Abstract
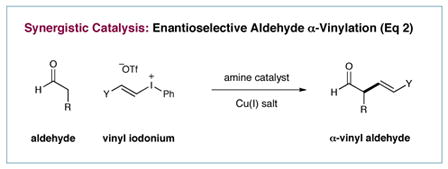
The enantioselective α-vinylation of aldehydes using vinyl iodonium triflate salts has been accomplished via the synergistic combination of copper and chiral amine catalysis. These mild catalytic conditions provide a direct route for the enantioselective construction of enolizable α-formyl vinylic stereocenters without racemization or olefin transposition. These high-value coupling adducts are readily converted into a variety of useful olefin synthons.
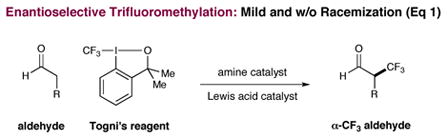
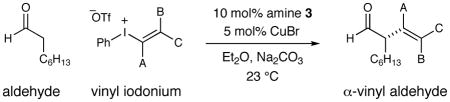
While mono-catalysis strategies have successfully delivered vast numbers of new reactions over many decades, multi-catalysis concepts that allow access to many elusive or unattainable transformations have only recently begun to evolve. In particular, synergistic catalysis, wherein two catalysts and two catalytic cycles work in concert to create a single new bond, has emerged as a powerful mechanistic approach to asymmetric reaction engineering.1–3 In this context, our laboratory has recently described the enantioselective α-trifluoromethylation and α-arylation of aldehydes via the synergistic combination of copper catalysis, organocatalysis and iodonium salts (eq 1), two protocols that enable the production of structural motifs of high value to the pharmaceutical sciences.4–6 Continuing this theme, we questioned whether this multi-catalysis strategy might be extended to the enantioselective α-vinylation of carbonyls, an important yet complex synthetic task given the capacity of such adducts to readily undergo olefin-carbonyl conjugation or product racemization.7 Herein we describe the successful execution of these ideals via the combination of two discrete catalytic cycles (chiral amine–enamine, Cu(I)–Cu(III)) that enables the enantioselective construction of a wide variety of α-substituted-β,γ-unsaturated aldehydes without olefin isomerization or stereochemical ablation (eq 2).8–11
 |
 |
 |
Design Plan
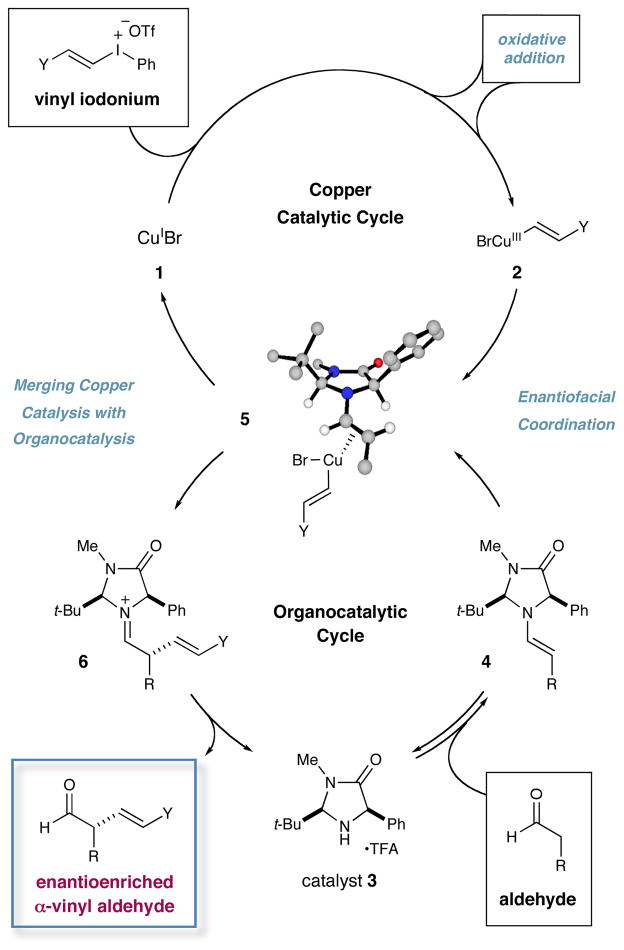
As shown in Scheme 1, we postulated that a Cu(I) catalyst 1 (e.g. CuBr), in the presence of a vinyl iodonium triflate, will undergo chemoselective oxidative addition into the weak alkenyl-iodine bond to generate a highly electrophilic Cu(III)-vinyl complex 2.12,13 In a simultaneous organocatalytic cycle, condensation of chiral amine catalyst 3 with an aldehyde should furnish the transient yet nucleophilic enamine 4. Computational studies predict that the Si-face of this enamine will be shielded by the phenyl ring of the imidazolidinone framework, leaving the Re-face accessible to electrophilic attack (see DFT-5).14 On this basis, complexation of the enamine π-system with Cu(III)-vinyl complex 2 is expected to lead to η1-iminium organocopper species (not shown), which, upon reductive elimination, will forge a carbon–carbon bond and reconstitute the active Cu(I) catalyst (thereby completing the metal cycle). Hydrolysis of the resulting iminium 6 will also release amine 3 to complete the organocatalysis cycle while furnishing the desired enantioenriched α-vinyl aldehyde.
Scheme 1.
Proposed Synergistic Cycles for Aldehyde α-Vinylation
Results
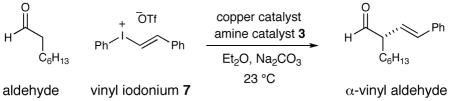
Our investigation began with an examination of the coupling of octanal with styrenyl iodonium triflate 7 in the presence of chiral amine catalyst 3 and various Cu(I) catalysts. As expected, no product formation was observed in the absence of copper salts (Table 1, entry 1). However, upon exposure to a catalytic amount of CuOAc, the desired α-vinyl aldehyde was produced with moderate yield and with excellent levels of asymmetric induction (entry 2, 67% yield, 97% ee). While the use of CuI resulted in diminished yields (entry 4, 23% yield), CuCl was found to be a competent coupling catalyst (entry 3, 82% yield, 97% ee). Optimal efficiency and enantiocontrol was achieved, however, using CuBr to afford the desired α-vinyl aldehyde adduct in 98% yield and 97% ee (entry 5). Importantly, lowering the copper and chiral amine catalyst loadings to 5 mol% and 10 mol%, respectively, had little impact on the overall performance of this multi-catalysis protocol (entry 6, 97% yield, 97% ee).
Table 1.
Impact of Amine and Copper Catalysts on Vinylation
| ||||
|---|---|---|---|---|
| entry | catalyst 3 mol% | copper catalyst (mol %) | yield (%)a | ee (%)b |
| 1 | 20 mol% | None | 0 | -- |
| 2 | 20 mol% | CuOAc (10 mol%) | 67 | 97 |
| 3 | 20 mol% | CuCl (10 mol%) | 82 | 97 |
| 4 | 20 mol% | CuI (10 mol%) | 23 | 97 |
| 5 | 20 mol% | CuBr (10 mol%) | 98 | 97 |
| 6 | 10 mol% | CuBr (5 mol%) | 97 | 97 |
1H NMR yield.
Determined by chiral SFC analysis of the corresponding alcohol, absolute configuration determined by chemical correlation.
With optimal conditions in hand, we next examined the structural diversity of the aldehydic coupling partner (Table 2). Notably, sterically demanding β-branched aldehydes were found to be competent substrates, providing α-vinylated products with excellent levels of optical purity (entries 3, 4 and 6, 73–79% yield, 96–99% ee). Moreover, the presence of pendent heteroatom functionalities does not have a deleterious impact on the reaction performance (entries 2, 5 and 6). It should be noted that the two cycles involved are sufficiently mild to maintain configurational integrity of the pendent Z-alkene (entry 1, 69% yield, 96% ee). More importantly, aldehydes bearing β-stereogenicity can be routinely employed to enable the highly diastereoselective construction of both syn- and anti-α,β-disubstituted adducts (entries 7 and 8, 81–84% yield, ≥20:1 dr). As a salient indicator of the dominance of catalyst versus substrate control in these processes, both β-stereocenter containing antipodes undergo α-olefination with the same sense of induction (as dictated by the amine catalyst).
Table 2.
| |||
|---|---|---|---|
| 1 |
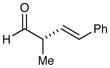 82% yield 94% ee 82% yield 94% ee |
2d,e |
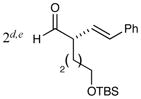 83% yield 96% ee 83% yield 96% ee |
| 3 |
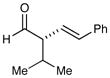 75% yield 99% ee 75% yield 99% ee |
4 |
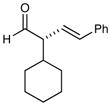 79% yield 99% ee 79% yield 99% ee |
| 5 |
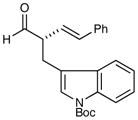 81% yield 91% ee 81% yield 91% ee |
6 |
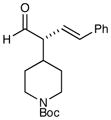 73% yield 96% ee 73% yield 96% ee |
| 7 |
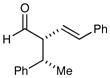 84% yield ≥20:1 dr 84% yield ≥20:1 dr |
8f |
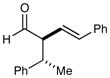 81% yield ≥20:1 dr 81% yield ≥20:1 dr |
Absolute configuration assigned by chemical correlation or analogy.
Isolated yield of the corresponding alcohol.
Enantiomeric excesss determined by chiral HPLC analysis of the corresponding alcohol.
Using 20 mol% 3.
Using 20 mol% CuBr.
Using (S,S)-3 catalyst.
Next we turned our attention to the scope of the vinyl coupling partner. As demonstrated in Table 3, these merged catalysis conditions are amenable to a broad range of iodonium triflates. Both electron-neutral and electron-poor styrenes (entries 1 and 2) as well as alkylvinyl groups (entries 3, 4 and 7) are transferred with high levels of enantiocontrol (≥93% ee).15 Traditionally metal-sensitive structural motifs such as allyl chlorides and allyl ethers (entries 5 and 6, 94–95% ee) are also readily incorporated to deliver useful synthons for subsequent elaboration. Moreover, this novel transformation is not limited to 1,2-disubstituted alkenyl group couplings but can also be applied to trisubtituted carbocycles (entries 9 and 10, 71–77% yield, 94–96% ee).16 The selective transfer of sterically demanding trisubstituted olefins can also be accomplished; however, this system requires the use of the corresponding mesityliodonium salt to avoid a competing aryl group transfer (entry 8).17
Table 3.
| |||
|---|---|---|---|
| 1 |
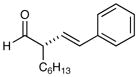 84% yield, 97% ee |
2 |
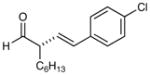 87% yield, 96% ee |
| 3 |
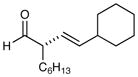 76% yield, 97% ee |
4 |
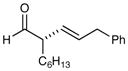 89% yield, 97% ee |
| 5d |
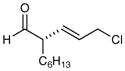 91% yield, 94% ee |
6 |
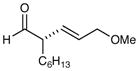 73% yield, 95% ee |
| 7 |
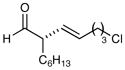 81% yield, 93% ee |
8e,f,g |
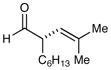 73% yield, 97% ee |
| 9e |
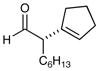 77% yield, 94% ee |
10e |
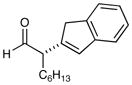 71% yield, 96% ee |
Absolute configuration assigned by chemical correlation or analogy.
Isolated yield of the corresponding alcohol.
Enantiomeric excess determined by chiral HPLC or SFC analysis of the corresponding alcohol.
1H NMR yield.
Using 20 mol% 3.
Using 30 mol% CuBr.
Using the corresponding mesitylvinyliodonium triflate.
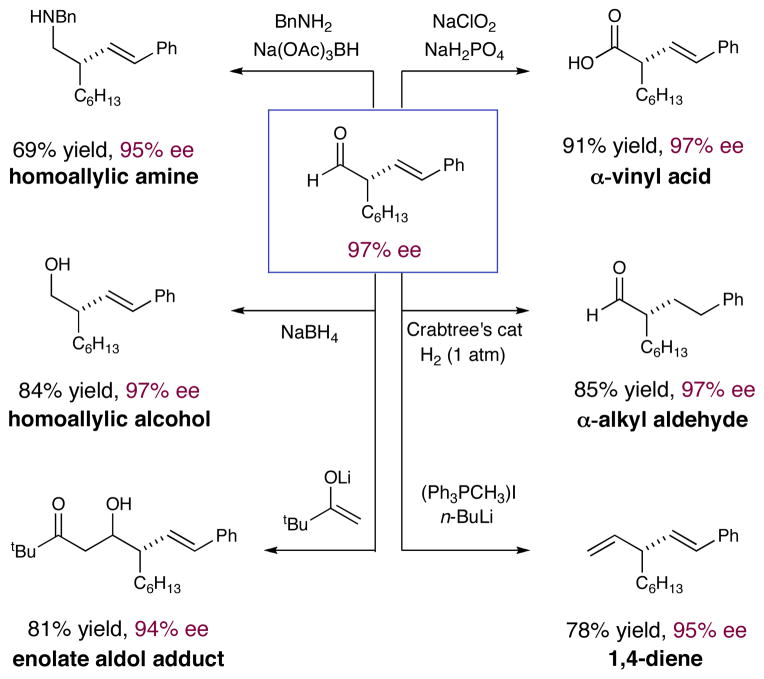
To demonstrate the utility of this new enantioselective vinylation reaction, we performed a series of subsequent synthetic manipulations to demonstrate that this protocol can lead to a diverse series of synthons (Scheme 2). For example, Pinnick oxidation of the formyl group provides an α-olefin carboxylic acid in high yield with complete preservation of enantiomeric purity. Moreover, hydrogenation of the alkene group using Crabtree’s catalyst leads to α-alkyl aldehyde products, again without loss in optical purity. Importantly, the formyl group can be utilized in a reductive amination sequence to afford a homoallylic amine scaffold in 95% ee, a remarkable outcome given that an iminium intermediate is formed in this process. As a related system, homoallylic alcohols can also be readily accessed via NaBH4 reduction, to generate adducts that should be of value for subsequent diastereoselective reactions such as epoxidation, cyclopropanation etc.18 The addition of metal enolates to these vinylation adducts can also be accomplished to generate aldol products with high efficiency. Lastly, the use of traditional Wittig conditions leads to 1,4-diene formation without loss of enantipurity at the sterogenic 3-position, again demonstrating the versatility of these 1,3-formyl-olefin adducts.
Scheme 2.
Access to Diverse Enantioenriched Vinyl Synthons
In conclusion, we have developed a highly enantioselective protocol for the α-vinylation of aldehydes with vinyl iodonium triflates using copper(I) and chiral amine catalysis in a synergistic coupling mechanism. This operationally simple, room temperature and mild protocol should also be cost effective given the substrates, catalysts and catalyst loadings employed. We fully expect that the α-formyl olefin products generated in this study will be of high value for practitioners of chemical synthesis and medicinal chemistry.
Supplementary Material
Acknowledgments
Financial support was provided by the NIHGMS (R01 GM103558-01) and kind gifts from Merck, Amgen and Abbott. E.S. thanks NIHGMS for a postdoctoral fellowship (5F32 GM096738-01).
Footnotes
Supporting Information Available. Experimental procedures and spectral data are provided.
References
- 1.For reviews on synergistic catalysis, see: Shao Z, Zhang H. Chem Soc Rev. 2009;38:2745. doi: 10.1039/b901258n.Zhong C, Shi X. Eur J Org Chem. 2010:2999.Allen AE, MacMillan DWC. Chem Sci. 2011:633. doi: 10.1039/C2SC00907B.Patil NT, Shinde VS, Gajula B. Org Biomol Chem. 2012;10:211. doi: 10.1039/c1ob06432k.
- 2.It should be noted that synergistic catalysis can lead to the formation of multiple new bonds, as in the case of cycloaddition processes.
- 3.For selected examples of synergistic catalysis, see: Sawamura M, Sudoh M, Ito Y. J Am Chem Soc. 1996;118:3309.Nakoji M, Kanayama T, Okino T, Takemoto Y. Org Lett. 2001;3:3329. doi: 10.1021/ol016567h.Kamijo S, Yamamoto Y. Angew Chem, Int Ed. 2002;41:3230. doi: 10.1002/1521-3773(20020902)41:17<3230::AID-ANIE3230>3.0.CO;2-W.Sammis GM, Danjo H, Jacobsen EN. J Am Chem Soc. 2004;126:9928. doi: 10.1021/ja046653n.Ibrahem I, Córdova A. Angew Chem, Int Ed. 2006;45:1952. doi: 10.1002/anie.200504021.Komanduri V, Krische MJ. J Am Chem Soc. 2006;128:16448. doi: 10.1021/ja0673027.Mukherjee S, List B. J Am Chem Soc. 2007;129:11336. doi: 10.1021/ja074678r.Hu W, Xu X, Zhou J, Liu W, Huang H, Hu J, Yang L, Gong L. J Am Chem Soc. 2008;130:7782. doi: 10.1021/ja801755z.Nicewicz D, MacMillan DWC. Science. 2008;322:77. doi: 10.1126/science.1161976.Shi Y, Roth KE, Ramgren SD, Blum SA. J Am Chem Soc. 2009;131:18022. doi: 10.1021/ja9068497.Ikeda M, Miyake Y, Nishibayashi Y. Angew Chem, Int Ed. 2010;49:7289. doi: 10.1002/anie.201002591.Raup DEA, Cardinal-David B, Holte D, Scheidt KA. Nature Chemistry. 2010;2:766. doi: 10.1038/nchem.727.Trost BM, Luan X, Miller Y. J Am Chem Soc. 2011;133:12824. doi: 10.1021/ja204817y.Ibrahem I, Santoro S, Himo F, Córdova A. Adv Synth Catal. 2011;353:245.Simonovich SP, Van Humbeck JF, MacMillan DWC. Chem Sci. 2012;3:58. doi: 10.1039/c1sc00556a.
- 4.Allen AE, MacMillan DWC. J Am Chem Soc. 2010;132:4986. doi: 10.1021/ja100748y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen AE, MacMillan DWC. J Am Chem Soc. 2011;133:4260. doi: 10.1021/ja2008906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The combination of copper catalysts and aryliodonium salts has been elegantly employed to generate arylated indoles via a Cu(III) species: Phipps RJ, Grimster NP, Gaunt MJ. J Am Chem Soc. 2008;130:8172. doi: 10.1021/ja801767s.
- 7.For transition metal catalyzed enolate vinylations, see: Chieffi A, Kamikawa K, Ahman J, Fox JM, Buchwald SL. Org Lett. 2001;3:1897. doi: 10.1021/ol0159470.Poulsen TB, Bernardi L, Bell M, Jørgensen KA. Angew Chem, Int Ed. 2006;45:6551. doi: 10.1002/anie.200602275.Taylor AM, Altman RA, Buchwald SL. J Am Chem Soc. 2009;131:9900. doi: 10.1021/ja903880q.
- 8.For transition metal catalyzed vinylation of α-halo carbonyl compounds, see: Dai X, Strotman A, Fu GC. J Am Chem Soc. 2008;130:3302. doi: 10.1021/ja8009428.Lou S, Fu GC. J Am Chem Soc. 2010;132:5010. doi: 10.1021/ja1017046.
- 9.For enantioselective organocatalytic SOMO vinylation of aldehydes, see: Kim H, MacMillan DWC. J Am Chem Soc. 2008;130:398. doi: 10.1021/ja077212h.
- 10.For reviews on iodonium salts, see: Varvoglis A. The Organic Chemistry of Polycoordinated Iodine. VCH Publishers; New York: 1992. Zhdakin VV, Stang PJ. Chem Rev. 2002;102:2523. doi: 10.1021/cr010003+.Stang PJ. J Org Chem. 2003;68:2997. doi: 10.1021/jo030022e.Zhdakin VV, Stang PJ. Chem Rev. 2008;108:5299. doi: 10.1021/cr800332c.
- 11.For carbonyl α-vinylation with iodonium salts, see: Beringer FM, Galton SA. J Org Chem. 1965;30:1930. doi: 10.1021/jo00826a001.Ochiai M, Sumi K, Takaoka Y, Kunushima M, Nagao Y, Shiro M, Fujita E. Tetrahedron. 1988;44:4095.Ochiai M, Shu T, Nagaoka T, Kitagawa Y. J Org Chem. 1997;62:2130. doi: 10.1021/jo962007y.
- 12.For studies on the mechanism of copper-catalyzed arylation, see: Beringer FM, Geering EJ, Kuntz I, Mausner M. J Phys Chem. 1956;60:141.Lockhart TP. J Am Chem Soc. 1983;105:1940.
- 13.The phenyliodonio group is a great nucleofuge, some 106 times more reactive than triflate, see: Okuyama T, Takino T, Sueada T. J Am Chem Soc. 1995;117:3360.Okuyama T. Acc Chem Res. 2002;35:12. doi: 10.1021/ar0100374.
- 14.DFT calculations for the enamine were performed with the use of B3LYP/6-311+G(2d,2p)//B3LYP/6-31G(d).
- 15.Electron rich styrenyliodonium salts exhibit poor stability, see; Thielges S, Bisseret P, Eustache J. Org Lett. 2005;7:681. doi: 10.1021/ol047516y.
- 16.This result is complementary to enantioselective α-vinylation studies using organo-SOMO catalysis, wherein carbocyclic olefinic substrates are not readily employed, see ref 7.
- 17.(a) Kalyani D, Deprez NR, Desai LV, Sanford MS. J Am Chem Soc. 2005;127:7330. doi: 10.1021/ja051402f. [DOI] [PubMed] [Google Scholar]; (b) Deprez NR, Sanford MS. J Am Chem Soc. 2009;131:11234. doi: 10.1021/ja904116k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoveyda AH, Evans DA, Fu GC. Chem Rev. 1993;93:1307. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.