Abstract
Background
Major depressive disorder as well as the use of serotonin reuptake inhibitors in pregnancy have been associated with preterm birth. Studies that have attempted to separate effects of illness from treatment have been inconclusive. We sought to explore the separate effects of serotonin reuptake inhibitor use and major depressive episodes in pregnancy on risk of preterm birth.
Methods
We conducted a prospective cohort study of 2793 pregnant women, oversampled for a recent episode of major depression or use of a serotonin reuptake inhibitor. We extracted data on birth outcomes from hospital charts and used binary logistic regression to model preterm birth (<37 weeks’ gestation). We used ordered logistic regression to model early (<34 weeks’ gestation) or late (34-36 weeks) preterm birth, and we used nominal logistic regression to model preterm birth antecedents (spontaneous preterm labor/preterm premature rupture of membranes/preterm for medical indications/term).
Results
Use of a serotonin reuptake inhibitor, both with (odds ratio=2.1 [95% confidence interval=1.0—4.6]) and without (1.6=[1.0—2.5]) a major depressive episode, was associated with preterm birth. A major depressive episode without serotonin reuptake inhibitor use (1.2; [0.68—2.1]) had no clear effect on preterm risk. None of these exposures was associated with early preterm birth. Use of serotonin reuptake inhibitors in pregnancy was associated with increases in spontaneous but not medically indicated preterm birth.
Conclusions
Serotonin reuptake inhibitor use increased risk of preterm birth. Although the effect of a major depressive episode alone was unclear, symptomatic women undergoing antidepressant treatment had elevated risk.
A number of prior investigations,1-5,6 ,7-10 but not all,11-13 find that use of serotonin reuptake inhibitor medication in pregnancy is associated with preterm birth. Other research14-19 finds that depressive symptoms or a major depressive episode in pregnancy increases the risk of preterm birth, although there is substantial disagreement in the literature.9,20-28 The few pregnancy studies that have assessed both serotonin reuptake inhibitor exposure and a major depressive episode have been either small9,28 or based on registry data for diagnoses and were less precise.29 Furthermore, no studies have explored the effects of a major depressive episode in pregnancy or use of a serotonin reuptake inhibitor on risk of early preterm birth (<34 weeks gestation), a more worrisome birth outcome than late preterm birth.
There are few investigations of the antecedents to preterm birth among women who experience an episode of major depression20 or who use a serotonin reuptake inhibitor in pregnancy.5,24 Preterm birth can be classified as subsequent to spontaneous preterm labor, subsequent to preterm premature rupture of membranes, or for fetal/maternal indications. Registry studies5,10 have suggested an association between the use of serotonin reuptake inhibitors and premature rupture of membranes, while a cohort study found that psychotropic medication use in pregnancy was associated with medically indicated but not spontaneous birth.24 A clear link between exposures and antecedents could help illuminate possible biologic mechanisms of preterm birth.
We conducted a prospective cohort study to explore the associations of a major depressive episode and use of serotonin reuptake inhibitor medication in pregnancy with risk for preterm birth or early preterm birth. Our a priori hypothesis was that serotonin reuptake inhibitors and major depressive episodes in pregnancy independently increase risk for both preterm and early preterm birth. As an exploratory analysis, we also examined associations between major depressive episodes, serotonin reuptake inhibitor medication, and antecedents of preterm birth.
METHODS
Study Design
Methods for this study have been described previously.30 This prospective cohort study was conducted to explore the relationship between a major depressive episode and antidepressant treatment in pregnancy as risk factors for preterm birth. Pregnant women were enrolled between March 2005 and May 2009; follow-up continued until September 2009. Yale University School of Medicine, as well as affiliated hospitals, provided human subjects approval for the study.
Inclusion/Exclusion Criteria
Respondents were eligible if they were at least 18 years of age, had not yet reached their 17th week of pregnancy and were willing to provide informed consent. We deemed women ineligible if they: (1) had a known multi-fetal pregnancy, (2) suffered from insulin-dependent diabetes, (3) did not speak English or Spanish, (4) did not have access to a telephone, (5) had plans to relocate, or (6) intended to terminate their pregnancy.
Recruitment and Assessment Procedures
We recruited participants from 137 obstetrical practices and hospital-based clinics throughout Connecticut and Western Massachusetts. While attending an early prenatal visit, pregnant patients were given a letter inviting them to participate. Signed letters were delivered to the central data collection site, from which research staff contacted potential participants by phone and obtained consent for screening. Staff administered a structured screening questionnaire that collected information about pregnancy dates; current mood (depressed, sad or discouraged, or loss of interest in usual activities); history of depressive episodes; antidepressant treatment; presence of diabetes or a multi-fetal gestation; and plans to relocate or terminate the pregnancy. We invited every potentially eligible woman who had either used an antidepressant or experienced a major depressive episode in the last five years to participate. We also randomly selected one-third of potentially eligible women without these characteristics, and invited them to participate in the study. We obtained written consent for the interviews and medical record review during initial home interviews that occurred before completion of the first 17 weeks of gestation. We re-interviewed participants by phone at 28 (± 2) weeks’ gestation (“monitoring” interview) and again 8 (± 4) weeks after delivery (“postpartum” phone interview). Women who were found to be in a major depressive episode were offered treatment referrals or, if there was a question of safety, a psychiatric assessment.
Exposure and Outcome Measures
At each assessment point, we determined major depressive episodes by administration of the depression module from the World Mental Health Composite International Diagnostic Interview v2.1 WMH-CIDI (CIDI). This interview is a valid and reliable, fully structured lay interview instrument31 that has been administered to more than 150,000 people from 28 countries. Although the interview has not been specifically validated for use in pregnant women, such women have been well represented among those interviewed in prior studies. Nearly 500 pregnant women were included in the National Comorbidity Survey32 and its replication study 33 (personal communication, R Kessler). In a validity study, the interview had high concordance with a semi-structured clinical psychiatric interview (the Structured Clinical Interview for DSM-IV) for 12-month period prevalence.34 The area under the receiver operating curve between the semi-structured clinical interview and our interview was between 0.8 and 0.9 for a depressive disorder. The specificity for any depressive disorder in the prior 12 months was 97% (standard error=0.9) and the sensitivity was 69% (11.8). The interview is similarly reliable when administered over the telephone.34 The time frame was adjusted to ask about symptoms on a monthly basis during pregnancy. We used a standard algorithm engineered by the developers to determine whether a participant met criteria for a major depressive episode during any given month of pregnancy. If a participant was depressed during any month of a trimester, she was designated as depressed in that trimester.
For women at the home interview who reported medication use, we asked to see the medication bottles. If the bottle was unavailable, we showed respondents pictures of various types of pills and capsules to aid their recall. Participants were asked again at subsequent interviews about the use of recent and current medication. While we requested (with consent) records from outpatient behavioral health clinicians to confirm diagnoses and medication prescriptions, many clinicians declined to provide information. Missing data made this source inadequate for analysis.
Data on preterm birth were obtained from hospital records by medical-record reviewers who were masked to a woman’s depression and medication status. Preterm birth was defined as delivery prior to 37 completed weeks of pregnancy. Early preterm birth included deliveries that occurred prior to 34 completed weeks of pregnancy. To corroborate gestational age we used a first-trimester ultrasound. If that was unavailable, we used the participant’s stated last menstrual period or due date given by her doctor. If both were unreliable, we used an ultrasound conducted later in pregnancy. To determine type of preterm birth, we used data from the medical record review, including 1) the hour that the participant started regular contractions, 2) if and when the fetal membranes ruptured, 3) whether and when she received a medication to precipitate labor, and 4) whether she underwent a cesarean delivery. We defined preterm labor as an antecedent to preterm delivery if the participant experienced regular phasic uterine contractions increasing in frequency and intensity leading to effacement and dilatation of the cervix. For a designation of preterm premature rupture of membranes, we required standard confirmation of rupture of the fetal membranes prior to uterine contractions. Data for women who delivered preterm were further reviewed by two physicians (including an obstetrician specialized in maternal-fetal medicine), to verify whether preterm birth was secondary to premature rupture of membranes, secondary to spontaneous preterm labor, or performed for fetal/maternal indications.
Potential Confounding Variables
The CIDI modules for panic disorder and generalized anxiety disorder were administered along with the module for major depressive episode. In order to determine a likely diagnosis of posttraumatic stress disorder, we used the modified posttraumatic Stress Disorder Symptom Scale.35 This is a self-reported measure administered to subjects to obviate possible literacy problems. It has 17 items and demonstrates good overall consistency, concurrent validity and internal consistency (alphas of 0.96 for a treatment sample and 0.97 for a community sample).35 It performs adequately when compared with the posttraumatic stress disorder module from the Structured Clinical Interview for DSM-IV, with a sensitivity of 93% and specificity of 62%.35
Information about the amount and dates of cigarette smoking, alcohol use, illicit drug use, and other medications was obtained at each visit. Information about prior pregnancies, including prior preterm delivery, was obtained at intake.
Interviewers and Quality Control
Interviewers received extensive training, which included a minimum of four days of instruction; review of training tapes that illustrated interview techniques and administration of the interview; and completion of at least six practice interviews and four supervised interviews before becoming eligible to conduct independent interviews. We audiotaped interviews with permission of participants and randomly selected a subset of tapes for quality-control assessment. In 5% of interviews, supervising staff called the participant and confirmed demographic and other key information that to verify accuracy of the interview. For an additional 5%, the entire interview tape was reviewed for quality of data collection. Finally, all interviews were reviewed by second-and third-level coders. Any inconsistencies, unresolved questions or missing information triggered review of the audiotape or a call-back to the participant. We used the same reliability procedures for phone interviews.
Study Enrollment
Study enrollment is illustrated in the Figure. An initial 9525 women volunteered to be screened, of whom 1905 (20%) met entrance criteria and either screened positive for a depressive episode within the last five years or were undergoing antidepressant treatment. An additional 4533 (48%) women met entrance criteria and screened negative for a depressive episode and antidepressant treatment. We invited all women who screened positive to participate, as well as a randomly selected 1612 (36%) who screened negative. Of these 3517 potential participants, we interviewed 2793, 283 women declined, 360 women could not be re-contacted, and 81 women were no longer eligible (typically because they had miscarried or could not be interviewed by the cut-off date). Only 37 (1%) women withdrew and did not consent to a medical chart review, or were lost to follow-up and did not have a chart that could be located. We included only singleton live births (n=2654). Women who miscarried, terminated the pregnancy, or had a stillborn infant were excluded. Of participants with a singleton live birth, 2487 (94%) completed at least one of the two remaining interviews, and 2208 women (83%) completed both. Data on birth outcomes were available for 99%.
Figure 1.
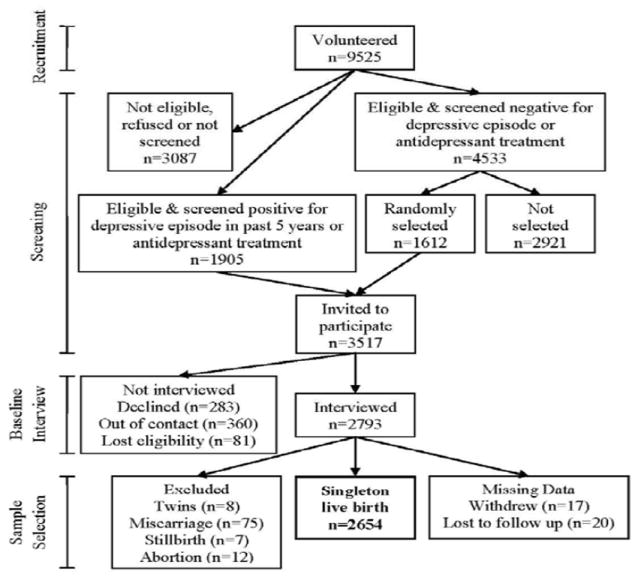
Study enrollment is illustrated in the Figure 1
Statistical Procedures
We used binary logistic regression to model risk of preterm birth. The exposure was classified into four levels: (1) women who had a major depressive episode and used serotonin reuptake inhibitors in pregnancy (depressive illness and medication); (2) women who had a major depressive episode and did not use a serotonin reuptake inhibitor (depressive illness only); (3) women who did not have a major depressive episode but used a serotonin reuptake inhibitor (medication only); and (4) women who had no major depressive episode and used no serotonin reuptake inhibitors (the reference category). If a woman was missing data due to missed interviews and was negative for a major depressive episode at observed time points, we considered her as negative in pregnancy. Partial data for serotonin reuptake inhibitor use were handled analogously. Possible confounding demographic factors (chosen a priori) included mother’s age, education (as a measure of socioeconomic status), race, smoking, illicit drug use, and history of preterm birth. These factors were included in all adjusted models.
We were concerned that the effect for serotonin reuptake inhibitor use might be confounded by illness severity. To address this possibility, we performed a second adjusted analysis that included factors for psychiatric illness history and severity (age of illness onset, number of hospitalizations, number of depressive episodes, and suicidal ideation), as well as concurrent diagnoses (posttraumatic stress disorder, generalized anxiety disorder, and panic disorder).
We used ordered logistic regression to model risk of early or late preterm birth. This outcome was categorized as birth before 34 completed weeks of gestation (early preterm), birth at 34 to 36 weeks (late preterm), and birth at 37 weeks or later (term, the outcome reference category). As a secondary outcome, we used nominal logistic regression to examine the antecedents of preterm birth: spontaneous premature labor, preterm premature rupture of membranes, and indicated preterm birth. In both ordinal and nominal logistic models, the exposure was defined as analogous to the binary logistic model. The group with neither medication nor depressive illness remained the exposure reference. All data were analyzed using SAS version 9.2 (SAS Institute, Cary, NC). We computed odds ratios (ORs) and confidence intervals (CIs).
To clarify the differences in exposure groups, we also tabulated preterm birth according to timing of depressive illness and medication use. Timing factors were categorized as 1) none in pregnancy, 2) early (first trimester) only, 3) late (second or third trimester) only, and 4) both early and late. Many of the combinations of depressive-illness and medication timing were rare in given trimesters, precluding a statistical analysis, but we present unadjusted data and rates of preterm birth for each combination.
RESULTS
Table 1 presents demographic and other characteristics for all participants, as well as those who delivered term and preterm. Not shown in Table 1 are demographic differences among exposure groups, especially between the medication-only and depressive illness-only groups. The medication-only women were predominantly (91%) white, while 73% of the both-medication-and-illness group was white. The illness-only group had a high percentage of black (21%) and Hispanic (29%) participants. In the neither-illness-nor-treatment group, 74% were white. The proportion with 16 or more years of education was similar (56-69%) for all groups except the illness-only group, where it was 28%. Serotonin reuptake inhibitors used by study participants included citalopram (n=26), fluoxetine (n=68), escitalopram (n=47), paroxetine (n=21), sertraline (n=121), duloxetine (n=8) and venlafaxine (n=29).
Table 1.
Main Exposures, Demographic Characteristics and Psychiatric Illness Factors by Preterm Birth
| Characteristic | Total (n=2654) No. (%) | Term (n=2429) No. (%) | Preterm (nN=225) No. (%) |
|---|---|---|---|
| Age (years) | |||
| <25 | 440 (17) | 397 (16) | 43 (19) |
| 25-34 | 1509 (57) | 1405 (58) | 104 (46) |
| 35+ | 705 (27) | 627 (26) | 78 (35) |
| Race/Ethnicity | |||
| White | 1957 (74) | 1799 (74) | 158 (70) |
| Black | 195 (7) | 172 (7) | 23 (10) |
| Hispanic | 383 (14) | 348 (14) | 35 (16) |
| Other | 119 (5) | 110 (5) | 9 (4) |
| Education (years) | |||
| <12 | 172 (6) | 158 (7) | 14 (6) |
| 12 | 382 (14) | 345 (14) | 37 (16) |
| 13-15 | 599 (23) | 538 (22) | 61 (27) |
| 16+ | 1501 (57) | 1388 (57) | 113 (50) |
| Smoking | |||
| None in pregnancy | 2265 (85) | 2074 (85) | 191 (85) |
| Trimester 1 only | 158 (6) | 145 (6) | 13 (6) |
| After trimester 1 | 231 (9) | 210 (9) | 21 (9) |
| Illicit Drugs | |||
| None since 1 month before pregnancy | 2245 (92) | 2244 (92) | 201 (89) |
| Marijuana only | 153 (6) | 141 (6) | 12 (5) |
| Other drugs | 56 (2) | 44 (2) | 12 (5) |
| Pregnancy history | |||
| No previous live births | 1141 (43) | 1037 (43) | 104 (46) |
| Term births only | 1305 (49) | 1235 (51) | 70 (31) |
| At least 1 preterm birth | 208 (8) | 157 (6) | 51 (23) |
| Post-traumatic stress disorder in pregnancy | 129 (5) | 106 (4) | 23 (10) |
| Generalized anxiety disorder in pregnancy | 252 (10) | 225 (9) | 27 (12) |
| Panic disorder in pregnancy | 98 (4) | 82 (3) | 16 (7) |
| Suicidal thoughts in pregnancy | 90 (3) | 78 (3) | 12 (5) |
| Number of depressive episodes prior to pregnancya | |||
| 0 | 1772 (67) | 1643 (68) | 129 (57) |
| 1 | 212 (8) | 184 (8) | 28 (12) |
| 2 | 175 (7) | 155 (6) | 20 (9) |
| 3 | 128 (5) | 115 (5) | 13 (6) |
| 4+ | 362 (14) | 327 (13) | 35 (16) |
| Unknown | 5 (<1) | 5 (<1) | 0 (0) |
| Age of onset of first depressive episode (years) | |||
| Number of depressive episodes | 1756 (66) | 1628 (67) | 128 (57) |
| <14 | 122 (5) | 112 (5) | 10 (4) |
| 14-17 | 184 (7) | 156 (6) | 28 (12) |
| 18-25 | 334 (13) | 306 (13) | 28 (12) |
| 26+ | 252 (10) | 222 (9) | 30 (13) |
| Unknown | 6 (<1) | 5 (<1) | 1 (<1) |
| Number of hospitalizations for depression | |||
| 0 | 2479 (93) | 2277 (94) | 202 (90) |
| 1 | 101 (4) | 90 (4) | 11 (5) |
| 2-3 | 51 (2) | 43 (2) | 8 (4) |
| 4 | 22 (1) | 18 (1) | 4 (2) |
| Unknown | 1 (<1) | 1 (<1) | 0 (0) |
Sixteen women experienced their first depressive episode during the first trimester of pregnancy
Estimates for risk of preterm birth by a major depressive episode and use of a serotonin reuptake inhibitor are shown in Table 2. This table presents two adjusted analyses, one that accounted for demographic factors as well as substance misuse and another that included these factors in conjunction with illness-severity factors. After accounting for demographic factors and substance misuse, women who experienced a major depressive episode and used serotonin reuptake inhibitors in pregnancy (depressive illness and medication), and women who used serotonin reuptake inhibitors without a major depressive episode (medication-only) had increased risk of preterm birth. A major depressive episode without serotonin reuptake inhibitor use (illness-only) was only weakly associated with preterm birth, if at all. Compared with unadjusted estimates, the adjusted risk for the medication-only group was higher, which likely reflects consideration of the demographic differences between groups mentioned above.
Table 2.
Risk of Preterm Birth by Major Depressive Episode and Serotonin Reuptake Inhibitor (SRI) Use During Pregnancy
| Exposure Major Depressive Episode | Unadjusted | Adjusted1a | Additionally Adjustedb | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| SRI Use | Term (n=2429) No. (%) | Preterm (m=225) No. (%) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | |
| Yes | Yes | 46 (84) | 9 (16) | 2.3 | (1.11-4.8) | 2.14 | (0.99-4.6) | 1.51 | (0.60-3.8) |
| Yes | No | 150 (90) | 17 (10) | 1.3 | (0.79-2.3) | 1.19 | (0.68-2.1) | 0.86 | (0.44-1.7) |
| No | Yes | 211 (89) | 27 (11) | 1.5 | (0.98-2.3) | 1.62 | (1.0-2.5) | 1.50 | (0.94-2.4) |
| No | No | 2022 (92) | 172 (8) | 1.0 | 1.0 | 1.0 | |||
Adjusted for age, education, race, smoking, illicit drug use and pregnancy history;
Adjusted for age, education, race, smoking, illicit drug use and pregnancy history, number of lifetime hospitalizations, age of depressive onset, number of prior depressive episodes, post-traumatic stress disorder, generalized anxiety disorder, panic disorder in pregnancy, and suicidal thoughts in pregnancy
Reference category.
Of particular concern was the possibility that the effect of serotonin reuptake inhibitors was confounded by psychiatric illness. The additionally adjusted model 2 addresses this possibility by including factors for other psychiatric disorders and indices of lifetime psychiatric illness burden. After accounting for these factors, both the illness-plus-medication group and the medication-only group continued to show elevated risk of preterm birth.
Risk of early and late preterm birth is shown in Table 3. Early preterm birth was uncommon, and the risk was relatively similar among the exposure groups, while differences were pronounced in risk of late preterm birth. The odds ratios were highest for late preterm birth among women who both had a major depressive episode and used a serotonin reuptake inhibitor during pregnancy (adjusted OR=3.2 [95% CI=1.6-6.8]).
Table 3.
Risk of Early and Late Preterm Birth by Major Depressive Episode and Serotonin Reuptake Inhibitor Use During Pregnancy
| Exposure Major Depressive Episode | Unadjusted | Adjusteda | ||||
|---|---|---|---|---|---|---|
| SRI Use | No. (%) With Outcome | OR | (95% CI) | OR | (95% CI) | |
| Early Preterm Birth (n=59) | ||||||
| Yes | Yes | 0 (0) | N/A | N/A | ||
| Yes | No | 4 (2) | 1.4 | (0.79-2.6) | 0.86 | (0.29-2.6) |
| No | Yes | 5 (2) | 0.96 | (0.38-2.4) | 0.93 | (0.35-2.4) |
| No | No | 50 (2) | 1.0 | 1.0 | ||
| Late Preterm Birth (n=166) | ||||||
| Yes | Yes | 9 (16) | 3.2 | (1.6-6.8) | 3.14 | (1.5-6.8) |
| Yes | No | 13 (8) | 1.4 | (0.79-2.6) | 1.34 | (0.71-2.5) |
| No | Yes | 22 (9) | 1.7 | (1.1-2.8) | 1.93 | (1.2-3.2) |
| 122 (6) | 1.0 | 1.0 | ||||
Adjusted for age, education, race, smoking, illicit drug use and pregnancy history
Early preterm was defined as <34 completed weeks gestation and late preterm as 34-36 completed weeks gestation
Reference category.
The likelihood that either a major depressive episode or use of a serotonin reuptake inhibitor is associated with specific antecedents for preterm birth is shown in Table 4. Although this analysis was exploratory in nature, and many of the combinations of exposure and outcome were relatively rare, an interesting picture emerged among women who took serotonin reuptake inhibitors without a major depressive episode. These women had a high risk of spontaneous preterm birth due to premature labor or premature rupture of membranes and low risk of preterm birth for a medical indication.
Table 4.
Preterm Birth Antecedenta by Major Depressive Episode and Serotonin Reuptake Inhibitor Use During Pregnancy
| Exposure Major Depressive Episode | Unadjusted | Adjustedb | ||||
|---|---|---|---|---|---|---|
| SRI USe | No. (%) with outcome | OR | (95% CI) | OR | (95% CI) | |
| Spontaneous Due to Premature Labor (n=73) | ||||||
| Major depressive episode and serotonin reuptake inhibitors in pregnancy | 2 (4) | 1.6 | (0.39-6.9) | 1.7 | (0.38-7.2) | |
| Major depressive episode in pregnancy only | 6 (4) | 1.5 | (0.63-3.5) | 1.3 | (0.53-3.2) | |
| Serotonin reuptake inhibitors in pregnancy only | 11 (5) | 2.0 | (1.0-3.8) | 2.1 | (1.1-4.3) | |
| No major depressive episode and no serotonin reuptake inhibitors in pregnancyc | 54 (2) | 1.0 | 1.0 | |||
| Major depressive episode and serotonin reuptake inhibitors in pregnancy | 5 (9) | 3.4 | (1.3-8.8) | 2.9 | (1.0-8.1) | |
| Major depressive episode in pregnancy only | 4 (2) | 0.83 | (0.30-2.3) | 0.85 | (0.30-2.5) | |
| Serotonin reuptake inhibitors in pregnancy only | 12 (5) | 1.8 | (0.94-3.3) | 1.8 | (0.95-3.5) | |
| No major depressive episode and no serotonin reuptake inhibitors in pregnancy c | 65 (3) | 1.0 | 1.0 | |||
| Non-spontaneous Medical Indication (n=59) | ||||||
| Major depressive episode and serotonin reuptake inhibitors in pregnancy | 55 | 2 (4) | 1.8 | (0.43-7.8) | 1.9 | (0.42-8.0) |
| Major depressive episode in pregnancy only | 166 | 6 (4) | 1.7 | (0.71-4.0) | 1.4 | (0.54-3.5) |
| Serotonin reuptake inhibitors in pregnancy only | 237 | 3 (1) | 0.60 | (0.19-1.9) | 0.68 | (0.21-2.2) |
| No major depressive episode and no serotonin reuptake inhibitors in pregnancy c | 2189 | 48 (2) | 1.0 | 1.0 | ||
7 women were excluded due to unknown antecedent;
Adjusted for age, education, race, smoking, illicit drug use and pregnancy history
Reference category.
We examined timing of depressive episodes and serotonin reuptake inhibitor use in Table 5. Although cell sizes are small, it appears that the risk of preterm birth with serotonin reuptake inhibitors is driven by women who used these medications throughout pregnancy. Women who used SRIs only early or only late in pregnancy had relatively low rates of preterm birth
Table 5.
Timing of Major Depressive Episodes and Use of SRI Medication During Pregnancy
| Serotonin reuptake inhibitors | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early only | Late only | Early and late | None during pregnancy | Total | |||||||||||
| Major depressive episode | Total No. | Preterm No. (%) | Total No. | Preterm No. (%) | Total No. | Preterm No. (%) | Total No. | Preterm No. (%) | |||||||
| Early only | 12 | 1 | (8) | 5 | 1 | (20) | 14 | 2 | (14) | 107 | 12 | (11) | 138 | 16 | (12) |
| Late only | 2 | 0 | (0) | 7 | 0 | (0) | 5 | 3 | (60) | 37 | 2 | (5) | 51 | 5 | (10) |
| Early and late | 2 | 0 | (0) | 3 | 0 | (0) | 5 | 2 | (40) | 23 | 3 | (13) | 33 | 5 | (15) |
| None during pregnancy | 100 | 11 | (11) | 21 | 1 | (5) | 117 | 15 | (13) | 2194 | 172 | (8) | 2432 | 199 | (8) |
| Total | 116 | 12 | (10) | 36 | 2 | (6) | 141 | 22 | (16) | 2361 | 189 | (8) | |||
DISCUSSION
In our cohort, the risk of preterm birth associated with major depressive disorder was uncertain, whereas serotonin reuptake inhibitor use, separate from illness, was a clear risk factor. Neither serotonin reuptake inhibitors nor major depressive episodes were associated with early preterm birth. Exploratory data suggest that serotonin reuptake inhibitors may be associated with spontaneous types of preterm birth, and that the risk is primarily with continuous use throughout pregnancy.
A recent quantitative review36 found a relative risk of 1.18 (95% CI=1.08-1.28) for preterm birth among women with elevated depressive symptoms. We estimated a similar risk (1.19[95% CI=0.68—2.09]) associated with depressive illness only. Women in the illness-plus-medication group may have been at higher risk than medication-only women in the model adjusted for demographic factors. Although this suggests a possible effect of a major depressive episode, the association may be explained by other factors.
The use of a serotonin reuptake inhibitor in pregnancy has been previously associated with preterm birth,1,3,5,37,38 although most studies have not included information on the underlying illness that required treatment. Even when data for both illness and medication are available, it is challenging to make inferences about independent effects because use of serotonin reuptake inhibitor occurs in the context of mental illness. A randomized clinical trial assigning medication treatment or no medication treatment would be required to eliminate confounding, but such a trial is infeasible. Our data do not suggest a perfect correlation between sickness and medication treatment. It appears that some women may not have had access to, or were unwilling to undergo, treatment; there may be other reasons why women with major depressive disorder are not taking SRIs. Although it is not possible to include every possible measure of illness severity in the adjusted model, the effect for serotonin reuptake inhibitor changed only a small amount when measures of illness severity were added to the model (Table 2). This suggests that differences in illness severity explain only part of the estimated risk found with use of a serotonin reuptake inhibitor.
Some support for our findings is found in a study that linked various registries for depressive illness and treatment with a birth outcome registry.29 This investigation found that overall preterm birth was higher in women treated with serotonin reuptake inhibitors compared with women having a depressive illness only, although effects were mostly explained by differences in illness severity. After controlling for these factors, associations between medication use and birth weight remained, while an effect on preterm birth was attenuated.
A possible mechanism for the association observed in our data is suggested by analyses of preterm type and early or late preterm birth: it appears serotonin reuptake inhibitors may be linked specifically with spontaneous preterm birth at 34 to 36 weeks. A possible relationship between serotonin reuptake inhibitors and particular antecedents of preterm birth has received less attention. Several registry studies5,10 reported an association between the use of a serotonin reuptake inhibitor and premature rupture of membranes, although the authors did not stipulate whether this occurred specifically with preterm births. Others found the opposite, that psychotropic medication use in pregnancy was associated with medically indicated but not spontaneous birth,24 or that risk of spontaneous preterm birth was reduced when women with depressive symptoms were treated with psychotropic medications.14 The latter two reports grouped antidepressant with sedative medication use, and neither evaluated the effects of serotonin reuptake inhibitors specifically. Our findings may be due in part to the precision with which we defined preterm-birth classifications. We performed chart reviews with the specific purpose of identifying the immediate antecedent of preterm birth, and we considered many sources of information including timing of delivery, hospital admission, labor onset, and rupture of membranes. A relationship between use of a serotonin reuptake inhibitor and spontaneous birth is biologically plausible, because serotonin reuptake inhibitors increase levels of selected cytokines39 that may play a role in the cascade toward spontaneous birth.40 Such findings may inform future studies that investigate the biological relationship between serotonin reuptake inhibitors and spontaneous preterm birth.
We had limited data on exposure timing. Because it appears that women who took medication only early or only late in pregnancy had somewhat lower risk compared with women who took medication throughout pregnancy, timing may be less important than overall amount or duration. The aforementioned study that linked psychiatric illness, medication use and birth outcomes also found that duration of exposure to a serotonin reuptake inhibitor influenced the duration of gestation.41
Our investigation has several limitations. The diagnosis from a structured interview can differ from that obtained by a thorough clinical assessment. As in the current study, others have found that a variety of potential confounding factors (including drug misuse, smoking, age, education, race/ethnicity and prior preterm birth) are differentially distributed among pregnant women who suffer from mood and anxiety disorders as compared with those without psychiatric illness.5 We adjusted for these factors in our analyses but cannot rule out residual confounding from factors measured imprecisely or incompletely. We cannot comment on the role of psychotherapy because our data do not include timing of onset of a depressive episode and initiation of psychotherapy. This leaves open the possibility of confounding by indication. Finally, with regard to use of serotonin reuptake inhibitors, we relied on participant report rather than blood levels. It is possible that participants underreported use of serotonin reuptake inhibitor medication.
Our study adds to a growing body of research suggesting that serotonin reuptake inhibitors during pregnancy may increase a woman’s risk of early delivery. In assessing the implications of this risk for clinical practice, care must be taken to balance the health implications for the mother with those for the fetus.
Acknowledgments
In the development of this report, we adhered to the uniform requirements for preparation of manuscripts and the guidelines set out in STROBE. All of the authors participated in the conduct of the study and writing or editing the manuscript. All authors approved the final copy. Dr. Yonkers is the lead author and discloses royalties from Up To Date and support from Pfizer via study medication for an NIMH trial. Drs. Norwitz and Lockwood also have received royalties from Up To Date. Remaining authors have no disclosures.
Financial Support: This study was supported by Grant R01 HD045735 from National Institute of Child Health and Human Development; Dr. Smith was supported by K12 DA031050 from the National Institute on Drug Abuse, National Institute on Alcohol Abuse and Alcoholism and Office of Research on Women’s Health.
References
- 1.Chambers C, Johnson K, Dick L, Felix R, Jones K. Birth outcomes in pregnant women taking fluoxetine. New England Journal of Medicine. 1996;335:1010–1015. doi: 10.1056/NEJM199610033351402. [DOI] [PubMed] [Google Scholar]
- 2.Davis RL, Rubanowice D, McPhillips H, Raebel MA, Andrade SE, Smith D, Yood MU, Platt R. Risks of congenital malformations and perinatal events among infants exposed to antidepressant medications during pregnancy. Pharmacoepidemiology and Drug Safety. 2007;16(10):1086–1094. doi: 10.1002/pds.1462. [DOI] [PubMed] [Google Scholar]
- 3.Kallen B. Neonate characteristics after maternal use of antidepressants in late pregnancy. Archives of Pediatrics & Adolescent Medicine. 2004;158(4):312–6. doi: 10.1001/archpedi.158.4.312. [DOI] [PubMed] [Google Scholar]
- 4.Lund N, Pedersen LH, Henriksen TB. Selective serotonin reuptake inhibitor exposure in utero and pregnancy outcomes. Archives of Pediatrics & Adolescent Medicine. 2009;163(10):949–54. doi: 10.1001/archpediatrics.2009.164. [DOI] [PubMed] [Google Scholar]
- 5.Reis M, Kallen B. Delivery outcome after maternal use of antidepressant drugs in pregnancy: an update using Swedish data. Psychological Medicine. 2010;40(10):1723–33. doi: 10.1017/S0033291709992194. [DOI] [PubMed] [Google Scholar]
- 6.Simon G, Cunningham M, Davis R. Outcomes of prenatal antidepressant exposure. The American Journal of Psychiatry. 2002;159:2055–2061. doi: 10.1176/appi.ajp.159.12.2055. [DOI] [PubMed] [Google Scholar]
- 7.Toh SS, Mitchell AAMD, Louik CS, Werler MMS, Chambers CDPMPH, Hernandez-Diaz SMDD. Antidepressant Use During Pregnancy and the Risk of Preterm Delivery and Fetal Growth Restriction. Journal of Clinical Psychopharmacology. 2009;29(6):555–560. doi: 10.1097/JCP.0b013e3181bf344c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wen SW, Yang Q, Garner P, Fraser W, Olatunbosun O, Nimrod C, Walker M. Selective serotonin reuptake inhibitors and adverse pregnancy outcomes. American Journal of Obstetrics and Gynecology. 2006;194(4):961. doi: 10.1016/j.ajog.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Wisner KL, Sit DK, Hanusa BH, Moses-Kolko EL, Bogen DL, Hunker DF, Perel JM, Jones-Ivy S, Bodnar LM, Singer LT. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. American Journal of Psychiatry. 2009;166(5):557–66. doi: 10.1176/appi.ajp.2008.08081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colvin L, Slack-Smith L, Stanley FJ, Bower C. Dispensing patterns and pregnancy outcomes for women dispensed selective serotonin reuptake inhibitors in pregnancy. Birth Defects Research Part A: Clinical and Molecular Teratology. 2011;91(3):142–152. doi: 10.1002/bdra.20773. [DOI] [PubMed] [Google Scholar]
- 11.Kulin NA, Pastuszak A, Sage SR, Schick-Boschetto B, Spivey G, Feldkamp M, Ormond K, Matsui D, Stein-Schechman AK, Cook L, Brochu J, Rieder M, Koren G. Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors. Journal of American Medical Association. 1998;279(8):609–610. doi: 10.1001/jama.279.8.609. [DOI] [PubMed] [Google Scholar]
- 12.Malm H, Klaukka T, Neuvonen PJ. Risks associated with selective serotonin reuptake inhibitors in pregnancy. Obstetrics & Gynecology. 2005;106(6):1289–96. doi: 10.1097/01.AOG.0000187302.61812.53. [DOI] [PubMed] [Google Scholar]
- 13.Pastuszak A, Schick-Boschetto B, Zuber C, Feldkamp M, Pinelli M, Sihn S, Donnenfeld A, McCormack M, Leen-Mitchell M, Woodland C, Gardner A, Hom M, Koren G. Pregnancy outcome following first-trimester exposure to fluoxetine (Prozac) Journal of American Medical Association. 1993;269(17):2246–2248. [PubMed] [Google Scholar]
- 14.Dayan J, Creveuil C, Marks MN, Conroy S, Herlicoviez M, Dreyfus M, Tordjman S. Prenatal depression, prenatal anxiety, and spontaneous preterm birth: A prospective cohort study among women with early and regular care. Psychosomatic Medicine. 2006;68(6):938–946. doi: 10.1097/01.psy.0000244025.20549.bd. [DOI] [PubMed] [Google Scholar]
- 15.Hedegaard M, Henriksen TB, Sabroe S, Secher NJ. Psychological distress in pregnancy and preterm delivery. British Medical Journal. 1993;307(6898):234–239. doi: 10.1136/bmj.307.6898.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jesse DE, Seaver W, Wallace DC. Maternal psychosocial risks predict preterm birth in a group of women from Appalachia. Midwifery. 2003;19(3):191–202. doi: 10.1016/s0266-6138(03)00031-7. [DOI] [PubMed] [Google Scholar]
- 17.Neggers Y, Goldenberg R, Cliver S, Hauth J. Effects of domestic violence on preterm birth and low birth weight. Acta Obstetricia et Gynecologica Scandinavica. 2004;83:455–460. doi: 10.1111/j.0001-6349.2004.00458.x. [DOI] [PubMed] [Google Scholar]
- 18.Orr S, James S, Prince CB. Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, MD. American Journal of Epidemiology. 2002;156(9):797–802. doi: 10.1093/aje/kwf131. [DOI] [PubMed] [Google Scholar]
- 19.Rondo P, Ferreira R, Nogueira F, Ribeiro M, Lobert H, Artes R. Maternal psychological stress and distress as predictors of low birth weight, prematurity and intrauterine growth retardation. European Journal of Clinical Nutrition. 2003;57:266–272. doi: 10.1038/sj.ejcn.1601526. [DOI] [PubMed] [Google Scholar]
- 20.Andersson L, Sundstrom-Poromaa I, Wulff M, Astrom M, Bixo M. Neonatal outcome following maternal antenatal depression and anxiety: A population-based study. American Journal of Epidemiology. 2004;159(9):872–881. doi: 10.1093/aje/kwh122. [DOI] [PubMed] [Google Scholar]
- 21.Copper R, Goldenberg RL, Das A, Elder N, Swain M, Norman G, Ramsey R, Cotroneo P, Collins B, Johnson F, Jones P, Meier A. The preterm prediction study: Maternal stress is associated with spontaneous preterm birth at less than thirty-five weeks’ gestation. American Journal of Obstetrics and Gynecology. 1996;175(5):1286–1292. doi: 10.1016/s0002-9378(96)70042-x. [DOI] [PubMed] [Google Scholar]
- 22.Diego MA, Field T, Hernandez-Reif M, Schanberg S, Kuhn C, Gonzalez-Quintero VH. Prenatal depression restricts fetal growth. Early Human Development. 2009;85(1):65–70. doi: 10.1016/j.earlhumdev.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dole N, Savitz D, Hertz-Picciotto I, Siega-Riz A, McMahon M, Buekens P. Maternal stress and preterm birth. American Journal of Epidemiology. 2003;157:14–24. doi: 10.1093/aje/kwf176. [DOI] [PubMed] [Google Scholar]
- 24.Gavin AR, Holzman C, Siefert K, Tian Y. Maternal depressive symptoms, depression, and psychiatric medication use in relation to risk of preterm delivery. Womens Health Issues. 2009;19(5):325–34. doi: 10.1016/j.whi.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haas JS, Fuentes-Afflick E, Stewart AL, Jackson RA, Dean ML, Brawarsky P, Escobar GJ. Prepregnancy health status and the risk of preterm delivery. Archives of Pediatrics & Adolescent Medicine. 2005;159(1):58–63. doi: 10.1001/archpedi.159.1.58. [DOI] [PubMed] [Google Scholar]
- 26.Li D, Liu L, Odouli R. Presence of depressive symptoms during early pregnancy and the risk of preterm delivery: a prospective cohort study. Human Reproduction. 2009;24(1):146–53. doi: 10.1093/humrep/den342. [DOI] [PubMed] [Google Scholar]
- 27.Perkin M, Bland J, Peacock J, Anderson H. The effect of anxiety and depression during pregnancy on obstetric complications. British Journal of Obstetrics and Gynaecology. 1993;100:629–634. doi: 10.1111/j.1471-0528.1993.tb14228.x. [DOI] [PubMed] [Google Scholar]
- 28.Suri R, Altshuler L, Hellemann G, Burt VK, Aquino A, Mintz J. Effects of antenatal depression and antidepressant treatment on gestational age at birth and risk of preterm birth. American Journal of Psychiatry. 2007;164(8):1206–13. doi: 10.1176/appi.ajp.2007.06071172. [DOI] [PubMed] [Google Scholar]
- 29.Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Archives of General Psychiatry. 2006;63(8):898–906. doi: 10.1001/archpsyc.63.8.898. [DOI] [PubMed] [Google Scholar]
- 30.Yonkers KA, Gotman N, Smith MV, Forray A, Belanger K, Brunetto WL, Lin H, Burkman RT, Zelop CM, Lockwood CJ. Does Antidepressant Use Attenuate the Risk of a Major Depressive Episode in Pregnancy? Epidemiology. 2011;22(6):848–854. doi: 10.1097/EDE.0b013e3182306847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wittchen H-U. Reliability and validity studies of the WHO Composite International Diagnostic Interview (CIDI): A critical review. Journal of Psychiatric Research. 1994;28:57–84. doi: 10.1016/0022-3956(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 32.Kessler R, McGonagle K, Zhao S, Nelson C, Hughes M, Eshleman S, Wittchen H, Kendler K. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 33.Kessler R, Nelson C, McGonagle K, Liu J, Swartz M, Blazer D. Comorbidity of DSM-III-R major depressive disorder in the general population: results from the US National Comorbidity Survey. The The British Journal of Psychiatry. 1996;168(30):17–30. [PubMed] [Google Scholar]
- 34.Kessler RC, Avenevoli S, Costello EJ, Green JG, Gruber MJ, Heeringa S, Merikangas KR, Pennell B-E, Sampson NA, Zaslavsky AM. National Comorbidity Survey Replication Adolescent supplement (NCS-A): II. Overview and design. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48(4):380–5. doi: 10.1097/CHI.0b013e3181999705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falsetti S, Resnick H, Pesick P, Kilpatrick D. The modified PTSD symptom scale: A brief self-report measure of posttraumatic stress disorder. Behavior Therapist. 1993;16:161–162. [Google Scholar]
- 36.Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Archives of General Psychiatry. 2010;67(10):1012–24. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Einarson A, Choi J, Einarson TR, Koren G. Adverse effects of antidepressant use in pregnancy: an evaluation of fetal growth and preterm birth. Depression & Anxiety. 2010;27(1):35–8. doi: 10.1002/da.20598. [DOI] [PubMed] [Google Scholar]
- 38.Calderon-Margalit R, Qiu C, Ornoy A, Siscovick DS, Williams MA. Risk of preterm delivery and other adverse perinatal outcomes in relation to maternal use of psychotropic medications during pregnancy. American Journal of Obstetrics & Gynecology. 2009;201(6):579.e1–8. doi: 10.1016/j.ajog.2009.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warner-Schmidt JL, Vanover KE, Chen EY, Marshall JJ, Greengard P. Antidepressant effects of selective serotonin reuptake inhibitors (SSRIs) are attenuated by antiinflammatory drugs in mice and humans. Proceedings of the National Academy of Sciences. 2011;108(22):9262–9267. doi: 10.1073/pnas.1104836108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norwitz E, Lye S. Biology of Parturition. In: Creasy R, Resnick R, Iams J, Lockwood C, Moore T, editors. Creasy & Resnick’s Maternal-Fetal Medicine. 6. Philadelphia, PA: Elsevier, Inc; 2009. pp. 69–85. [Google Scholar]
- 41.Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C. Effects of timing and duration of gestational exposure to serotonin reuptake inhibitor antidepressants: population-based study. British Journal of Psychiatry. 2008;192(5):338–43. doi: 10.1192/bjp.bp.107.037101. [DOI] [PubMed] [Google Scholar]


