Abstract
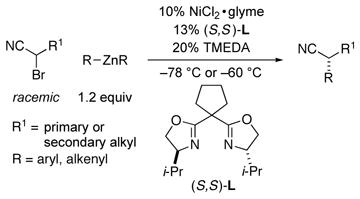
The first method for the stereoconvergent cross-coupling of racemic α-halonitriles is described, specifically, nickel-catalyzed Negishi arylations and alkenylations that furnish an array of enantioenriched α-arylnitriles and allylic nitriles, respectively. Noteworthy features of this investigation include: the highly enantioselective synthesis of α-alkyl-α-aryl nitriles that bear secondary α-alkyl substituents; the first examples of the use of alkenylzinc reagents in stereoconvergent Negishi reactions of alkyl electrophiles; demonstration of the utility of a new family of ligands for asymmetric Negishi cross-couplings (a bidentate bis(oxazoline), rather than a tridentate pybox); in the case of arylzinc reagents, carbon–carbon bond formation at a remarkably low temperature (−78 °C), the lowest reported to date for an enantioselective cross-coupling of an alkyl electrophile; a mechanistic dichotomy between Negishi reactions of an unactivated versus an activated secondary alkyl bromide.
Many bioactive compounds bear a cyano group attached to a stereogenic carbon.1 Furthermore, nitriles serve as versatile precursors to a diverse array of molecules, including heterocycles, aldehydes, ketones, amides, carboxylic acids, and amines.2 Enantioenriched α-alkyl-α-arylnitriles are particularly noteworthy targets, since they can be transformed into α-aryl carboxylic acids such as α-arylpropionic acids, which are widely used as non-steroidal anti-inflammatory drugs (e.g., naproxen).3
A variety of methods have been described for the catalytic asymmetric synthesis of α-alkyl-α-arylnitriles.4,5,6 For example, nickel-catalyzed hydrocyanation of vinylarenes can generate α-methyl-α-arylnitriles with excellent ee; however, there have been no reports of corresponding highly enantioselective hydrocyanations of substituted styrenes to produce nitriles that bear α-alkyl substituents larger than methyl. The conjugate addition of H–CN to β-aryl-α,β-unsaturated carbonyl compounds provides an alternate route to α-alkyl-α-arylnitriles, although current methods generally only furnish products with α-alkyl groups that are primary.
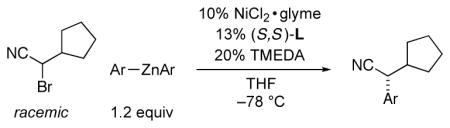
We have been interested in exploring the catalytic asymmetric synthesis of secondary α-arylnitriles through the reaction of an aryl nucleophile with a nitrile that bears a leaving group geminal to the cyano substituent; to the best of our knowledge, there is no precedent for this transformation using a chiral catalyst.7 However, while our study was underway, Falck reported an important investigation of Suzuki arylations of α-(sulfonyl)nitriles in the presence of an achiral palladium catalyst.7c In the case of two enantioenriched electrophiles (one unhindered and one doubly activated), he established that carbon–carbon bond formation proceeds with inversion of stereochemistry with little or no erosion in ee; on the other hand, Falck did not describe the stereochemical outcome for Suzuki couplings of electrophiles that bear a secondary alkyl substituent. In this report, we provide a complementary approach wherein a chiral catalyst achieves stereoconvergent Negishi cross-couplings of racemic electrophiles with aryl, as well as with alkenyl, nucleophiles (eq 1).
 |
(1) |
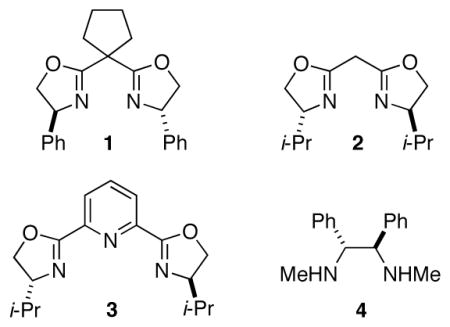
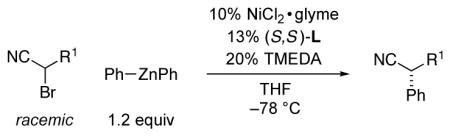
Negishi reactions are an especially attractive family of cross-coupling processes, due to the compatibility of organozinc reagents with a wide range of functional groups and the typical lack of a need for a stoichiometric quantity of a Brønsted-basic additive.8 In previous studies, we determined that several classes of activated electrophiles are suitable coupling partners in asymmetric Negishi alkylations or arylations; in every instance, nickel with a pybox ligand proved to be the catalyst of choice.9,10 Unfortunately, however, when we applied any of the arylation methods to the phenylation of an α-bromonitrile, we obtained disappointing results (<40% ee).
We decided to look beyond tridentate pybox ligands, and after substantial effort we determined that the Negishi phenylation of an α-bromonitrile can be achieved in good ee and yield in the presence of NiCl2•glyme and a new, readily available bis(oxazoline) ligand11 (94% ee, 98% yield; Table 1, entry 1).12 The reaction proceeds at −78 °C, which is, to the best of our knowledge, the lowest temperature that has been employed for a cross-coupling of an alkyl electrophile.13
Table 1.
Stereoconvergent Negishi Phenylation of a Racemic α-Bromonitrile: Effect of Reaction Parametersa

| |||
|---|---|---|---|
| entry | variation from the “standard” conditions | ee (%) | yield (%)b |
| 1 | none | 94 | 98 |
| 2 | no NiCl2 • glyme | – | <2 |
| 3 | no L | <2 | 3 |
| 4 | PhZnX, instead of Ph2Zn | 90 | 6 |
| 5 | r.t., instead of −78 °C | 4 | 16 |
| 6 | 1, instead of L | 84 | 89 |
| 7 | 2, instead of L | 8 | 88 |
| 8 | 3, instead of L | 8 | 32 |
| 9 | 4, instead of L | 32 | 25 |
| 10 | 0.6 equiv Ph2Zn | 93 | 48 |
| 11 | 5% NiCl2 • glyme, 6.5% (S,S )-L | 93 | 68 |
All data are the average of two experiments.
Yield determined by GC analysis versus a calibrated internal standard.
As illustrated in Table 1, essentially no cross-coupling of the α-bromonitrile is observed in the absence of NiCl2•glyme (entry 2) or of bis(oxazoline) L (entry 3), as well as when an arylzinc halide is employed as the nucleophilic reaction partner (entry 4). The reaction proceeds in poor ee and yield when conducted at room temperature (entry 5), and ligands other than L provide modestly (bis(oxazoline) 1; entry 6) or substantially (bis(oxazoline) 2, pybox 3, and 1,2-diamine 4 (entries 7–9)) inferior results. Use of less Ph2Zn (entry 10) or less catalyst (entry 11) leads to a decrease in yield, but no loss in enantioselectivity.
With our optimized method, asymmetric Negishi cross-couplings of Ph2Zn with a range of racemic α-bromonitriles proceed efficiently, furnishing α-phenylnitriles in good ee and yield (Table 2).14 A wide variety of functional groups are compatible with the reaction conditions, including an ether (entry 5), a carbamate (entry 6), an amide and a heterocycle (entry 7), a sulfonamide (entries 8 and 9), an unactivated alkyl chloride (entry 9), and an alkene (entries 11 and 12). In view of the limitations of other methods for generating highly enantioenriched α-alkyl-α-arylnitriles in the case of branched alkyl groups (e.g., via asymmetric hydrocyanation; vide supra), our high ee’s for Negishi cross-couplings of hindered electrophiles (entries 1–9) are particularly noteworthy.
Table 2.
Stereoconvergent Negishi Phenylations of Racemic α-Bromonitrilesa
| |||
|---|---|---|---|
| entry | R1 | ee (%) | yield (%) b |
| 1 | i-Pr | 92 | 77 |
| 2 |
|
92 | 98 |
| 3 |
|
92 | 92 |
| 4c |
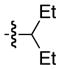
|
92 | 92 |
| 5 |
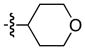
|
92 | 94 |
| 6 |
|
90 | 96 |
| 7 |
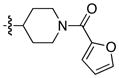
|
85 | 95 |
| 8 |
|
91 | 94 |
| 9 |
|
90 | 94 |
| 10 | Me | 82 | 67 (83)d |
| 11 |
|
78 | 88 |
| 12 |
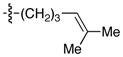
|
76 | 94 |
All data are the average of two experiments.
Yield of purified product.
Reaction temperature: −60 °C.
Yield determined by GC analysis versus a calibrated internal standard.
This process for the Negishi arylation of α-bromonitriles is not limited to phenylation reactions. Thus, we can cross-couple with a range of nucleophiles in uniformly high ee and yield (Table 3), regardless of whether the aryl group is electron-rich (entries 1–3) or electron-poor (entry 4).15
Table 3.
Stereoconvergent Negishi Arylations of Racemic Bromonitrilesa
All data are the average of two experiments.
Yield of purified product.
Reaction temperature: −60 °C.
Although we have previously described examples of stereoconvergent Negishi reactions of activated electrophiles with alkyl- and arylzinc reagents, we have never been able to achieve corresponding cross-couplings with alkenylzinc reagents.9 We were therefore pleased to determine that we can apply the conditions (except reaction temperature16) that we developed for enantioselective Negishi arylations (Table 3) of α-bromonitriles to alkenylations (Table 4).17 Thus, as depicted in Table 4, we can accomplish an array of asymmetric alkenylations with good ee, thereby providing ready access to enantioenriched allylic nitriles.18 No racemization or isomerization (to the conjugated nitrile) of the allylic nitrile is observed under these mild conditions.
Table 4.
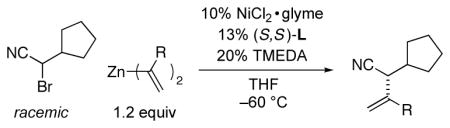
Stereoconvergent Negishi Alkenylations of Racemic α-Bromonitrilesa
| |||
|---|---|---|---|
| entry | R | ee (%) | yield (%) b |
| 1 | (CH2)3Ph | 80 | 64 |
| 2 |
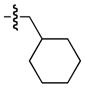
|
86 | 59 |
| 3 |
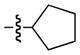
|
89 | 78 |
| 4 | Ph | 91 | 94 |
| 5 |
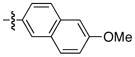
|
92 | 92 |
All data are the average of two experiments.
Yield of purified product.
The enantioenriched allylic nitriles are suitable substrates for stereoselective functionalizations. For example, osmium-catalyzed dihydroxylation proceeds with good diastereoselectivity (eq 2).
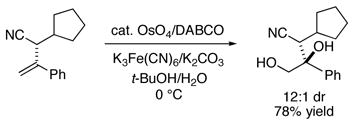 |
(2) |
We have previously proposed that a radical intermediate may be involved in the oxidative-addition step of certain nickel-catalyzed cross-couplings of unactivated secondary alkyl halides.19 One observation consistent with this hypothesis is the stereochemistry of cyclization/cross-couplings of electrophiles that bear pendant olefins. We have now determined that, as for Hiyama and Suzuki reactions,20 Negishi reactions of secondary bromides 5 and 6 proceed with the same diastereoselectivities as observed for reductive cyclizations of these substrates by Bu3SnH, which is consistent with a common intermediate (I) in the cyclization step for all of these processes (eq 3).
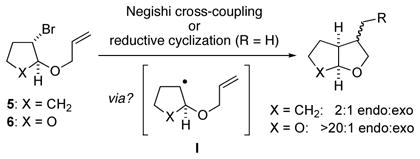 |
(3) |
In this regard, it is interesting to note that the substrates depicted in entries 11 and 12 of Table 2, which have the potential to cyclize/cross-couple to furnish five-membered rings, afford only acyclic coupling products. To gain insight into whether cyclization of a radical intermediate might be occurring, but reversibly, in enantioselective cross-couplings of α-bromonitriles that bear pendant olefins, we examined the Negishi reaction of the electrophile illustrated in eq 4. If radical cyclization is occurring, but the acyclic radical preferentially proceeds to the cross-coupling product (Curtin–Hammett scenario), then the Z stereochemistry of the starting material would not be preserved in the acyclic product. However, we observe only the Z isomer, which suggests that radical cyclization is not occurring in this Negishi reaction of an α-bromonitrile, in contrast to our previous studies of cross-couplings of unactivated secondary alkyl bromides.21
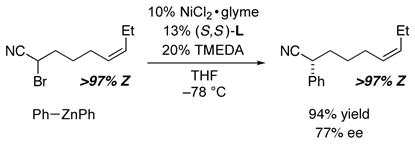 |
(4) |
In summary, we have developed the first method for the stereoconvergent cross-coupling of racemic α-halonitriles, specifically, nickel-catalyzed Negishi arylations and alkenylations that generate a variety of useful enantioenriched secondary α-arylnitriles and allylic nitriles, respectively. With regard to accessing secondary α-alkyl-α-arylnitriles, this work complements other prominent approaches, which are most effective for the synthesis of products in which the alkyl group is methyl (hydrocyanation of olefins) or a primary alkyl substituent (conjugate addition of HCN). For the first time for studies of asymmetric Negishi cross-couplings, an alkenylzinc reagent can serve as an effective nucleophilic partner, and a ligand other than a tridentate pybox is the ligand of choice; this latter observation is significant for future explorations of enantioselective Negishi reactions. Couplings with arylzinc reagents occur at a remarkably low temperature (−78 °C), the lowest temperature reported to date for such asymmetric carbon–carbon bond-forming processes. Finally, a mechanistic study provides a contrast between nickel-catalyzed Negishi reactions of unactivated vs. activated secondary alkyl bromides. An array of additional investigations of enantioselective cross-couplings of alkyl electrophiles are underway.
Supplementary Material
Acknowledgments
Support has been provided by the National Institutes of Health (National Institute of General Medical Sciences, grant R01–GM62871) and the Kwanjeong Educational Foundation (fellowship to J.C.). We thank Ashraf Wilsily for assistance in the preparation of substrates
Footnotes
Supporting Information Available: Experimental procedures and compound characterization data (PDF). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For leading references, see: Fleming FF. Nat Prod Rep. 1999;16:597–606.Fleming FF, Yao L, Ravikumar PC, Funk L, Shook BC. J Med Chem. 2010;53:7902–7917. doi: 10.1021/jm100762r.
- 2.For leading references, see: Murahashi S-I, editor. Science of Synthesis. Vol. 19 Georg Thieme Verlag; Stuttgart, Germany: 2004.
- 3.For leading references, see: Landoni MF, Soraci A. Current Drug Metabolism. 2001;2:37–51. doi: 10.2174/1389200013338810.Kumaresan C. Int J Curr Pharm Res. 2010;2:1–3.Casalnuovo AL, Rajanbabu TV. In: Chirality in Industry II. Collins AN, Sheldrake GN, Crosby J, editors. Chapter 15 John Wiley & Sons; New York: 1997.
- 4.To the best of our knowledge, effective methods for the catalytic enantioselective synthesis of secondary nitriles via C–H → C–Ar α-arylations have not yet been reported.
- 5.For reviews of catalytic asymmetric hydrocyanations of olefins, see: van Leeuwen PWN. In: Science of Synthesis, Stereoselective Synthesis. De Vries JG, Molander GA, Evans PA, editors. Vol. 1. Georg Thieme Verlag; Stuttgart, Germany: 2011. pp. 409–475.RajanBabu TV, Casalnuovo AL. In: Comprehensive Asymmetric Catalysis. Jacobsen EN, Pfaltz A, Yamamoto H, editors. Vol. 1. Springer; New York: 1999. pp. 267–378.
- 6.For examples of catalytic asymmetric conjugate additions of H–CN to generate α-alkyl-α-arylnitriles, see: α substituent = primary alkyl group: Mita T, Sasaki K, Kanai M, Shibasaki M. J Am Chem Soc. 2005;127:514–515. doi: 10.1021/ja043424s.Mazet C, Jacobsen EN. Angew Chem, Int Ed. 2008;47:1762–1765. doi: 10.1002/anie.200704461.Kurono N, Nu N, Sakaguchi Y, Uemura M, Ohkuma T. Angew Chem, Int Ed. 2011;50:5541–5544. doi: 10.1002/anie.201100939.α substituent = secondary alkyl group: Wang J, Li W, Liu Y, Chu Y, Lin L, Liu X, Feng X. Org Lett. 2010;12:1280–1283. doi: 10.1021/ol100169r.
- 7.We are aware of only a few reports of cross-couplings of this type even with achiral catalysts: Negishi reactions: Frejd T, Klingstedt T. Synthesis. 1987:40–42.Hiyama reactions: Strotman NA, Sommer S, Fu GC. Angew Chem, Int Ed. 2007;46:3556–3558. doi: 10.1002/anie.200700440.Suzuki reactions: He A, Falck JR. J Am Chem Soc. 2010;132:2524–2525. doi: 10.1021/ja910582n.Suzuki reactions: Yang Y, Tang S, Liu C, Zhang H, Sun Z, Lei A. Org Biomol Chem. 2011;9:5343–5345. doi: 10.1039/c1ob05326d.
- 8.For a review, see: Negishi E-i, Hu Q, Huang Z, Wang G, Yin N. In: The Chemistry of Organozinc Compounds. Rappoport Z, Marek I, editors. Chapter 11 Wiley; New York: 2006.
- 9.Alkylations of α-haloamides: Fischer C, Fu GC. J Am Chem Soc. 2005;127:4594–4595. doi: 10.1021/ja0506509.Alkylations of benzylic halides: Arp FO, Fu GC. J Am Chem Soc. 2005;127:10482–10483. doi: 10.1021/ja053751f.Alkylations of allylic halides: Son S, Fu GC. J Am Chem Soc. 2008;130:2756–2757. doi: 10.1021/ja800103z.Arylations of propargylic halides and carbonates: Smith SW, Fu GC. J Am Chem Soc. 2008;130:12645–12647. doi: 10.1021/ja805165y.Oelke AJ, Sun J, Fu GCJ. Am Chem Soc. 2012;134:2966–2969. doi: 10.1021/ja300031w.Arylations of α-haloketones: Lundin PM, Esquivias J, Fu GC. Angew Chem, Int Ed. 2009;48:154–156. doi: 10.1002/anie.200804888.
- 10.For a very recent application of our asymmetric allylation method in the total synthesis of a natural product (carolacton), see: Schmidt T, Kischning A. Angew Chem, Int Ed. 2012;51:1063–1066. doi: 10.1002/anie.201106762.
- 11.Bis(oxazoline) L can be prepared in one step from commercially available 1,1-cyclopentanedicarbonitrile and valinol.
- 12.All of the diorganozinc compounds are generated from Grignard reagents and Zn(OMe)2 (for a report of the use of Zn(OMe)2 to generate organozinc reagents, see: Côté A, Charette AB. J Am Chem Soc. 2008;130:2771–2773. doi: 10.1021/ja710864p.When ZnX2 (X=Cl, Br, or I) is employed instead of Zn(OMe)2, somewhat lower ee and/or yield are observed.
- 13.For enantioselective Kumada reactions of α-haloketones that proceed at −60 °C, see: Lou S, Fu GC. J Am Chem Soc. 2010;132:1264–1266. doi: 10.1021/ja909689t.
- 14.Notes: Under our standard conditions: on a gram-scale, the asymmetric Negishi reaction illustrated in entry 8 of Table 2 proceeds in 90% ee and 93% yield; if PhMgBr is employed as the nucleophile (Table 2, entry 2), Kumada coupling occurs in 88% ee and 6% yield; there is no erosion in the enantiomeric excess of the product during the course of a cross-coupling; an α-chloronitrile and an α-iodonitrile cross-couple in significantly lower ee (chloride) and/or yield (chloride and iodide); diethylzinc and alkylzinc halides are not suitable cross-coupling partners.
- 15.Under our standard reaction conditions, essentially no cross-coupling is observed when (o-tol)2Zn is employed as the nucleophile.
- 16.The cross-coupling proceeds in high ee, but somewhat slowly, at −78 °C.
- 17.Under our standard reaction conditions, divinylzinc cross-couples in 55% ee and 80% yield.
- 18.There have been a few reports of catalytic asymmetric hydrocyanations of 1,3-dienes to generate allylic nitriles (up to 86% ee): Wilting J, Janssen M, Müller C, Vogt D. J Am Chem Soc. 2006;128:11374–11375. doi: 10.1021/ja064378u.Saha B, RajanBabu TV. Org Lett. 2006;8:4657–4659. doi: 10.1021/ol062002f.
- 19.For early suggestions, see: Zhou J, Fu GC. J Am Chem Soc. 2004;126:1340–1341. doi: 10.1021/ja039889k.Powell DA, Fu GC. J Am Chem Soc. 2004;126:7788–7789. doi: 10.1021/ja047433c.For a recent discussion and leading references, see: Zultanski SL, Fu GC. J Am Chem Soc. 2011;133:15362–15364. doi: 10.1021/ja2079515.For some noteworthy reports by others, see: Jones GD, Martin JL, McFarland C, Allen OR, Hall RE, Haley AD, Brandon RJ, Konovalova T, Desrochers PJ, Pulay P, Vicic DA. J Am Chem Soc. 2006;128:13175–13183. doi: 10.1021/ja063334i.Phapale VB, Buñuel E, García-Iglesias M, Cárdenas DJ. Angew Chem, Int Ed. 2007;46:8790–8795. doi: 10.1002/anie.200702528.Lin X, Phillips DLJ. Org Chem. 2008;73:3680–3688. doi: 10.1021/jo702497p.
- 20.For studies of: Hiyama reactions: Powell DA, Maki T, Fu GC. J Am Chem Soc. 2005;127:510–511. doi: 10.1021/ja0436300.Suzuki reactions: González-Bobes F, Fu GC. J Am Chem Soc. 2006;128:5360–5361. doi: 10.1021/ja0613761.
- 21.It is possible that, in the case of certain activated electrophiles, oxidative addition may not proceed via an initial SH2-like abstraction of the halide by nickel to generate an alkyl radical.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.