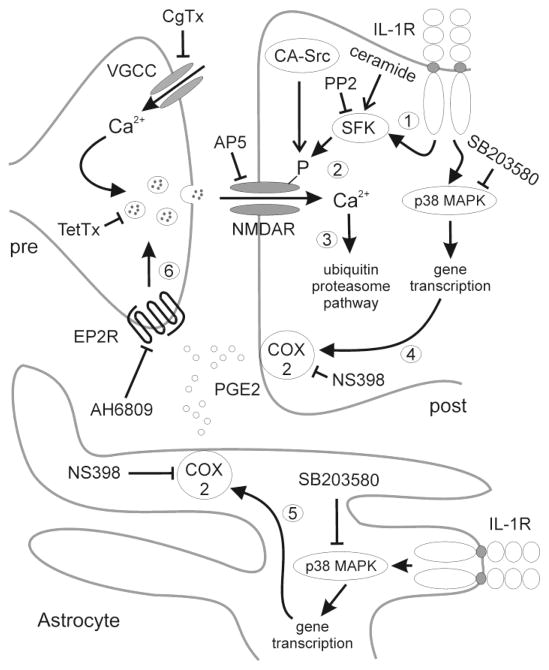
Fig. 6.
Schematic displays hypothesized pre- and post-synaptic signaling pathways activated by IL-1β to induce synapse loss. Blunt lines and arrows, respectively denote conclusions based on the inhibitory and activating treatments tested in this study. Aspects of the model based on published reports are labeled numerically. 1) IL-1R activation results in the activation of Src family kinases (SFK) (Viviani et al., 2006;(Weber et al., 2010). 2) SFKs phosphorylate and increase the sensitivity of NMDA receptors to glutamate (Salter et al., 2009). 3) Increased Ca2+ influx via NMDA receptors activates the ubiquitin-proteasome pathway to produce synapse loss (Kim et al., 2008; Waataja et al., 2008). IL1-R activation in neurons (4) (Samad et al., 2001) and astrocytes (5) (Hartung et al., 1989) leads to p38 MAPK mediated transcription of the COX2 gene. 6) PGE2 activation of presynaptic EP2 receptors increases the release of glutamate (Sang et al., 2005).
The signaling pathways that mediate the IL-1β induced synapse loss described here are consistent with signaling cascades known to be activated by the IL-1 receptor. The activated IL-1 receptor forms a signaling complex that activates the p38 MAPK pathway and the AP-1 transcription factor to increase the expression of proinflammatory genes including COX-2 (Weber et al., 2010). IL-1β up-regulates COX-2 in neuronal cells (Samad et al., 2001) and in astrocytes (Hartung et al., 1989), consistent with the prevention of synapse loss by the COX-2 inhibitor NS398. In hippocampal cultures PGE2 increases the frequency of miniature excitatory postsynaptic currents (Sang et al., 2005), a presynaptic effect that is consistent with the prevention of synapse loss by the EP2 receptor antagonist AH6809 and the presynaptic toxins TetTx and CgTx. Astrocytes respond to IL-1β with increases in glutamate release (Sama et al., 2008), although the sensitivity to toxins described here suggests that presynaptic terminals were the source of glutamate for inducing synapse loss. Glutamate released from astrocytes may play a more prominent role in activating cell death pathways via extrasynaptic NMDA receptors (D’Ascenzo et al., 2007; Gouix et al., 2009). IL-1 receptor activation stimulates N-sphingomyelinase to increase ceramide production with subsequent activation of Src in hypothalamic neurons (Sanchez-Alavez et al., 2006). Src phosphorylation of the NMDA receptor sensitizes the receptor to glutamate and increases its cell surface expression (Salter et al., 2009). We found that the Src family kinase inhibitor PP2 prevented IL-1β-induced synapse loss. PGE2 did not induce synapse loss when applied alone to the hippocampal cultures. Only when postsynaptic Src was also activated by expression of CA-Src or by simultaneous application of the Src activating ligand C2-ceramide did PGE2 induce synapse loss. Thus our data, when considered with previous reports (Sang et al., 2005; Salter et al., 2009), are consistent with a model in which IL-1β-induced a coincident increase in glutamate release via the prostaglandin pathway and sensitization of postsynaptic NMDA receptors via the Src pathway to evoke synapse loss.
The loss of excitatory synapses may be a mechanism to protect from over stimulation (Kim et al., 2008). Excessive loss of synaptic connections is deleterious to network function, but this may be a necessary cost for the improved survival afforded by reduced excitation. The timing and concentration of IL-1β relative to insult determine whether it exacerbates or attenuates neurotoxicity (Fogal and Hewett, 2008). Thus, synapse loss may underlie the protective actions of IL-1β (Carlson et al., 1999) whereas sustained, strong activation of NMDA receptors may contribute to the toxic effects of IL-1β (Allan et al., 2005). We have shown previously that the release of IL-1β mediates synapse loss and neuronal death evoked by the HIV envelope protein gp120 (Kim et al., 2011). In that study, we demonstrated that both processes required NMDA receptor activation. However, the ubiquitin proteasome pathway mediated synapse loss and death was induced by nitric oxide synthase. Synapse loss preceded cell death by at least 24 h. Thus, IL-1β activates two pathways via the NMDA receptor: one improves survival at the cost of impaired network function and the other leads to cell death.
The activation of IL-1 receptors on hypothalamic neurons mediates the fever response that accompanies host response to infection (Sanchez-Alavez et al., 2006) and contributes to the synaptic activation that underlies some forms of epilepsy (Vezzani et al., 2011). IL-1β is required for long-term potentiation, a strengthening of excitatory synapses that participates in memory formation (Schneider et al., 1998). The IL-1β-induced synapse loss described here may act to reduce pathological increases in synaptic activity that accompany the inflammatory response and counteract physiological increases in synaptic strength to protect from excitotoxicity that results from over stimulation of glutamatergic synaptic networks.
NMDA induced synapse loss is reversible (Waataja et al., 2008). Thus, we hypothesize that the synapse loss induced by IL-1β is not part of the agonal event but is instead a coping mechanism. Clearly transient impairment of the network is preferable to neuronal death. However, in the context of chronic neuroinflammatory conditions such as NeuroAIDS, synapse loss may contribute to cognitive decline (Sa et al., 2004). The compensation for excess excitation afforded by synapse loss might also reduce the ability of the network to cope with additional stressors. Indeed evidence suggests that elderly HIV patients are at increased risk for developing Alzheimer’s disease pathology and cognitive impairment (Jayadev and Garden, 2009).
We conclude that IL-1β-induced loss of synaptic connections requires coincident pre- and post-synaptic mechanisms. We hypothesize that a COX-2 pathway increased glutamate release and a Src family kinase sensitized NMDA receptors to glutamate. This novel mechanism may play a more prominent role in synapse loss relative to neuronal death induced by IL-1β. IL-1β mediated changes in synaptic number enable excitatory networks to adapt to neuroinflammatory conditions, suggesting that synapse loss associated with cognitive decline in neurodegenerative disorders may be a neuroprotective mechanism gone awry. These findings provide a foundation for developing drugs that might improve function in neurodegenerative disease.
Acknowledgments
The National Institute on Drug Abuse (grants DA07304 and DA11806) and the National Science Foundation (grant IOS0814549) supported this work.
Footnotes
Disclaimers: none
Contributor Information
Anjuli Mishra, Department of Pharmacology, University of Minnesota Medical School, 321 Church Street SE, 6-120 Jackson Hall, Minneapolis, MN 55455, USA.
Hee Jung Kim, Department of Pharmacology, University of Minnesota Medical School, 321 Church Street SE, 6-120 Jackson Hall, Minneapolis, MN 55455, USA. Department of Physiology, College of Medicine, Dankook University, San#29, Anseo-dong, Dongnam-gu, Cheonan-si, Chungnam, 330-714, Korea.
Angela H. Shin, Department of Pharmacology, University of Minnesota Medical School, 321 Church Street SE, 6-120 Jackson Hall, Minneapolis, MN 55455, USA
Stanley A. Thayer, Email: sathayer@umn.edu, Department of Pharmacology, University of Minnesota Medical School, 321 Church Street SE, 6-120 Jackson Hall, Minneapolis, MN 55455, USA; phone: (612) 626-7049; fax: (612) 625-8408
References
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- Boutin H, LeFeuvre RA, Horai R, Asano M, Iwakura Y, Rothwell NJ. Role of IL-1alpha and IL-1beta in ischemic brain damage. J Neurosci. 2001;21:5528–5534. doi: 10.1523/JNEUROSCI.21-15-05528.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson NG, Wieggel WA, Chen J, Bacchi A, Rogers SW, Gahring LC. Inflammatory cytokines IL-1 alpha, IL-1 beta, IL-6, and TNF-alpha impart neuroprotection to an excitotoxin through distinct pathways. J Immunol. 1999;163:3963–3968. [PubMed] [Google Scholar]
- D’Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP, Moss SJ, Haydon PG. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci U S A. 2007;104:1995–2000. doi: 10.1073/pnas.0609408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogal B, Hewett SJ. Interleukin-1beta: A Bridge between Inflammation and Excitotoxicity? J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05315.x. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Fredrik Jarskog L, Vadlamudi S, Lauder JM. Prenatal infection and risk for schizophrenia: IL-1beta, IL-6, and TNFalpha inhibit cortical neuron dendrite development. Neuropsychopharmacol. 2004;29:1221–1229. doi: 10.1038/sj.npp.1300446. [DOI] [PubMed] [Google Scholar]
- Gouix E, Leveille F, Nicole O, Melon C, Had-Aissouni L, Buisson A. Reverse glial glutamate uptake triggers neuronal cell death through extrasynaptic NMDA receptor activation. Mol Cell Neurosci. 2009;40:463–473. doi: 10.1016/j.mcn.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Hartung HP, Schafer B, Heininger K, Toyka KV. Recombinant interleukin-1 beta stimulates eicosanoid production in rat primary culture astrocytes. Brain Res. 1989;489:113–119. doi: 10.1016/0006-8993(89)90013-9. [DOI] [PubMed] [Google Scholar]
- Jayadev S, Garden G. Host and Viral Factors Influencing the Pathogenesis of HIV-Associated Neurocognitive Disorders. Journal of Neuroimmune Pharmacology. 2009;4:175–189. doi: 10.1007/s11481-009-9154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki H, Nakai S, Hirai Y, Akaji K, Kiso Y, Satoh M. Interleukin-1 beta inhibits long-term potentiation in the CA3 region of mouse hippocampal slices. Eur J Pharmacol. 1990;181:323–326. doi: 10.1016/0014-2999(90)90099-r. [DOI] [PubMed] [Google Scholar]
- Kelly A, Vereker E, Nolan Y, Brady M, Barry C, Loscher CE, Mills KH, Lynch MA. Activation of p38 plays a pivotal role in the inhibitory effect of lipopolysaccharide and interleukin-1 beta on long term potentiation in rat dentate gyrus. J Biol Chem. 2003;278:19453–19462. doi: 10.1074/jbc.M301938200. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Thayer SA. Lithium increases synapse formation between hippocampal neurons by depleting phosphoinositides. Mol Pharmacol. 2009;75:1021–1030. doi: 10.1124/mol.108.052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Martemyanov KA, Thayer SA. Human Immunodeficiency Virus Protein Tat Induces Synapse Loss via a Reversible Process That Is Distinct from Cell Death. J Neurosci. 2008;28:12604–12613. doi: 10.1523/JNEUROSCI.2958-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Shin AH, Thayer SA. Activation of Cannabinoid Type 2 Receptors Inhibits HIV-1 Envelope Glycoprotein gp120-Induced Synapse Loss. Mol Pharmacol. 2011;80:357–366. doi: 10.1124/mol.111.071647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Liu L, Barger SW, Griffin WST. Interleukin-1 Mediates Pathological Effects of Microglia on Tau Phosphorylation and on Synaptophysin Synthesis in Cortical Neurons through a p38-MAPK Pathway. J Neurosci. 2003;23:1605–1611. doi: 10.1523/JNEUROSCI.23-05-01605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty V, El Hachmane M, Amedee T. Dual modulation of synaptic transmission in the nucleus tractus solitarius by prostaglandin E2 synthesized downstream of IL-1beta. Eur J Neurosci. 2008;27:3132–3150. doi: 10.1111/j.1460-9568.2008.06296.x. [DOI] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. Neuroinflammation in Alzheimer’s Disease and Mild Cognitive Impairment: A Field in Its Infancy. Journal of Alzheimer’s Disease. 2011;19:355–361. doi: 10.3233/JAD-2010-1219. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Buttini M, Limoges J, Bock P, Gendelman HE. An analysis of HIV-1-associated inflammatory products in brain tissue of humans and SCID mice with HIV-1 encephalitis. J Neurovirol. 1997;3:401–416. doi: 10.3109/13550289709031186. [DOI] [PubMed] [Google Scholar]
- Pinteaux E, Trotter P, Simi A. Cell-specific and concentration-dependent actions of interleukin-1 in acute brain inflammation. Cytokine. 2009;45:1–7. doi: 10.1016/j.cyto.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Luheshi GN. Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 2000;23:618–625. doi: 10.1016/s0166-2236(00)01661-1. [DOI] [PubMed] [Google Scholar]
- Sa MJ, Madeira MD, Ruela C, Volk B, Mota-Miranda A, Paula-Barbosa MM. Dendritic changes in the hippocampal formation of AIDS patients: a quantitative Golgi study. Acta Neuropathol (Berl) 2004;107:97–110. doi: 10.1007/s00401-003-0781-3. [DOI] [PubMed] [Google Scholar]
- Salter M, YD, LVK, XJL, GP . Regulation of NMDA Receptors by Kinases and Phosphatases. In: Van Dongen AMe., editor. Biology of the NMDA Receptor. Boca Raton (FL): CRC Press; 2009. [PubMed] [Google Scholar]
- Sama MA, Mathis DM, Furman JL, Abdul HM, Artiushin IA, Kraner SD, Norris CM. Interleukin-1beta-dependent signaling between astrocytes and neurons depends critically on astrocytic calcineurin/NFAT activity. J Biol Chem. 2008;283:21953–21964. doi: 10.1074/jbc.M800148200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- Sanchez-Alavez M, Tabarean IV, Behrens MM, Bartfai T. Ceramide mediates the rapid phase of febrile response to IL-1beta. Proc Natl Acad Sci U S A. 2006;103:2904–2908. doi: 10.1073/pnas.0510960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang N, Zhang J, Marcheselli V, Bazan NG, Chen C. Postsynaptically synthesized prostaglandin E2 (PGE2) modulates hippocampal synaptic transmission via a presynaptic PGE2 EP2 receptor. J Neurosci. 2005;25:9858–9870. doi: 10.1523/JNEUROSCI.2392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H, Pitossi F, Balschun D, Wagner A, del Rey A, Besedovsky HO. A neuromodulatory role of interleukin-1beta in the hippocampus. Proc Natl Acad Sci U S A. 1998;95:7778–7783. doi: 10.1073/pnas.95.13.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, Maroso M, Balosso S, Sanchez MA, Bartfai T. IL-1 receptor/Toll-like receptor signaling in infection, inflammation, stress and neurodegeneration couples hyperexcitability and seizures. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Viviani B, Gardoni F, Bartesaghi S, Corsini E, Facchi A, Galli CL, Di Luca M, Marinovich M. Interleukin-1beta Released by gp120 Drives Neural Death through Tyrosine Phosphorylation and Trafficking of NMDA Receptors. J Biol Chem. 2006;281:30212–30222. doi: 10.1074/jbc.M602156200. [DOI] [PubMed] [Google Scholar]
- Waataja JJ, Kim HJ, Roloff AM, Thayer SA. Excitotoxic loss of post-synaptic sites is distinct temporally and mechanistically from neuronal death. J Neurochem. 2008;104:364–375. doi: 10.1111/j.1471-4159.2007.04973.x. [DOI] [PubMed] [Google Scholar]
- Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal. 2010;3:cm1. doi: 10.1126/scisignal.3105cm1. [DOI] [PubMed] [Google Scholar]