Abstract
dnTGFβRII mice, expressing a dominant negative form of TGFβ receptor II under control of the CD4 promoter, develop autoimmune colitis and cholangitis . We previously observed that deficiency in IL-12p40 led to a marked diminution of inflammation in both the colon and the liver. To distinguish whether IL-12p40 mediated protection acted via the IL-12 or IL-23 pathways, we generated an IL-23p19−/− dnTGFβRII strain deficient in IL-23 but not in IL-12; mice were longitudinally followed for changes in the natural history of disease and immune responses. Interestingly, IL-23p19−/− mice demonstrate dramatic improvement in their colitis but no changes in biliary pathology; mice also manifest reduced Th17 cell populations and unchanged IFN-γ levels. We submit that the IL-12/Th1 pathway is essential for biliary disease pathogenesis, while the IL-23/Th17 pathway mediates colitis. To further assess the mechanism of the IL-23 mediated protection from colitis, we generated an IL-17A−/− dnTGFβRII strain deficient in IL-17, a major effector cytokine produced by IL-23-dependent Th17 cells. Deletion of the IL-17A gene did not affect the severity of either cholangitis or colitis, suggesting that the IL-23/Th17 pathway contributes to the colon disease in an IL-17-independent manner. These results affirm that the IL-12/Th1 pathway is critical to biliary pathology in dnTGFβRII mice while the colitis is caused by a direct effect of IL-23.
Keywords: Primary biliary cirrhosis, murine models, autoimmunity, cholangitis, colitis
Introduction
Murine strains with a deficiency in specific cytokine pathways are important tools for investigating the mechanism of immunopathogenesis of autoimmunity. Mice transgenic for directed expression of a dominant negative form of transforming growth factor beta receptor type II (dnTGFβRII), under the control of the CD4 promoter lacking the CD8 silencer, spontaneously develop an inflammatory bowel disease (IBD) (1). In addition, dnTGFβRII mice develop an autoimmune biliary ductular disease with strong similarity to human primary biliary cirrhosis (PBC), an organ-specific autoimmune disease characterized by destruction of intrahepatic small bile duct biliary epithelial cells (2, 3). Deletion of IL-12p40 in dnTGFβRII mice, which results in deficiency of both IL-12 and IL-23, leads to marked diminution of inflammation in both the liver and the colon (4). In efforts to distinguish between the roles of the cytokine pathways mediated by IL-12 and IL-23 in the pathogenesis of the liver and colon diseases in dnTGFβRII mice, we generated two new mutant strains of dnTGFβRII mice: an IL-23p19−/− strain, which is deficient in IL-23 but not other members of the IL-12 family; and an IL-17A−/− strain, which is deficient in IL-17, a major effector cytokine produced by IL-23-dependent Th17 cells (5). The results of our study demonstrate that while deletion of IL-23p19 eliminates colitis but not cholangitis, the deletion of IL-17A had no significant effect on either cholangitis or colitis. Therefore, the IL-12/Th1, but not the IL-23/Th17, pathway, is important for autoimmune cholangitis. Our data also suggest that the IL-23/Th17 pathway contributes to the colon disease in an IL-17-independent manner.
Materials and Methods
Animals
The dnTGFβRII colony on a B6 background (B6.Cg-Tg(Cd4-TGFBR2)16Flv/J) was maintained at the University of California at Davis animal facility (Davis, CA) (3). The B6 (IL-17A−/−) mice and the B6 (IL-23p19−/−) mice were generous gifts from Dr. Yoichiro Iwakura (University of Tokyo, Tokyo, Japan) and Dr. Frederic J. de Sauvage (Genetech, South San Francisco, CA), respectively (6). IL-23p19−/− dnTGFβRII mice were generated as previously described (3, 4). Briefly, male dnTGFβRII mice were mated with female IL-23p19−/− mice to obtain IL-23p19+/− dnTGFβRII mice, which were subsequently back-crossed with female IL-23p19−/− mice to obtain IL-23p19−/− dnTGFβRII mice. The parental dnTGFβRII and the derived IL-23p19−/− dnTGFβRII mice were genotyped at 3 to 4 weeks of age to confirm the dnTGFβRII and IL-23p19−/− genes in their genomic DNA (3). IL-17A−/− dnTGFβRII mice were similarly generated. All mice were fed sterile rodent Helicobacter Medicated Dosing System (three-drug combination) diets (Bio-Serv, Frenchtown, NJ) and maintained in individually ventilated cages under specific pathogen-free conditions. Sulfatrim (Hi-tech Pharmacal, Amityville, NY) was delivered through drinking water. At 24 weeks of age, animals were sacrificed to collect sera, spleen, liver and colon tissues. The experimental protocols were approved by the University of California Animal Care and Use Committee.
Histopathology
The liver and colon from sacrificed mice were fixed in 4% paraformaldehyde, embedded in paraffin, cut into 4 μm sections, deparaffinized, stained with hematoxylin and eosin (H&E), and evaluated using light microscopy (4). The liver histopathology was graded as: 0, no inflammation (or bile duct damage); 1, mild inflammation (or bile duct damage); 2, moderate inflammation (or bile duct damage); and 3, severe inflammation (or bile duct damage) (4). The colon histopathology was graded as: 0, no significant changes; 1, minimal scattered mucosal inflammatory cell infiltrates, with or without minimal epithelial hyperplasia; 2, mild scattered to diffuse inflammatory cell infiltrates, sometimes extending into the submucosa and associated with erosions, with mild to moderate epithelial hyperplasia and mild to moderate mucin depletion from goblet cells; 3, moderate inflammatory cell infiltrates that were sometimes transmural, with moderate to severe epithelial hyperplasia and mucin depletion; and 4, marked inflammatory cell infiltrates that were often transmural and associated with crypt abscesses and occasional ulceration, with marked epithelial hyperplasia, mucin depletion, and loss of intestinal glands (7).
Immunohistochemistry
To monitor neutrophil infiltration, sections of colon were stained for myeloperoxidase (MPO) as previously described (8). The colon sections were blocked using 20% (v/v) normal swine serum in TBS for 30 min, stained for MPO using a rabbit anti-MPO Ab (0398; DAKO), followed by staining with biotin labeled anti-rabbit Ab (E0413; DAKO). Avidin-biotin peroxidase (K0377; DAKO) and histogreen (Linaris) were used for color development.
Flow Cytometry
Liver and colon infiltrating mononuclear cells (MNCs) were isolated as described (9, 10). The cells were re-suspended in staining buffer (0.2% BSA, 0.04% EDTA and 0.05% sodium azide in PBS), divided into 25μl aliquots, and incubated with anti-mouse FcR blocking reagent (eBioscience) for 15 minutes at 4°C. Cells were washed and stained for 30 minutes at 4°C with cocktails containing combinations of fluorochrome conjugated mAb for the cell surface markers CD4, CD8a, CD44, CD69, NK1.1 (Biolegend, San Diego, CA), and TCR-β (eBioscience, San Diego, CA). To determine T cell activation, mAbs for CD44 and CD69 were used (1, 11). IgG isotype antibodies with matching conjugates were used in parallel as negative controls. The cells were then washed with PBS containing 0.2% BSA. A FACScan flow cytometer (BD Immunocytometry Systems, San Jose, CA) upgraded for detection of 5 colors by Cytek Development (Fremont, CA) was used to acquire data, which were analyzed with Cellquest PRO software (BD Immunocytometry Systems).
Serum Immunoglobulins (Ig), anti-mitochondrial antibodies (AMA), and antinuclear antibodies (ANA, Gp210/Sp100)
Levels of serum IgG, IgM, and IgA were determined using a murine IgG, IgM, and IgA enzyme-linked immunosorbent assay quantitation kit (Bethyl Laboratories, Montgomery, TX). Serum AMAs were detected using an enzyme-linked immunosorbent assay based on recombinant murine PDC-E2 as described (12). Immunoreactivity was determined by measuring the optical density at 450 nm after incubation with 100 μL of tetramethylbenzidine substrate (BD Biosciences, San Jose, CA) for 30 minutes. Serum ANAs (Gp210/Sp100) were measured by QUANTA Lite Gp210/Sp100 (INOVA Diagnostics, San Diego, CA).
Cytokine Analysis
For analysis of cytokines secreted from cultured CD4 T cells, CD4T cells were isolated from spleen MNCs with CD4 (L3T4) MicroBeads (Miltenyi Biotec Inc., Auburn, CA). Aliquots of 2.0 x 105 CD4 T cells were cultured in 96-well round-bottom plates in 200μl of RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS) (GIBCO-Invitrogen Corp., Grand Island, NY), 100 μg/mL streptomycin, 100 U/mL penicillin, and 0.5 μg/mL each of anti-CD3 (BioLegend) and anti-CD28 (BioLegend). The cultures were incubated for 72 hours at 37°C in a humidified 5% CO2 incubator, then centrifuged to collect supernatants.
For analysis of cytokine levels in tissue, total protein was extracted from 30 mg of frozen liver or colon tissues by homogenization in T-Per® Tissue Protein Extraction buffer (Thermo, Rockford, IL) containing a protease inhibitor cocktail (Roche, Indianapolis, IN). The homogenized tissue suspension was centrifuged at 12,000 g for 20 minutes at 4°C and the supernatant was stored at −80°C until use. The total protein concentration of each sample was measured using the BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA).
Levels of IL-17A, TNF-α, IL-6, IL-10, IL-4, IL-2, and IFN-γ in sera, cell culture supernatant and tissue lysates were measured with a cytokine bead array assay using the Mouse Th1/Th2/Th17 Cytokine Kit (BD Biosciences). Levels of IL-22 and MIP-2/CXCL2 were measured using the Quantikine mouse Mouse/Rat IL-22 Immunoassay kit and mouse CXCL2/MIP-2 kit (R&D Systems, Minneapolis, MN).
For measuring levels of cytokine gene mRNA, total RNA was extracted from frozen colon tissues using RNeasyR Plus Mini Kit (QIAGEN), and cDNA was synthesized by Superscript III reverse-transcriptase (Invitrogen) according to the manufacturer’s protocols. The real-time PCR system (ViiATM 7; Applied Biosystems) was used for quantitative PCR. The primers used were as follows: 5'-TCCAGAAGGCCCTCAGACTA-3' (forward) and 5'-AGCATCTTCTCGACCCTGAA-3' (reverse) for mouse IL-17A, 5′-TAGCCAAGACTGTGATTGCGG-3′ (forward) and 5′-AGACATCTCCTCCCATCAGCAG-3′ (reverse) for mouse IFN-γ, 5'-AAGCCTGTAGCCCACGTCGTA-3' (forward) and 5'-AGGTACAACCCATCGGCTGG- 3' (reverse) for mouse TNF-α, 5'-TCCATCCAGTTGCCTTCTTG-3' (forward) and 5'-TTCCACGATTTCCCAGAGAAC-3' (reverse) for mouse IL-6, 5'-CATGGCCTTCCGTGTTCCTA-3' (forward) and 5'-CCTGCTTCACCACCTTCTTGAT-3' (reverse) for mouse Glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Amplification was performed for 40 cycles in a total volume of 16 μL and products detected using SYBR Green. The relative expression level of each target gene was determined by normalizing its mRNA level to the internal control gene GAPDH.
Statistical analysis
Two-tailed unpaired Mann-Whitney test, one-way analysis of variance (ANOVA) followed by a Bonferroni multiple comparisons test, or Fisher's exact test were used for different analyses as appropriate. P values < 0.05 were considered statistically significant.
Results
Depletion of IL-23p19 ameliorated colitis in dnTGFβRII mice
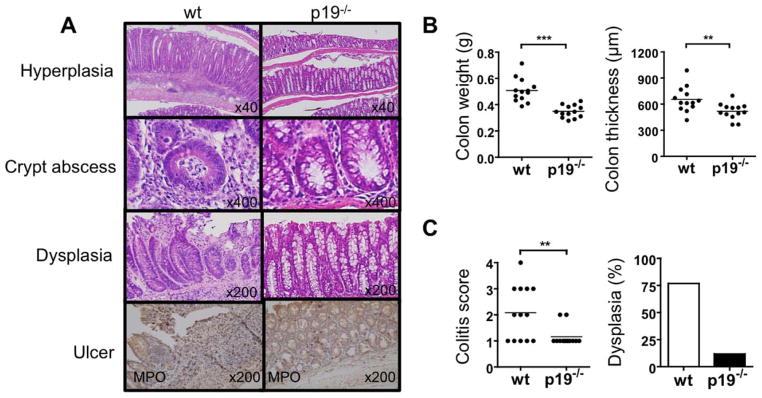
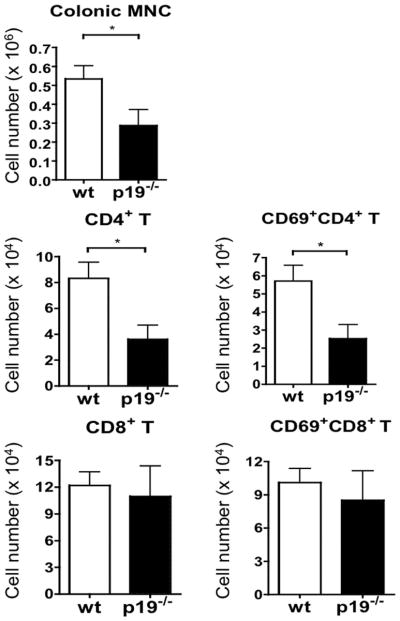
Since 5-month old dnTGFβRII mice develop IBD, we examined IL-23p19−/− dnTGFβRII mice for colitis at 24 weeks of age. Colonic hyperplasia, crypt abscesses, and epithelial ulcers were readily observed in dnTGFβRII mice but not in IL-23p19−/− mice (Fig. 1A). Colon weight and thickness, which correlates with severity of colitis, were significantly decreased in IL-23p19−/− dnTGFβRII mice compared to the age-matched dnTGFβRII mice (Fig. 1B). Colonic infiltration of total mononuclear cells, as well as total and activated CD4 T cells, was significantly decreased in IL-23p19−/− mice compared to dnTGFβRII mice, while no differences were observed in the levels of infiltrating CD8 T cell populations (Fig 2). MPO+ cells appeared to accumulate around the ulcer region in the dnTGFβRII mice, whereas only a few of these cells were observed in the colon mucosal layer of IL-23p19−/− dnTGFβRII (Fig. 1A). In addition, a relatively higher incidence of dysplasia was observed in the dnTGFβRII mice than the IL-23p19−/− mice (Fig. 1A and 1C).
Figure 1.
Colitis is improved in IL-23p19−/− dnTGFβRII mice compared to parental dnTGFβRII mice. A. Representative histological staining of colon sections. Colonic hyperplasia, crypt abscess and dysplasia were frequently observed in dnTGFβRII mice (wt), but not IL-23p19−/− dnTGFβRII mice (p19−/− ). MPO+ cells accumulated around the ulcer region in dnTGFβRII mice, while few foci of MPO+ cells were noted within the mucosal layer in the IL-23p19−/− dnTGFβRII mice. B. Colon weight and colon wall thickness. C. Colitis score and incidence rate of dysplasia. **, P < 0.01; ***, P < 0.001; determined using two-tailed unpaired Mann-Whitney test.
Figure 2.
The numbers of total MNCs, CD4 T cells, and CD8 T cells in Colon tissues from IL-23p19−/− mice (n=6) and parental dnTGFβRII mice (n=6), determined by flow cytometry. *, P < 0.05; determined using two-tailed unpaired Mann-Whitney test.
Depletion of IL-23p19 did not suppress autoimmune cholangitis in dnTGFβRII mice
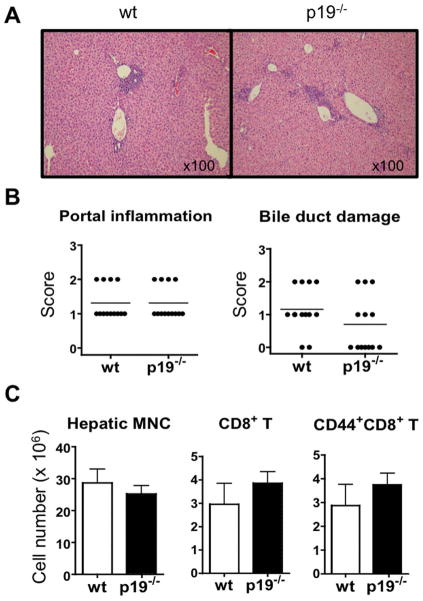
We next compared liver histology in IL-23p19−/− dnTGFβRII mice and dnTGFβRII mice at 24 weeks of age. There was no significant difference in the levels of inflammatory portal lymphoid cell infiltration and bile duct damage between the two mouse strains (Fig. 3A and 3B). In addition, the numbers of intra-hepatic T cells, including the total CD8 T cell population and activated CD8 T cells (defined by CD69+ and CD44+ phenotypes (1, 11), known to be pathogenic in the liver disease of dnTGFβRII mice (13), did not differ significantly between the two mouse strains (Fig. 3C). These results indicate that the deficiency in IL-23p19 did not protect dnTGFβRII mice from developing liver disease.
Figure 3.
Cholangitis in the livers of IL-23p19−/− mice and dnTGFβRII mice. A. H&E-stained liver sections. B. Liver portal inflammation and bile duct damage scores in IL-23p19−/− mice (n=13) and parental dnTGFβRII mice (n=13). C. Numbers of total MNCs, CD8 T cells, and CD44+CD8 T cells in the liver of dnTGFβRII mice (n=7) and IL-23p19−/− dnTGFβRII mice (n=7). No significant difference was found in any of these comparisons between the two strains (two-tailed unpaired Mann-Whitney test).
Serum levels of Ig, AMA and ANA in IL-23p19−/− dnTGFβRII mice
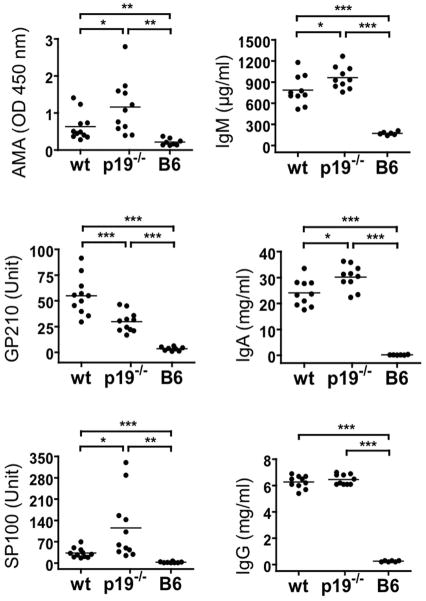
To address if IL-23 has a role in autoantibody induction, serum levels of AMA and ANA as well as those for total IgG, IgM, and IgA were measured by ELISA. As shown in Fig. 4, the level of IgG in the IL-23p19−/− dnTGFβRII mice was higher than in normal B6 mice but were comparable with those of dnTGFβRII mice. In contrast, the levels of IgM and IgA in IL-23p19−/− mice were significantly higher than that of dnTGFβRII mice. In the IL-23p19−/− dnTGFβRII mice, the levels of AMA and anti-SP100 ANA were significantly higher than that of the dnTGFβRII mice (p<0.05), while the levels of anti-GP210 ANA were significantly lower than that of the dnTGFβRII mice (p<0.001) but still significantly higher than that of B6 mice (p<0.001) (Fig. 4). These data indicate that in this model of autoimmunity, IL-23 is in general not critical for autoantibody production, but has opposite effects on the levels of different autoantibodies.
Figure 4.
Serum levels of AMA (PDC-E2), ANA (GP210 and SP100), and total Ig in dnTGFβRII mice (n=11) and IL-23p19−/− dnTGFβRII mice (n=11), as well as normal B6 mice (n=8) served as the negative control. Horizontal bars represent median values. *, P < 0.05; **, P < 0.01; ***, P < 0.001; by one-way ANOVA followed by a Bonferroni multiple comparisons test.
Effect of IL-23p19 depletion on serum cytokine levels
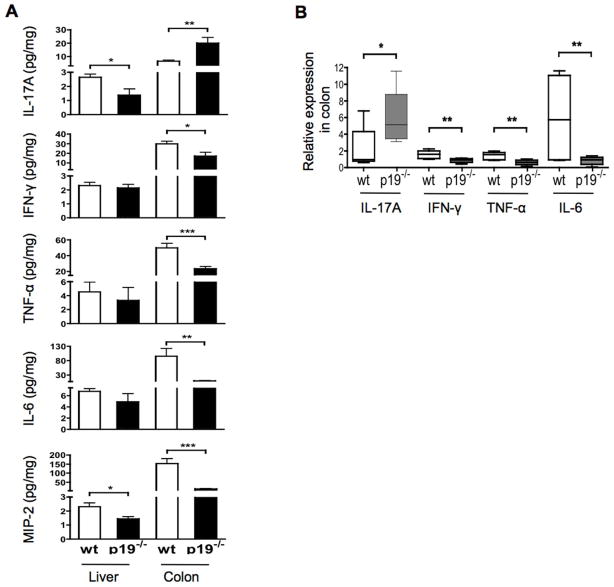
We measured a panel of pro-inflammatory cytokines in the sera from the IL-23p19−/− and the parental dnTGFβRII mice. As shown in Table 1, sera from the IL-23p19−/− mice as compared with dnTGFβRII sera contained significantly lower levels of most of the cytokines tested which included IL-17A, TNF-α, IL-6, IL-22, IL-10, IL-4, IL-2, and MIP-2/CXCL2 (macrophage inflammatory protein-2). The only exception was IFN-γ, which was not reduced in the IL-23p19−/− mice, indicating that deletion of IL-23p19 does not affect the differentiation of Th1 cells.
Table 1.
Serum levels of inflammatory cytokines
| Cytokine | Concentration (pg/ml)
|
P value | |
|---|---|---|---|
| wt | p19−/− | ||
| IL-17A | 34.4±8.0 | 8.1±3.1 | ** |
| IFN-γ | 37.7±8.9 | 43.5±19.7 | |
| TNF-α | 61.6±7.3 | 28.0±6.6 | ** |
| IL-6 | 73.3±11.5 | 12.9±2.3 | *** |
| MIP-2 | 63.4±11.0 | 32.4±3.7 | * |
| IL-22 | 7.6±1.4 | 2.5±1.2 | * |
| IL-10 | 151.2±35.5 | 42.5±16.2 | * |
| IL-4 | 14.9±3.0 | 5.3±1.3 | ** |
| IL-2 | 11.5±3.1 | 2.7±0.8 | * |
Serum samples from IL-23p19−/− dnTGFβRII mice(n=10) and dnTGFβ RII mice (n=10) were tested. Data are presented as an average ± SD for each group.
P < 0.05,
P < 0.01,
P < 0.001, by two-tailed unpaired Mann-Whitney test.
Decreased Th17 cell population in IL-23p19−/− dnTGFβRII mice
To determine whether deletion of the IL-23p19 gene influences the generation of cytokine based Th1, Th2 and Th17 cell populations, CD4 T cells isolated from the spleen of the IL-23p19−/− and the parental dnTGFβRII mice were cultured with anti-CD3/CD28 antibody for 3 days and the levels of secreted IFN-γ, IL-4and IL-17A measured in the supernatant fluid. The levels of secreted IL-17A were significantly reduced in IL-23p19−/− mice compared to dnTGFβRII mice (22.4 ± 3.6 pg/ml in IL-23p19−/− mice versus 4.7 ± 0.9 pg/ml in dnTGFβRII mice (p<0.01); the levels of IL-4 and IFN-γ were not significantly different between the two strains. These data suggest that deletion of IL-23p19 reduced the population of Th17 cells but not Th1 or Th2 cells in the spleen.
Deletion of IL-23p19 reduced IFN-γ, TNF-α and IL-6 in colon but not in liver
We next compared the levels of select inflammatory cytokines in colon and liver tissues from the IL-23p19−/− and the parental dnTGFβRII mice. As shown in Fig. 5A, while deletion of IL-23p19 resulted in a significant decrease in the levels of IFN-γ, TNF-α and IL-6 in the colon, there was no detectable change of these cytokines in the liver. In contrast, the levels of IL-17A were increased in the colon but decreased in the liver tissues from the IL-23p19−/− mice. The different cytokine levels in the colon of these two mouse strains are in agreement with their mRNA levels in the colon (Fig. 5B).
Figure 5.
Expression of cytokines in the liver and colon tissues. A. Cytokines in whole protein lysates of liver and colon tissues from dnTGFβRII mice (n= 12) and IL-23p19−/− mice (n= 12). B. Relative levels of cytokine gene mRNA in colon tissues of dnTGFβRII mice (n= 6) and IL-23p19−/− mice (n= 6). *, P < 0.05, **, P < 0.01, ***, P < 0.001, determined using two-tailed unpaired Mann-Whitney test.
Depletion of IL-17A did not affect the severity of autoimmune cholangitis or colitis in dnTGFβRII mice
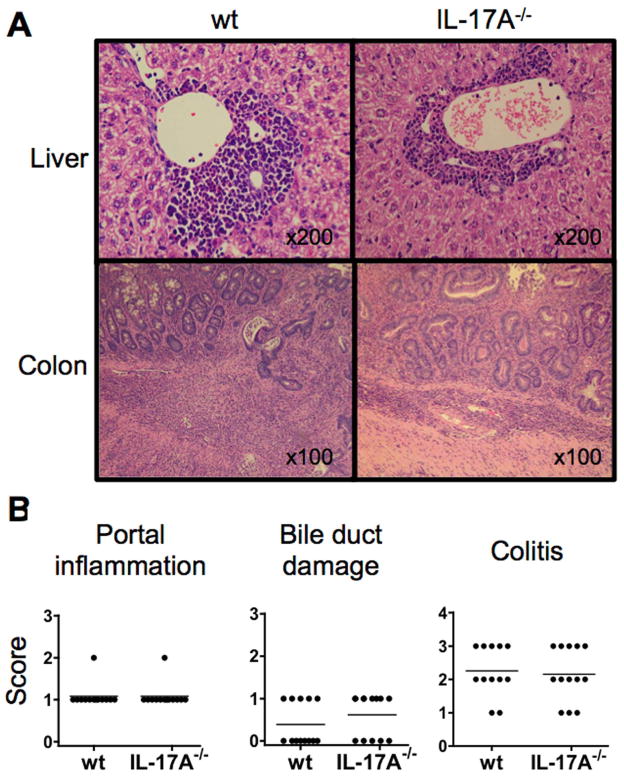
To determine if IL-17 was critical for the pathogenesis of autoimmune liver or colon diseases in the dnTGFβRII mice, we generated IL-17A−/− dnTGFβRII mice. Histological examination of the liver and colon sections detected no significant differences in either the levels of lymphoid cell infiltration and/or tissue damage in both the liver and colon tissues between the IL-17A−/− mice and the paternal dnTGFβRII mice (Fig. 6A and 6B). These results indicate that IL-17A is not critical for the spontaneous development of either autoimmune cholangitis or colitis in the dnTGFβRII mice.
Figure 6.
Histological evidence of cholangitis and colitis in IL-17A−/− dnTGFβRII mice and parental dnTGFβRII mice. A. Representative Hematoxylin-eosin (H&E)-stained liver and colon sections. The upper panels demonstrate lymphoid cell infiltration within the portal tract area around a damaged bile duct. The lower panels demonstrate diffused lymphoid cell infiltration from the mucosal layer into the muscular layer. B. Scoring of liver portal inflammation, bile duct damage, and colitis in IL-17A−/− dnTGFβRII mice (IL-17A−/− ) compared with the parental dnTGFβRII mice (wt). No significant differences were detected in any of the comparisons (two-tailed unpaired Mann-Whitney test).
Discussion
Previously we observed that deletion of IL-12p40 protected the dnTGFβRII mice from the spontaneous development of autoimmune disease in both the liver and the colon (4). Since the IL-12p40 is a subunit shared by IL-12 and IL-23, deletion of this subunit disrupts both the IL-12/Th1 pathway and IL-23/Th17 pathway. The first objective of this study was to examine the role of IL-23 in the liver and colon diseases of the dnTGFβRII mouse model by deleting p19 of the IL-23 heterodimer which is unique to this cytokine in the IL-12 family. While IL-12 is required for the development of IFN-γ producing Th1 cells, IL-23 induces the differentiation of naive CD4T cells into a highly pathogenic helper T cell population, termed Th17, that produces IL-17A, IL-17F, IL-6, and TNF but not IFN-γ or IL-4 (5). Several previous studies have suggested a potential link between IL-17 and PBC (14) (15, 16). Therefore the second strategy we used in the current study was to delete the gene encoding IL-17A in efforts to examine if this cytokine contributes to the autoimmune pathogenesis in the dnTGFβRII mice.
Results from these studies demonstrate that disrupting the IL-23/Th17 pathway by deleting IL-23p19 abolished colitis but had no detectable effect on the severity of cholangitis in the dnTGFβRII mice, indicating that the IL-23/Th17 axis is involved in the pathogenesis of autoimmune colitis but not in the cholangitis of this mouse model. However, deletion of the IL-17 gene from dnTGFβRII did not affect either colitis or cholangitis, indicating that IL-17 is not a key factor in the pathogenic IL-23/Th17 axis in the spontaneous development of colon disease of the dnTGFβRII mouse strain. Of note, deletion of IL-23 resulted in increased levels of AMA and anti-SP100 but decreased levels of anti-GP210. The mechanism for these differential effects of IL-23 should be addressed in future studies.
The autoimmune cholangitis that developed in the IL-23p19−/− mice was associated with an intact IL-12/Th1 pathway, as indicated by the high levels of IFN-γ in this mouse strain. In contrast cholangitis did not develop in IL-12p40−/− mice that lack the IL-12/Th1 pathway (4). Taken together these results confirm that the IL-12/Th1 immunity is necessary and sufficient for the development of cholangitis in dnTGFβRII mice. We have recently reported that adoptive transfer of CD8 T cells from dnTGFβRII into B6/Rag1−/− mice led to liver histopathology similar to that in the donor mice. In contrast, adoptive transfer of CD4 T cells predominantly induced IBD in the recipient mice (13). These data demonstrated that in the dnTGFβRII mice, CD8T cells are the major pathogenic effector of the cholangitis, while CD4 T cells are involved in the IBD. This is in agreement with our current finding that while comparable levels of CD8 T cells are present in the liver tissues of the IL-23p19−/− and dnTGFβRII mice, both develop cholangitis, and that protection against colitis in the IL-23p19−/− mice was associated with reduced numbers of total and activated CD4 T cells but not CD8 T cells in the colon. These findings further support the organ-specific pathogenic role of CD4 and CD8 T cells in the dnTGFβRII mice. The dnTGFβRII mice were completely protected from the autoimmune diseases in both the liver and the colon only when the IL-12/Th1 pathway was eliminated by deletion of IL-12p40 (4).
It has been previously shown that the IL-23/Th17 pathway plays a key role in T cell-mediated IBD and other autoimmune diseases in murine models that either involved cytokine gene knock-outs or antibody treatment in mice (17–26). Our current study in dnTGFββRII mice showed that deletion of the IL-23p19 gene resulted in a marked reduction of the Th17 population in the spleen, which is associated with prevention of colitis. However deletion of IL-17 gene did not prevent colitis, suggesting that the pathogenic effect in the colon of the dnTGFβRII mice was not mediated by IL-17. Actually the levels of IL-17 cytokine and mRNA in the colon of IL-23p19−/− mice was even higher than those in the dnTGFβRII mice, despite the fact that colitis was present in the latter but not in the former. It is important to note that in addition to the synthesis of IL-17, Th17 cells are also a major source for a number of other cytokines including IL-6 (5).
One of the most prominent features in the cytokine profile of IL-23p19−/− mice is the significant decrease in the levels of IL-6 in both the serum and colon (Table 1 and Figure 6). This is in agreement with previously reported role of IL-23-dependent IL-6 in development of colon inflammation as shown in other animal models of IBD (19, 27–30). It was recently observed that IL-6 levels were elevated in active IBD patients at diagnosis and during therapy (28). It has also been suggested that IL-23 might directly activate a subset of macrophages and dendritic cells expressing the IL-23R, resulting in the production of inflammatory mediators, such as TNF-α, IL-6, and IL-1 (25). Of note, using our dnTGFβRII mice model, we recently reported that depletion of IL-6 significantly improved colitis but exacerbated autoimmune cholangitis in liver (31). These studies indicate that the role of IL-6 in the pathogenesis of organ-specific autoimmune diseases is also different between the liver and colon. These data should become a major consideration in the use of anti-cytokine therapy in the treatment of organ-specific autoimmune diseases. We note recent data from our laboratory on therapeutic manipulation of this and similar models of autoimmune cholangitis (12).
It has been known for some time that individuals with IBD have a 10- to 40-fold increased risk of developing colorectal cancer compared with the general population. This is in agreement with the fact that colitis-associated cancer frequently develops from persistently inflamed mucosa and progresses through dysplasia to adenocarcinoma, following an “inflammation-dysplasia-carcinoma sequence” that contrasts the “adenoma-carcinoma sequence” of sporadic colorectal cancer. Therefore, effective anti-inflammatory treatment, such as infliximab therapy, could reduce the development of colorectal dysplasia and cancer in IBD (32–34). While colonic dysplasia was frequently observed in the dnTGFβRII mice (Fig. 1A), deletion of IL-23p19 reduced the incidence of dysplasia (Fig. 1C), suggesting that immunotherapies aimed at blocking the IL-23 pathway (26) could prevent IBD-related colon cancer.
In summary, our studies demonstrate that deletion of IL-23p19 improved colitis and reduced the rate of colonic dysplasia, but had no effect on liver cholangitis, in the dnTGFβRII mice. These findings confirm that in this mouse model, the IL-12/Th1 pathway is critical to biliary pathology, while the colitis is caused by a direct effect of IL-23. This study demonstrates that disruption of a pathway with a global effect, such as TGFβ signaling in CD4 T cells, leads to pathogenesis in different sites with distinct immune mechanisms. Therefore, care needs to be taken prior to the institution of immunotherapeutic strategies for organ-specific autoimmune diseases which should be tailored to address different targets in each disease.
Acknowledgments
Financial support provided by National Institutes of Health grant DK090019.
The authors thank Katsunori Yoshida, Thomas P Kenny, Hajime Tanaka, and Chen-yen Yang for technical support in this experiment. We also thank Ms. Nikki Phipps for support in preparing this article.
Abbreviations
- PBC
Primary biliary cirrhosis
- AMA
anti-mitochondrial auto-antibodies
- PDC-E2
pyruvate dehydrogenase E2 complex
- ANA
antinuclear antibody
- dnTGFβRII
dominant negative form of transforming growth factor beta receptor type II
- MNCs
mononuclear cells
- IBD
inflammatory bowel disease
- MPO
Myeloperoxidase
- CD
Crohn’s disease
- UC
ulcerative colitis
Contributor Information
Yugo Ando, Email: yugo.ando@gmail.com.
Guo-Xiang Yang, Email: gxyang@ucdavis.edu.
Masanobu Tsuda, Email: tsuda.masanobu@gmail.com.
Kazuhito Kawata, Email: kkawata@ucdavis.edu.
Weici Zhang, Email: ddzhang@ucdavis.edu.
Takahiko Nakajima, Email: tnkjm@med.u-toyama.ac.jp.
Koichi Tsuneyama, Email: ktsune@med.u-toyama.ac.jp.
Patrick Leung, Email: psleung@ucdavis.edu.
Zhe-Xiong Lian, Email: zxlian1@ustc.edu.cn.
Kazuichi Okazaki, Email: okazaki@hirakata.kmu.ac.jp.
William M. Ridgway, Email: ridgwawm@ucmail.uc.edu.
Gary L. Norman, Email: glnorman@inovadx.com.
Aftab A. Ansari, Email: pathaaa@emory.edu.
Xiao-Song He, Email: xiaosong@stanford.edu.
Ross L. Coppel, Email: ross.coppel@monash.edu.
M. Eric Gershwin, Email: megershwin@ucdavis.edu.
References
- 1.Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 2.Gershwin ME, Mackay IR. The causes of primary biliary cirrhosis: Convenient and inconvenient truths. Hepatology. 2008;47:737–745. doi: 10.1002/hep.22042. [DOI] [PubMed] [Google Scholar]
- 3.Oertelt S, Lian ZX, Cheng CM, Chuang YH, Padgett KA, He XS, Ridgway WM, et al. Anti-mitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. J Immunol. 2006;177:1655–1660. doi: 10.4049/jimmunol.177.3.1655. [DOI] [PubMed] [Google Scholar]
- 4.Yoshida K, Yang GX, Zhang W, Tsuda M, Tsuneyama K, Moritoki Y, Ansari AA, et al. Deletion of interleukin-12p40 suppresses autoimmune cholangitis in dominant negative transforming growth factor beta receptor type II mice. Hepatology. 2009;50:1494–1500. doi: 10.1002/hep.23132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghilardi N, Kljavin N, Chen Q, Lucas S, Gurney AL, De Sauvage FJ. Compromised humoral and delayed-type hypersensitivity responses in IL-23- deficient mice. J Immunol. 2004;172:2827–2833. doi: 10.4049/jimmunol.172.5.2827. [DOI] [PubMed] [Google Scholar]
- 7.Kamanaka M, Huber S, Zenewicz LA, Gagliani N, Rathinam C, O'Connor W, Jr, Wan YY, et al. Memory/effector (CD45RB(lo)) CD4 T cells are controlled directly by IL-10 and cause IL-22-dependent intestinal pathology. J Exp Med. 2011;208:1027–1040. doi: 10.1084/jem.20102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haegens A, Heeringa P, van Suylen RJ, Steele C, Aratani Y, O'Donoghue RJ, Mutsaers SE, et al. Myeloperoxidase deficiency attenuates lipopolysaccharide-induced acute lung inflammation and subsequent cytokine and chemokine production. J Immunol. 2009;182:7990–7996. doi: 10.4049/jimmunol.0800377. [DOI] [PubMed] [Google Scholar]
- 9.Lian ZX, Okada T, He XS, Kita H, Liu YJ, Ansari AA, Kikuchi K, et al. Heterogeneity of dendritic cells in the mouse liver: identification and characterization of four distinct populations. J Immunol. 2003;170:2323–2330. doi: 10.4049/jimmunol.170.5.2323. [DOI] [PubMed] [Google Scholar]
- 10.Matsuda JL, Gapin L, Sydora BC, Byrne F, Binder S, Kronenberg M, Aranda R. Systemic activation and antigen-driven oligoclonal expansion of T cells in a mouse model of colitis. J Immunol. 2000;164:2797–2806. doi: 10.4049/jimmunol.164.5.2797. [DOI] [PubMed] [Google Scholar]
- 11.Robertson AK, Rudling M, Zhou X, Gorelik L, Flavell RA, Hansson GK. Disruption of TGF-beta signaling in T cells accelerates atherosclerosis. J Clin Invest. 2003;112:1342–1350. doi: 10.1172/JCI18607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moritoki Y, Lian ZX, Lindor K, Tuscano J, Tsuneyama K, Zhang W, Ueno Y, et al. B-cell depletion with anti-CD20 ameliorates autoimmune cholangitis but exacerbates colitis in transforming growth factor-beta receptor II dominant negative mice. Hepatology. 2009;50:1893–1903. doi: 10.1002/hep.23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang GX, Lian ZX, Chuang YH, Moritoki Y, Lan RY, Wakabayashi K, Ansari AA, et al. Adoptive transfer of CD8(+) T cells from transforming growth factor beta receptor type II (dominant negative form) induces autoimmune cholangitis in mice. Hepatology. 2008;47:1974–1982. doi: 10.1002/hep.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lan RY, Salunga TL, Tsuneyama K, Lian ZX, Yang GX, Hsu W, Moritoki Y, et al. Hepatic IL-17 responses in human and murine primary biliary cirrhosis. J Autoimmun. 2009;32:43–51. doi: 10.1016/j.jaut.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harada K, Shimoda S, Sato Y, Isse K, Ikeda H, Nakanuma Y. Periductal interleukin-17 production in association with biliary innate immunity contributes to the pathogenesis of cholangiopathy in primary biliary cirrhosis. Clin Exp Immunol. 2009;157:261–270. doi: 10.1111/j.1365-2249.2009.03947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rong G, Zhou Y, Xiong Y, Zhou L, Geng H, Jiang T, Zhu Y, et al. Imbalance between T helper type 17 and T regulatory cells in patients with primary biliary cirrhosis: the serum cytokine profile and peripheral cell population. Clin Exp Immunol. 2009;156:217–225. doi: 10.1111/j.1365-2249.2009.03898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 18.Kullberg MC, Jankovic D, Feng CG, Hue S, Gorelick PL, McKenzie BS, Cua DJ, et al. IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J Exp Med. 2006;203:2485–2494. doi: 10.1084/jem.20061082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrison PJ, Ballantyne SJ, Kullberg MC. Interleukin-23 and T helper 17-type responses in intestinal inflammation: from cytokines to T-cell plasticity. Immunology. 2011;133:397–408. doi: 10.1111/j.1365-2567.2011.03454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abraham C, Cho JH. IL-23 and autoimmunity: new insights into the pathogenesis of inflammatory bowel disease. Annu Rev Med. 2009;60:97–110. doi: 10.1146/annurev.med.60.051407.123757. [DOI] [PubMed] [Google Scholar]
- 22.Neurath MF. IL-23: a master regulator in Crohn disease. Nat Med. 2007;13:26–28. doi: 10.1038/nm0107-26. [DOI] [PubMed] [Google Scholar]
- 23.Holtta V, Klemetti P, Sipponen T, Westerholm-Ormio M, Kociubinski G, Salo H, Rasanen L, et al. IL-23/IL-17 immunity as a hallmark of Crohn's disease. Inflamm Bowel Dis. 2008;14:1175–1184. doi: 10.1002/ibd.20475. [DOI] [PubMed] [Google Scholar]
- 24.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 25.Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W, et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pizarro TT, De La Rue SA, Cominelli F. Role of interleukin 6 in a murine model of Crohn's ileitis: are cytokine/anticytokine strategies the future for IBD therapies? Gut. 2006;55:1226–1227. doi: 10.1136/gut.2005.083121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carey R, Jurickova I, Ballard E, Bonkowski E, Han X, Xu H, Denson LA. Activation of an IL-6:STAT3-dependent transcriptome in pediatric-onset inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:446–457. doi: 10.1002/ibd.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishimoto N, Kishimoto T. Interleukin 6: from bench to bedside. Nat Clin Pract Rheumatol. 2006;2:619–626. doi: 10.1038/ncprheum0338. [DOI] [PubMed] [Google Scholar]
- 30.Nishimoto N, Kishimoto T. Humanized antihuman IL-6 receptor antibody, tocilizumab. Handb Exp Pharmacol. 2008:151–160. doi: 10.1007/978-3-540-73259-4_7. [DOI] [PubMed] [Google Scholar]
- 31.Zhang W, Tsuda M, Yang GX, Tsuneyama K, Rong G, Ridgway WM, Ansari AA, et al. Deletion of interleukin-6 in mice with the dominant negative form of transforming growth factor beta receptor II improves colitis but exacerbates autoimmune cholangitis. Hepatology. 2010;52:215–222. doi: 10.1002/hep.23664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korelitz BI. Considerations of surveillance, dysplasia, and carcinoma of the colon in the management of ulcerative colitis and Crohn's disease. Med Clin North Am. 1990;74:189–199. doi: 10.1016/s0025-7125(16)30595-8. [DOI] [PubMed] [Google Scholar]
- 33.Rosenstock E, Farmer RG, Petras R, Sivak MV, Jr, Rankin GB, Sullivan BH. Surveillance for colonic carcinoma in ulcerative colitis. Gastroenterology. 1985;89:1342–1346. doi: 10.1016/0016-5085(85)90653-5. [DOI] [PubMed] [Google Scholar]
- 34.Kim YJ, Hong KS, Chung JW, Kim JH, Hahm KB. Prevention of colitis-associated carcinogenesis with infliximab. Cancer Prev Res (Phila) 2010;3:1314–1333. doi: 10.1158/1940-6207.CAPR-09-0272. [DOI] [PubMed] [Google Scholar]