Abstract
Background
Borderline personality disorder (BPD) is characterized by an inability to regulate emotional responses. The amygdala is important in learning about the valence (goodness and badness) of stimuli and has been reported to function abnormally in BPD.
Methods
Event-related functional MRI (fMRI) was employed in three groups: unmedicated BPD (n=33) and schizotypal personality disorder (SPD;n=28) participants and healthy controls (n=32) during a task involving an intermixed series of unpleasant, neutral, and pleasant pictures each presented twice within their respective trial block/run. The amygdala was hand-traced on each participant’s structural-MRI scan which was co-registered to their BOLD-scan. Amygdala responses were examined with a mixed-model MANOVA with repeated measures.
Results
Compared with both control groups, BPD patients showed greater amygdala activation, particularly to the repeated emotional but not neutral pictures and a prolonged return to baseline for the overall BOLD response averaged across all pictures. Despite amygdala overactivation, BPD patients showed a blunted response on the self-report ratings of emotional but not neutral pictures. Fewer dissociative symptoms in both patient groups were associated with greater amygdala activation to repeated unpleasant pictures.
Conclusions
The increased amygdala response to the repeated emotional pictures observed in BPD was not observed in SPD patients suggesting diagnostic specificity. This BPD-related abnormality is consistent with the well-documented clinical feature of high sensitivity to emotional stimuli with unusually strong and long-lasting reactions. The finding of a mismatch between physiological and self-report measures of emotion reactivity in BPD patients suggests they may benefit from treatments which help them recognize emotions.
Keywords: borderline personality disorder, schizotypal personality disorder, amygdala, emotion, fMRI, arousal, valence
Deficits in emotion regulation are a core feature of borderline personality disorder (BPD)(1–4). Patients with BPD have suicide rates 50 times the general population (5), utilize more mental health resources than individuals with other psychiatric disorders (6–7) and because BPD is present in approximately 2–5.9% of the general population, it is at least as prevalent as schizophrenia and bipolar I disorder (8–10). Affective instability in BPD is characterized as an inability to regulate emotional responses (11–12) with a high sensitivity to emotional stimuli and unusually strong and long-lasting reactions (2,13). This phenomenological description suggests that BPD patients may have an abnormality in their decrement of response to repeatedly presented emotional stimuli. Habituation is defined as the decrease in physiological responsivity that occurs to a repeated presentation of the same stimulus (14–15). Understanding the neutral substrates of emotion-processing deficits in BPD may ultimately help target biological or psychological treatments and predict which individuals with BPD respond best to a specific type of treatment. This strategy has shown promise in predicting response to cognitive behavioral therapy in depressed patients, e.g.,(16).
The amygdala plays an important role in modulating attention/vigilance particularly in potentially threatening social situations and perceiving the valence of events/objects and emotional expressions of others (17–19). Translational neuroscience animal models indicate the amygdala plays a central role in learning about unpleasant- and pleasantly-valenced stimuli (20). Given BPD patients exhibit emotion dysregulation, it is not surprising that the amygdala is the most investigated brain structure in fMRI studies of this disorder and the majority employed standardized-photographic images from the International Affective Picture Show (IAPS)(21). Yet, regardless of whether pictures, faces, or scripts were used, studies primarily show amygdala overactivity in BPD during the processing of unpleasant stimuli (13,22–27).
One possible mechanism accounting for amygdala overactivity in BPD is impaired habituation. Habituation is one of the most documented and fundamental forms of nervous system plasticity (28). The initial response to a novel stimulus involves a rapid shift of attentional processes (i.e. an orienting response), but with one or more repeated presentations without meaningful consequences, response amplitude is reduced. Prior studies in healthy adults document strong evidence of a decrement in the amygdala BOLD response to repeatedly presented emotional stimuli, e.g.,(18,29). Animal models (30–31) and human work (32–33) also pinpoint the amygdala as an important component of the system involved in the acquisition and memory storage of unpleasant stimuli. The present study is the first to examine whether BPD patients show abnormal amygdala habituation and/or differences in the shape, amplitude, and habituation of the BOLD response to repeated-emotional stimuli.
We (34) and others (35–37) have shown that BPD patients exhibit exaggerated affective startle to borderline-salient stimuli compared with HCs which may be mediated by symptom severity (e.g., 37). Animal models indicate whole-body startle is modulated by the amygdala in the context of fear-conditioning (20) and we examined startle-eyeblink amplitude, a component of whole-body startle during the processing of borderline-salient (e.g., suicidal) and neutral (e.g., coin) words. Compared with HCs, BPD patients showed exaggerated startle amplitude during unpleasant but not neutral words (34). In contrast, on self-report, the BPD patients showed a blunted response by rating the unpleasant words as less unpleasant but did not differ from the HCs for the neutral-word condition. This mismatch between the physiological and subjective response to emotional stimuli is consistent with a psychophysiological ambulatory monitoring study which also reported an inability to label emotions (35) and fMRI studies reporting a mismatch between amygdala activation and self-report ratings in BPD (22). The present study further examines the concept that BPD is characterized by a mismatch between physiological and self-report responses to emotional stimuli.
This fMRI study addresses several key issues unresolved by prior BPD work. First, given unpleasant pictures are highly arousing compared with neutral pictures or a resting state, we controlled for both valence and arousal levels in our study by including three picture conditions (unpleasant/high arousal-vs.-neutral/low arousal-vs.-pleasant/high arousal) using the standardized-IAPS library (21). Second, as a reliability check, subjective emotion was measured using self-report ratings of the pictures both in the magnet and following the session. Third, the diagnostic specificity of amygdala dysfunction in BPD was addressed by including BPD patients with no schizotypal personality disorder (SPD) traits and a psychiatric-control group of SPD patients without BPD traits. Fourth, the shape of the amygdala BOLD-response curve and its change over time was examined during novel and repeated presentations of the emotional and neutral pictures.
To examine the hypothesis that BPD patients exhibit high sensitivity to emotional stimuli and unusually strong and long-lasting reactions as measured by amygdala activation, we presented each of the pictures twice within their respective trial block/run. This allowed an examination of changes in the BOLD response from the novel- to the repeated-picture presentation. Additionally, we examined the time-course of the amygdala BOLD response curves. Compared with both control groups, we hypothesized that BPD patients would show a (a) protracted amygdala BOLD response (i.e. slower return to baseline), particularly following emotional pictures; (b) pattern of greater amygdala activation to repeated compared with novel emotional but not neutral pictures; and (c) mismatch between their amygdala and self-report response to emotional pictures. Exploratory correlations between amygdala activation to the repeated-unpleasant pictures and self-reported symptom severity scales were conducted separately for the patient groups. We used the strict criteria of Vul et al (38) to conduct our correlational analysis which involved the mean BOLD response (AUC) for all voxels within our amygdala region-of-interest (i.e. aggregated data) which was traced on structural-MRI for each participant blind to their diagnosis and functional-imaging data. A standard whole-brain analysis using FSL(4.1)(39) was also conducted to confirm our amygdala region-of-interest results and explore other regions.
Methods
Participants
Thirty-three patients with BPD, 28 patients with SPD and 32 HCs were included (Table-1; Table-S1) for additional demographic/clinical/exclusionary-criteria details). The groups did not significantly differ in age, gender, or education and all patients met DSM-IV criteria. All patients were unmedicated at the time of their fMRI scan (>6 weeks) and most were never-previously medicated. Patients with a history of schizophrenia, psychotic disorder, bipolar (Type I) affective disorder, or current major depressive disorder (MDD; episode occurring within 2 months of the scan) were excluded. Healthy control participants had no Axis I or II diagnosis and no Axis I disorder in any first-degree family member. All participants provided written informed consent in accordance with the Mount Sinai School of Medicine Institutional Review Board guidelines.
Table 1.
Demographics for Healthy Control, Borderline Personality (BPD), and Schizotypal Personality Disorder (SPD) Groups
| Characteristic | Healthy Controls (n=32) | BPD patients (n=33) | SPD patients (n=28) | t value | p value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | Age | ||
|
| |||||||||||
| Age (years): | 32.8 | 9.7 | 22–57 | 31.6 | 9.1 | 18–51 | 35.9 | 11.0 | 20–55 | N vs. BPD: t(63)= 0.52 | 0.61 |
| Education*: | 4.53 | 2.78 | 1–9 | 3.85 | 2.29 | 1–8 | 4.0 | 2.05 | 1–8 | N vs. SPD: t(59)= −1.16 | 0.25 |
| Sex: | N | % | N | % | N | % | N | BPD vs. SPD: t(58)= −1.67 | 0.10 | ||
|
|
|||||||||||
| Male | 12 | 38% | 13 | 39% | 16 | 57% | 12 | Education
|
|||
| Female | 20 | 62% | 20 | 61% | 12 | 43% | 20 | N vs. BPD: t(63)= 1.08 | 0.28 | ||
| Handedness: | N | % | N | % | N | % | N vs. SPD: t(59)= −0.27 | 0.79 | |||
| Right | 29 | 91% | 29 | 88% | 24 | 86% | BPD vs. SPD: t(58)= 0.83 | 0.41 | |||
|
|
|||||||||||
| Left | 2 | 6% | 4 | 12% | 3 | 11% | Sex
|
||||
| Both | 1 | 3% | 0 | 0% | 1 | 3% | N vs. BPD: t(63)= 0.15 | 0.88 | |||
| Symptom Severity **: | - | - | 7.77 | 1.26 | 5.5–10 | 7.27 | 1.15 | 5–10.5 | N vs. SPD: t(59)= 1.53 | 0.13 | |
| Past MDD***: | N | % | N | % | N | % | BPD vs. SPD: t(58)= 1.38 | 0.17 | |||
|
|
|||||||||||
| - | - | 21 | 64% | 6 | 21% | Handedness
|
|||||
| Psychoactive Meds: | N | % | N | % | N | % | N vs. BPD: t(63)= 0.04 | 0.97 | |||
| Never Medicated | - | - | 16 | 48% | 23 | 82% | N vs. SPD: t(59)= −0.46 | 0.65 | |||
| Previously Medicated | - | - | 17a | 52% | 5b | 18% | BPD vs. SPD: t(58)= −0.55 | 0.58 | |||
All diagnoses were made using a structured-diagnostic interview conducted by a psychologist using the Structured Clinical Interview for DSM-IV Axis I (74) and the Structured Interview for DSM-IV Personality Disorders (75), followed by a consensus meeting. Only BPD (kappa=0.82) and SPD (kappa=0.73) patients without diagnostic comorbidity were included (i.e. BPD patients without SPD traits and SPD patients without BPD traits). Exclusion criteria for all participants included severe medical or neurological illness, head injury, any prior substance dependence or substance abuse during the past six months. Healthy controls (100%) and patients (90%) were recruited through advertisements in local newspapers and the remaining patients (10%) from psychiatric outpatient clinic referrals at Mount Sinai Hospital. All participants were right handed and had a negative urine toxicology screen for drugs of abuse, and women, a negative pregnancy test on scan day.
Education = highest degree earned: 1=no high school diploma; 2=GED; 3=high school diploma; 4=technical training; 5=some college, no degree; 6=associate degree; 7=bachelor’s degree; 8=master’s degree; 9=MD/PhD/JD/PharmD.
Symptom severity: For each patient, each of the DSM-IV criteria for each personality disorder was rated on a 4-point scale (0 = absent, 0.5 = somewhat present, 1.0 = definitely present/prototypic, 2.0 = severe, pervasive). As required for a DSM-IV diagnosis of BPD, these patients met at least five of the nine DSM-IV criteria. BPD patients were allowed no more than three SPD criteria with two items rated as 1.0 and one item rated as 0.5. As required for a DSM-IV diagnosis of SPD, these patients met at least five of the nine SPD criteria with a rating ≥ 1.0. SPD patients were allowed no more than three BPD criteria with two items rated as 1.0 and one item rated as 0.5 in order to control for comorbidity and/or traits. To quantify the level of clinical symptom severity, we added up the individual symptom ratings for each diagnostic criterion.
MDD = past major depressive disorder (MDD) was defined as prior episode occurring > two months from the time of fMRI scan.
Of the 17 BPD patients who previously received psychoactive medications, 14 received antidepressants, 2 received antipsychotics, 2 received benzodiazepines, 3 received stimulants, and 2 received mood stabilizers.
Of the 5 SPD patients who previously received psychoactive medications, 1 received an antipsychotic, 5 received antidepressants, and 2 received stimulants.
With a few exceptions noted in Table-S1, all participants completed psychometric self-report measures of aggression (Buss-Perry Aggression Questionnaire; BPAQ;40), impulsivity (Barratt Impulsiveness Scale-11; BIS11;41), affective lability (Affective Lability Scale; ALS;42), dissociative symptoms (Dissociative Experiences Scale; DES;43), affective intensity (Affective Intensity Measure; AIM;44), and childhood abuse and neglect (Childhood Trauma Questionnaire; CTQ;45). One-way between-group (HC-vs.-BPD-vs.-SPD) ANOVAs were conducted on the total scores and follow-up t-tests were conducted to determine which groups differed (Table-S1).
Functional and Structural MRI Acquisition
The MRI scan procedure was conducted on a Siemens-Allegra head-dedicated 3T scanner and included a T2, EPI, and T1-weighted structural MP-RAGE (Magnetization-Prepared-Rapid-Gradient-Echo scan). See Figure S1 in the Supplement for scan-parameter details.
Event-related fMRI affective picture processing task
During the fMRI scan, participants viewed unpleasant, neutral, and pleasant photographic pictures (from the IAPS (21); see Supplement). A total of 96 intermixed unpleasant, neutral, and pleasant photographic pictures were presented a. E-Prime software (46) was used for the design and presentation of all stimuli in the scanner. The 96 pictures were presented twice within their respective run for a total of 192 picture trials. Each trial was 8-sec long and included either (a) the presentation of a picture (for 6-sec) followed by a 3-choice button press response prompt (for 2-sec; described in detail below) or (b) a fixation cross (8-sec). The presentation of either a picture or fixation cross was semi-randomized with the number of consecutive trials varying from 1–6 for pictures and 1–3 for fixation trials. Each run contained 24 unique pictures (8 unpleasant, 8 neutral, 8 pleasant) which were repeated once (48 picture events) and 16 non-picture (fixation cross) events (total=64 contiguous trials per run). The total scan time was 38-min, 12-sec which was divided into four runs with 30-sec before and 31-sec after each run (30+(8*64)+31=573 sec;4 runs=2292 sec).
We chose predominantly social pictures including faces and social interactions. Across the four runs, the unpleasant and pleasant pictures were matched based on the picture ratings from the standardized IAPS manual for arousal (all p>0.28) and for valence they were equally divergent from neutral. The neutral pictures were matched across each of the four runs on arousal and valence. All participants viewed the same stimulus sequence.
Participants were instructed to attend to the pictures and think about their meaning for them personally. Immediately following the offset of each picture, a cartoon-like picture of a right hand with the pointer finger labeled as pleasant, middle finger labeled as neutral, and the ring finger labeled as unpleasant appeared for 2-sec. As soon as participants saw the hand prompt, they made a 3-choice response with their right hand using a BrainLogics fiber optic button system (Psychology Software Tools, Inc., Pittsburgh, PA). The responses following each picture were recorded on a desk-top computer and helped to ensure that participants were continuously engaged in the task. Immediately following the scan, participants viewed the same 96 pictures again outside the magnet on a laptop and rated them using the Self-Assessment Manikin scale (SAM;9-point scale)(47).
Image processing
We conducted two kinds of event-related analyses of the functional-imaging data: (a) amygdala BOLD response time-series analysis based on hand-traced regions-of-interest and (b) a standard whole-brain GLM-analysis using FSL.
For both approaches, FSL’s fMRI Expert Analysis Tool (39) was used for image processing. The BOLD data were preprocessed with motion correction using MCFLIRT(48), non-brain removal using BET(49), spatial smoothing (FWHM=5 mm) and a high-pass temporal filter (cutoff=70 sec). The MP-RAGE and EPI images were co-registered with a 7-degrees-of-freedom (DOF) linear transformation followed by alignment to the MNI brain template using a 12-DOF linear fit.
Amygdala delineation and region-of-interest analysis
For each participant, we traced the amygdala volume on anterior commissure-posterior commissure positioned structural/MP-RAGE images using our published methods (Figure S1 in the Supplement). Following the coregistration, we obtained the mean BOLD response time-series values (1–11 with each 3-sec epoch beginning at picture onset and continuing to 33-sec; 11x3=33 sec) for the fMRI hemodynamic response curves averaged across the voxels within each hemisphere of the amygdala region-of-interest for each of the key stimulus conditions (novel/unpleasant/startle, novel/unpleasant/no startle, repeated/unpleasant/startle, repeated/unpleasant/no startle, novel/pleasant/startle, novel/pleasant/no startle, repeated/pleasant/startle, repeated/pleasant/no startle, novel/neutral/startle, novel/neutral/no startle, repeated/neutral/startle, repeated/neutral/no startle) averaged across all runs. We have previously published (50) our methods for using a similar time-series approach for the BOLD response.
We conducted a Group (HC-vs.-BPD-vs.-SPD) × Picture type (U, N, P) × Picture repetition (novel, repeated) × Startle stimulus (startle presented 4000 msec following picture onset, no startle stimulus) × Hemisphere (left, right) × Time (1 to 11: 3-sec, 6, 9…33-sec following picture onset) MANOVA using Statistica (51). Diagnostic group was the between-group factor and the remaining factors were all repeated-measures. We report multivariate F-values (Wilks Lambda) or univariate F with Huynh-Feldt adjusted p-values. Significant effects with Group, were followed-up using Fisher’s LSD tests (shown in figures).
To limit the number of exploratory clinical correlations, Pearson correlation coefficients were calculated between BOLD activation (area under the curve) in the amygdala (averaged across hemisphere, startle/no startle, and time) during the repeated unpleasant, neutral, and pleasant pictures for each patient group.
Standard whole-brain GLM analysis
Image-processing steps for the whole-brain analysis are described in Figure-3. The statistical images were thresholded using clusters determined by Z>2.0 and a corrected-cluster significance threshold of p<0.05 (52) with a color bar showing p<0.05 (corrected). We also report the z-score and cluster size for the significant regions (Table-S2). For the amygdala clusters, we used the amygdala mask from the Harvard-Oxford atlas included in FSL.
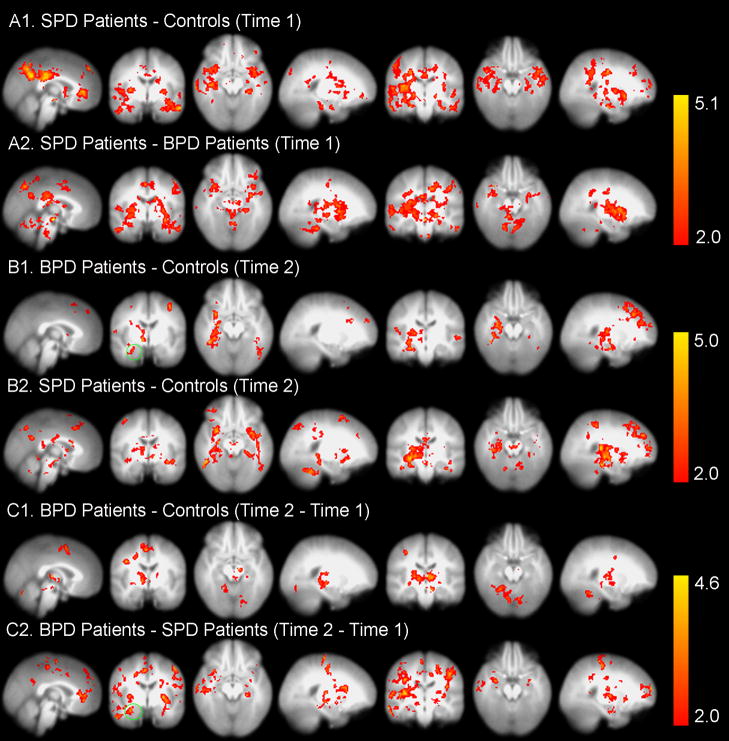
Figure 3.
Statistical probability maps from FSL(4.1)(39) for the between-group effects in response to unpleasant pictures are shown. The maps are thresholded at Z>2.0 (or p<0.05, corrected) and the color bar shows p<0.05 (corrected). See Table-S2 for the size and location of the clusters.
A1–2. These SPM results demonstrate increased amygdala activity in SPD patients compared with HCs (A1) and in the SPD patients compared with BPD patients (A2) in response to the novel or first (i.e. Time 1) presentation of the unpleasant pictures.
B1–2. With the repeated presentation of unpleasant pictures, the whole-brain analysis showed increased activity in the right amygdala (region inside the green circle) and left fusiform gyrus (BA37) in the BPD patients compared with the HCs (B1) and in regions including the bilateral amygdala and right dorsolateral prefrontal cortex (DLPFC; BA10) in the SPD patients compared with the HCs (B2).
C1–2. Between-group effects for (Time 2 (repeated) – Time 1 (novel)) differences. C1. Activity was increased in brain areas including the right fusiform gyrus (BA37), anterior cingulate (BA24), and inferior frontal gyrus (BA44) in BPD patients compared with HCs. C2. Activity was also increased in BPD compared with SPD patients in regions including left and right (green circle) amygdala and right DLPFC (BA10).
Standard whole-brain GLM analysis. GLM analysis was first carried out on the preprocessed fMRI data for each single subject with FILM (FMRIB’s Improved Linear Model) with six contrasts set for unpleasant, neutral, and pleasant pictures at novel presentation (Time-1) and repeated presentation (Time-2). Next, single-subject statistics were fed into second-level multi-session, multi-subject analysis. The between-group t-test analysis was performed on the three separate groups with FLAME (FMRIB’s Local Analysis of Mixed Effects).
Results
Amygdala activation during picture processing
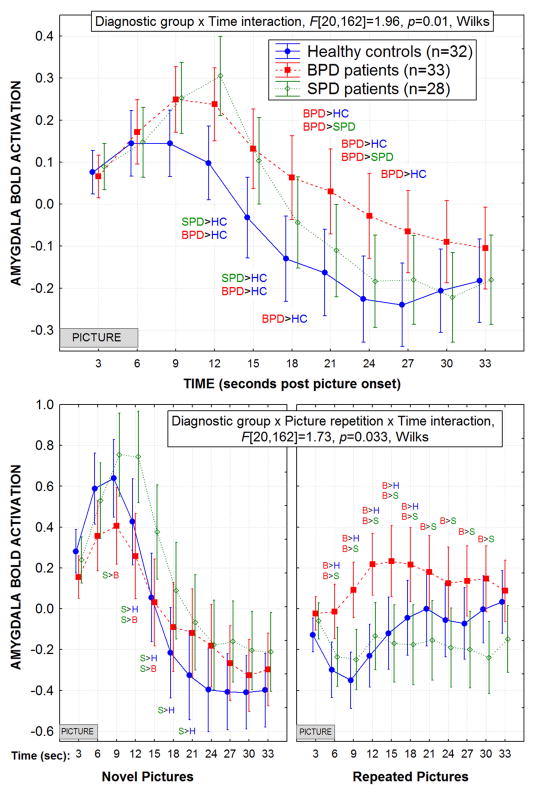
Following picture onset, the BPD patients exhibited an overall amygdala BOLD response curve (averaged across all repeated-measures except Time) with a much slower return to baseline compared with the HC and SPD groups (Figure-1-Top). The HCs showed the smallest amygdala BOLD response peak while the SPD group showed the greatest, and the BPD patients were intermediate. The HCs also had the fastest peak latency, SPD patients showed the slowest, and BPD patients were intermediate, Group × Time interaction, F[20,162]=1.96, p=0.012, Wilks. The BPD group showed the smallest overall peak response in the amygdala during the novel pictures yet, the greatest overall peak response during the repeated pictures, Group × Picture repetition (novel, repeated) × Time interaction, F[20,162]=1.73, p=0.033, Wilks (Figure-1-Bottom).
Figure 1.
Top: The amygdala BOLD response curve is shown for each of the three groups averaged across all repeated measures (picture type, repetition, startle/no startle, and hemisphere) except for Time (3, 6, 9…33 sec following picture onset). The amygdala was hand traced on each individual study participant’s structural MRI (MP-RAGE) and co-registered to their EPI scan. The borderline personality disorder (BPD) patients showed an amygdala response curve which peaked later than the healthy controls and took longer to return to baseline compared with both healthy controls and schizotypal personality disorder (SPD) patients. This BPD-related pattern of a protracted amygdala response is consistent with the concept that BPD patients have long-lasting responses to emotional stimuli. The SPD patients showed the longest peak latency and highest peak response in the amygdala while the controls showed the shortest peak response and lower overall BOLD activation in the amygdala. As shown, this Group × Time interaction was significant. For both graphs, the significant post-hoc Fisher’s LSD tests, p<0.05 are noted and the standard error bars are provided.
Bottom: The BPD group showed a greater overall BOLD response in the amygdala during the repeated pictures compared with both the healthy controls and the SPD patients. In contrast, the SPD group showed a higher peak during the novel pictures. As shown, this Group × Picture repetition (novel, repeated) × Time interaction was significant.
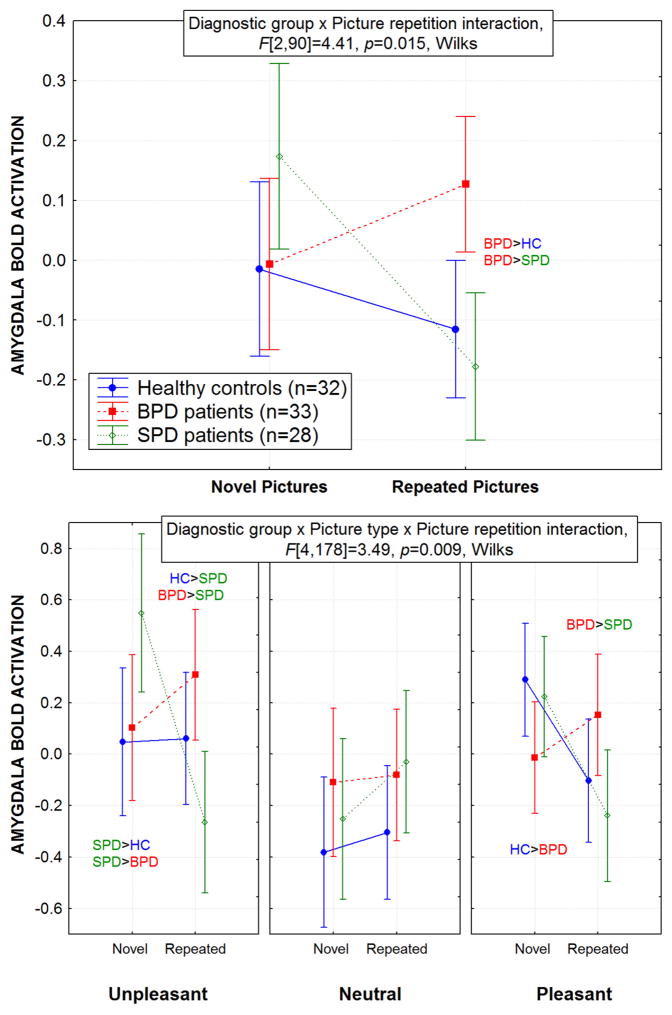
Averaged across the Time factor discussed above, the BPD patients exhibited a normal pattern of overall amygdala activation during novel pictures but greater amygdala activation during repeated pictures compared with both HCs and SPD patients (Figure-2-Top). Interestingly, the SPD group showed an opposite pattern from BPD with greater amygdala activation during the novel pictures and normal activation during the repeated pictures, Group × Picture repetition interaction, F[2,90]=4.41, p=0.015.
Figure 2.
Top: Overall, the BPD group showed greater amygdala activation during the repeated pictures compared with the healthy control and SPD groups. In contrast, the SPD group showed greater amygdala activation during the novel picture presentation. As shown, this Group × Picture repetition (novel, repeat) interaction was significant. Significant post-hoc Fisher’s LSD tests, p<0.05 and standard error bars are shown for both graphs.
Bottom: For each of the groups, the mean amygdala BOLD response is shown during the novel and repeated picture presentations for each of the three picture types. Compared with healthy controls and schizotypal personality disorder (SPD) patients, the borderline personality disorder (BPD) patients showed an increase in their mean amygdala response from the novel to the repeated emotional (both the unpleasant and pleasant pictures) pictures. This Group × Picture type (unpleasant, neutral, pleasant) × Picture repetition (novel, repeat) interaction was significant.
Of greatest interest, Figure-2-Bottom shows that compared with HCs and SPD patients, the BPD patients exhibited a pattern of greater amygdala BOLD activation to the emotional (both unpleasant and pleasant) but not the neutral pictures when they were repeated. In striking contrast, the SPD patients showed greater amygdala activation to the neutral pictures when repeated, compared with the HCs and BPD patients. HCs showed greater amygdala activation than BPD patients to the novel-pleasant pictures while the BPD group showed greater activation to the repeated-pleasant pictures. This complex pattern was significant, Group × Picture type (U, N, P) × Picture repetition (novel, repeat) interaction, F[4,178]=3.49, p=0.009, Wilks. The startle/no startle and hemisphere factors did not interact with Group and none of the other interactions with Group reached significance.
None of the BPD patients studied had a current MDD diagnosis. But in order to address the issue of whether our findings are related to a vulnerability to depression rather than BPD per se, we compared patients with a past history of MDD to those without any history of MDD. This two-group MANOVA failed to show a main effect of Group or any interaction with Group indicating these BPD subgroups did not significantly differ from each other in terms of their amygdala BOLD response pattern (p-values>0.41).
Whole-brain activation during picture processing
The amygdala results revealed in the whole-brain analysis were very consistent with the results of our primary region-of-interest amygdala analysis (Figure-3).
Like the amygdala region-of-interest analysis, the comparison between patients with a past history of MDD and those without any history of MDD failed to reach significance for the whole-brain analysis.
Self-report ratings of picture valence
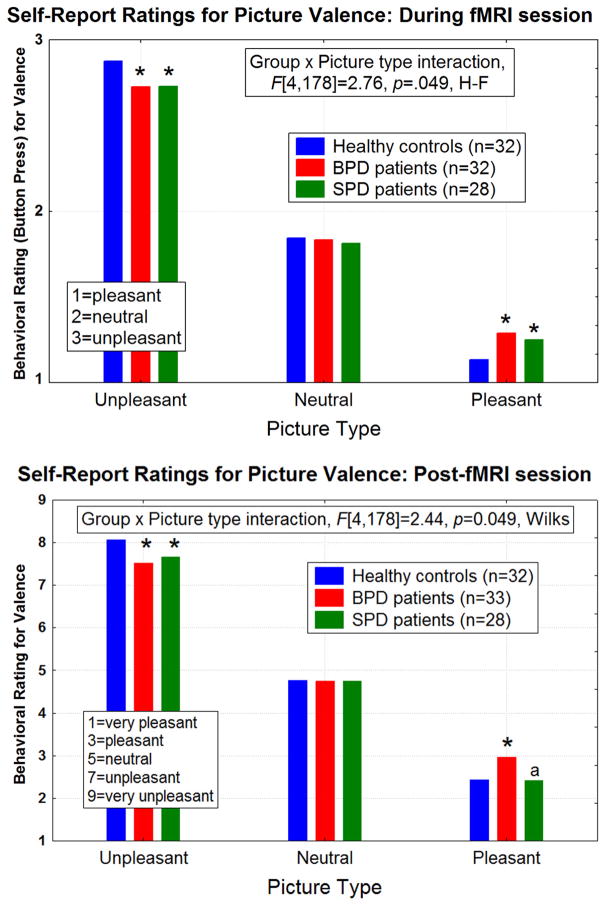
During fMRI session
Compared with HCs, both BPD and SPD patients showed a blunted response on self-report ratings of emotional picture valence, i.e. rating unpleasant pictures as less unpleasant and pleasant pictures as less pleasant (Group × Picture type interaction, F[4,178]=2.76, p=0.049 H-F; Figure-4-Top). There were no between-group differences for the neutral-picture ratings. Follow-up post-hoc tests revealed that both patient groups showed a pattern of a general-blunted response to the emotional but not the neutral pictures (all p-values<0.007).
Figure 4.
Group means for the self-report ratings of picture valence are shown for the pictures viewed in the magnet (Top graph) and following the fMRI session (Bottom graph using the Self-Assessment Manikin (SAM) scale (47)). There were no between-group differences for the neutral picture ratings. Both Group × Picture type interactions are significant as shown.
Top: During the fMRI session, both the borderline personality disorder (BPD) and schizotypal personality disorder (SPD) groups showed blunted (i.e. rated unpleasant and pleasant as more neutral) self-report ratings of the emotional pictures compared with healthy controls. Asterisks denote significant differences from the healthy controls (follow-up Fisher’s LSD tests), all p-values<0.007.
Bottom: Similarly, the post-fMRI session self-report ratings show that compared with the healthy control group, both the BPD and SPD groups exhibited a blunted response pattern to the unpleasant pictures. BPD patients also showed a blunted response for the pleasant pictures compared with healthy controls and SPD patients. Asterisks denote significant differences from the healthy controls (Fisher’s LSD tests, all p-values<0.007). The “a” represents BPD>SPD, p=0.0002.
Post-fMRI session
The mean self-report valence ratings are shown in Figure-4-Bottom and as expected, looked very similar to the 3-point ratings obtained during the fMRI (described above). Compared with the HC group, both patient groups showed a blunted response on self-report ratings of the unpleasant pictures. The BPD patients also showed a blunted response for the pleasant condition compared with HCs and SPD patients (Group × Picture type interaction, F[4,178]=2.44, p=0.049, H-F; follow-up tests were significant with p-values<0.007).
Symptom correlations
Correlational analyses showed that among the BPD group, greater amygdala BOLD activation to repeated unpleasant (r=0.36, p=0.04; r=0.37, p=0.03) and neutral (r=0.39, p=0.03; r=0.40, p=0.02) pictures was associated with higher aggression and affective lability (measured by the BPAQ and ALS, respectively).
Among both patient groups, greater BOLD activation during the repeated-unpleasant pictures was associated with fewer dissociative symptoms (BPD: r=−0.36, p=0.047; SPD: r=−0.40, p=0.042; see Figure S2 in the Supplement). SPD patients who showed greater BOLD activation during the repeated-pleasant pictures also showed fewer dissociative symptoms (r=−0.43, p=0.03). The BIS, AIM, and CTQ scores were not correlated with amygdala responses. Spearman’s correlations produced the same pattern of significance.
Discussion
Our study has two novel findings reflecting BPD-related abnormalities in amygdala function. First, BPD patients showed reduced overall habituation (time-series) in terms of their BOLD-response curve returning to baseline following picture onset. That is, averaged across the three picture conditions (unpleasant, neutral, and pleasant), BPD patients showed a prolonged amygdala response compared with the HC and SPD groups. The region-of-interest time-series showed and the whole-brain analysis confirmed that in the BPD group, the amygdala was more strongly activated when the same emotional but not neutral pictures were presented the second time. This pattern is consistent with the concept that BPD patients exhibit unusually long-lasting reactions to emotional cues (2,13).
Secondly, BPD patients showed a potentiated amygdala response to emotional pictures when repeated relative to the first (novel) presentation. In contrast, HCs and SPD patients showed either the same level of amygdala response or a decrement in their amygdala response to the repeated emotional pictures, consistent with a pattern of amygdala habituation or emotional learning. This BPD pattern of increased amygdala activation to repeated emotional pictures was not observed in our psychiatric control group of SPD patients, suggesting it has diagnostic specificity. The potentiated amygdala response to the repeated-emotional pictures in BPD indicates an abnormality in a very basic function which has important evolutionary relevance because it allows for optimal allocation of information processing resources, away from emotional stimuli not associated with real threat or reward (29). Our HC finding of similar levels of amygdala activation to novel and repeated unpleasant pictures and a pattern of habituation for pleasant pictures is consistent with other normative studies using the IAPS, (e.g., 53). However, our HCs showed less difference between novel and repeated unpleasant pictures than reported in Phan et al (53). This is likely due to paradigmatic differences, e.g., we compared novel to repeated pictures presented within their respective run while Phan et al examined differences across early and late runs which would likely evince more significant habituation.
In order to differentiate between an abnormality in general arousal vs. valence, the unpleasant and pleasant pictures employed were matched on high arousal level, yet opposite in valence while the neutral pictures were low on arousal level. Thus, our finding of exaggerated and prolonged amygdala response to emotional but not neutral pictures in BPD is consistent with a general arousal deficit rather than an abnormal response to unpleasant/negative stimuli per se. This arousal-deficit interpretation is consistent with psychophysiological work showing that BPD patients exhibit greater-than-normal skin conductance responsivity—a measure of autonomic arousal—while imagining scripts with highly unpleasant BPD-salient themes (abandonment/rejection), as well as, positive themes (36). Future fMRI studies comparing unpleasant stimuli varying in arousal level (e.g., highly-vs.-generally unpleasant pictures) are needed to further understand the neural substrates of this BPD-related arousal deficit.
The BPD patients exhibited a mismatch between their physiological (overactive amygdala) and subjective experience (blunted self-report response) of emotion. This finding is consistent with prior imaging (22,26) and affective startle (an amygdala-based psychophysiological measure)(34) studies showing a disconnect between the subjective experience of emotion and the physiological response in BPD. A blunted response to emotional pictures is consistent with prior work indicating impaired emotion recognition abilities (54–56) and reduced pain perception (e.g., 57) and our results suggest abnormalities in amygdala function may play an important role.
Among all patients, those reporting fewer dissociative symptoms showed greater amygdala activation with repeated unpleasant picture viewing while those reporting more dissociative symptoms showed less amygdala activation. It seems counterintuitive that patients with greater symptom severity would show more normal amygdala habituation yet, this finding suggests that dissociation may serve as a defensive response for coping with unpleasant stimuli. This is consistent with studies showing that BPD patients with low present-state dissociation exhibited larger amplitude startle responses compared to those with high present-state dissociation (58). BPD patients with high state-dissociative experiences also show poor emotional learning evidenced by no increase in self-report valence ratings or a skin conductance measure of arousal to a conditioned stimulus (59).
To our knowledge, this is the first study to examine amygdala function during an emotion paradigm in SPD. Consistent with our finding that the SPD group showed greater overall amygdala activation averaged across all picture types, prior work shows that schizophrenia patients demonstrate greater skin conductance reactivity to emotional and neutral films (60). Unlike the BPD patients, the SPD patients in our study did exhibit habituation of the amygdala response to repeated-emotional pictures. However, they differed from both HCs and BPD patients by showing a pattern of greater activation to the repeated compared with novel-neutral pictures. One possibility is that because a key feature of SPD is paranoid ideation, the patients perceived the neutral pictures as more threatening. We found a blunted self-report response in SPD to the unpleasant but not pleasant pictures (9-point-SAM scale). This is consistent with work showing that individuals with high scores on the suspiciousness subscale of the Schizotypal-Personality Questionnaire (61) demonstrate reduced perception of affect in body posture (62).
Study limitations
While this BPD study has clear strengths including a large sample of unmedicated patients, personality disorder control group of SPD patients, and gold-standard tracing of the amygdala, several limitations merit comment. There are many ways to examine habituation and we examined only two: the neural response to a repeated picture within the same run to minimize scanner drift issues that occur across runs and the habituation (time-series) of the BOLD response. Future fMRI research should design experiments to examine other types of habituation to affective stimuli in BPD. The current study, our prior affective-startle study (34), and other recent work (36) employed stimuli with primarily social and borderline-salient contexts (e.g., picture of a man hitting a woman, the word “alone”, scripts describing scenes of abandonment) and found significant normal-BPD differences. However, it is unclear whether HC-BPD group differences would be observed using non-social stimuli. An experimental manipulation of social-vs.-non-social stimulus content in BPD would be useful.
No significant differences in brain activation were observed between BPD patients with and without a past history of MDD suggesting that our results are not related to the vulnerability to depression. It will be important to replicate this finding in a larger sample. Recent work indicates that compared with HCs, MDD patients show greater amygdala responses to masked sad faces but smaller responses to happy faces (63) suggesting a MDD-related trait-like bias toward excessive processing of unpleasant stimuli. In contrast to MDD, our findings indicate that BPD patients show excessive amygdala activation to both unpleasant and pleasant pictures, consistent with a general arousal deficit.
Summary and Possible implications
We provide evidence that BPD patients exhibit an exaggerated amygdala response when exposed to repeated-emotional pictures and a blunted subjective experience of emotion. This finding may have important clinical implications for the type of treatment—specifically, therapies that focus on developing the skill to recognize one’s own emotional responses—that might be particularly helpful in BPD. The concept that there is a deficit in “mentalization” in BPD has long been recognized by clinicians developing psychotherapeutic treatment for BPD(64). In fact, the psychotherapies that have been empirically demonstrated to be most effective in BPD focus on learning the skill of recognizing one’s own emotions, including mentalization-based therapy (65–66) and Dialectical Behavior Therapy (67–70).
Our findings also have implications for pharmacotherapy treatment given evidence from animal models demonstrating the possibility of altering emotional memories via disruption of reconsolidation at the level of the amygdala (71). There is some evidence that glucocorticoids directly facilitate extinction learning based on their actions in potentiating effects of the glutamatergic N-methyl-d-aspartate (NMDA) receptors in the amygdala (72–73). Future work in this area has direct implications for the treatment of BPD with excellent potential to further refine diagnostic specificity, provide new targets for therapeutic interventions, and glean a useful biological predictor of treatment response in BPD.
Supplementary Material
Acknowledgments
This research was supported by NIMH grant R01MH073911 to E.A.H. and grant UL1RR029887 from the National Center for Research Resources, National Institutes of Health. The authors thank Dr. Monte Buchsbaum for use of the Multi-Image-Processing-Software (MIPS) used to trace the amygdala on structural MRI scans and Dr. Jin Fan for assisting with the E-prime program for the fMRI experiment.
Footnotes
On half of the novel (initial) and repeated-picture presentations, participants heard a brief static noise-burst (50-msec duration, 105dB) through headphones which they were instructed to ignore. A subset of the fixation trials also contained the noiseburst to ensure unpredicatability across trial types. The rationale for presenting the noiseburst during the scan was so that the fMRI paradigm closely resembled our psychophysiological paradigm involving affective startle eyeblink which is conducted outside the scanner.
Financial disclosures
None of the authors report any biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gunderson JG, Phillips KA. A current view of the interface between borderline personality disorder and depression. Am J Psychiatry. 1991;148:967–975. doi: 10.1176/ajp.148.8.967. [DOI] [PubMed] [Google Scholar]
- 2.Linehan MM. Cognitive behavioral treatment of borderline personality disorder. New York: The Guilford Press; 1993. [Google Scholar]
- 3.Skodol AE, Gunderson JG, Pfohl B, Widiger TA, Livesley WJ, Siever LJ. The borderline diagnosis I: psychopathology, comorbidity, and personality structure. Biol Psychiatry. 2002;51:936–950. doi: 10.1016/s0006-3223(02)01324-0. [DOI] [PubMed] [Google Scholar]
- 4.Siever LJ, Torgersen S, Gunderson JG, Livesley WJ, Kendler KS. The borderline diagnosis III: identifying endophenotypes for genetic studies. Biol Psychiatry. 2002;51:964–968. doi: 10.1016/s0006-3223(02)01326-4. [DOI] [PubMed] [Google Scholar]
- 5.Skodol AE, Gunderson JG, McGlashan TH, Dyck IR, Stout RL, Bender DS, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry. 2002;159:276–283. doi: 10.1176/appi.ajp.159.2.276. [DOI] [PubMed] [Google Scholar]
- 6.Zanarini MC, Frankenburg FR, Khera GS, Bleichmar J. Treatment histories of borderline inpatients. Comprehen Psychiatry. 2001;42:144–150. doi: 10.1053/comp.2001.19749. [DOI] [PubMed] [Google Scholar]
- 7.Lieb K, Zanarini MC, Schmahl C, Linehan MM, Bohus M. Borderline personality disorder. Lancet. 2004;364:453–461. doi: 10.1016/S0140-6736(04)16770-6. [DOI] [PubMed] [Google Scholar]
- 8.Coid J, Yang M, Tyrer P, Roberts A, Ullrich S. Prevalence and correlates of personality disorder in Great Britain. Brit J Psychiatry. 2006;188:423–431. doi: 10.1192/bjp.188.5.423. [DOI] [PubMed] [Google Scholar]
- 9.Lenzenweger MF, Lane MC, Loranger AW, Kessler RC. DSM-IV personality disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;62:553–564. doi: 10.1016/j.biopsych.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant BF, Chou SP, Goldstein RB, Huang B, Stinson FS, Saha TD, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. Journal Clin Psychiatry. 2008;69:533–545. doi: 10.4088/jcp.v69n0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunderson JG, Zanarini MC. Pathogenesis in borderline personality. In: Tasman A, Hales RE, Frances AJ, editors. Review of Psychiatry. Washington, D.C: American Psychiatric Press; 1989. pp. 25–48. [Google Scholar]
- 12.Linehan MM. Understanding Borderline Personality Disorder. New York: The Guilford Press; 1995. [Google Scholar]
- 13.Donegan NH, Sanislow CA, Blumberg HP, Fulbright RK, Lacadie C, Skudlarski P, et al. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biol Psychiatry. 2003;54:1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- 14.Andreassi JL. Psychophysiology: Human behavior and physiological response. 2. Hillsdale, N.J: Lawrence Erlbaum Associates; 1989. [Google Scholar]
- 15.Stern RM, Ray WJ, Quigley KS. Psychophysiological Recording. 2. New York, N.Y: Oxford University Press; 2001. [Google Scholar]
- 16.Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am J Psychiatry. 2006;163:735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- 17.Amaral DG. The primate amygdala and the neurobiology of social behavior: implications for understanding social anxiety. Biol Psychiatry. 2002;51:11–17. doi: 10.1016/s0006-3223(01)01307-5. [DOI] [PubMed] [Google Scholar]
- 18.Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J Neuroscience. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Ann Rev Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 20.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 21.Lang PJ, Bradley MM, Cuthbert BN. Technical report A-6. University of Florida; Gainseville, FL: 2005. International affective picture system (IAPS): Instruction manual and affective ratings. [Google Scholar]
- 22.Herpertz SC, Dietrich TM, Wenning B, Krings T, Erberich SG, Willmes K, et al. Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biol Psychiatry. 2001;50:292–298. doi: 10.1016/s0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- 23.Driessen M, Beblo T, Mertens M, Piefke M, Rullkoetter N, Silva-Saavedra A, et al. Posttraumatic stress disorder and fMRI activation patterns of traumatic memory in patients with borderline personality disorder. Biol Psychiatry. 2004;55:603–611. doi: 10.1016/j.biopsych.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 24.Minzenberg MJ, Fan J, New AS, Tang CY, Siever LJ. Fronto-limbic dysfunction in response to facial emotion in borderline personality disorder: an event-related fMRI study. Psychiatry Res. 2007;155:231–243. doi: 10.1016/j.pscychresns.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koenigsberg HW, Siever LJ, Lee H, Pizzarello S, New AS, Goodman M, et al. Neural correlates of emotion processing in borderline personality disorder. Psychiatry Res. 2009;172:192–199. doi: 10.1016/j.pscychresns.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koenigsberg HW, Fan J, Ochsner KN, Liu X, Guise KG, Pizzarello S, et al. Neural correlates of the use of psychological distancing to regulate responses to negative social cues: A study of patients with borderline personality disorder. Biol Psychiatry. 2009;66:854–63. doi: 10.1016/j.biopsych.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulze L, Domes G, Kruger A, Berger C, Fleischer M, Prehn K, et al. Neuronal correlates of cognitive reappraisal in borderline patients with affective instability. Biol Psychiatry. 2011;69:564–573. doi: 10.1016/j.biopsych.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 28.Siddle DA. Orienting, habituation, and resource allocation: an associative analysis. Psychophysiology. 1991;28:245–259. doi: 10.1111/j.1469-8986.1991.tb02190.x. [DOI] [PubMed] [Google Scholar]
- 29.Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. NeuroReport. 2001;12:379–383. doi: 10.1097/00001756-200102120-00039. [DOI] [PubMed] [Google Scholar]
- 30.LeDoux J. Emotional networks and motor control: a fearful view. Prog Brain Res. 1996;107:437–446. doi: 10.1016/s0079-6123(08)61880-4. [DOI] [PubMed] [Google Scholar]
- 31.Davis M. The role of the amygdala in fear and anxiety. Ann Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 32.Canli T, Zhao Z, Brewer J, Gabrieli JD, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. J Neurosci. 2000;20:RC99. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature reviews. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- 34.Hazlett EA, Speiser LJ, Goodman M, Roy M, Carrizal M, Wynn JK, et al. Exaggerated affect-modulated startle during unpleasant stimuli in borderline personality disorder. Biol Psychiatry. 2007;62:250–255. doi: 10.1016/j.biopsych.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 35.Ebner-Priemer UW, Kuo J, Schlotz W, Kleindienst N, Rosenthal MZ, Detterer L, et al. Distress and affective dysregulation in patients with borderline personality disorder: a psychophysiological ambulatory monitoring study. J Nerv Ment Dis. 2008;196:314–320. doi: 10.1097/NMD.0b013e31816a493f. [DOI] [PubMed] [Google Scholar]
- 36.Limberg A, Barnow S, Freyberger HJ, Hamm AO. Emotional vulnerability in borderline personality disorder is cue specific and modulated by traumatization. Biol Psychiatry. 2011;69:574–582. doi: 10.1016/j.biopsych.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 37.Barnow S, Limberg A, Stopsack M, Spitzer C, Grabe HJ, Freyberger HJ, Hamm A. Dissociation and emotion regulation in borderline personality disorder. Psychol Med Nov. 2011;9:1–12. doi: 10.1017/S0033291711001917. [DOI] [PubMed] [Google Scholar]
- 38.Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect Psychol Sci. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- 39.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 40.Buss AHPM. The aggression questionnaire. J Pers Soc Psychol. 1992;63:452–459. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- 41.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 42.Harvey PD, Greenberg BR, Serper MR. The affective lability scales: development, reliability, and validity. J Clin Psychol. 1989;45:786–793. doi: 10.1002/1097-4679(198909)45:5<786::aid-jclp2270450515>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 43.Bernstein-Carlson P, FW An update on the Dissociative Experiences Scale. Dissociation. 1993;6:16–27. [Google Scholar]
- 44.Larsen RJ, Diener E. A multitrait-multimethod examination of affect structure: hedonic level and emotional intensity. Person Individ Diff. 1985;6:631–636. [Google Scholar]
- 45.Bernstein D, Fink L. Childhood Trauma Questionnaire: A Retrospective Self-Report. San Antonia, TX: Psychological Corporation; 1998. [Google Scholar]
- 46.E-Prime. E-Prime. 1.1. Pittsburgh: Psychology Software Tools; 2002. [Google Scholar]
- 47.Bradley MM, Lang PJ. Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. J Behav Ther Exp Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 48.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 49.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hazlett EA, Buchsbaum MS, Zhang J, Newmark RE, Glanton CF, Zelmanova Y, et al. Frontal-striatal-thalamic mediodorsal nucleus dysfunction in schizophrenia-spectrum patients during sensorimotor gating. Neuroimage. 2008;42:1164–1177. doi: 10.1016/j.neuroimage.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.StatSoft, Inc. STATISTICA (data analysis software system), version 9.1. Tulsa, OK: 2010. www.statsoft.com. [Google Scholar]
- 52.Worsley KJ. Statistical analysis of activation images. In: Jezzard PPMM, Smith SM, editors. Functional MRI: An Introduction to Methods. OUP; 2001. [Google Scholar]
- 53.Phan KL, Liberzon I, Welsh RC, Britton JC, Taylor SF. Habituation of rostral anterior cingulate cortex to repeated emotionally salient pictures. Neuropsychopharm. 2003;28:1344–1350. doi: 10.1038/sj.npp.1300186. [DOI] [PubMed] [Google Scholar]
- 54.Dyck M, Habel U, Slodczyk J, Schlummer J, Backes V, Schneider F, et al. Negative bias in fast emotion discrimination in borderline personality disorder. Psychol Med. 2008;39:855–64. doi: 10.1017/S0033291708004273. [DOI] [PubMed] [Google Scholar]
- 55.Dziobek I, Preibler S, Grozdanovic Z, Heuser I, Heekeren HR, Roepke S. Neuronal correlates of altered empathy and social cognition in borderline personality disorder. Neuroimage. 2011;57:539–48. doi: 10.1016/j.neuroimage.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 56.New AS, aan het Rot M, Ripoll LS, Perez-Rodriguez M, Lazarus S, Zipursky E, et al. Empathy and alexithymia in borderline personality disorder: Clinical and laboratory measures. J Personality Dis. doi: 10.1521/pedi.2012.26.5.660. (in press) [DOI] [PubMed] [Google Scholar]
- 57.Schmahl C, Bohus M, Esposito F, Treede RD, Di Salle F, Greffrath W. Neural correlates of antinociception in borderline personality disorder. Arch Gen Psychiatry. 2006;63:659–667. doi: 10.1001/archpsyc.63.6.659. [DOI] [PubMed] [Google Scholar]
- 58.Ebner-Priemer UW, Badeck S, Beckmann C, Wagner A, Feige B, Weiss I, et al. Affective dysregulation and dissociative experience in female patients with borderline personality disorder: a startle response study. J Psychiatr Res. 2005;39:85–92. doi: 10.1016/j.jpsychires.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 59.Ebner-Priemer UW, Mauchnik J, Kleindienst N, Schmahl C, Peper M, Rosenthal MZ, et al. Emotional learning during dissociative states in borderline personality disorder. J Psychiatry Neurosci. 2009;34:214–222. [PMC free article] [PubMed] [Google Scholar]
- 60.Kring AM, Neale JM. Do schizophrenic patients show a disjunctive relationship among expressive, experiential, and psychophysiological components of emotion? J Abn Psychology. 1996;105:249–257. doi: 10.1037//0021-843x.105.2.249. [DOI] [PubMed] [Google Scholar]
- 61.Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17:555–564. doi: 10.1093/schbul/17.4.555. [DOI] [PubMed] [Google Scholar]
- 62.Shean G, Bell E, Cameron CD. Recognition of nonverbal affect and schizotypy. J Psychol. 2007;141:281–291. doi: 10.3200/JRLP.141.3.281-292. [DOI] [PubMed] [Google Scholar]
- 63.Victor TA, Furey ML, Fromm SJ, Ohman A, Drevits WC. Relationship between amygdala response to masked faces and mood state and treatment in major depression. Arch Gen Psychiatry. 2010;67:1128–1138. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bateman AW, Fonagy P. Mentalization-based treatment of BPD. J Personality Dis. 2004;18:36–51. doi: 10.1521/pedi.18.1.36.32772. [DOI] [PubMed] [Google Scholar]
- 65.Bateman A, Fonagy P. 8-year follow-up of patients treated for borderline personality disorder: mentalization-based treatment versus treatment as usual. Am J Psychiatry. 2008;165:631–638. doi: 10.1176/appi.ajp.2007.07040636. [DOI] [PubMed] [Google Scholar]
- 66.Bateman AW, Ryle A, Fonagy P, Kerr IB. Psychotherapy for borderline personality disorder: mentalization based therapy and cognitive analytic therapy compared. Int Rev Psychiatry. 2007;19:51–62. doi: 10.1080/09540260601109422. [DOI] [PubMed] [Google Scholar]
- 67.Linehan MM, Comtois KA, Murray AM, Brown MZ, Gallop RJ, Heard HL, et al. Two-year randomized controlled trial and follow-up of dialectical behavior therapy vs therapy by experts for suicidal behaviors and borderline personality disorder. Arch Gen Psychiatry. 2006;63:757–766. doi: 10.1001/archpsyc.63.7.757. [DOI] [PubMed] [Google Scholar]
- 68.Lynch TR, Trost WT, Salsman N, Linehan MM. Dialetical behavior therapy for borderline personality disorder. Ann Rev Clin Psychology. 2007;3:181–205. doi: 10.1146/annurev.clinpsy.2.022305.095229. [DOI] [PubMed] [Google Scholar]
- 69.Scheel KR. The empirical basis of dialectical behavior therapy: summary, critique, and implications. Clin Psychology. 2000;7:68–86. [Google Scholar]
- 70.Linehan MM. The empirical basis of dialectical behavior therapy: Development of new treatments versus evaluation of existing treatments. Clin Psychology: Science and Practice. 2000;7:113–119. [Google Scholar]
- 71.Debiec J, Ledoux JE. Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neurosci. 2004;129:267–272. doi: 10.1016/j.neuroscience.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 72.Yang YL, Chao PK, Lu KT. Systemic and intra-amygdala administration of glucocorticoid agonist and antagonist modulate extinction of conditioned fear. Neuropsychopharm. 2006;31:912–924. doi: 10.1038/sj.npp.1300899. [DOI] [PubMed] [Google Scholar]
- 73.Yang YL, Chao PK, Ro LS, Wo YY, Lu KT. Glutamate NMDA receptors within the amygdala participate in the modulatory effect of glucocorticoids on extinction of conditioned fear in rats. Neuropsychopharm. 2007;32:1042–1051. doi: 10.1038/sj.npp.1301215. [DOI] [PubMed] [Google Scholar]
- 74.First MB, Spitzer R, Gibbon M, Williams J. Structured clinical interview for Axis I disorders-patient edition. New York: Biometrics Research, New York State Psychiatric Institute; 1996. [Google Scholar]
- 75.Pfohl B, Blum N, Zimmerman M. Structured clinical interview for DSM-IV personality (SIDP-IV) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 76.Haznedar MM, Buchsbaum MS, Wei TC, Hof PR, Cartwright C, Bienstock CA, et al. Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. Am J Psychiatry. 2000;157:1994–2001. doi: 10.1176/appi.ajp.157.12.1994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.