Abstract
Our recent studies indicate that the longer peripheral persistence of naive CD4 T cells that occurs with age is necessary for the development of the key aging defects that lead to compromised responses to vaccination and to new pathogens or new strains of circulating infectious agents. This longer persistence is in turn is linked to the decrease in development of new thymic emigrants and thymic involution that occur at adolescence. Therefore the process of development of naïve CD4 aging defects, is closely tied to the homeostasis of T cells and the shifts that occur in their homeostasis with age. Here we review this connection between age-related changes in T cell homeostasis and the development of T cell defects and discuss the implication for approaches to better vaccinating the elderly.
Keywords: immunosenescence, homeostasis, aging, vaccines, T cells
1. Nature of Naïve T Cells Defects in Function
Naïve CD4 cells develop intrinsic age-associated defects that include a longer cellular lifespan, lower Ca+ flux in response to T cell receptor (TCR)-triggering, and an altered production of cytokines, including reduced IL-2 production (reviewed in [1–3]). We find that in addition, aged naïve T cells make increased IFNγ and osteopontin (OPN) during the primary response (Zhang and Swain unpublished). In vitro, aged naïve cells expand less to antigenic stimulation unless exogenous cytokines that bind the IL-2Rγ are added or conditions that enhance autocrine IL-2 are achieved, as discussed below. Aged effector populations that develop when IL-2 is enhanced seem to function well and, in fact, when polarizing cytokines are added, effectors generated can be polarized to Th1 and Th2-like subsets, producing appropriate cytokines [4, 5]. However, in aged mice in vivo and among aged naïve CD4 T cells that respond in young mice, development of optimum helper function for long-lived production of neutralizing antibody is compromised. Effectors from aged naïve cells transition to memory phenotype and are retained at least as well as those from the young [4]. Thus it seems more appropriate to conclude that there is a selective loss of some functions (proliferation, cognate help) while others are maintained or even enhanced (IFNγ and OPN and pro-inflammatory functions). Most studies of the defects in primary naïve CD4 T cells have been carried out in mice with a few exceptions such as one report indicating the differentiation of naive CD4 to Th17 In aging humans is increased compared to young people [6]. Some of the most definitive studies have used naïve CD4 T cells rigorously purified from T cell receptor transgenic mice which were then studied in vitro or transferred to young hosts so that intrinsic defects in the aged naïve cells could be studied [7]. It is very important to analyze purified naïve or memory CD4 subsets because as animals age the T cell populations undergo major shifts in subsets, with the naïve population decreasing and cells of memory phenotype increasing. As indicated bellow, this occurs even when no overt antigen stimulation is present.
Importantly, the more profound defects in CD4 T cell function are found when aged naïve CD4 T cells that transition to memory cells re-encounter antigen [4, 8]. These memory cells generated from aged naïve CD4 T cells secrete a restricted pattern of cytokines and expand little after restimulation. In the mouse, in vitro studies showed that these memory cells make little IL-2, very low levels of Th2 cytokines (IL-4, IL-5, IL-13) but normal or higher levels of IFNγ, TNF and IL-10. This shift is seen even when Th2 polarized effectors become memory [4, 8]. Importantly, the analysis of antigen-specific memory populations, that are developed by priming in vitro or in vivo and thus are known to be bona fide memory cells, has facilitated these analyses. The helper response of memory cells generated from aged naïve cells is dramatically reduced even when the primary effector populations generated from young and aged naive CD4 T cells are equivalent due to the addition of exogenous cytokines. This holds true for both Th1 and Th2 effector populations transferred into young hosts, which were then vaccinated 4 weeks later and for memory cells generated in vitro from such effectors. Since these studies involved the adoptive transfer of the same number of young or aged memory cells, the impact of aging on a per cell basis could be investigated. Both the expansion of antigen-specific B cells, as well as the titers of IgG, were significantly lower when memory cells generated from aged naive CD4 T cells provided help compared to memory cells generated from young naive CD4 T cells [4, 8]. Interestingly, memory cells that were generated from young naive CD4 T cells that were then allowed to age function quite well for at least one year following primary stimulation [8]. This indicates that it is not just how long a cell has been in the periphery, but rather the age of the cell at initial priming that determines the quality of the resulting memory cells.
Thus, the naïve T cells seem to develop intrinsic, heritable defects that impact selective aspects of their initial response and cause the memory cells they become to respond poorly. This poor memory response, especially the loss of cognate helper function among CD4 T cells, is likely a major cause of the poor efficacy of vaccines that are given to the elderly [18]. A number of studies in humans have supported the concept that the memory T cells, both CD4 and CD8, found in the elderly following vaccination are less responsive than those from the young [19].
We suggest that the shifts in immune response function of aged naïve CD4 T cells, that intensify with the transition to memory, could be explained by epigenetic changes in aged T cells that occur with their prolonged lifespan and could be intensified as they further differentiate to memory cells. The consequences of the shifts are profound. The loss of helper activity and poor proliferative response of memory cells derived from aged naïve CD4 T cells, suggests that following immunization for a new pathogen, the aged will develop neither a robust B response and long-lived Ab or long-lived Ab-forming plasma cells nor a population of highly functional memory CD4 T cells, two of the most important mediators of immunity. Thus, a key issue is whether strategies can be identified that either enhance or circumvent the age-associated defects in naïve CD4 T cell response and if each strategy results in better immunity to reinfection.
It is important here to distinguish between the age-associated defects that develop progressively in T cells and the severe collapse of T cell function seen at very advanced age in mice and humans [20], which are likely due to distinct mechanisms. The collapse at the end of life involves severe repertoire contraction of CD8 T cells and CD8 monoclonal expansions and a severe loss of functions of CD4 cells and accumulation of cells that have a severe senescence that cannot replicate and are severely deficient in production of all cytokines (Zhang and Swain, unpublished.) Once such a state occurs it is hard to imagine it is reversible since the rapidly declining elderly are likely to have multiple problems. We focus here on defects in T cell immunity that begin to accumulate much earlier, develop gradually, and are characterized by modest reductions in proliferative response and shifts in development of effectors, with selective, more pronounced defects in memory cells and helper and Th2 activities.
2. T Cell Homeostasis and the Development of Age-Associated Defects
The dynamics of T cell generation and its regulation are complex and the features of the process give T cells a unique homeostasis, which is achieved by a tightly regulated balance of cell division and death. Naïve CD4 T cells are generated in the thymus, with positive and negative selection giving rise to a repertoire selected to recognize self MHC/self peptide with low affinity. This allows recognition of self MHC and self peptides by naïve cells in the periphery to deliver mild, but critical, survival signals. Once generated, the naive CD4 and CD8 T cells leave the thymus and emigrate to the periphery. They leave the thymus as recent thymic emigrants (RTE), which are a unique population [21] that still maintains markers associated with thymus selection, such as heightened levels of the recombination activating gene (RAG) recombinase [19] and they can thus be identified [22]. Within a short time, 1–2 weeks, these transition into mature naïve T cells [23]. The size of the naïve T cell compartments are regulated by the pace of generation naïve T cells in the thymus, the lifespan of the naïve T cells and whatever homeostatic turnover of T cells occurs in the periphery. Starting in adolescence, there is a pronounced onset of thymic involution that is linked to the appearance of sex hormones and reinforced by decreases in growth hormones [24–27]. Progressive thymic involution with age causes a steady decline in new thymic emigrants, which drop approximately 10-fold compared to that in pre-adolescent animals over several months and RTE then remain at a stable, low level [22, 28]. Importantly, this one log decline in new T cell production does not result in a proportional loss of naïve T cells, which decline more slowly, resulting in a level in old age of approximately one third the number in adolescents [28–31]. This homeostasis is likely achieved by a set of feedback mechanisms that senses naïve CD4 T cell number and acts to preserve it.
When memory cells develop they are longer-lived than naïve and, all else being equal, they would eventually totally dominate the peripheral pool. Thus, homeostatic mechanisms must be in place to maintain naïve T cell numbers in the periphery [32]. Naïve T cell survival is achieved in large part by T cell receptor (TCR) engagement with self-peptide/MHC [33, 34] and homeostatic survival factors such as IL-7 and IL-15 [35, 36]. There is also potential turnover of the naïve population and occasional division of naïve CD8 T cells has been described, but naïve CD4 T cells show very little such division [10]. Another important component regulating peripheral T homeostasis is cellular lifespan, which has often been considered a static cell-intrinsic property. The balance of cell division and death due to cellular aging, combined with the rate of new emigrants entering the pool will determine the naïve population size. Since the naïve T cell population size decreases with age at a rate less than the decline in RTE, either homeostatic expansion due to division or a longer lifespan or some combination of the two is predicted with age.
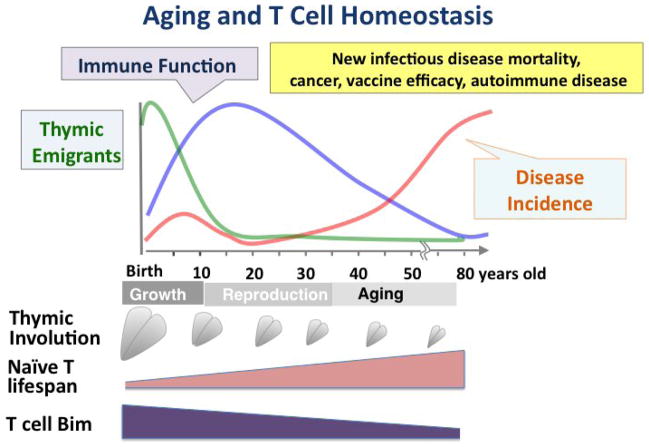
As mentioned above, turnover of CD8 T cells in mice is seen at significant levels, but that of CD4 is less. Turnover of naïve T cells is also detectable in humans and non-human primates [31, 37], where it has been suggested that it could explain the retention of a naïve population. We tested the other possibility, namely that with age, presumably in response to the decreased egress of RTE, naïve CD4 T cells in the periphery might increase their lifespan to help maintain the population [38]. Indeed, when naïve cells were isolated from aged mice and their lifespan determined following transfer to a young, intact host, the aged naïve cells had a much longer survival time than their young counterparts, leading to their selective accumulation in the peripheral secondary lymphoid organs over several weeks [38]. Moreover, the naïve CD4 T cells did not divide over the several weeks and the increase in lifespan was clearly seen in both naïve CD4 T cells from TCR transgenic animals as well as non-transgenic ones [38]. This and further studies [38, 39] provided strong evidence that increased lifespan of naïve CD4 T cells occurs progressively with age and is cell intrinsic. Our studies suggest that the increase in lifespan is mediated by the age-related progressive downregulation of the pro-apoptotic protein Bim [39], a known regulator of T cell survival [40]. Thus, we conclude that the increase of lifespan in the naïve cells in older mice is not accompanied by increased turnover and is a major factor in the maintenance of a sizable naïve CD4 population with age [38]. This is illustrated in Figure 1.
Figure 1. Aging and T Cell Homeostasis.
The cartoon, displays approximate relative levels in production of recent thymic emigrants (RTE) (green line) and immune function (blue line) as well as incidence of diseases that increase with aging (orange line) on a lifeline for humans. Below depicts thymic involution, increase in the lifespan of naïve T cells and decrease in Bim expression.
3. How are increased T cell lifespan and development of age-related functional changes linked?
We have suggested previously that losses in CD4 function were linked to the chronological age of the CD4 T cells [41]. In several studies in mouse models, we used several treatments that lead to newly generated naïve CD4 T cells including irradiation and bone marrow reconstitution or selective depletion of peripheral CD4 T cells, to ask if newly generated T cells in aged animals would be more functional. After depletion of peripheral CD4 T cells, those newly generated T cells in aged animals were fully functional. Likewise when aged bone marrow was transferred into irradiated hosts, the newly generated naïve CD4 T cells did not display aging defects. This suggested that aging defects only developed as the cells themselves aged. We also found that if we increased the “age” of naïve T cells by thymectomizing mice to cut off the flow of new thymic emigrants, they developed defects more quickly [32]. An additional study indicated that naïve CD4 T cells bearing a TCR Vβ5 transgene for an Ovalbumin peptide (OT-II), which are deleted in the periphery by interaction with a MMTV superantigen [42], also fail to develop most age-associated defects, presumably as a consequence of their lack of long-term persistence [43]. Furthermore in support of this concept, was the finding that when memory CD4 cells were generated in young mice and then allowed to age, the cells retained excellent function [8].
This hypothesis that defects develop in naïve T cells with cell age recently received strong support. When we found that CD4 T cells developed an extended lifespan with age and that adult thymectomy accelerated the pace of development of increases in lifespan. The increased lifespan was strongly correlated with progressively lower levels of Bim expression by the T cells. Importantly, these T cells with an increased lifespan due to Bim reduction, developed age-associated defects at an accelerated pace [39]. Further support for the hypothesis that functional changes are linked to cellular aging came from studies demonstrating that in newly generated naïve CD4 T cells derived from aged bone marrow, Bim levels were high and these cells had the functional profiles of young CD4 T cells. The CD4 T cells only developed reduced Bim expression and functional defects with time. Reduction of Bim in Bim+/− or Bim−/− CD4 T cells led to immediate proportional increase in lifespan, but the development of functional changes required a number of months, about 6–7 in the heterozygous naïve CD4 T cells [39]. Thus reduced Bim did not itself cause defects, it only enhanced the persistence of the naïve cells. In fact, there was a close correlation between peripheral persistence of the naïve CD4 T cells and reduced function, including IL-2 production and expansion of naïve cells, as well as the ability to support isotype-switched antibody production and generation of functional memory. In addition, naïve CD4 T cells exhibiting reduced Bim were poor helpers and made dysfunctional memory cells. Taken together these findings provide strong evidence that with increasing age, naïve CD4 T cells down-regulate Bim which results in a longer lifespan that in turn favors development of age-associated defects.
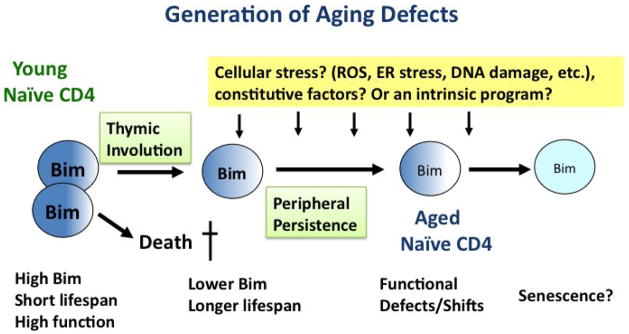
Similar reductions have been observed for aging naïve CD8 T cells in the mouse (Zhang and Swain, unpublished results). Furthermore, with our encouragement, John Wherry and Doug Dolfi examined Bim expression by young and old human T cells and found a similar decrease with age (Wherry and Dolfi, personal communication). This indicates that the down-regulation of Bim expression with age is a general T cell phenomenon preserved across species. While a compelling model, this still does not identify the specific mechanism(s) that causes the persisting cells to become age-challenged. However it does provide important clues. In Bim deficient naïve CD4 T cells (+/− and −/−), including bone marrow chimeras, aging defects in the Bim+/− are seen within 6–8 months, a time at which the animal is quite young. This suggests that defects are not dependent on signals unique to the aged but not younger host environment, such as high levels of inflammatory mediators. In addition, the reversal of defects in newly generated naïve CD4 from aged mice with aged bone marrow, suggests the aged environment during naïve T cell generation is not sufficient to impose the changes. Together this suggests the mechanisms that dictate this set of age-associated defects works specifically on the naïve T cell population. The results we see are highly consistent from mouse to mouse, and independent of our animal facilities when we re-located. This suggests that the signal(s) responsible for the induction are constitutive. We are currently analyzing some constitutive factors that could regulate naïve CD4 homeostasis to see if any obvious candidates are involved. Figure 2 depicts the process of naïve CD4 aging, the involvement of Bim, enhanced lifespan and theoretical other factors.
Figure 2. Generation of Aging Defects.
We purpose that the decrease in RTE associated with adolescence, induces pathways that mediate reduction in Bim and that this reduction in Bim increases naïve CD4 lifespan. Our data supports the model that the longer persistence of the naïve T cells facilitates the development of age-associated changes. The factors directly responsible for those changes are not known, but the fact that defects can develop in 6–8 months in mice with reduced Bim, indicate that a highly aged environment is not necessary and point to a role for constitutive factors or a intrinsic program of some sort.
Evidence has accumulated that the phenomenon of progressive age-related changes is somehow linked to sharply reduced thymic output of emigrants that occurs concurrent with the onset of sex hormone increase during adolescence. The sex hormones contribute to thymic atrophy, which ultimately results in the reduction in RTE [44]. We postulate this reduction, in turn, causes a homeostatic feedback mechanism induced to keep the peripheral naïve CD4 population from excessive contraction. A key part of this feedback mechanism leads to reduced Bim expression, that induces longer lifespan of naïve CD4 T cells and which thus achieves homeostasis of the naïve CD4 T cell population, which decreases much more slowly than the number of RTE and is maintained at a substantial, though slowly decreasing size, throughout life. This is a dynamic equilibrium maintained for a long period of time, since as mentioned above, complete removal of CD4 T cells or all T cells in aged animals results in the production of new T cells that are shorter rather than longer-lived. There could also be a two-way feedback mechanism at work since, following depletion of peripheral T cells, there were similar repopulation kinetics in both young and aged animals [41]. The relationship of decreased Bim expression, increased lifespan and progressively reduced function is illustrated in Figure 2.
4. Can the progressive age-related changes in T cells be overcome to promote efficacy of vaccines?
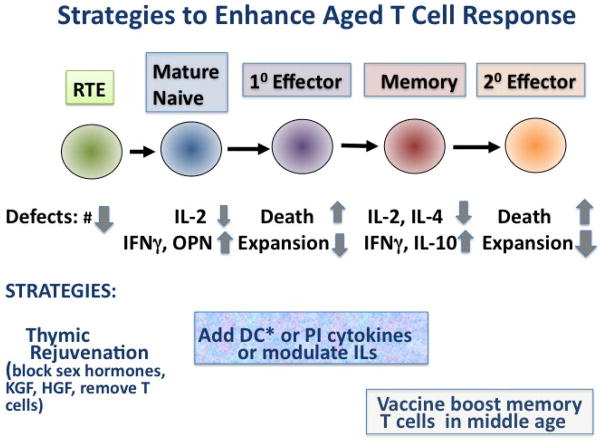
We consider several strategies that are suggested by these results and might promote vaccine responses in the elderly. These are summarized in Figure 3.
Figure 3. Strategies to Enhance Aged T Cell Response.
Depiction of some of the key deficiencies in the different stages of T cell response. Some strategies to reverse or overcome those deficiencies are indicated. Several approaches could be considered for enhancing vaccine responses in the elderly Thus, it has been suggested that the reduced number of functional naïve T cells could be approached by thymic rejuvenation to transiently increase production of RTE. This could be timed before a vaccine to generate a new naïve response. The lowered production of IL-2 by naïve and memory T cells and increased production of IFNγ and OPN could be modulated during vaccination or response to a pathogen by several approaches including direct addition of IL-2 and PI cytokines and/or blocking IFNγ and OPN. We suggest using TLR agonist-activated DC (DC*) as APC in can accomplish these cytokine shifts in a vaccine setting in a more targeted and physiological fashion. Increases in PI cytokine and IL-2 also would reduce cell death and therefore promote expansion leading to more effectors. In addition giving vaccines to boost those memory T cell populations generated in early age, should enhance protective responses to recurrent pathogens.
4.1. Middle-aged booster vaccines
The studies above suggest that age-related T cell changes are a consequence of the way T cell differentiation is regulated and thus it is highly unlikely that major interference with the process leading to the changes, is either feasible or desirable. Therefore, we consider other approaches. First, in terms of enhancing T cell immunity in the aged to known infectious threats, optimizing early and middle–age vaccines would be beneficial, and this seems a very reasonable strategy with few clear drawbacks. Boosts of childhood vaccines in middle age, designed to boost the frequency of memory T cells so sufficient numbers can be retained into older ages, could selective increase the proportion of memory T cells to known pathogens that frequently cause disease outbreaks. Strategies to avoid the blocking effects of circulating neutralizing antibodies would need to be developed, since these are likely to interfere with T cell boosting. For instance vaccines for middle-age should avoid those proteins containing dominant B cell epitopes.
However, when a completely new pathogen or strain emerges for which no previous immunity is present, the hope for improving vaccines in our minds lies in either transiently increasing the supply of RTE to allow a window for vaccination or somehow overcoming or merely enhancing the response of the aged naïve CD4 T cell population.
4.2. Thymic Rejuvenation
One strategy, thymic rejuvenation, while attractive, probably carries significant risks even if it could be achieved. The principle would be that depletion of a substantial fraction of the peripheral naïve CD4 population and/or re-balancing of sex and growth hormones should facilitate a temporary spurt in newly generated naive CD4 T cells that could be exploited by a selective vaccination in concert with the new burst of naïve T cells for those infectious agents that have emerged during a patients’ adulthood. The details of achieving this require extensive further investigation. A number of investigators are studying the possible effects of sex hormone depletion [25, 44–46] and growth factors that stimulate thymopoiesis [47–50] that might enhance the generation of new naïve RTE. We suggest that depletion of effete naïve CD4 T cells to induce production of new ones, coupled with these other approaches could possibly be considered. Still this would involve a great deal of intervention and thus is not particularly attractive. If strategies to induce improved response of the naïve T cells in the elderly could be successful, they most likely would be more attractive.
4.3. Cytokine-mediated Enhancement of Naïve T Cell Response
One of the main defects of aged CD4 T cells is that they produce significantly less IL-2 upon TCR stimulation when compared to young cells. This results in the aged cells undergoing fewer rounds of cell division, exhibiting less clonal expansion and expressing a less differentiated phenotype [5]. Importantly, this age-related defect in the aged naive T cells can be reversed by the addition of exogenous IL-2. This leads to the aged cells proliferating much like the young cells and exhibiting enhanced subsequent IL-2 production. In addition, aged T cells stimulated in the presence of exogenous IL-2 also exhibit a more activated cell surface phenotype, including enhanced expression of CD25 and down regulation of CD62L when compared to aged cells stimulated without exogenous IL-2. Finally, the addition of exogenous IL-2 to aged CD4 T cells could enhance their differentiation to both Th1 and Th2 subsets, such that their cytokine production was similar to young T cells [5]. These results demonstrated that one functionally significant defect of aged naive CD4 T cells was their inability to produce enough IL-2, and did not involve defects in response to exogenously provided IL-2. Unfortunately, once these aged effectors generated in the presence of exogenous IL-2 returned to a resting state, they re-exhibited age-related defects such as reduced clonal expansion and cytokine production, indicating that the IL-2 enhancement was only transient [4]. This indicates that defects other than reduced IL-2 production contribute to the age-associated changes in naïve CD4 T cells.
Since it has been appreciated for some time that inflammatory cytokines can provide a “third” signal that enhances the costimulation of naive CD4 T cells [51], we went on to determine if these cytokines could boost the responses of aged CD4 T cells. For these studies, a mixture of the pro-inflammatory (PI) cytokines IL-1, IL-6 and TNF was employed. PI cytokines added to cultures enhanced the clonal expansion and IL-2 production by antigen stimulated aged CD4 T cells via a mechanism involving the enhanced activation of the transcription factor nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) [52]. This is quite important since NFκB activation is important for T cell activation, cytokine production and migration (reviewed in [53]).
Importantly, in vivo, a similar enhancement of donor aged naïve CD4 expansion was achieved by treatment of young host recipients with a cocktail of PI cytokines. A key impairment especially relevant to vaccine ineffectiveness, is that intrinsic age-related defects in naive CD4 T cells result in them exhibiting very poor cognate helper function for humoral responses following vaccination with antigen in an alum adjuvant in an adoptive transfer model [54]. The addition of PI cytokines to the vaccination protocol significantly enhanced the in vivo clonal expansion of the aged CD4 T cells [52]. Importantly, vaccination with antigen plus PI cytokines was shown to also dramatically enhance the in vivo cognate helper activity of aged CD4 T cells, including enhanced expansion of antigen-specific B cell populations as well as enhanced germinal center formation and IgG production [55]. We have also determined that toll-like receptor (TLR) agonists, such as poly I:C, used as an adjuvant can significantly boost the in vivo cognate helper function of aged CD4 T cells, leading to enhanced humoral responses [55]. Thus the combination of increased IL-2 and PI cytokines has the potential to substantially enhance the responses of aged naïve CD4 T cells so they approach levels of those with young cells. Recently our colleagues have found that the combination of IL-6 with IL-2 promotes the response of aged memory phenotype T cells from older humans [56]. This suggest that the pathways to enhance the impair responses of aged T cells may in large part be shared across species and in both CD4 and CD8 T cells.
4.4. Enhanced Responses Induced by TLR-activated DC
One highly efficient and antigen-specific way to deliver PI cytokines to naïve T cells would be to induce dendritic cells that make such cytokines to produce them during cognate interaction with the responding T cells. This would have the very substantial advantage that the PI cytokines would be targeted to the relevant, antigen-specific T cells and would not be systemic. It is known that TLR agonists activate dendritic cells (DC) and other APC, to produce PI cytokines. However, TLR agonist ligands initiate a wide range of responses in many cell types in vivo, so systemic treatment is likely to cause multiple, unintended, negative effects. Therefore we tested if DC pretreated with TLR agonists would be better APC for aged naïve CD4 T cells. TLR agonist treatment of bone marrow-derived DC caused the DC to produce very high levels of IL-6 and other PI cytokines when they were used to stimulate aged naïve CD4 T cells. This effect was IL-6 dependent and resulted in enhanced proliferation, increased IL-2 production, improved viability, and promotion of the development of an expanded population of aged effectors [57]. Importantly, the memory cells derived from effectors generated with the activated DC also had greater potential for secondary expansion following re-encounter with antigen.
These studies have also been extended to an in vivo model demonstrating that TLR agonist-treated DC promoted greater expansion of aged naïve CD4 T cells in adoptive hosts (Brahmakshatriya and Swain, unpublished). This is heartening from the perspectives of enhancing vaccine efficacy. It is important to note that the enhancing effect of activated DC is also seen on young naïve CD4 T cells, indicating this pathway is available in T cells regardless of age and it has not been impaired by age-associated changes. It is not yet clear whether activated DC can enhance all functions that are altered by aging, but those studies should soon be accomplished.
These findings have clinical importance since the elderly exhibit significantly reduced vaccine efficacy such as that for influenza [58]. We have determined that strategies to boost “costimulatory” PI cytokines by the use of TLR adjuvants can enhance aged T cell function and this has now been examined in a human model. Significantly, a new study has recently shown that a TLR4 agonist adjuvant can boost the T cell responses to influenza vaccination in older adults [59]. The findings of this study are very encouraging and have the potential to greatly improve vaccine-mediated protection against influenza in the elderly. Several studies suggest DC functions are well-preserved with age [60, 61], which is in agreement with our studies [57]. Thus strategies to target TLR agonists to DC might be one approach that could be taken.
5. Summary
It is clear that T cells become significantly less able to engage in effective immune responses and to become functional memory cells as we age. We suggest that the one main mechanism leading to this impairment lies in the basic principles governing T cell development across species, i.e. that the thymus involutes in adolescence and produces many fewer naïve T cells. We postulate this lower production of emigrants is balanced by a longer lifespan of naïve T cells to maintain a naïve T cell pool, and that this longer lifespan facilitates development of age-associated defects among naïve CD4, and likely CD8 T cells. Thus it is unlikely we can prevent the impairment of naïve response.
However there are a number of approaches that could potentially circumvent the naïve CD4 ineffectiveness. These include middle-aged vaccines to boost existing memory, strategies to transiently augment naïve T cell production and use of approaches that enhance the response of aged naïve T cells in an antigen-specific way. These hold promise to greatly improve the effectiveness of vaccines to newly emerging pathogens that need to be given late in life.
Highlights.
Naive T cells become less able to engage in effective immune responses with aging.
Reduced production of naive T cells with age is balanced by a longer lifespan.
Reduction in Bim expression is associated with extended lifespan of naive T cells.
A longer lifespan facilitates development of age-related defects among naïve T cells.
Alternative vaccination strategies may be able to overcome these defects.
Acknowledgments
This work was supported by public health service grants AG02154 (L.H.), AG02160 (L.H. and S.L.S.) and AG025805 (S.L.S) and funding from the Trudeau Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haynes L, Lefebvre JS. Age-related deficiencies in antigen-specific CD4 T cell responses: Lessons from mouse models. Aging and Disease. 2011;2:374–381. [PMC free article] [PubMed] [Google Scholar]
- 2.Linton P-J, Haynes L, Tsui L, Zhang X, Swain S. From naive to effector-alterations with aging. Immunol Reviews. 1997;160:9–18. doi: 10.1111/j.1600-065x.1997.tb01023.x. [DOI] [PubMed] [Google Scholar]
- 3.Miller RA, Garcia G, Kirk CJ, Witkowski JM. Early activation defects in T lymphocytes from aged mice. Immunol Rev. 1997;160:79–90. doi: 10.1111/j.1600-065x.1997.tb01029.x. [DOI] [PubMed] [Google Scholar]
- 4.Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc Natl Acad Sci U S A. 2003;100:15053–15058. doi: 10.1073/pnas.2433717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haynes L, Linton P-J, Eaton SM, Tonkonogy SL, Swain SL. IL-2, but not other common γ chain (γc)-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med. 1999;190:1013–1023. doi: 10.1084/jem.190.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JS, Lee WW, Kim SH, Kang Y, Lee N, Shin MS, et al. Age-associated alteration in naive and memory Th17 cell response in humans. Clin Immunol. 2011;140:84–91. doi: 10.1016/j.clim.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haynes L, Swain SL. Why aging T cells fail: implications for vaccination. Immunity. 2006;24:663–666. doi: 10.1016/j.immuni.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eaton SM, Maue AC, Swain SL, Haynes L. Bone Marrow Precursor Cells from Aged Mice Generate CD4 T Cells That Function Well in Primary and Memory Responses. J Immunol. 2008;181:4825–4831. doi: 10.4049/jimmunol.181.7.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li SP, Cai Z, Shi W, Brunmark A, Jackson M, Linton PJ. Early antigen-specific response by naive CD8 T cells is not altered with aging. J Immunol. 2002;168:6120–6127. doi: 10.4049/jimmunol.168.12.6120. [DOI] [PubMed] [Google Scholar]
- 12.Ely KH, Ahmed M, Kohlmeier JE, Roberts AD, Wittmer ST, Blackman MA, et al. Antigen-specific CD8+ T cell clonal expansions develop from memory T cell pools established by acute respiratory virus infections. J Immunol. 2007;179:3535–3542. doi: 10.4049/jimmunol.179.6.3535. [DOI] [PubMed] [Google Scholar]
- 13.Ely KH, Roberts AD, Kohlmeier JE, Blackman MA, Woodland DL. Aging and CD8+ T cell immunity to respiratory virus infections. Exp Gerontol. 2007;42:427–431. doi: 10.1016/j.exger.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Decman V, Laidlaw BJ, Doering TA, Leng J, Ertl HC, Goldstein DR, et al. Defective CD8 T cell responses in aged mice are due to quantitative and qualitative changes in virus-specific precursors. J Immunol. 2012;188:1933–1941. doi: 10.4049/jimmunol.1101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed M, Lanzer KG, Yager EJ, Adams PS, Johnson LL, Blackman MA. Clonal expansions and loss of receptor diversity in the naive CD8 T cell repertoire of aged mice. J Immunol. 2009;182:784–792. doi: 10.4049/jimmunol.182.2.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med. 2008;205:711–723. doi: 10.1084/jem.20071140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Messaoudi I, Lemaoult J, Guevara-Patino JA, Metzner BM, Nikolich-Zugich J. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J Exp Med. 2004;200:1347–1358. doi: 10.1084/jem.20040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McElhaney JE. Influenza vaccine responses in older adults. Ageing Res Rev. 2011;10:379–388. doi: 10.1016/j.arr.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou X, McElhaney JE. Age-related changes in memory and effector T cells responding to influenza A/H3N2 and pandemic A/H1N1 strains in humans. Vaccine. 2011;29:2169–2177. doi: 10.1016/j.vaccine.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goronzy JJ, Lee WW, Weyand CM. Aging and T-cell diversity. Exp Gerontol. 2007;42:400–406. doi: 10.1016/j.exger.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fink PJ, Hendricks DW. Post-thymic maturation: young T cells assert their individuality. Nat Rev Immunol. 2011;11:544–549. doi: 10.1038/nri3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc Natl Acad Sci U S A. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nat Immunol. 2004;5:418–425. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 24.Chidgey AP, Boyd RL. Stemming the tide of thymic aging. Nat Immunol. 2006;7:1013–1016. doi: 10.1038/ni1006-1013. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg GL, Alpdogan O, Muriglan SJ, Hammett MV, Milton MK, Eng JM, et al. Enhanced immune reconstitution by sex steroid ablation following allogeneic hemopoietic stem cell transplantation. J Immunol. 2007;178:7473–7484. doi: 10.4049/jimmunol.178.11.7473. [DOI] [PubMed] [Google Scholar]
- 26.Napolitano LA, Schmidt D, Gotway MB, Ameli N, Filbert EL, Ng MM, et al. Growth hormone enhances thymic function in HIV-1-infected adults. J Clin Invest. 2008;118:1085–1098. doi: 10.1172/JCI32830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rossi S, Blazar BR, Farrell CL, Danilenko DM, Lacey DL, Weinberg KI, et al. Keratinocyte growth factor preserves normal thymopoiesis and thymic microenvironment during experimental graft-versus-host disease. Blood. 2002;100:682–691. doi: 10.1182/blood.v100.2.682. [DOI] [PubMed] [Google Scholar]
- 28.Sempowski GD, Gooding ME, Liao HX, Le PT, Haynes BF. T cell receptor excision circle assessment of thymopoiesis in aging mice. Mol Immunol. 2002;38:841–848. doi: 10.1016/s0161-5890(01)00122-5. [DOI] [PubMed] [Google Scholar]
- 29.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 30.Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 31.Kilpatrick RD, Rickabaugh T, Hultin LE, Hultin P, Hausner MA, Detels R, et al. Homeostasis of the naive CD4+ T cell compartment during aging. J Immunol. 2008;180:1499–1507. doi: 10.4049/jimmunol.180.3.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swain S, Clise-Dwyer K, Haynes L. Homeostasis and the age-associated defect of CD4 T cells. Semin Immunol. 2005;17:370–377. doi: 10.1016/j.smim.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boursalian TE, Bottomly K. Survival of naive CD4 T cells: roles of restricting versus selecting MHC class II and cytokine milieu. J Immunol. 1999;162:3795–3801. [PubMed] [Google Scholar]
- 34.Martin B, Becourt C, Bienvenu B, Lucas B. Self-recognition is crucial for maintaining the peripheral CD4+ T-cell pool in a nonlymphopenic environment. Blood. 2006;108:270–277. doi: 10.1182/blood-2006-01-0017. [DOI] [PubMed] [Google Scholar]
- 35.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 36.Vella A, Teague TK, Ihle J, Kappler J, Marrack P. Interleukin 4 (IL-4) or IL-7 prevents the death of resting T cells: stat6 is probably not required for the effect of IL-4. J Exp Med. 1997;186:325–330. doi: 10.1084/jem.186.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cicin-Sain L, Messaoudi I, Park B, Currier N, Planer S, Fischer M, et al. Dramatic increase in naive T cell turnover is linked to loss of naive T cells from old primates. Proc Natl Acad Sci U S A. 2007;104:19960–19965. doi: 10.1073/pnas.0705905104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsukamoto H, Clise-Dwyer K, Huston GE, Duso DK, Buck AL, Johnson LL, et al. Age-associated increase in lifespan of naive CD4 T cells contributes to T-cell homeostasis but facilitates development of functional defects. Proc Natl Acad Sci U S A. 2009;106:18333–18338. doi: 10.1073/pnas.0910139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsukamoto H, Huston GE, Dibble J, Duso DK, Swain SL. Bim dictates naive CD4 T cell lifespan and the development of age-associated functional defects. J Immunol. 2010;185:4535–4544. doi: 10.4049/jimmunol.1001668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 41.Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. Newly generated CD4 T cells in aged animals do not exhibit age-related defects in response to antigen. J Exp Med. 2005;201:845–851. doi: 10.1084/jem.20041933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fink PJ, Fang CA, Turk GL. The induction of peripheral tolerance by the chronic activation and deletion of CD4+V beta 5+ cells. J Immunol. 1994;152:4270–4281. [PubMed] [Google Scholar]
- 43.Jones SC, Clise-Dwyer K, Huston G, Dibble J, Eaton S, Haynes L, et al. Impact of post-thymic cellular longevity on the development of age-associated CD4+ T cell defects. J Immunol. 2008;180:4465–4475. doi: 10.4049/jimmunol.180.7.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heng TS, Chidgey AP, Boyd RL. Getting back at nature: understanding thymic development and overcoming its atrophy. Curr Opin Pharmacol. 2010;10:425–433. doi: 10.1016/j.coph.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 45.Goldberg GL, Sutherland JS, Hammet MV, Milton MK, Heng TS, Chidgey AP, et al. Sex steroid ablation enhances lymphoid recovery following autologous hematopoietic stem cell transplantation. Transplantation. 2005;80:1604–1613. doi: 10.1097/01.tp.0000183962.64777.da. [DOI] [PubMed] [Google Scholar]
- 46.Heng TS, Goldberg GL, Gray DH, Sutherland JS, Chidgey AP, Boyd RL. Effects of castration on thymocyte development in two different models of thymic involution. J Immunol. 2005;175:2982–2993. doi: 10.4049/jimmunol.175.5.2982. [DOI] [PubMed] [Google Scholar]
- 47.Chen BJ, Cui X, Sempowski GD, Chao NJ. Growth hormone accelerates immune recovery following allogeneic T-cell-depleted bone marrow transplantation in mice. Exp Hematol. 2003;31:953–958. doi: 10.1016/s0301-472x(03)00196-6. [DOI] [PubMed] [Google Scholar]
- 48.Chen BJ, Deoliveira D, Spasojevic I, Sempowski GD, Jiang C, Owzar K, et al. Growth hormone mitigates against lethal irradiation and enhances hematologic and immune recovery in mice and nonhuman primates. PLoS One. 2010;5:e11056. doi: 10.1371/journal.pone.0011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lynch HE, Goldberg GL, Chidgey A, Van den Brink MR, Boyd R, Sempowski GD. Thymic involution and immune reconstitution. Trends Immunol. 2009;30:366–373. doi: 10.1016/j.it.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holland AM, van den Brink MR. Rejuvenation of the aging T cell compartment. Curr Opin Immunol. 2009;21:454–459. doi: 10.1016/j.coi.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, et al. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- 52.Haynes L, Eaton SM, Burns EM, Rincon M, Swain SL. Inflammatory cytokines overcome age-related defects in CD4 T cell responses in vivo. J Immunol. 2004;172:5194–5199. doi: 10.4049/jimmunol.172.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 54.Eaton SM, Burns EM, Kusser K, Randall TD, Haynes L. Age-related defects in CD4 T cell cognate helper function lead to reductions in humoral responses. J Exp Med. 2004;200:1613–1622. doi: 10.1084/jem.20041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maue AC, Eaton SM, Lanthier PA, Sweet KB, Blumerman SL, Haynes L. Proinflammatory adjuvants enhance the cognate helper activity of aged CD4 T cells. J Immunol. 2009;182:6129–6135. doi: 10.4049/jimmunol.0804226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou X, Behzad H, Haynes L, Reed SG, Swain SL, McElhaney JE. IL-2 and IL-6 cooperate to enhance the generation of effectors from aged human CD8 T cells to live influenza virus. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones SC, Brahmakshatriya V, Huston G, Dibble J, Swain SL. TLR-activated dendritic cells enhance the response of aged naive CD4 T cells via an IL-6-dependent mechanism. J Immunol. 2010;185:6783–6794. doi: 10.4049/jimmunol.0901296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monto AS, Ansaldi F, Aspinall R, McElhaney JE, Montano LF, Nichol KL, et al. Influenza control in the 21st century: Optimizing protection of older adults. Vaccine. 2009;27:5043–5053. doi: 10.1016/j.vaccine.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 59.Behzad H, Huckriede AL, Haynes L, Gentleman B, Coyle K, Wilschut JC, et al. GLA-SE, a Synthetic Toll-like Receptor 4 Agonist, Enhances T-Cell Responses to Influenza Vaccine in Older Adults. J Infect Dis. 2012;205:466–473. doi: 10.1093/infdis/jir769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tan SY, Cavanagh LL, d’Advigor W, Shackel N, de St Groth BF, Weninger W. Phenotype and functions of conventional dendritic cells are not compromised in aged mice. Immunol Cell Biol. 2012 doi: 10.1038/icb.2011.104. [DOI] [PubMed] [Google Scholar]
- 61.Tesar BM, Walker WE, Unternaehrer J, Joshi NS, Chandele A, Haynes L, et al. Murine myeloid dendritic cell-dependent toll-like receptor immunity is preserved with aging. Aging Cell. 2006;5:473–486. doi: 10.1111/j.1474-9726.2006.00245.x. [DOI] [PubMed] [Google Scholar]