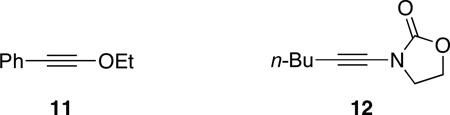Abstract
A highly effective silver-catalyzed formal inverse electron-demand Diels-Alder reaction of 1,2-diazines and siloxy alkynes has been developed. The reactions provide ready access to a wide range of siloxy naphthalenes and anthracenes, which are formed in good to high yields, under mild reaction conditions, using low catalyst loadings.
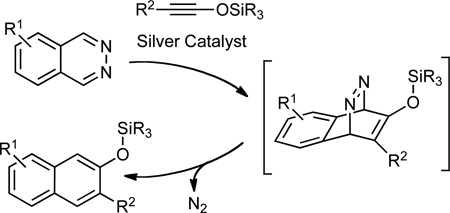
The prominent role played by the Diels-Alder (DA) reaction in organic synthesis is due in no small measure to the discovery of Lewis acid catalysis of this reaction.1,2,3,4 However, such activation has proven successful primarily for the acceleration of normal electron-demand DA reactions, those between electron-rich dienes and electron-deficient dienophiles. Lewis acid activation of the complementary process, the inverse electron-demand Diels-Alder (IEDDA) reaction, between an electron-deficient diene and an electron-rich dienophile, has met with limited success.5 The IEDDA reaction of heterocyclic azadienes, including diazines,6,7 triazines,8 and tetrazines,9 provides access to a wide range of useful heterocycles and carbocycles.10 The reported IEDDA reactions of such azadienes are nearly all thermal processes, often requiring high reaction temperatures. For example, reactions of phthalazine with enamines or ynamines typically require heating to 130 °C or proceed only when electron-withdrawing groups are installed on the 1,2-diazines.6d To the best of our knowledge, the only reported examples of Lewis acid catalyzed IEDDA reactions of heterocyclic azadienes are those by Wegner and coworkers. These researchers employed a novel diboraanthracene for bidentate activation of phthalazine towards cycloadditions with enamines and enol ethers, allowing reactions to take place at 40–170 °C.11,12 We describe the development of silver-catalyzed cycloaddition between phthalazines and siloxy alkynes,13,14 corresponding to a formal IEDDA reaction, that take place with concomitant loss of nitrogen to afford a wide range of silyl-protected 2-naphthols (Eq. 1).15 The reactions proceed at room temperature and give the products in good to high yields, typically using only 1.0–2.0 mol% of the silver catalyst. It is noteworthy that 3-substituted 2-naphthols are important precursors to the widely used axially chiral 2,2'-binols.16,17
 |
(1) |
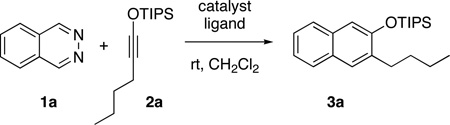
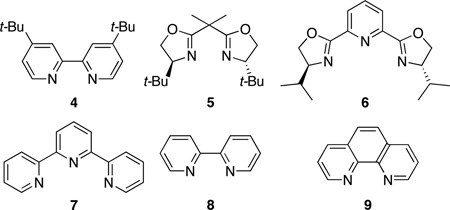
In initial studies, we found phthalazine 1a to be unreactive towards siloxy alkyne 2a at room temperature, and gave the expected cycloadduct in only 5% yield after 24 h at 150 °C (0.2 M in mesitylene). Likewise, little or none of the cycloadduct was observed when the reaction was carried out in the presence of common Lewis acids (MgCl2, ZnBr2, TiCl4, SnCl4, Sc(OTf)3 and BF3•OEt2). The low reactivity observed with traditional Lewis acids, prompted us to examine catalysts known to have π-acidic properties. Among the different metals considered, gold and silver based catalysts appeared promising, given their known high affinity for alkynes. When the reaction of 1a and 2a was carried out in the presence of 10 mol% AuCl, it produced the desired siloxy naphthalene 3a in 38% yield after 24 h at room temperature (Table 1, entry 1). The related gold salt [Au(PPh3)NTf2] also catalyzed the cycloaddition, albeit less effectively (14%). Better results were obtained with silver based catalysts. The use of AgOTf (5 mol%) promoted a rapid reaction, giving the product in 57% yield (entry 2). The reaction conditions were developed further by taking into account the coordination chemistry of silver complexes of diazines. The literature records examples of 1,2-diazines such as pyridazine and phthalazine functioning as non-chelating, bridging ligands for Ag+ ions, giving rise to polymeric complexes in some instances.18 Considering that such coordination of phthalazine to silver might be required for the observed reactivity, we hypothesized that a multidentate, chelating ligand might still enable the required activation but through a monomeric complex rather than polymeric or oligomeric structures. With that in mind, we examined t-Bubipy 4 as a bidentate ligand and were pleased to see the yield rise to 79% (entry 3). Increasing the ligand:silver ratio to 2.2 did not affect the yield, but it slowed the reaction slightly. The reaction proceeded in lower yields in CH3CN and THF, and was essentially shut down in ether and toluene. A further examination of ligands showed that BOX ligand 5 as well as the tridentate ligands pyBOX 6 and terpyridine 7 were lower yielding (entries 4–6). On the other hand, both bipyridine 8 and phenanthroline 9 were highly efficacious, affording the siloxy naphthalene product in 90 and 81% yields, respectively (entries 7 and 8). Further studies showed that pyridine and Hunig’s base had almost no effect on the yield vis-à-vis the ligand free conditions (entries 9 and 10). Additionally, the strongly coordinating ligand DMAP stopped the activity of AgOTf completely when used in a 2.2:1 ratio (entry 11). Interestingly, the tertiary amine-based, bidentate ligand TMEDA proved to be highly effective, giving the product in 81% yield (entry 12). These results, when considered together, underscore the importance of bidentate chelating ligands for effective catalysis. Given its simplicity, bipyridine 8 was selected for further study. With 8 as the ligand, just 1 mol% loading of the AgOTf was sufficient to promote the reaction, providing the product in essentially the same yield as with 5 mol% loading (Cf. entries 7 and 13). Comparable results were obtained, but with a shorter reaction time, when AgNTf2 was used as the catalyst (entry 14).
Table 1.
Reaction Development and Optimizationa
 | ||||
|---|---|---|---|---|
| Entry | Catalyst (mol%) |
Ligand (mol%) |
Time (h) |
Yieldb (%) |
| 1c | AuCl (10) | - | 24 | 38 |
| 2 | AgOTf (5) | - | 2 | 57 |
| 3 | AgOTf (5) | 4 (5.5) | 1.25 | 79 |
| 4 | AgOTf (5) | 5 (5.5) | 2 | 49 |
| 5 | AgOTf (5) | 6 (5.5) | 2 | 37 |
| 6 | AgOTf (5) | 7 (5.5) | 2 | 49 |
| 7d | AgOTf (5) | 8 (5.5) | 2 | 90 |
| 8d | AgOTf (5) | 9 (5.5) | 2 | 81 |
| 9d | AgOTf (5) | pyridine (11) | 2 | 63 |
| 10d | AgOTf (5) | iPr2NEt (11) | 2 | 58 |
| 11 | AgOTf (5) | DMAP (11) | 2 | <1 |
| 12d | AgOTf (5) | TMEDA (5.5) | 2 | 81 |
| 13e | AgOTf (1) | 8 (1.1) | 3 | 88 |
| 14e | AgNTf2 (1) | 8 (1.1) | 1.5 | 89 |
 | ||||
Reactions were carried out using 0.2 mmol of 1a and 0.3 mmol of 2a in CH2Cl2 (1.0 mL/0.1 mmol 1a).
Determined by 1H-NMR spectroscopy using 1,3,5-trimethoxybenzene as an internal standard.
1.0 mL of CH2Cl2/0.2 mmol 1a.
0.4 mmol of 1a was used.
0.4 mmol of 1a and 0.8 mL of CH2Cl2 were used.
We next investigated the scope of the catalyzed cycloaddition reaction under the optimized conditions (Table 2). Phthalazine and benzo[g]phthalazine gave the corresponding cycloadducts 3a and 3b in 82 and 72% isolated yields, respectively (entries 1–2). The latter result demonstrates that the current method can be applied equally to the preparation of siloxy anthracenes in addition to naphthalenes. Dichloro- and dimethyl-substituted phthalazines afforded the tetrasubstituted naphthalenes in good yields (entries 3–4). The reaction of 1-chlorophthalazine was highly regioselective, giving 3e as the only regioisomer (entry 5). 5-Nitrophthalazine gave the cycloadduct in good yield but with lower regioselectivity (entry 6). We have also examined the scope of the cycloaddition reaction with regard to changes in the substituents on the siloxy alkyne (entries 7–11). The sterically hindered tert-butyl substituted siloxy alkyne reacted more slowly under the standard conditions, but gave the expected product in good yield using 10 mol% of the catalyst in refluxing dichloromethane (entries 12 and 13). Siloxy alkyne having a phenyl substituent was particularly reactive and afforded the corresponding 3-phenyl-substituted naphthol product in 92% isolated yield with just 0.5 mol% catalyst loading (entries 14 and 15). The higher reactivity of the phenyl-substituted alkyne was confirmed through its reactions with other phthalazine derivatives. In all cases the corresponding products were obtained in high yields with 1 mol% catalyst loading (entries 16–18). It was found that pyridazine and pyrido[2,3-d]pyridazine (10) gave no reaction when subjected to the above protocol.19 Finally, to assess the preparative value of the present cycloaddition methodology, we carried out the reaction between phthalazine 1a and siloxy alkyne 2a on a 20 mmol scale using 1 mol% of the catalyst and obtained the siloxy naphthalene product 3a in 74% isolated yield (5.26 g). To further demonstrate the scalability of the method, the tert-butyl substituted siloxy alkyne was reacted with phthalazine 1a on a 10 mmol scale and gave the cycloadduct 3l in 83% isolated yield (2.97 g). It is noteworthy that 3-substituted 2-naphthols, prepared through this silver catalyzed process, are not readily accessible, as Friedel-Crafts reactions of 2-naphthols give 1-substituted products with small electrophiles and a mixture of 3- and 6-substituted products with large electrophiles.20
Table 2.
Scope of AgNTf2-Catalyzed Cycloaddition Reactionsa
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Catalyst mol% |
Time (h) |
Product | Yieldb (%) |
Entry | Catalyst mol% |
Time (h) |
Product | Yieldb (%) |
| 1 | 1.0 | 2 |  |
82 | 9 | 1.0 | 1 |  |
81 |
| 2 | 2.0 | 1.5 | 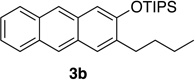 |
72 | 10d | 2.0 | 3 | 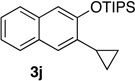 |
75 |
| 3 | 1.0 | 7 | 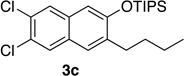 |
78 | 11e | 2.0 | 3 | 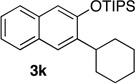 |
83 |
| 4 | 2.0 | 3 | 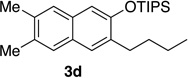 |
84 | 12d | 10 | 22 | 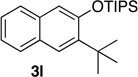 |
67 |
| 13d,f | 10 | 7 | 80 | ||||||
| 5c | 2.0 | 4 | 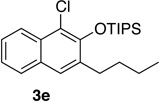 |
67 | 14 | 1.0 | 1 | 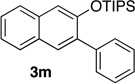 |
95 |
| 15 | 0.5 | 3 | 92 | ||||||
| 6c | 2.0 | 4 | 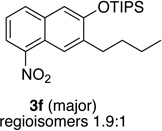 |
74 | 16 | 1.0 | 3 | 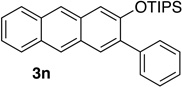 |
84 |
| 7d | 2.0 | 3 | 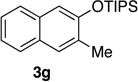 |
73 | 17 | 1.0 | 2 | 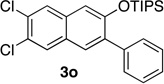 |
85 |
| 8e | 2.0 | 1 | 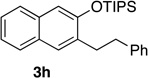 |
70 | 18 | 1.0 | 6 | 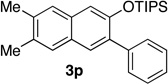 |
94 |
Reactions were carried out using 0.5 or 1.0 mmol of diazine and 1.3 equiv of the siloxy alkyne with 8AgNTf2 ratio of 1.1:1.
Isolated yields.
For the determination of the regiochemistry of the product, see the Supporting Information.
2.0 equiv of siloxy alkyne was used.
1.5 equiv of siloxy alkyne was used.
The reaction was carried out in refluxing CH2Cl2.
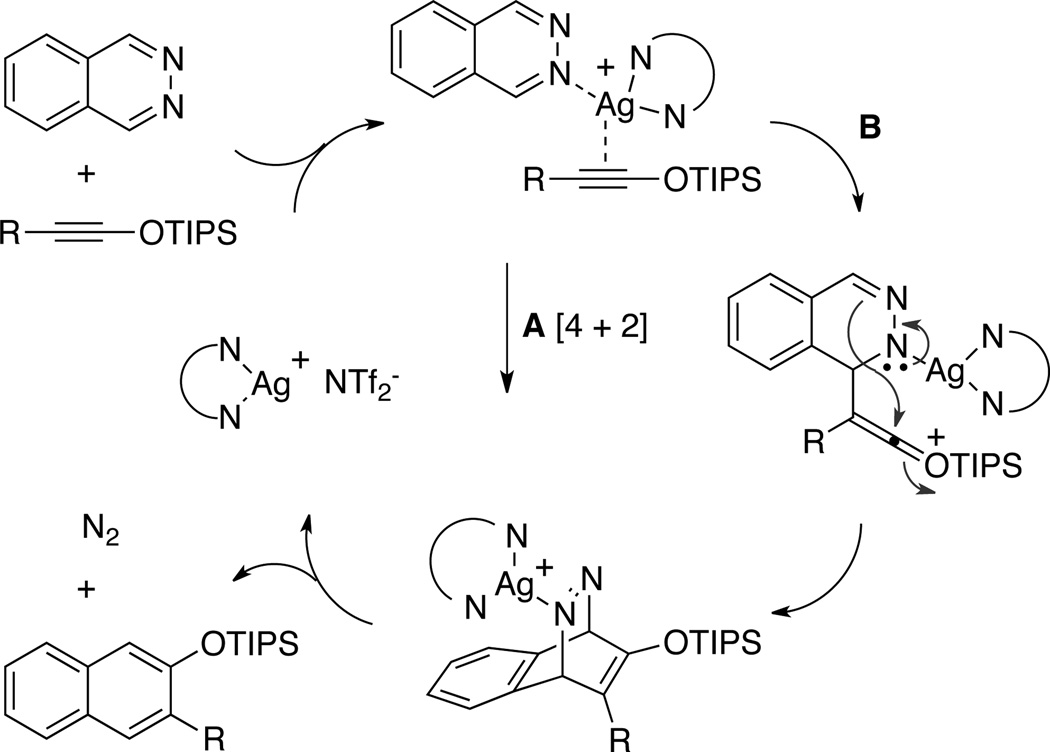
A mechanistic rationale for the phthalazine-siloxy alkyne cycloaddition is presented in Scheme 1. One possibility is that silver ion coordination of the phthalazine renders it more electron-deficient, lowering the activation energy for a concerted IEDDA reaction (path A). We have found, however, that ethoxy alkyne 11 and ynamide 12, both of which are comparably electron-rich to siloxy alkyne 2a, gave none of the expected cycloaddition products with phthalazine under the same reaction conditions. These results when considered with the ineffectiveness of the other Lewis acids tested (vide supra) suggest that the role of silver may not simply be to activate phthalazine. Indeed, 1H-NMR studies have previously shown that siloxy alkynes interact with AgNTf2.13a In light of these factors, one can also consider a step-wise mechanism to explain the formal cycloaddition reaction (path B). Coordination of the Ag+ to phthalazine21 and siloxy alkyne would not only bring the two reaction partners in proximity but would also activate the two reactants. Nucleophilic attack of the siloxy alkyne to phthalazine would produce a diaza-enolate intermediate along with a highly reactive silylketenium. Intramolecular addition of the diaza-enolate to the silylketenium would give a bicyclic azo-intermediate that upon spontaneous extrusion of N2 would afford the siloxy naphthalene product along with the initial catalyst.22,23
Scheme 1.
Proposed Reaction Mechanism
In summary, we have developed a Ag-catalyzed formal inverse electron-demand Diels-Alder reaction of phthalazines with siloxy alkynes to afford a broad range of silyl protected 2-naphthols. The cycloadditions proceed at room temperature and give the products in good to high yields, typically with just 1.0–2.0 mol% catalyst loadings. We believe that the catalysis of IEDDA reactions of various other heterocyclic azadienes presents a fertile ground for the discovery of useful new synthetic methods.
Supplementary Material
ACKNOWLEDGMENT
This work was funded by the National Institutes of Health (P50 GM086145) and the Chicago Biomedical Consortium with support from the Searle Funds at the Chicago Community Trust.
ABBREVIATIONS
- TIPS
triisopropylsilyl
- Tf
trifluoromethanesulfonyl
- DMAP
4-dimethylaminopyridine
- TMEDA
tetramethylethylenediamine
- Ph
phenyl
Footnotes
ASSOCIATED CONTENT
Supporting Information. Detailed experimental procedures and full compound characterization. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Yates P, Eaton P. J. Am. Chem. Soc. 1960;82:4436. [Google Scholar]
- 2.Oppolzer W. Comprehensive Organic Synthesis. Vol 5. New York: Pergamon Press; 1991. Intermolecular Diels-Alder Reactions; p. 315. [Google Scholar]
- 3.Much effort has been devoted to enantioselective catalysis of the DA reaction. See: Evans DA, Johnson JS. In: Comprehensive Asymmetric Catalysis. Jacobsen EN, Pfaltz A, Yamamoto H, editors. Vol. 3. New York: Springer; 1999. 1177 and references therein. Corey EJ. Angew. Chem. Int. Ed. 2002;41:1650. doi: 10.1002/1521-3773(20020517)41:10<1650::aid-anie1650>3.0.co;2-b. Merino P, Marqués-López E, Tejero T, Herrera RP. Synthesis. 2010:1.
- 4.For applications of the DA reaction to natural product synthesis see: Nicolaou KC, Snyder SA, Montagnon T, Vassilikogiannakis G. Angew. Chem. Int. Ed. 2002;41:1668. doi: 10.1002/1521-3773(20020517)41:10<1668::aid-anie1668>3.0.co;2-z. Takao K, Munakata R, Tadano K. Chem. Rev. 2005;105:4779. doi: 10.1021/cr040632u.
- 5.For selected examples see: Gademann K, Chavez DE, Jacobsen EN. Angew. Chem. Int. Ed. 2002;41:3059. doi: 10.1002/1521-3773(20020816)41:16<3059::AID-ANIE3059>3.0.CO;2-I. Li P, Yamamoto H. J. Am. Chem. Soc. 2009;131:16628. doi: 10.1021/ja908127f.
- 6.Selected papers on 1,2-diazines: Neunhoeffer H, Werner G. Tetrahedron Lett. 1972;16:1517. Neunhoeffer H, Werner G. Liebigs Ann. Chem. 1973:437. Gruseck U, Heuschmann M. Tetrahedron Lett. 1987;28:6027. Oishi E, Taido N, Iwamoto K, Miyashita A, Higashino T. Chem. Pharm. Bull. 1990;38:3268.
- 7.For intramolecular IEDDA reactions of 1,2-diazines see: Jojima T, Takeshiba H, Konotsune T. Chem. Pharm. Bull. 1972;20:2191. Boger DL, Coleman RS. J. Org. Chem. 1984;49:2240. Boger DL, Sakya MS. J. Org. Chem. 1988;53:1415. For an elegant application to natural product synthesis see: Boger DL, Coleman RS. J. Am. Chem. Soc. 1987;109:2717.
- 8.Selected papers on 1,2,3-Triazines: Neunhoeffer H, Clausen M, Vötter HD, Ohl H, Krüger C, Angermund K. Liebigs Ann. Chem. 1985:1732. Anderson ED, Boger DL. J. Am. Chem. Soc. 2011;133:12285. doi: 10.1021/ja204856a. 1,2,4-Triazines: Neunhoeffer H, Frühauf HW. Tetrahedron Lett. 1969;10:3151. Dittmar W, Sauer J, Steigel A. Tetrahedron Lett. 1969;10:5171. Boger DL, Panek JS. J. Am. Chem. Soc. 1985;107:5745.
- 9.Selected papers on tetrazines: Carboni RA, Lindsey RV., Jr J. Am. Chem. Soc. 1959;81:4342. Boger DL, Coleman RS, Panek JS, Yohannes D. J. Org. Chem. 1984;49:4405. Sauer J, Heldmann DK, Hetzenegger J, Krauthan J, Sichert H, Schuster J. Eur. J. Org. Chem. 1998:2885. For application of tetrazines in bioconjugation reactions see: Blackman ML, Royzen M, Fox JM. J. Am. Chem. Soc. 2008;130:13518. doi: 10.1021/ja8053805. Taylor MT, Blackman ML, Dmitrenko O, Fox JM. J. Am. Chem. Soc. 2011;133:9646. doi: 10.1021/ja201844c.
- 10.(a) Boger DL. Tetrahedron. 1983;39:2869. [Google Scholar]; (b) Boger DL. Chem. Rev. 1986;86:781. [Google Scholar]; (c) Fringuelli F, Taticchi A. Dienes in the Diels-Alder Reaction. New York: Wiley; 1990. [Google Scholar]
- 11.(a) Kessler SN, Wegner HA. Org. Lett. 2010;12:4062. doi: 10.1021/ol101701z. [DOI] [PubMed] [Google Scholar]; (b) Kessler SN, Neuburger M, Wegner HA. Eur. J. Org. Chem. 2011:3238. [Google Scholar]
- 12.(a) For an independent report on complexes between diboraanthracene and diazenes see: Lorbach A, Bolte M, Lerner H-W, Wagner M. Chem. Commun. 2010;46:3592. doi: 10.1039/c001803a. (b) The corresponding indium based Lewis acid has also been reported: Gabbaï FP, Schier A, Riede J, Hynes MJ. Chem. Commun. 1998:897.
- 13.(a) Sweis RF, Schramm MP, Kozmin SA. J. Am. Chem. Soc. 2004;126:7442. doi: 10.1021/ja048251l. [DOI] [PubMed] [Google Scholar]; (b) Zhang LM, Kozmin SA. J. Am. Chem. Soc. 2004;126:10204. doi: 10.1021/ja046586x. [DOI] [PubMed] [Google Scholar]; (c) Zhang LM, Kozmin SA. J. Am. Chem. Soc. 2004;126:11806. doi: 10.1021/ja046112y. [DOI] [PubMed] [Google Scholar]; (d) Sun J, Kozmin SA. Angew. Chem. Int. Ed. 2006;45:4991. doi: 10.1002/anie.200601276. [DOI] [PubMed] [Google Scholar]; (e) Sun J, Keller VA, Meyer ST, Kozmin SA. Adv. Synth. Catal. 2010;352:839. doi: 10.1002/adsc.200900835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siloxy alkynes and alkynoates have been used as formal dienophiles. See: Danheiser RL, Brisbois RG, Kowalczyk JL, Miller RF. J. Am. Chem. Soc. 1990;112:3093. Austin WF, Zhang Y, Danheiser RL. Org. Lett. 2005;7:3905. doi: 10.1021/ol051307b. Austin WF, Zhang Y, Danheiser RL. Tetrahedron. 2008;64:915. doi: 10.1016/j.tet.2007.10.113. Shindo M, Sato Y, Shishido K. J. Org. Chem. 2001;66:7818. doi: 10.1021/jo015929w. Review: Shindo M. Tetrahedron. 2007;63:10. doi: 10.1016/j.tet.2007.07.033.
- 15.For recent examples for the synthesis of substituted 2-naphthols see: Juteau H, Gareau Y, Lachance H. Tetrahedron Lett. 2005;46:4547. Zhang X, Sarkar S, Larock RC. J. Org. Chem. 2006;71:236. doi: 10.1021/jo051948k. Dai Y, Feng X, Liu H, Jiang H, Bao M. J. Org. Chem. 2011;76:10068. doi: 10.1021/jo201907t. For reviews on the synthesis of substituted naphthalenes see: Katritzky AR, Li J, Xie L. Tetrahedron. 1999;55:8263. de Koning CB, Rousseau A, van Otterlo WAL. Tetrahedron. 2003;59:7.
- 16.Review on the asymmetric oxidative coupling of 2-naphthol derivatives: Wang H. Chirality. 2010;22:827. doi: 10.1002/chir.20843.
- 17.Brunel JM. Chem. Rev. 2005;105:857. doi: 10.1021/cr040079g. [DOI] [PubMed] [Google Scholar]
- 18.(a) Tsuda T, Ohba S, Takahashi M, Ito M. Acta Crystallogr. Sect. C. 1989;45:887. [Google Scholar]; (b) Whitcomb DR, Rogers RD. J. Chem. Cryst. 1995;25:137. [Google Scholar]; (c) Whitcomb DR, Rogers RD. Inorg. Chim. Acta. 1997;256:263. [Google Scholar]; (d) Carlucci L, Ciani G, Proserpio DM, Sironi A. Inorg. Chem. 1998;37:5941. doi: 10.1021/ic970043p. [DOI] [PubMed] [Google Scholar]; (e) Whitcomb DR, Rajeswaran M. Inorg. Chim. Acta. 2008;361:1357. [Google Scholar]
- 19.See the Supporting Information (SI) for details on the preparation of pyrido[2,3-d]pyridazine.
- 20.(a) Das B, Reddy CR, Sindhu C, Sudhakar C, Nagendra S. Synthesis. 2010:3731. [Google Scholar]; (b) Layer RW. Tetrahedron Lett. 1974;15:3459. [Google Scholar]
- 21.The coordination of Ag+ to phthalazine in the presence of 2,2'-bipyridine was investigated by 1H- and 13C-NMR experiments. See the SI for details.
- 22.The involvement of N-Si interactions may play a role in the activation of siloxy alkynes. We thank one of the reviewers for suggesting this possibility.
- 23.Mild Brønsted acids promote a different cycloaddition reaction between phthalazines and siloxy alkynes. These findings will be reported in due course.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.