Abstract
Immune cells and hematopoietic progenitors express estrogen receptors (ER). As ligand-activated transcription factors that modulate chromatin structure, ER regulate transcriptional programs that direct the development or functional responses of immune cells. ER-regulated immune responses likely contribute to significant sex biases in infection, autoimmunity and other inflammatory diseases, and changes in immune function during the female hormonal cycle and pregnancy. Here we summarize our own and others’ studies showing that ERα signaling regulates the development of dendritic cells (DCs), antigen-presenting cells crucial for initiation of innate and adaptive immunity. During inflammation, elevated GM-CSF directs the development of new DCs from monocytes or other precursors that infiltrate tissues and lymphoid organs, and these de novo populations of inflammatory DCs have critical roles in programming T cell-mediated responses during infection and autoimmunity. Estradiol acting via ERα, but not ERβ, promotes the GM-CSF-mediated inflammatory pathway of DC differentiation, leading to the development of DCs with increased functional capacity. Estradiol/ERα signaling acts directly in GM-CSF-stimulated myeloid progenitors to induce elevated levels of IRF4, a transcription factor that directs a developmental program underlying CD11b+ DC differentiation. In contrast, during homeostatic Flt3 Ligand-driven DC development, ERα signaling decreases numbers of myeloid progenitors and differentiated DCs, yet promotes more functionally competent DCs. Thus ERα signaling regulates the response of DC progenitors to the external cytokine environment, thereby altering the strength or integrity of DC developmental pathways. The development of increased numbers of DCs during inflammation will likely increase the magnitude of DC-mediated functional responses including cytokine production, processing and MHC-mediated presentation of antigens, and activation and polarization of T and B lymphocytes; these functions also may be regulated directly by ERα signaling. In sum, via profound effects on DC development and ensuing functional responses, ERα signaling can regulate the quality of the adaptive immune responses and influence the resolution of infection or chronic inflammatory diseases.
Keywords: Estrogen, Estrogen Receptor, Sex Hormone, SERM, Immunoendocrinology, Dendritic Cells, Antigen-presenting cells, Cellular differentiation, Inflammation, Hematopoiesis
1. Introduction
Novel roles of nuclear hormone receptors in immune cell biology have been elucidated in recent years. Through their action as ligand-activated transcription factors, nuclear receptors (e.g., for estrogen, androgen, vitamin D, retinoic acid) regulate gene expression programs that direct immune cell development or function (Moro et al., 2008; Olsen and Kovacs, 1996; Fish, 2008). Immune cells and their progenitors in females and males express estrogen receptors (ERs). Accordingly, effects of estrogens or ERα on normal or pathological immune responses, or immune cell development, have been identified (Straub, 2007; Kovats and Carreras, 2008). An understanding of how ER signaling regulates immunity is clinically important. Autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis and multiple sclerosis are strongly female-biased and modulated by sex steroid levels and pregnancy, yet the cellular and molecular basis for this is not completely understood (Whitacre, 2001). Furthermore, sex differences including sex hormone levels are one crucial factor that modulates susceptibility and immune responses to virus infection (Klein et al., 2011). Thus the study of mechanisms of ER-regulated immunity will lay the foundation for new therapeutic approaches to these sex-biased immune diseases. Here, I begin with a description of ER signaling mechanisms and an overview of DC development and function, followed by a review of studies that provide evidence for the ER-mediated regulation of the development and function of dendritic cells (DCs) in rodents and humans.
2. ER signaling mechanisms
ER are ligand-dependent transcription factors that regulate chromatin structure and gene expression by forming complexes with chromatin-modifying coregulators and other transcription factors (Mann et al., 2011). Estrogens also elicit rapid (within minutes) changes in cell signaling pathways, and although currently there is no consensus as to whether these rapid responses involve the classical ER, the signaling pathways activated can lead to histone modifications and altered chromatin structure near estrogen-regulated genes (Mann et al., 2011; Heldring et al., 2007). Thus a complete understanding of how estrogens modulate immune responses will ultimately stem from investigation of mechanisms by which ligand-bound ER modulate gene expression programs in immune cells or their precursors.
ER α and β proteins are members of the nuclear receptor super family; single ER chains form αα, ββ and αβ dimers, each of which may be functionally distinct (Heldring et al., 2007). ERα, and in some cases ERβ, expression by mature immune cells in murine lymphoid organs and human blood has been reported [reviewed in (Kovats et al., 2010)]. ERα appears to be ubiquitously expressed by immune cells, based on reports of expression in T and B lymphocytes, DCs, macrophages, monocytes, natural killer cells and mast cells. ERβ is less universally expressed. Within human peripheral blood mononuclear cells (PBMC), CD4+ T cells express higher amounts of ERα than ERβ, CD8+ T cells express low amounts of both ER, and B cells express more ERβ than ERα (Phiel et al., 2005). Human monocytes and monocyte-derived DCs, and blood myeloid and plasmacytoid DCs, express ER and respond to estrogens (Mor et al., 2003; Escribese et al., 2008; Seillet et al., 2012). In mice, splenic DCs and peritoneal macrophages express ERα but not ERβ. However, some populations of DCs in vivo, such as DCs infiltrating the central nervous system during experimental autoimmune encephalomyelitis (EAE), do express ERβ (Du et al., 2011).
Hematopoietic progenitors in human and murine bone marrow also express ER. CD34+ hematopoietic progenitors in human adult bone marrow, but not cord blood, express both ERα and ERβ (Igarashi et al., 2001). In mice, ERα is expressed by adult bone marrow hematopoietic progenitors, but not by fetal liver progenitors (Igarashi et al., 2001; Carreras et al., 2008). These data suggest that ER expression in hematopoietic progenitors coincides with development of the mature immune system in neonatal life.
Blood and tissues contain variable levels of both endogenous and exogenous ER ligands, which are likely to impact DC differentiation and function during homeostasis and inflammation. Endogenous estrogens include 17-β-estradiol, the major form present in adult females and males, and estriol, which is produced at high levels during pregnancy. The KD of the ER for estradiol is 0.1–1.0 nM (27–272 pg/ml). This is consistent with serum levels of estradiol in cycling female mice: ~25–35 pg/ml during diestrus and ~70–200 pg/ml during estrus, and in male mice: ~8–15 pg/ml (Foster et al., 1983). Serum estradiol levels in humans peak at 200–500 pg/ml, while levels at the term of pregnancy reach 16,000 – 30,000 pg/ml (Askanase and Buyon, 2002). The body also is exposed to ill-defined amounts of exogenous ER ligands, including phytoestrogens and environmental endocrine disruptors such as bisphenol A. A class of pharmaceuticals termed selective ER modulators (SERM), such as tamoxifen and raloxifene, also may modulate immune function. SERM have tissue-specific agonist or antagonist properties and are used to treat breast cancer and osteoporosis, and contemplated for treatment of autoimmune disease (Dutertre and Smith, 2000). Recently identified estrogen receptor subtype agonists are a new class of drugs planned for treatment of clinical conditions secondary to menopause, but also may be useful for immune modulation (Leitman et al., 2010).
Upon ligand binding, cytosolic ER dimers are released from stabilizing heat shock proteins and translocate to the nucleus. Nuclear ER directly bind estrogen response sequence elements proximal to genes, or are tethered indirectly to DNA by forming complexes with other transcription factors that bind DNA via their own consensus sequences (O’Lone et al., 2004). ER often bind to DNA associated with transcription factors (e.g. NF-κB, Sp1, AP-1, C/EBPβ) that are important for immune cell function (Leitman et al., 2010). Each structurally distinct ligand imparts a specific unique conformation to ER dimers, which then dictates recruitment of distinct profiles of coregulators and histone-modifying enzymes into multi-protein transcription complexes (Heldring et al., 2007). The complex of ligand-bound ERα, coregulators and histone-modifying enzymes leads to post-translational histone modifications (acetylation, phosphorylation, methylation) that alter chromatin structure (Mann et al., 2011). Transcriptional coregulators may act as coactivators or corepressors (or both), remodeling chromatin in configurations that are permissive or inhibitory for transcription. For example, some ER coactivators have histone acetyltransferase activity (e.g. SRC1), or they can interact with acetyltransferases such as p300/CBP, leading to transcriptional activation. ER corepressors such as NCOR can complex with histone deacetylases, leading to gene repression. Although cell type specific responses to ER ligands are dictated by the cellular complement of coregulators, little is known about coregulator expression in immune cells.
Ligation of ER may lead to disparate patterns of gene expression in different cell types, depending on ligand form and concentration, the relative cellular expression of the two ER, and the availability of coactivators or corepressors that vary between cell types (Frasor et al., 2003). ERα and ERβ have distinct and common target genes (Leitman et al., 2010). Exclusive ligation of ERα or ERβ results in distinct molecular outcomes in several model systems, and when expressed by the same cell, ERβ action modulates gene expression networks regulated by ERα, often opposing them. Thus, it will be important to define a role for either ERα or ERβ (or both) in particular immune pathways.
3. Dendritic cell function in innate and adaptive immunity
DCs are antigen-presenting cells whose function bridges innate and adaptive immunity. In their role as immune system sentinels, DCs are activated by conserved pathogen molecules, such as lipopolysaccharide, peptidoglycan or single-stranded viral RNA, that bind to several classes of cell surface or intracellular pattern recognition receptors. Pattern recognition receptors include the Toll-like receptors (TLR) as well as the NOD-like receptors (NLR) and inflammasomes, C-type lectin receptors (CLR) and the RIG-I-like receptors (RLR) (Kawai and Akira, 2010; Franchi et al., 2009; Rathinam and Fitzgerald, 2011). Pattern recognition receptors also are triggered by self-molecules (e.g. nucleic acids, extracellular ATP, uric acid) released by damaged tissue, a process likely involved in the initiation of autoimmunity. Upon binding ligand, pattern recognition receptors initiate signal transduction cascades, leading to activation of transcription factors such as NF-κB that induce expression of genes involved in DC functional responses. Once activated via pattern recognition receptors, DC functions during innate and adaptive immunity include: (i) production of soluble mediators including cytokines, chemokines, and reactive oxygen species; (ii) antigen acquisition, degradation and MHC-mediated presentation to T cells; (iii) upregulation of costimulatory molecules involved in lymphocyte activation; (iv) migration from peripheral tissues to lymph nodes; and (v) induction of the activation and differentiation of naïve, effecter and memory B and T lymphocyte populations (Mellman and Steinman, 2001).
Resident tissue DCs are primarily involved in the initial sensing of pathogens or damaged self (Helft et al., 2010). These DCs produce proinflammatory cytokines and chemokines, migrate to regional lymph nodes and activate populations of T and B lymphocytes to initiate or perpetuate immune responses.
De novo development of DCs also occurs during infection, autoimmune disease and other chronic inflammatory conditions (Shi and Pamer, 2011). During inflammation, elevated GM-CSF directs the development of new DCs from monocytes or other proliferating precursors that infiltrate tissues and secondary lymphoid organs (Schmid et al., 2011; Hamilton, 2008). Via their functional responses that promote innate and adaptive immunity, the newly developed DCs are often central to programming the quality of the lymphocyte-mediated immune response during autoimmunity and infection (Nakano et al., 2009; Serbina et al., 2003; Ballesteros-Tato et al., 2010; King et al., 2009).
We and others have determined that estradiol and ERα signaling promote the GM-CSF-mediated differentiation of DCs from murine hematopoietic progenitors or human and rat monocytes (Paharkova-Vatchkova et al., 2004; Mao et al., 2005; Carreras et al., 2008; Carreras et al., 2010; Douin-Echinard et al., 2008; Siracusa et al., 2008; Zhang et al., 2004; Komi and Lassila, 2000). These studies are described in detail below. Augmentation of the inflammatory pathway of DC development by ERα signaling may lead to increased numbers of inflammatory DCs in tissues and lymphoid organs that can direct innate and adaptive immune responses. Furthermore, DC development in the presence of ER signaling may alter gene expression programs that affect DC functional responses.
4. Overview of DC development
DC subsets in vivo are defined based on location, surface markers, and function, and if they are present in homeostasis or develop during inflammation (Hashimoto et al., 2011; Shortman and Naik, 2007; Geissmann et al., 2010). Most DC populations have a short lifespan in vivo (2–10 days) and thus are continually being replenished during homeostasis and inflammation. Furthermore, upon activation by inflammatory stimuli, DCs die within 1–2 days. Thus it is probable that DC developmental pathways and DC numbers will be impacted by physiological changes in the host, including fluctuations in sex hormone levels. I will first describe DC developmental pathways that occur during homeostasis and inflammation; then I will describe how ERα regulates these pathways of DC differentiation.
4.1. Homeostasis
In homeostasis, DC populations in murine lymphoid organs include conventional CD11chi DCs, composed of CD11b+ and CD8α+ subsets, and plasmacytoid DCs. Peripheral tissues such as lung, intestinal lamina propia and dermis also contain multiple DC subsets, the most abundant of which are phenotypically defined as CD11b+ or CD103+. These tissue DC populations constitutively migrate into local lymph nodes, where they likely participate in mechanisms of self-tolerance. Analogous populations of lymph node and tissue DCs in humans are being defined (Segura et al., 2012).
The phenotypically defined lymphoid organ and migratory tissue DC subsets also are distinguished by their developmental dependence on specific precursors, particular combinations of transcription factors and one or more of the cytokines Fms-like tyrosine kinase 3 Ligand (Flt3L), granulocyte-macrophage colony-stimulating factor (GM-CSF) or macrophage colony-stimulating factor (M-CSF) (Merad and Manz, 2009; Geissmann et al., 2010; Helft et al., 2010). Multiple cell types produce Flt3L including bone marrow stromal cells (Lyman and Jacobsen, 1998). Flt3L stimulates Flt3/CD135, a receptor tyrosine kinase expressed on early hematopoietic progenitors, leading to STAT3 signaling which also is essential for DC development (Laouar et al., 2003). Multiple cell types constitutively produce M-CSF. The M-CSF receptor (CD115) is a receptor tyrosine kinase that signals via a domain encoded by the Fms proto-oncogene to activate multiple downstream pathways (Mouchemore and Pixley, 2012). GM-CSF is produced at nearly undetectable levels during homeostasis and acts via the GM-CSF receptor, which stimulates the JAK-STAT, MAPK, PI3K and NF-κB pathways (van de Laar et al., 2012). Analyses of mice lacking these cytokines or their receptors revealed that the Flt3L-driven pathway of DC development has the more pronounced effect on DC development during homeostasis in vivo, although M-CSF and GM-CSF also contribute to development of particular subsets of tissue DCs in the steady state (Merad and Manz, 2009; Helft et al., 2010; Sathe and Wu, 2011). As described below, estradiol/ERα signaling regulates both GM-CSF- and Flt3L-mediated DC differentiation from hematopoietic progenitors of myeloid cells.
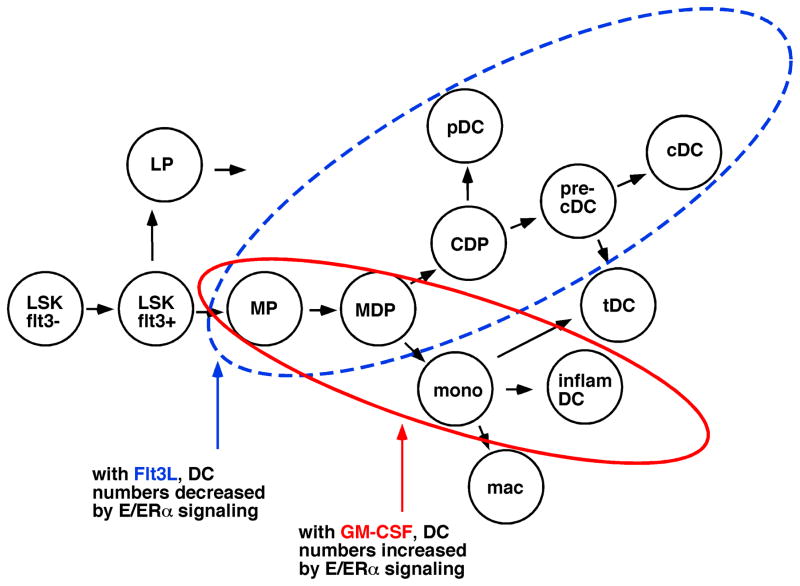
DCs arise from one of multiple hematopoietic progenitors during homeostasis, progressing through intermediate cell stages before becoming terminally differentiated DCs (Fig. 1) (Merad and Manz, 2009; Sathe and Wu, 2011). Hematopoietic progenitors are contained within a bone marrow population of lineage marker negative (Lin−) cells that lack surface proteins expressed by mature myeloid, lymphoid or erythroid cells. The Lin− Sca1+ c-kit+ (LSK) fraction contains long term repopulating progenitors, which give rise to LSK-flt3+ short term repopulating progenitors that are the precursors of the common myeloid progenitor and the common lymphoid progenitors (Schmid et al., 2010). DCs develop from the flt3+ (CD135+) fractions of myeloid progenitors or lymphoid progenitors (Merad and Manz, 2009; Sathe and Wu, 2011).
Fig. 1. Hematopoietic progenitors of DC are differentially regulated by ERα signaling in Flt3L and GM-CSF mediated developmental pathways.
Long-term repopulating hematopoietic progenitors (LSK-flt3−) progress through LSK-flt3+ progenitors which branch into myeloid (MP) and lymphoid (LP) progenitors. For DC development, MP yield the macrophage-DC progenitor (MDP) and common DC progenitor (CDP). In Flt3L-mediated DC development during homeostasis, the CDP yields plasmacytoid DC (pDC) and pre-cDC, the immediate precursors of conventional DC (cDC) and some tissue DC (tDC). Estradiol (E)/ERα signaling decreases the number of DC that develop along this pathway (blue dashed circle). In the GM-CSF-driven pathway, MP give rise to MDP and monocytes that differentiate to populations of inflammatory DC and some tDC. Estradiol/ERα signaling promotes this developmental pathway (red solid circle). LP also yield some DC populations in vivo (not shown). This figure was adapted from (Schmid et al., 2010).
Downstream of the myeloid progenitor, developmental intermediates in bone marrow include the common DC progenitor (CDP) with restricted potential for conventional (cDC) and plasmacytoid (pDC) DCs (Sathe and Wu, 2011), and the macrophage-DC progenitor (MDP) with potential for macrophages and cDCs (Fogg et al., 2006). A later intermediate, pre-cDCs, which develop into conventional tissue DCs, is present in blood and lymphoid organs (Liu et al., 2009). Blood-borne monocytes also give rise to populations of CD11b+ conventional tissue DCs in lymphoid organs and tissues during homeostasis (Jakubzick et al., 2008). We and others have demonstrated that bone marrow populations of LSK progenitors and myeloid progenitors express ERα but not ERβ, indicating that these progenitors will be regulated by ERα signaling (Igarashi et al., 2001; Carreras et al., 2008).
4.2. Inflammation
Inflammation associated with autoimmunity and infection leads to increased numbers of DCs as well as changes in the composition and phenotype of DC populations in tissues and lymph nodes. Because DC lifespan is short, during the course of inflammation, newly developed DC populations will rapidly replace older resident DC populations and thus participate in adaptive immune responses. In response to an initial insult perceived via pattern recognition receptors, resident tissue DCs activated in situ produce cytokines and chemokines that recruit other immune cells to the affected tissue. These cytokine-producing DCs also migrate to local lymph nodes where they contribute to an inflammatory environment and present their acquired antigens to T cells. Once the inflammation is established, blood-borne DC precursors including monocytes and CDPs are activated via pattern recognition receptor signaling and recruited to inflamed lymph nodes or tissues. Monocytes differentiate to inflammatory DCs in lymph nodes or tissues, while CDPs give rise to increased numbers of conventional and plasmacytoid DCs in lymph nodes (Shortman and Naik, 2007; Schmid et al., 2011).
A specialized population of inflammatory Gr-1hi monocytes migrates preferentially to inflamed tissues or local lymph nodes and differentiates to de novo populations of phenotypically distinct inflammatory DCs that are not present in homeostasis (Shi and Pamer, 2011). Monocyte recruitment and inflammatory DC differentiation occurs in murine autoimmune disease models such as EAE and in human diseases such as multiple sclerosis, psoriasis and atherosclerosis (Serbina et al., 2008). Monocyte recruitment and differentiation also play an important role in human microbial and parasite infections including Toxoplasmosis, Mycobacterium tuberculosis, and Salmonella, and have been well studied in murine Listeria monocytogenes infection (Serbina et al., 2008).
The new DC development during inflammation is often driven by elevated GM-CSF (Shortman and Naik, 2007; Hamilton, 2002; Varol et al., 2009). GM-CSF does not circulate at detectable levels in homeostasis and is more readily detected upon induction by IL-1 and TNFα associated with bacterial or viral infection during infection (Hamilton, 2008). GM-CSF levels are increased during autoimmune diseases such as multiple sclerosis and rheumatoid arthritis, in which GM-CSF reaches high levels in inflamed synovial joints. Mice lacking GM-CSF or its receptor have been used to define a role for elevated GM-CSF in infections such as Listeria monocytogenes (Zhan et al., 1998) and autoimmune disease models such as collagen-induced arthritis and EAE (Hamilton, 2002).
Newly developed inflammatory DC populations play an important non-redundant role in the immune response during infection or autoimmunity. Recent murine studies have elucidated the importance of inflammatory monocyte to DC differentiation (either in LN or peripheral tissues) during the inflammation associated with autoimmunity (EAE model), pathogen infection (Listeria monocytogenes, Trypanosoma brucei, influenza virus) or complete Freund’s adjuvant/antigen immunization (Nakano et al., 2009; Serbina et al., 2003; Ballesteros-Tato et al., 2010; King et al., 2009; Bosschaerts et al., 2010). This de novo differentiation of populations of inflammatory and tissue DCs ultimately is critical to the ensuing immune response because these newly developed DCs are often the population that presents disease-related antigens, directs the differentiation of naïve T cells into antigen-specific effector cells, and produces proinflammatory cytokines or other soluble mediators that dictate the course of the immune response and disease. For example, during influenza virus infection, de novo populations of CD11b+ DC in lymph nodes uniquely express CD70 and are crucial to priming of a virus-specific CD8+ T cell response, and blood monocyte-derived CD11b+ Gr-1hi inflammatory DC produce IL-12p70 and uniquely induce differentiation of antigen-specific TH1 cells (Nakano et al., 2009; Ballesteros-Tato et al., 2010).
Thus an understanding of how environmental factors such as estrogens regulate de novo DC differentiation pathways will provide insight into disease pathogenesis. We and others have extensively characterized how estradiol-ERα signaling promotes GM-CSF-mediated DC differentiation in an ex vivo culture model (see below). The action of ERα signaling to increase numbers of new DCs during inflammation is one way in which estrogens may modulate DC-mediated functional responses in immunity and disease.
5. Regulation of GM-CSF-mediated DC development by estradiol/ERα signaling
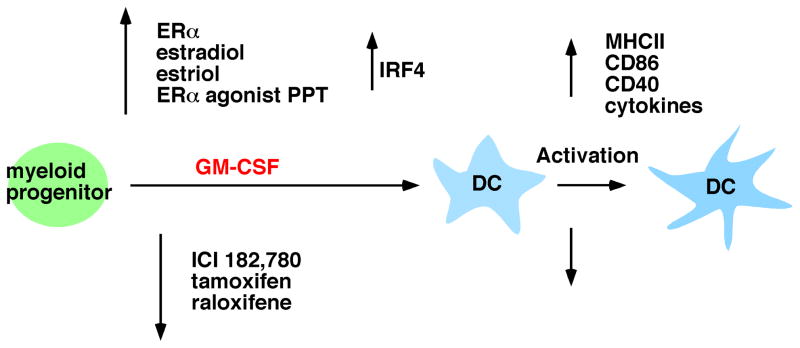
Bone marrow progenitors cultured with GM-CSF yield CD11b+ DCs that most closely resemble in vivo DC populations that are derived from inflammatory monocytes and select populations of langerin+ and CD11b+ tissue DCs. Multiple groups have shown that estradiol/ERα signaling promotes GM-CSF-mediated DC differentiation from murine bone marrow precursors (Paharkova-Vatchkova et al., 2004; Douin-Echinard et al., 2008; Siracusa et al., 2008). In this section, we summarize our results in this model, including the estrogen-promoted DC phenotype and functional capacity, ER α and β dependence, DC progenitors responsive to ER signaling, and a molecular target of ERα in progenitors (Fig. 2). While our studies were done with murine cells, estradiol also promotes human and rat monocyte to DC differentiation mediated by GM-CSF (Komi and Lassila, 2000; Zhang et al., 2004).
Fig. 2. Estradiol/ERα signaling promotes GM-CSF-driven DC differentiation and yields DC with increased functional capacity.
ERα signaling, in response to estradiol, estriol and the ERα selective agonist PPT, acts in GM-CSF-stimulated myeloid progenitors to increase transcription of IRF4 and promote DC differentiation. DC that differentiated in the presence of these ER ligands showed an increased response to activating stimuli in that they displayed higher levels of MHC class II and costimulatory molecules, activated T cells and produced greater amounts of inflammatory cytokines. The presence of the ER antagonist ICI 182,780 or the SERM tamoxifen and raloxifene inhibited DC differentiation and led to a DC population that was hyporesponsive to activating stimuli.
5.1. Estrogens promote GM-CSF-mediated DC differentiation via ERα
Differentiation of DCs occurs upon culture of primary murine bone marrow cells in GM-CSF; due to the asynchronous nature of the culture, significant numbers of DCs are observed and typically studied after 7–10 days (Inaba et al., 1992). Since fetal calf serum (FCS) contains estradiol and phenol red is a weak ER agonist (Berthois et al., 1986), we used steroid depleted (charcoal dextran stripped) FCS and phenol red-free culture (termed “hormone-deficient”) medium, lacking measurable levels of estradiol, to assess a requirement for estradiol in this model of DC differentiation. Beginning with total bone marrow cells, we found that differentiation of DCs requires physiological amounts (near the KD of the ER, 0.1 nM) of 17-β-estradiol in the culture medium, with the dose response peaking at 1 nM (Paharkova-Vatchkova et al., 2004; Carreras et al., 2008). DC differentiation from both female and male bone marrow cells is impaired in hormone-deficient medium and is restored by inclusion of ≥ 0.05–0.1 nM estradiol during the entire culture period. The steroidal ER antagonist ICI 182,780, an inhibitor of both ERα and ERβ, competitively inhibits the effect of estradiol in hormone-deficient medium, demonstrating that estradiol acts via ER. When included in bone marrow cultures containing regular tissue culture medium (with phenol red and regular FCS), ICI 182,780 reduces DC numbers, indicating that DC differentiation depends upon the ER ligands present in the tissue culture medium typically used for culturing immune cells. Estriol, a form of estrogen elevated during pregnancy, also promotes DC differentiation when present at 1 nM (S.K., unpublished observations). Testosterone or dihydrotestosterone did not restore DC differentiation in hormone deficient medium, indicating a specific effect of ER, but not AR, ligands (Paharkova-Vatchkova et al., 2004). This was consistent with the fact that DCs did not express aromatase and thus could not convert testosterone to estradiol (S.K., unpublished observations).
DC differentiation from ERα−/− but not ERβ−/− bone marrow cells is inhibited, indicating an essential role only for ERα in the effect of estradiol to promote GM-CSF-driven DC differentiation (Paharkova-Vatchkova et al., 2004; Carreras et al., 2008; Douin-Echinard et al., 2008). Consistent with this, the ERα-specific ligand PPT [4,4′,4″-(4-Propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol] at 1–10 nM, but not the ERβ-specific ligand DPN [2,3-bis(4-Hydroxyphenyl)-propionitrile] at 1–10 nM, induced DC differentiation in hormone-deficient medium (S.K., unpublished observations).
5.2 Estradiol promotes DC functional competence
The CD11c+ DCs that develop in the GM-CSF-driven bone marrow cultures may be divided into two distinct populations, CD11bint Ly6C− and CD11bhi Ly6C+, both of which have typical DC morphology (Mao et al., 2005). Estradiol/ERα signaling is preferentially required for the differentiation of the CD11bint Ly6C− DCs, although it also promotes increased numbers of CD11bhi Ly6C+ DCs. The estradiol-dependent CD11bint Ly6C− DCs preferentially express langerin (CD207) and contained Birbeck granules, suggesting that they correspond to in vivo populations of tissue langerin+ DC such as epidermal Langerhans cells or dermal CD103+ DCs (Mao et al., 2005).
Relative to CD11bhi Ly6C+ DCs, the estrogen-dependent CD11bint Ly6C− DCs display higher intrinsic levels of MHC class II and costimulatory molecules prior to activation, and show the greatest increase in MHC class II and CD80, CD83, and CD86 after activation by Toll-like receptor (TLR) ligands (Mao et al., 2005). DCs that differentiated in the presence of ER antagonists such as ICI182,780 are primarily CD11bhi Ly6C+ and express reduced levels of MHC class II and CD86 before and after activation via TLR (Paharkova-Vatchkova et al., 2004). In addition to increased expression of MHC class II, CD40 and CD86, DCs that developed in the presence of estradiol produce more IL-12 in response to TLR ligands (Siracusa et al., 2008) and more IL-12 and IL-6 upon CD40L stimulation (Douin-Echinard et al., 2008). The DCs that differentiated in estradiol-supplemented hormone-deficient medium are fully functional in the processing and presentation of self and foreign antigens via MHC class II molecules and stimulation of the proliferation of naïve CD4+ T cells (Paharkova-Vatchkova et al., 2004). Taken together, these data indicate that estradiol/ERα signaling promotes a functionally competent DC subset that is capable of antigen internalization and presentation, TLR signaling, upregulation of high levels of costimulatory molecules and stimulation of naïve T cells.
5.3. Selective ER modulators (SERM) modulate DC development and functional competence
SERM have tissue specific agonist or antagonist effects on ER signaling. The SERM raloxifene and tamoxifen are used in treatment of osteoporosis and breast cancer. We determined if these SERM, in amounts comparable to concentrations (2.5 nM raloxifene and 200 nM tamoxifen) detected in the plasma of humans taking oral SERMs, would modulate GM-CSF-mediated DC differentiation (Nalbandian et al., 2005). Neither raloxifene nor tamoxifen alone could act to promote DC development from bone marrow precursors in hormone-deficient medium, indicating that they are not acting as ER agonists on these cells. Raloxifene and tamoxifen each significantly inhibit the differentiation of estrogen-dependent DCs from bone marrow precursors ex vivo in competition experiments with physiological levels of estradiol, suggesting that the SERM exert their effects via ER. Similar studies have shown that the SERM tamoxifen and toremifene inhibit the GM-CSF and IL-4 supported differentiation of human DCs from peripheral blood monocytes and synovial fluid macrophages (Komi and Lassila, 2000; Komi et al., 2001).
DCs differentiated in the presence of SERM were assessed for their capacity to internalize antigens and respond to inflammatory stimuli by increasing surface expression of molecules important for APC function. While SERM-exposed DCs have increased ability to internalize antigens, they are hyporesponsive to bacterial LPS: relative to control DCs, they less efficiently upregulate surface MHC class II, CD86, CD80 and CD40 (Nalbandian et al., 2005). Human DCs differentiated in the presence of SERM also exhibit reduced production of IL-12 and decreased ability to stimulate proliferation of allogeneic T cells (Komi and Lassila, 2000). This phenotype indicates that these SERM act to maintain DCs in an immature state by inhibiting DC responsiveness to inflammatory stimuli. These data suggest that raloxifene and tamoxifen may decrease the strength of immune responses in vivo through modulation of DC differentiation and activation. Indeed, systemic tamoxifen exposure decreases autoimmunity in murine models (Behjati and Frank, 2009).
5.4. Estradiol acts directly via ERα in myeloid progenitors to promote GM-CSF-mediated DC differentiation
In DC differentiation cultures initiated from total bone marrow cells, it was possible that estradiol regulated DC differentiation by acting on one or more hematopoietic progenitors, or alternately, on mature immune cells in bone marrow that might produce factors necessary for DC differentiation. We tested whether estradiol acted directly on defined ERα+ ERβ− DC progenitors isolated from murine bone marrow. As mentioned above, the flt3+ fraction of the common myeloid progenitor, CMP (Lin− c-kit+ Sca-1− CD34+ FcγRlo IL-7Rα−) can develop into all DC subsets when transferred in vivo. We found that estradiol acts in highly purified ERα+ ERβ− myeloid progenitors (Lin− c-kit+ Sca-1− Flt3+ IL-7Rα−) to promote GM-CSF-mediated DC differentiation (Carreras et al., 2008). Thus estradiol acts directly in myeloid progenitors via ERα, but not ERβ. Kinetic experiments showed that the bulk of the effect of estradiol to promote differentiation of myeloid progenitors occurs within the first 24 hours of culture, suggesting an effect on initiation of DC differentiation (Carreras et al., 2010).
5.5. The transcription factor IRF4 is induced by ERα in myeloid progenitors
To identify genes induced by estradiol/ERα signaling, we performed gene expression profiling in which myeloid progenitors were incubated with GM-CSF and vehicle or estradiol (1 nM) for 6–24 hr. This experiment identified interferon regulatory factor 4 (Irf4) as an estradiol-induced gene (Carreras et al., 2010). Estradiol increased Irf4 RNA only in the context of GM-CSF signaling which induces Irf4, and thus can be considered a cofactor that amplifies the GM-CSF response in myeloid progenitors. We focused on Irf4 since it was previously reported to be induced by GM-CSF and critical for development of CD11c+ CD11b+ splenic DCs in vivo (Tamura et al., 2005; Suzuki et al., 2004; Lehtonen et al., 2005).
Our experiments showed that cell-intrinsic estradiol/ERα signaling in differentiating myeloid progenitors and CD11c− precursors increases and sustains Irf4 mRNA and protein expression, and promotes the development of the CD11cint Ly6C− DC subset expressing the highest levels of IRF4. Among transcription factors involved in DC differentiation, estradiol has a specific effect on IRF4, with no effect on the amount of Irf8, Pu.1, and Id2 mRNA. Enforced expression of Irf4 cDNA in differentiating ERα−/− BM cells restores the development of the estradiol/ERα-dependent CD11bint Ly6C− DC population, indicating that sufficiently high levels of IRF4 alleviate the requirement for ERα signaling during GM-CSF-mediated DC differentiation. Taken together, these data show that at an early stage in the myeloid progenitor response to GM-CSF, ERα signaling induces an elevated amount of IRF4, which leads to a developmental program underlying CD11b+ DC differentiation.
ERα may directly bind at the Irf4 locus and participate in protein complexes that open chromatin; indeed conserved consensus estrogen response elements (ERE) are present in the DNA 5′ and 3′ to the Irf4 locus. Alternately, ERα signaling may modulate expression or activity of an intermediate molecule such as NF-κB, which is known to be activated by GM-CSF and to induce IRF4 in monocytes and lymphocytes, and has been reported to interact with ER (Lehtonen et al., 2005; Grumont and Gerondakis, 2000; Kalaitzidis and Gilmore, 2005).
IRF4 and PU.1 act as dosage-sensitive regulators to instruct distinct cell differentiation programs (Sciammas et al., 2006; Dakic et al., 2007; Laslo et al., 2006). Therefore, elevation of IRF4 expression by ERα signaling may alter the ability of IRF4 to regulate target genes involved in DC differentiation. IRF4 binds DNA via interferon stimulated response elements (ISRE) or is recruited to composite IRF4/PU.1 binding sites by phosphorylated PU.1 (Marecki and Fenton, 2002). Our data show that in myeloid progenitors, Pu.1 mRNA changed little in response to GM-CSF or estradiol stimulation over the 24 hr time period in which Irf4 mRNA is significantly increased. Therefore, it is possible that increased amounts of IRF4 protein alter the recruitment of IRF4/PU.1 complexes to target genes involved in the DC developmental program.
5.6. ER expression promotes CD11b+ DC development during inflammation in vivo
Our results in the GM-CSF-driven culture model suggest that ERα signaling promotes new DC differentiation during inflammation in vivo. To determine the relative efficiency of DC differentiation from ERα+ and ERα−/− bone marrow precursors in vivo, we analyzed the CD11c+ CD11b+ DC subset present in bone marrow of mixed ERα+/ERα−/− bone marrow chimeric mice at an early time point (3 weeks) post-reconstitution when myeloid cells, but few lymphocytes, have developed. In this model, DC development occurs in the post-radiation inflammatory environment in which both GM-CSF and Flt3L may be elevated. In the bone marrow of these chimeric mice, newly differentiated CD11b+ DCs are derived preferentially from ERα+ donor bone marrow cells (~2:1 ratio of ERα+ to ERα−/− DCs) (Carreras et al., 2010). These data show that during radiation-induced inflammation in vivo, ERα signaling promotes CD11b+ DC development from short term repopulating bone marrow progenitors. Our ongoing experiments are using mixed bone marrow chimeras to address how ERα signaling regulates DC development in disease models.
New DC differentiation during inflammation also may be regulated by ERβ ligands. In the murine EAE model of multiple sclerosis, local development of new DC populations is driven by GM-CSF (Hesske et al., 2010). These include a population of anti-inflammatory DCs that differentiates from resident microglia cells, and a second population of pro-inflammatory DCs that differentiates from blood-borne monocytes. ERβ ligands are neuroprotective in the EAE model, and ERβ ligand treatment decreases the percentage of TNFα-producing DCs in the central nervous system, suggesting an effect of ERβ ligands on the differentiation of inflammatory DCs (Du et al., 2011). This work also shows that ERβ expression by DCs is required for reduction of disease severity when DCs from mice with EAE are transferred to naïve recipients. Thus ERβ may regulate both DC development and function in EAE.
6. Regulation of Flt3 Ligand-mediated DC development by estradiol/ERα signaling
Flt3L mediates DC differentiation during homeostasis in vivo and in culture models. Flt3L mediated DC differentiation from bone marrow cells leads to DCs that are phenotypically and functionally equivalent to CD11c+ B220+ SiglecH+ plasmacytoid DCs and B220− CD11c+ B220− conventional CD11bhi and CD11blow DCs that are present in lymphoid organs in homeostasis (Naik et al., 2005). To test the effect of estradiol and ER signaling in this culture model, total bone marrow cells were incubated in Flt3L-supplemented steroid deficient medium without or with estradiol. Relative to vehicle-treated cultures, inclusion of physiological levels (0.1 nM) of estradiol dramatically reduces the number of viable cells, leading to a decrease in development of all DC subsets (Carreras et al., 2008). Complementary results were observed in hormone-replete regular medium; inclusion of the ER antagonist ICI 182,780 increases the numbers of viable cells and differentiated DCs.
Experiments with bone marrow cells from ERα−/− and ERβ−/− mice showed that estradiol acts only via ERα to decrease Flt3L-mediated DC differentiation. However, in contrast to the effects on DC numbers, we noted that plasmacytoid and conventional DCs that developed in the presence of estradiol display higher levels of MHC class II and were more responsive to TLR ligands than those DCs that developed in the absence of estradiol. This suggests that estradiol exerts two distinct effects in these cultures. One is to inhibit progenitor numbers; the second is to promote a complete DC differentiation program resulting in functionally competent DCs. We also have observed similar independent effects of ERα signaling on progenitors and DC numbers and phenotype in vivo during homeostasis in mixed ERα+/ERα−/− bone marrow chimeric mice (S.K., manuscript in preparation).
As in the GM-CSF-driven model, estradiol/ERα signaling acts directly in myeloid progenitors to decrease cell numbers and DC differentiation in the Flt3L-driven model (Carreras et al., 2008). These data suggest that ERα signaling in the context of Flt3L acts to decrease either the survival or proliferation of myeloid progenitors, which then results in a reduction in differentiated DCs.
Our data are consistent with studies of pregnant or estradiol-treated mice that have shown that elevated systemic estrogen levels reduce numbers of hematopoietic progenitor populations in vivo (Kincade et al., 2000; Medina et al., 2001; Welner et al., 2007; Harman et al., 2006). Therefore, ER signaling regulates hematopoietic progenitor homeostasis, with agonist ER ligands such as estradiol serving to limit the number of myeloid and lymphoid progenitors in the steady state. Accordingly, during homeostasis, estradiol/ERα signaling may limit DC numbers in lymphoid organs to levels that are optimal for maintenance of immunity in adults.
7. ER signaling differentially regulates DC differentiation depending on the extracellular cytokine environment
Use of culture models with primary bone marrow cells has allowed us to dissect the clearly distinct effects of ER ligands on the two cytokine-driven pathways of differentiation. Our data show that physiological levels of estradiol can act on the same highly purified myeloid progenitor population to promote GM-CSF-mediated and decrease Flt3L-mediated DC differentiation. Therefore, depending on the extracellular cytokine environment, the same highly purified cell type can respond differently to estradiol or an ER antagonist. These data suggest that ER signaling in DC progenitors regulates cellular differentiation by differential interaction with cytokine receptor signals.
Our data are consistent with a recently proposed model in which cytokines instruct uncommitted DC progenitors, bearing receptors for multiple cytokines (e.g., Flt3L, GM-CSF, M-CSF) that promote DC development, to commit to a particular lineage (Schmid et al., 2010). In this signal strength model, signals that lead to DC differentiation would be integrated from the cytokine receptor profile, the availability of cytokines in specific niches, and other downstream signals (Schmid et al., 2010). These signals may differ during homeostasis and inflammation since Flt3L levels increase systemically during physiological stress, and GM-CSF is produced locally upon inflammation. We propose that ER signaling is one critical factor that modulates the response of DC progenitors to their cytokine environment, thereby regulating the strength or integrity of the developmental pathway. More studies are needed to resolve how the numbers and developmental potential of long term repopulating hematopoietic progenitors and short term DC precursors are regulated by endogenous levels of sex hormones during homeostasis and inflammation in vivo.
8. Effects of cell-intrinsic ER signaling on DC functional responses
Multiple studies have shown that manipulation of estrogen levels in vivo either promote or inhibit DC functional responses in diverse human and rodent model systems (reviewed in (Straub, 2007; Hughes and Clark, 2007; Kovats and Carreras, 2008)). For the most part, these experiments do not distinguish between direct and indirect effects of estrogens on DCs, although in some cases direct effects of estrogens on isolated DCs in vitro have been reported. Recent studies of interest show that levels of systemic estrogens at the higher end of the normal spectrum promote anti-inflammatory responses of DCs in mice. For example, estrus levels of estradiol reduce the ability of DCs to promote TH17 responses during Candida albicans infection (Relloso et al., 2012), and mice treated with estriol contain tolerogenic DCs that protect against inflammatory autoimmune EAE (Papenfuss et al., 2011). Most of these types of studies also do not distinguish between the potentially distinct effects of estradiol and ERs on the development vs. the function of DCs.
In addition to acting in DC precursors to regulate development, ER signaling in mature DC may regulate their function in at least two ways. (i) Development of DCs in the context of ER action may lead to epigenetic changes in DC precursors that alter gene expression programs governing mature DC functional responses. For example, in the context of high estrogen levels in pregnancy, ERs could act in DC precursors to open chromatin in a set of genes that promote a tolerogenic DC phenotype. (ii) Secondly, ERs may act acutely during mature DC activation to directly regulate gene expression. For example, upon DC stimulation, ERs may be recruited directly to cytokine promoters to promote or inhibit gene expression.
To address this issue, new models of mice bearing conditional alleles of ERα and specific promoter-driven Cre recombinase mice are being developed. These mice enable the study of cell type-specific ER deficiency in the context of normal cycling hormone levels in mice. In a recent exciting study, mice lacking ERα in CD11c+ DC were generated using this approach and used to study DC responses to TLR ligands (Seillet et al., 2012). Upon injection of TLR7 and TLR9 nucleic acid ligands, ERα-deficient pDCs present in females with intact ovaries showed significantly reduced IFNα production. This study indicates that ERα signaling acts directly in pDCs to promote IFNα synthesis after a TLR stimulus. This type of study, in which cell-intrinsic ERs are manipulated in the context of physiologically normal levels of ER ligands, will help to identify ER-regulated immune responses in specific cell types that are responsive to the distinct hormone environments that exist between the sexes or that occur during the female lifespan.
These murine studies have a human correlate. Studies that compared responses between sexes in humans showed that female PBMCs or pDCs also produce significantly more IFNα in response to viral nucleic acids or synthetic TLR7 ligands (Meier et al., 2009; Berghofer et al., 2006). Interestingly, the sex difference could not be reproduced by short-term in vitro exposure to estradiol, suggesting that long-term exposure to higher levels of estrogens in females leads to greater pDC function. More recently, a study showed that a 1-month treatment of post-menopausal women with estradiol leads to a greater fraction of blood pDC capable of producing IFNα upon stimulation via TLR7 or TLR9, providing evidence that the greater pDC function in pre-menopausal females is related to estradiol levels in vivo (Seillet et al., 2012). These data suggest the hypothesis that the action of estradiol during DC differentiation increases pDC functional capacity. Indeed, transfer of human female CD34+ hematopoietic stem cells into female or male immunodeficient mice showed that the human pDC that develop in female mice have a greater capacity to produce IFNα (Seillet et al., 2012). These studies help to explain differences in pDC function that occur between males and females, as well as in pre- and post-menopausal women.
9. Conclusion
The manifestation of autoimmunity or infection in humans is the sum of multiple relatively modest genetic, environmental and metabolic effects on the immune response. One way to understand human disease is to identify the physiological factors that regulate the function of immune cells. Levels of estrogens or other ER ligands potently influence the development and function of immune cells, and the magnitude of ER signaling may account for sex differences and the effects of pregnancy on autoimmunity and infection. As ligand-activated transcription factors, ER regulate gene expression programs that direct development or function of immune cells. Here we have focused on DCs, but the work of many others has revealed effects of estradiol and/or ER signaling on the development and function of B and T lymphocytes, NK cells, monocytes and macrophages (Straub, 2007; Cunningham and Gilkeson, 2011). We envision two distinct mechanisms by which ERα signaling might influence DC-mediated immune responses during inflammation in vivo: (1) regulation of de novo development of tissue and inflammatory DCs that appear in draining lymph nodes or inflamed tissues and are crucial for the adaptive immune response, and (2) regulation of the pro-inflammatory and T cell stimulatory responses of mature DCs.
Our own work, combined with studies of other groups, has demonstrated that ERα signaling promotes an inflammatory pathway of DC development and increases the functional competence of the DC that develop under the influence of ER signaling. Furthermore, we and others have shown that ER signaling regulates hematopoietic progenitor numbers and DC development during homeostasis. Thus, ERα-mediated regulation of DC developmental pathways will alter DC numbers in vivo, which is likely to modulate the magnitude or quality of innate and adaptive immune responses. Other groups have defined effects of estrogens and ERs on mature DC functional responses that also influence adaptive immunity. Together with direct effects of ER signaling on other immune cell types, these events will influence the resolution of infection or chronic inflammatory diseases.
Highlights.
Immune cells and hematopoietic progenitors express ER.
Estradiol/ERα signaling in the context of GM-CSF promotes inflammatory DC development.
ERα increases levels of the transcription factor IRF4 in GM-CSF-stimulated myeloid progenitors.
Estradiol/ERα signaling in the context of Flt3 Ligand decreases hematopoietic progenitor numbers.
ERα signaling during DC development promotes the functional competence of DC.
ERα signaling regulates mature DC functional responses.
Acknowledgments
SK would like to thank the present and former members of her laboratory for their participation in the work described in this review article: H. Agrawal, S. Bajaña, E. Carreras, A. Mao, G. Nalbandian, V. Paharkova-Vatchkova, K. Roach and S. Turner. SK was supported over the years by Oklahoma Center for the Advancement of Science & Technology grants HR06-157 and HR09-055, NIH grants AI063078, AI079616, AI083715, AI092511, the Alliance for Lupus Research, the American Heart Association and the Arthritis Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Askanase AD, Buyon JP. Reproductive health in SLE. Best Pract Res Clin Rheumatol. 2002;16:265–280. doi: 10.1053/berh.2002.0225. [DOI] [PubMed] [Google Scholar]
- Ballesteros-Tato A, Leon B, Lund FE, Randall TD. Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8(+) T cell responses to influenza. Nat Immunol. 2010;11:216–224. doi: 10.1038/ni.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behjati S, Frank MH. The effects of tamoxifen on immunity. Curr Med Chem. 2009;16:3076–3080. doi: 10.2174/092986709788803042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghofer B, Frommer T, Haley G, Fink L, Bein G, Hackstein H. TLR7 ligands induce higher IFN-alpha production in females. J Immunol. 2006;177:2088–2096. doi: 10.4049/jimmunol.177.4.2088. [DOI] [PubMed] [Google Scholar]
- Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986;83:2496–500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosschaerts T, Guilliams M, Stijlemans B, Morias Y, Engel D, Tacke F, Herin M, De Baetselier P, Beschin A. Tip-DC development during parasitic infection is regulated by IL-10 and requires CCL2/CCR2, IFN-gamma and MyD88 signaling. PLoS Pathog. 2010;6:e1001045. doi: 10.1371/journal.ppat.1001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreras E, Turner S, Frank MB, Knowlton N, Osban J, Centola M, Park CG, Simmons A, Alberola-Ila J, Kovats S. Estrogen receptor signaling promotes dendritic cell differentiation by increasing expression of the transcription factor IRF4. Blood. 2010;115:238–246. doi: 10.1182/blood-2009-08-236935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreras E, Turner S, Paharkova-Vatchkova V, Mao A, Dascher C, Kovats S. Estradiol Acts Directly on Bone Marrow Myeloid Progenitors to Differentially Regulate GM-CSF or Flt3 Ligand-Mediated Dendritic Cell Differentiation. J Immunol. 2008;180:727–738. doi: 10.4049/jimmunol.180.2.727. [DOI] [PubMed] [Google Scholar]
- Cunningham M, Gilkeson G. Estrogen receptors in immunity and autoimmunity. Clin Rev Allergy Immunol. 2011;40:66–73. doi: 10.1007/s12016-010-8203-5. [DOI] [PubMed] [Google Scholar]
- Dakic A, Wu L, Nutt SL. Is PU.1 a dosage-sensitive regulator of haemopoietic lineage commitment and leukaemogenesis? Trends Immunol. 2007;28:108–114. doi: 10.1016/j.it.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Douin-Echinard V, Laffont S, Seillet C, Delpy L, Krust A, Chambon P, Gourdy P, Arnal JF, Guery JC. Estrogen receptor alpha, but not beta, is required for optimal dendritic cell differentiation and [corrected] CD40-induced cytokine production. J Immunol. 2008;180:3661–3669. doi: 10.4049/jimmunol.180.6.3661. [DOI] [PubMed] [Google Scholar]
- Du S, Sandoval F, Trinh P, Umeda E, Voskuhl R. Estrogen receptor-beta ligand treatment modulates dendritic cells in the target organ during autoimmune demyelinating disease. Eur J Immunol. 2011;41:140–150. doi: 10.1002/eji.201040796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutertre M, Smith CL. Molecular mechanisms of selective estrogen receptor modulator (SERM) action. J Pharmacol Exp Ther. 2000;295:431–47. [PubMed] [Google Scholar]
- Escribese MM, Kraus T, Rhee E, Fernandez-Sesma A, Lopez CB, Moran TM. Estrogen inhibits dendritic cell maturation to RNA viruses. Blood. 2008;112:4574–4584. doi: 10.1182/blood-2008-04-148692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8:737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- Foster HL, Small JD, Fox JG. Normative biology, immunology and husbandry. Orlando, FL: Academic Press Inc; 1983. [Google Scholar]
- Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol. 2009;10:241–247. doi: 10.1038/ni.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology. 2003;144:4562–4574. doi: 10.1210/en.2003-0567. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumont RJ, Gerondakis S. Rel induces interferon regulatory factor 4 (IRF-4) expression in lymphocytes: modulation of interferon-regulated gene expression by rel/nuclear factor kappaB. J Exp Med. 2000;191:1281–1292. doi: 10.1084/jem.191.8.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton JA. GM-CSF in inflammation and autoimmunity. Trends Immunol. 2002;23:403–408. doi: 10.1016/s1471-4906(02)02260-3. [DOI] [PubMed] [Google Scholar]
- Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- Harman BC, Miller JP, Nikbakht N, Gerstein R, Allman D. Mouse plasmacytoid dendritic cells derive exclusively from estrogen-resistant myeloid progenitors. Blood. 2006;108:878–885. doi: 10.1182/blood-2005-11-4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35:323–335. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Strom A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87:905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- Helft J, Ginhoux F, Bogunovic M, Merad M. Origin and functional heterogeneity of non-lymphoid tissue dendritic cells in mice. Immunol Rev. 2010;234:55–75. doi: 10.1111/j.0105-2896.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- Hesske L, Vincenzetti C, Heikenwalder M, Prinz M, Reith W, Fontana A, Suter T. Induction of inhibitory central nervous system-derived and stimulatory blood-derived dendritic cells suggests a dual role for granulocyte-macrophage colony-stimulating factor in central nervous system inflammation. Brain. 2010;133:1637–1654. doi: 10.1093/brain/awq081. [DOI] [PubMed] [Google Scholar]
- Hughes GC, Clark EA. Regulation of dendritic cells by female sex steroids: relevance to immunity and autoimmunity. Autoimmunity. 2007;40:470–481. doi: 10.1080/08916930701464764. [DOI] [PubMed] [Google Scholar]
- Igarashi H, Kouro T, Yokota T, Comp PC, Kincade PW. Age and stage dependency of estrogen receptor expression by lymphocyte precursors. Proc Natl Acad Sci U S A. 2001;98:15131–1516. doi: 10.1073/pnas.011513098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubzick C, Bogunovic M, Bonito AJ, Kuan EL, Merad M, Randolph GJ. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. J Exp Med. 2008;205:2839–2850. doi: 10.1084/jem.20081430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaitzidis D, Gilmore TD. Transcription factor cross-talk: the estrogen receptor and NF-kappaB. Trends Endocrinol Metab. 2005;16:46–52. doi: 10.1016/j.tem.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kincade PW, Medina KL, Payne KJ, Rossi MI, Tudor KS, Yamashita Y, Kouro T. Early B-lymphocyte precursors and their regulation by sex steroids. Immunol Rev. 2000;175:128–37. [PubMed] [Google Scholar]
- King IL, Dickendesher TL, Segal BM. Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood. 2009;113:3190–3197. doi: 10.1182/blood-2008-07-168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Hodgson A, Robinson DP. Mechanisms of sex disparities in influenza pathogenesis. J Leukoc Biol. 2011 doi: 10.1189/jlb.0811427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komi J, Lassila O. Nonsteroidal anti-estrogens inhibit the functional differentiation of human monocyte-derived dendritic cells. Blood. 2000;95:2875–282. [PubMed] [Google Scholar]
- Komi J, Mottonen M, Luukkainen R, Lassila O. Non-steroidal anti-oestrogens inhibit the differentiation of synovial macrophages into dendritic cells. Rheumatology. 2001:185–91. doi: 10.1093/rheumatology/40.2.185. [DOI] [PubMed] [Google Scholar]
- Kovats S, Carreras E. Regulation of dendritic cell differentiation and function by estrogen receptor ligands. Cell Immunol. 2008;252:81–90. doi: 10.1016/j.cellimm.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovats S, Carreras E, Agrawal H. Sex steroid receptors in immune cells. In: Klein SL, Roberts CW, editors. Sex Hormones and Immunity to Infection. Springer; 2010. pp. 53–91. [Google Scholar]
- Laouar Y, Welte T, Fu XY, Flavell RA. STAT3 is required for Flt3L-dependent dendritic cell differentiation. Immunity. 2003;19:903–912. doi: 10.1016/s1074-7613(03)00332-7. [DOI] [PubMed] [Google Scholar]
- Laslo P, Spooner CJ, Warmflash A, Lancki DW, Lee HJ, Sciammas R, Gantner BN, Dinner AR, Singh H. Multilineage transcriptional priming and determination of alternate hematopoietic cell fates. Cell. 2006;126:755–766. doi: 10.1016/j.cell.2006.06.052. [DOI] [PubMed] [Google Scholar]
- Lehtonen A, Veckman V, Nikula T, Lahesmaa R, Kinnunen L, Matikainen S, Julkunen I. Differential expression of IFN regulatory factor 4 gene in human monocyte-derived dendritic cells and macrophages. J Immunol. 2005;175:6570–6579. doi: 10.4049/jimmunol.175.10.6570. [DOI] [PubMed] [Google Scholar]
- Leitman DC, Paruthiyil S, Vivar OI, Saunier EF, Herber CB, Cohen I, Tagliaferri M, Speed TP. Regulation of specific target genes and biological responses by estrogen receptor subtype agonists. Curr Opin Pharmacol. 2010;10:629–636. doi: 10.1016/j.coph.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman SD, Jacobsen SE. c-kit ligand and Flt3 ligand: stem/progenitor cell factors with overlapping yet distinct activities. Blood. 1998;91:1101–1134. [PubMed] [Google Scholar]
- Mann M, Cortez V, Vadlamudi RK. Epigenetics of Estrogen Receptor Signaling: Role in Hormonal Cancer Progression and Therapy. Cancers (Basel) 2011;3:1691–1707. doi: 10.3390/cancers3021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao A, Paharkova-Vatchkova V, Hardy J, Miller MM, Kovats S. Estrogen selectively promotes the differentiation of dendritic cells with characteristics of Langerhans cells. J Immunol. 2005;175:5146–5151. doi: 10.4049/jimmunol.175.8.5146. [DOI] [PubMed] [Google Scholar]
- Marecki S, Fenton MJ. The role of IRF-4 in transcriptional regulation. J Interferon Cytokine Res. 2002;22:121–133. doi: 10.1089/107999002753452737. [DOI] [PubMed] [Google Scholar]
- Medina KL, Garrett KP, Thompson LF, Rossi MI, Payne KJ, Kincade PW. Identification of very early lymphoid precursors in bone marrow and their regulation by estrogen. Nat Immunol. 2001;2:718–24. doi: 10.1038/90659. [DOI] [PubMed] [Google Scholar]
- Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, Wen TF, Lindsay RJ, Orellana L, Mildvan D, Bazner S, Streeck H, Alter G, Lifson JD, Carrington M, Bosch RJ, Robbins GK, Altfeld M. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15:955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–28. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- Merad M, Manz MG. Dendritic cell homeostasis. Blood. 2009;113:3418–3427. doi: 10.1182/blood-2008-12-180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor G, Sapi E, Abrahams VM, Rutherford T, Song J, Hao XY, Muzaffar S, Kohen F. Interaction of the estrogen receptors with the Fas ligand promoter in human monocytes. J Immunol. 2003;170:114–122. doi: 10.4049/jimmunol.170.1.114. [DOI] [PubMed] [Google Scholar]
- Moro JR, Iwata M, von Andriano UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouchemore KA, Pixley FJ. CSF-1 signaling in macrophages: pleiotrophy through phosphotyrosine-based signaling pathways. Crit Rev Clin Lab Sci. 2012;49:49–61. doi: 10.3109/10408363.2012.666845. [DOI] [PubMed] [Google Scholar]
- Naik SH, Proietto AI, Wilson NS, Dakic A, Schnorrer P, Fuchsberger M, Lahoud MH, O’Keeffe M, Shao QX, Chen WF, Villadangos JA, Shortman K, Wu L. Cutting edge: generation of splenic CD8+ and CD8- dendritic cell equivalents in Fms-like tyrosine kinase 3 ligand bone marrow cultures. J Immunol. 2005;174:6592–6597. doi: 10.4049/jimmunol.174.11.6592. [DOI] [PubMed] [Google Scholar]
- Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T, Gunn MD. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol. 2009;10:394–402. doi: 10.1038/ni.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian G, Paharkova-Vatchkova V, Mao A, Nale S, Kovats S. The selective estrogen receptor modulators, tamoxifen and raloxifene, impair dendritic cell differentiation and activation. Journal of Immunology. 2005;175:2666–2675. doi: 10.4049/jimmunol.175.4.2666. [DOI] [PubMed] [Google Scholar]
- O’Lone R, Frith MC, Karlsson EK, Hansen U. Genomic targets of nuclear estrogen receptors. Mol Endocrinol. 2004;18:1859–1875. doi: 10.1210/me.2003-0044. [DOI] [PubMed] [Google Scholar]
- Olsen NJ, Kovacs WJ. Gonadal steroids and immunity. Endocr Rev. 1996;17:369–84. doi: 10.1210/edrv-17-4-369. [DOI] [PubMed] [Google Scholar]
- Paharkova-Vatchkova V, Maldonado R, Kovats S. Estrogen preferentially promotes the differentiation of CD11c+ CD11b(intermediate) dendritic cells from bone marrow precursors. J Immunol. 2004;172:1426–1436. doi: 10.4049/jimmunol.172.3.1426. [DOI] [PubMed] [Google Scholar]
- Papenfuss TL, Powell ND, McClain MA, Bedarf A, Singh A, Gienapp IE, Shawler T, Whitacre CC. Estriol generates tolerogenic dendritic cells in vivo that protect against autoimmunity. J Immunol. 2011;186:3346–3355. doi: 10.4049/jimmunol.1001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phiel KL, Henderson RA, Adelman SJ, Elloso MM. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett. 2005;97:107–113. doi: 10.1016/j.imlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rathinam VA, Fitzgerald KA. Cytosolic surveillance and antiviral immunity. Curr Opin Virol. 2011;1:455–462. doi: 10.1016/j.coviro.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relloso M, Aragoneses-Fenoll L, Lasarte S, Bourgeois C, Romera G, Kuchler K, Corbi AL, Munoz-Fernandez MA, Nombela C, Rodriguez-Fernandez JL, Diez-Orejas R. Estradiol impairs the Th17 immune response against Candida albicans. J Leukoc Biol. 2012;91:159–165. doi: 10.1189/jlb.1110645. [DOI] [PubMed] [Google Scholar]
- Sathe P, Wu L. The network of cytokines, receptors and transcription factors governing the development of dendritic cell subsets. Protein Cell. 2011;2:620–630. doi: 10.1007/s13238-011-1088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid MA, Kingston D, Boddupalli S, Manz MG. Instructive cytokine signals in dendritic cell lineage commitment. Immunol Rev. 2010;234:32–44. doi: 10.1111/j.0105-2896.2009.00877.x. [DOI] [PubMed] [Google Scholar]
- Schmid MA, Takizawa H, Baumjohann DR, Saito Y, Manz MG. Bone marrow dendritic cell progenitors sense pathogens via Toll-like receptors and subsequently migrate to inflamed lymph nodes. Blood. 2011;118:4829–4840. doi: 10.1182/blood-2011-03-344960. [DOI] [PubMed] [Google Scholar]
- Sciammas R, Shaffer AL, Schatz JH, Zhao H, Staudt LM, Singh H. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity. 2006;25:225–236. doi: 10.1016/j.immuni.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Segura E, Valladeau-Guilemond J, Donnadieu MH, Sastre-Garau X, Soumelis V, Amigorena S. Characterization of resident and migratory dendritic cells in human lymph nodes. J Exp Med. 2012;209:653–660. doi: 10.1084/jem.20111457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seillet C, Laffont S, Tremollieres F, Rouquie N, Ribot C, Arnal JF, Douin-Echinard V, Gourdy P, Guery JC. The TLR-mediated response of plasmacytoid dendritic cells is positively regulated by estradiol in vivo through cell-intrinsic estrogen receptor alpha signaling. Blood. 2012;119:454–464. doi: 10.1182/blood-2011-08-371831. [DOI] [PubMed] [Google Scholar]
- Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- Siracusa MC, Overstreet MG, Housseau F, Scott AL, Klein SL. 17{beta}-Estradiol Alters the Activity of Conventional and IFN-Producing Killer Dendritic Cells. J Immunol. 2008;180:1423–1431. doi: 10.4049/jimmunol.180.3.1423. [DOI] [PubMed] [Google Scholar]
- Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Honma K, Matsuyama T, Suzuki K, Toriyama K, Akitoyo I, Yamamoto K, Suematsu T, Nakamura M, Yui K, Kumatori A. Critical roles of interferon regulatory factor 4 in CD11bhighCD8alpha- dendritic cell development. Proc Natl Acad Sci U S A. 2004;101:8981–8986. doi: 10.1073/pnas.0402139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T, Tailor P, Yamaoka K, Kong HJ, Tsujimura H, O’Shea JJ, Singh H, Ozato K. IFN regulatory factor-4 and -8 govern dendritic cell subset development and their functional diversity. J Immunol. 2005;174:2573–2581. doi: 10.4049/jimmunol.174.5.2573. [DOI] [PubMed] [Google Scholar]
- van de Laar L, Coffer PJ, Woltman AM. Regulation of dendritic cell development by GM-CSF: molecular control and implications for immune homeostasis and therapy. Blood. 2012;119:3383–3393. doi: 10.1182/blood-2011-11-370130. [DOI] [PubMed] [Google Scholar]
- Varol C, Yona S, Jung S. Origins and tissue-context-dependent fates of blood monocytes. Immunol Cell Biol. 2009;87:30–38. doi: 10.1038/icb.2008.90. [DOI] [PubMed] [Google Scholar]
- Welner RS, Pelayo R, Garrett KP, Chen X, Perry SS, Sun XH, Kee BL, Kincade PW. Interferon-producing killer dendritic cells (IKDCs) arise via a unique differentiation pathway from primitive c-kitHiCD62L+ lymphoid progenitors. Blood. 2007;109:4825–4931. doi: 10.1182/blood-2006-08-043810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitacre CC. Sex differences in autoimmune disease. Nat Immunol. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Lieschke GJ, Grail D, Dunn AR, Cheers C. Essential roles for granulocyte-macrophage colony-stimulating factor (GM-CSF) and G-CSF in the sustained hematopoietic response of Listeria monocytogenes-infected mice. Blood. 1998;91:863–869. [PubMed] [Google Scholar]
- Zhang QH, Hu YZ, Cao J, Zhong YQ, Zhao YF, Mei QB. Estrogen influences the differentiation, maturation and function of dendritic cells in rats with experimental autoimmune encephalomyelitis. Acta Pharmacol Sin. 2004;25:508–513. [PubMed] [Google Scholar]