Abstract
Introduction
Obstructive sleep apnea (OSA) is common among commercial drivers and associated with health/safety risks, leading several trucking firms to mandate OSA screening.
Methods
19,371 commercial drivers were screened for OSA with an on-line questionnaire (Somni-Sage®) through employer mandates. Questionnaire and polysomnography results were analyzed retrospectively.
Results
Screening categorized 5,908 (30%) drivers as higher risk. To date, employers have sent 2,103 higher risk drivers for polysomnography, demonstrating that 68% of high risk drivers tested had an apnea hypopnea index (AHI) >10 and 80% had an AHI ≥5. A conservative prevalence estimate for OSA (AHI >10) was 21% among the drivers studied.
Conclusion
Online screening followed by polysomnography for high-risk drivers demonstrates as many as 21% of commercial drivers may have OSA. Mandatory screening can have a high yield among commercial drivers.
Keywords: obstructive sleep apnea, screening questionnaire, polysomnography, commercial motor vehicle operators, truck drivers
Introduction
Obstructive sleep apnea (OSA) is common among commercial motor vehicle (CMV) operators with prevalence estimates ranging from 13–28%. (1–3). According to the Federal Motor Carrier Safety Administration, in 2010 there were 14 million commercial driver license (CDL) holders(4). Thus, using estimates of OSA prevalence among drivers (3, 5, 6) 1.8 to 3.9 million CDL holders are expected to have OSA - most of them undiagnosed and untreated. Untreated OSA is recognized to increase the risk of motor vehicle crashes (MVC) in drivers (7–11). Recent systematic reviews have found that OSA on average increases the risk of MVC by 1.2 to 4.9-fold (12–14).
Additionally, untreated OSA is associated with poor cardiovascular and metabolic health (15–23), added healthcare costs (24–26), and lost workdays (27). In general, it is estimated that untreated OSA doubles health care utilization and expenditures compared to those without OSA (28). However, effective treatment of OSA with positive airway pressure (PAP) significantly mitigates health expenditures (28, 29) and reduces elevated vehicular crash risk to close to that of drivers without OSA (30). Therefore, identifying commercial drivers with OSA and treating them effectively should decrease crash-related injuries and fatalities and improve drivers’ health.
On the basis of its accident investigations, the National Transportation Safety Board has urged the Federal Motor Carrier Safety Administration (FMCSA), the Federal Railroad Administration, US mass transit authorities and the Federal Aviation Administration to adopt mandatory OSA screening regulations for transportation operators (31). In parallel, efforts to educate occupational and sleep-related medical professionals have come through an extensive review published by the Joint Task Force (JTF) of the American College of Chest Physicians, the American College of Occupational and Environmental Medicine, and the National Sleep Foundation (32). This same review by the JTF also promulgated expert consensus guidelines for OSA screening (combining both self-reported symptoms, as well as a reliance on objectively measured criteria) during commercial driver medical examinations in the absence of federal guidelines. A 2008 study, however, demonstrated that while most occupational physicians performing commercial driver medical examinations (CDME) feel screening drivers for OSA is important, the majority of these physicians were not utilizing the consensus or other rigorous criteria for screening (33). In the absence of a Federal OSA screening mandate and with most CDME providers not screening on their own, some trucking companies have voluntarily implemented their own driver screening programs.
While most experts agree OSA screening of drivers is necessary, considerable disagreement over screening methods and criteria for triggering a sleep study referral remain (32, 34, 35). Moreover, the CMV operator population presents unique and specific challenges to the successful identification and treatment of those with OSA. These challenges include: high driver turnover, extreme driver mobility and driver fear and/or resistance to an OSA diagnosis because of the potential consequences on medical certification and employment. Inadequate health insurance coverage and confusion over employer and/or regulatory requirements also complicate this picture. Several investigations have demonstrated screening based on simple driver self-reports of OSA, snoring or excessive daytime sleepiness are unreliable and have low yields in conjunction with a commercial drivers’ medical examinations (CDME) or other occupational setting. (1, 34, 35)
Most prior studies concerning active OSA screening programs for commercial drivers have come from occupational medicine clinics conducting screening using the JTF consensus criteria during CDME or similar certification exams (1, 34, 36). This article describes a distinct approach. A patented computer-based screening instrument (Somni-Sage® Precision Pulmonary Diagnostics, Houston, TX, US 7,720,696 B1) was administered on-line to over 19,000 drivers at three major trucking firms through an employer-driven program.
Methods
Study Population
The study population was constituted exclusively by commercial motor vehicle drivers who completed an employer-mandated OSA screening questionnaire and were employees of one of three national trucking firms during the study period (April 1, 2006 through April 10, 2010). Retrospective analyses of anonymous de-identified data from these drivers were approved as an exempt research protocol by the Institutional Review Board of the Cambridge Health Alliance.
OSA Screening Instrument
Precision Pulmonary Diagnostics, LLC (PPD) developed a self-report, on-line screening questionnaire from a review of the available medical literature and an unpublished pilot study of 100 drivers using their complete medical records and polysomnogram (PSG) results. None of those drivers are included in the present data. The goal was to provide trucking companies with a simple, cost-effective tool for identifying drivers with an increased likelihood of having OSA. Higher risk drivers would then be candidates for PSG for diagnostic confirmation. This OSA screening instrument is trademarked as Somni-Sage®; and it has been awarded a US Patent (US 7,720,696 B1). A copy of the SomniSage component questions is provided in the Appendix (see Appendix Table 1, http://links.lww.com/JOM/A98).
During the study period, drivers were told the screen was mandatory and strongly encouraged to complete the SomniSage questionnaire form on-line. However, no penalties were administered for failing to complete the questionnaire. The questionnaire was completed online by each driver independently. There were no “helpers” assigned to assist the drivers in the process. The SomniSage questionnaire was exclusively done as an employer-based, on-line program and was not performed as part of the CDME or coupled with the drivers’ CDME.
The self-report, computerized screening instrument has mostly mandatory fields that must be completed (cannot be skipped). Non-mandatory fields were neck size for men and women (because most drivers don’t know their collar size) and for women whether or not they were on hormone replacement therapy. In addition to questions on symptoms, SomniSage also includes reliable surrogate measures of objective data (self-reports of height and weight and medical conditions) for formulating a prediction for OSA in any given driver. SomniSage incorporates weighted values for body mass index (BMI), presence of hypertension, presence or absence of heavy snoring, witnessed apneas, other symptoms and medical co-morbidities, and neck circumference, as well as excessive daytime sleepiness (EDS). The latter was considered positive if a driver had an Epworth Sleepiness Scale (ESS) ≥10 (37) and/or answered “often” to the following question: “Do you become drowsy while driving?”
Based on a patented statistical algorithm derived from all the questionnaire responses, drivers are then categorized as higher priority for PSG testing (Class 1) or lower priority for further testing with PSG, (Non-Class1). These SomniSage categorizations of OSA likelihood are referred to as “Higher Risk” and “Lower Risk” throughout the manuscript.
Diagnostic Confirmation
CMV drivers in the employer programs were not taken out of service in order to undergo confirmatory diagnostic testing, which was conducted within the PPD network of sleep clinics located near major participating long-haul carrier operating centers. Drivers received sleep studies for diagnostic confirmation by one of two largely independent mechanisms (See Figure 1). The vast majority was referred for PSG based on being categorized as Higher Risk on the SomniSage screening instrument (see above). Drivers’ risk status was communicated to the employers, and the employers had the sole discretion to refer Higher Risk drivers for PSG. These decisions were usually based on the driver’s schedule availability, participating network testing center (sleep lab) availability and proximity to a driver’s route, the employer’s budget (how many drivers/month could be tested) and the driver’s continued employment with the firm. A smaller number of drivers received PSG testing independent of their SomniSage risk status at the specific request of a physician performing a CDME. These sleep studies were also performed within the PPD network and therefore, their results were also available for the present study.
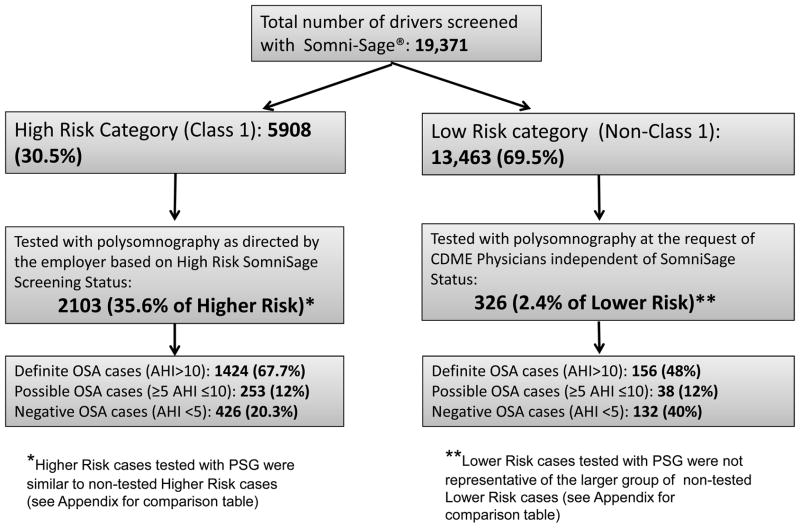
Figure 1.
Participant flow chart for the 19,371 CMV operators screened for OSA risk characterization using the OSA screening instrument
To date, all sleep studies have been full, multi-channel, in sleep laboratory, technician-attended PSGs or “Type 1” PSGs as defined the American Academy of Sleep Medicine (AASM). During the study period 1433 tests (68%) were performed in AASM accredited facilities and the remaining tests were performed in facilities whose applications for accreditation were pending during the study period. All sleep studies were interpreted by Board Certified sleep medicine physicians (84% of studies), or physicians qualified to sit for the certifying exam in sleep medicine (16% of studies).
Data Analysis
Retrospective analysis of the screening results was performed using a comprehensive database derived by merging the results of the on-line sleep apnea screening instrument (Somni-SageR) with records of overnight diagnostic sleep studies when they were performed. For comparisons considering self-reported values for age, BMI (calculated from height and weight self-reports) and neck circumference, we created acceptable ranges based on reasonably expected ranges for biologic plausibility and/or employer hiring practices (minimum age of 21 years). Individual values were considered potentially valid and included in the analyses within the following respective ranges: age- 21–75 years old, BMI- 17–65 kg/m2, and neck circumference- 14–23 inches. Age, BMI and Neck Circumference values falling outside of these ranges were defined as missing, but the driver’s remaining valid data for other SomniSage responses were included in the analyses of those variables.
The presence of OSA was defined on the basis of the apnea-hypopnea index (AHI) recorded at PSG, which provides the average number of apnea and hypopnea episodes per hour of sleep time during the recording. Definite OSA diagnoses were assigned to drivers with an AHI >10 and possible OSA to drivers with an AHI of 5–10.
All analyses were conducted using Stata version 11.1/SE (Stata Corp., College Station, TX). Statistical significance was defined as a two-sided p-value <0.05 for all tests. Comparisons between groups were performed with the Kruskal-Wallis test for continuous variables and the Fisher exact test for nominal variables, on the more conservative assumption that some data were not normally distributed.
Results
From April 1, 2006 through April 10, 2010, a total of 19,371 CMV operators have each been screened once for OSA risk characterization using SomniSage. The self-reported demographic and anthropometric characteristics of the study participants are presented in Table 1. The vast majority of participants (19,055 or 98%) reported both their age and BMI within the designated acceptable valid ranges (age 21–75 years and BMI 17–65 kg/m2). In contrast, many drivers skipped the neck circumference question or provided biologically implausible responses. Potentially valid responses for neck circumference were reported by 67% of male drivers and only 0.13% of female drivers. The driver population was over 90% male and half of both male and female drivers were obese (BMI ≥30 kg/m2). Increased neck circumference (≥17 inches) was also common among male drivers (53% of valid responses).
Table 1.
Demographic and anthropometric characteristics based on the Somni-Sage® self-reports of 19,371 professional drivers screened for OSA
| Baseline Characteristics | Gender | Mean (±SD) | Median (95% range) | Minimum | Maximum | Valid observations N (%) |
|---|---|---|---|---|---|---|
| Age | Male | 41.2 [11.2] | 41.3 (23.4–59.4) | 21.0 | 74.8 | 17,641 (91.1) |
| Female | 41.2 [9.7] | 42.7 (24.1–55.6) | 21.1 | 74.8 | 1477 (7.6) | |
| BMI | Male | 30.9 [6.6] | 29.9 (21.8–43.2) | 17.0 | 64.6 | 17,827 (92) |
| Female | 31.0 [7.4] | 30.0 (20.7–44.8) | 17.2 | 62.2 | 1478 (7.6) | |
| Neck size* | Male | 17.0 [1.6] | 17.0 (14.5–20.0) | 14 | 23 | 12,929 (66.7) |
AHI= apnea/hypopnea index; BMI= body mass index; SD= standard deviation
Insufficient female data for neck size
Comparisons of self-reported health conditions on the screening questionnaire according to OSA risk status are presented in Tables 2 (males) and 3 (females). Self-reported excessive daytime sleepiness (EDS), witnessed apneas, hypertension and diabetes were more prevalent in Higher Risk drivers (males and females) than Lower Risk drivers. Furthermore, Higher Risk drivers were significantly more obese than Lower Risk drivers.
Table 2.
Comparison of self-reported health conditions on the Somni-Sage®questionnaire, according to drivers’ Somni-Sage® prioritization category (male participants, n= 17,882)
| Somni-Sage® Parameter | Somni-Sage® prioritization* | p-values | |
|---|---|---|---|
| Higher Risk Class 1*(5728 drivers) n (%) | Lower Risk Non-class 1 (12,154 drivers) n (%) | ||
| EDS** | 944 (16.5) | 708 (5.8) | <0.001 |
| Witnessed Apneas** | 1085 (19) | 0 (0) | <0.001 |
| Urinate at night** | 1499 (26) | 762 (6) | <0.001 |
| Hypertension** | 2423 (42) | 946 (8) | <0.001 |
| Diabetes** | 991 (17) | 237 (2) | <0.001 |
| Heart Problem** | 278 (5) | 110 (0.9) | <0.001 |
| Heart Operation** | 356 (6) | 177(1.5) | <0.001 |
| Heart Burn** | 798 (14) | 757 (6) | <0.001 |
| COPD** | 29 (0.7) 3933 drivers |
30 (0.25) 11,957 drivers |
<0.001 |
| Family history of Heart Disease** | 408 (21) 1917 drivers |
547 (11.5) 4777 drivers |
<0.001 |
| Family history of Hypertension** | 1059 (55) 1917 drivers |
1280 (27) 4777 drivers |
<0.001 |
| Family history of Diabetes** | 812 (42) 1917 drivers |
1103 (23) 4777 drivers |
<0.001 |
| Family history of Sleep Apnea** | 241 (13) 1917 drivers |
70 (1.5) 4777 drivers |
<0.001 |
| Family history of Cancer** | 459 (24) 1917 drivers |
779 (16) 4777 drivers |
<0.001 |
| Age mean [SD] | 43.7 [10.7] 5656 drivers |
40.1 [11.2] 11985 |
<0.001 |
| BMI mean [SD] | 36.4 [6.4] 5718 drivers |
28.3 [5.0] 12,109 drivers |
<0.001 |
| AHI mean [SD]*** | 29 [29] 2014 drivers |
17 [23] 271 drivers |
<0.001 |
| ESS mean [SD] | 3.9 [3.3] 5470 drivers |
3.5 [3.1] 12,060 drivers |
<0.001 |
|
ESS >10 n (%) mean [SD] |
247 (4.5) 5470 drivers 12.8 [2.0] |
361 (3) 12,060 drivers 13.3 [2.4] |
<0.001 |
| Neck size mean [SD] | 18.0 [1.6] 4561 drivers |
16.4 [1.2] 8368 drivers |
<0.001 |
BMI = body mass index; COPD = chronic obstructive pulmonary disease; EDS =excessive daytime sleepiness; ESS = Epworth sleepiness scale;
Class 1= high priority for further testing (PSG); Non-class 1= lower priority for further testing (PSG)
Number and (%) of participants that answered, “yes” on the Somni-Sage® questionnaire
EDS was considered positive if a driver had an Epworth Sleepiness Scale (ESS) ≥10 and/or answered “often” to the following question: “Do you become drowsy while driving?”
Among all participating drivers, 5,908 were categorized as Higher Risk for OSA and 13,463 were classified as being Lower Risk for OSA. From the 5,908 Higher Risk drivers, employers sent 2,103 drivers for overnight PSGs during the study period with definite OSA (AHI>10/h) diagnosed in 1,424, possible OSA (5≤AHI≤10/h) in 253 and negative OSA (AHI<5/h) in 426. An additional 326 PSGs were performed on Lower Risk drivers based on concerns of medical providers conducting their commercial driver medical examinations (CDME) with definite OSA diagnosed in 156, possible OSA in 38 and negative OSA in 132 drivers (Figure 1). PSG tested and non-tested Higher Risk drivers were quite similar in terms of major clinical OSA predictors (age, BMI, hypertension and neck circumference). In contrast, Lower Risk drivers who underwent PSG were not representative of the much larger group of non PSG tested Lower Risk drivers. They were significantly older, more obese and had on average larger neck circumferences (please see Table 4 comparing PSG tested and non-tested drivers).
Table 4.
Comparison between high and low risk drivers that were tested with PSG versus the ones that were not yet tested with PSG.
| Parameters | High-risk Drivers (Class 1) n=5908 | Low-risk drivers (non-class 1) n= 13,463 | ||||
|---|---|---|---|---|---|---|
| Tested with PSG n=2103 | Not yet tested with PSG n=3805 | p-value | Tested with PSG n=326 | Not yet tested with PSG n=13,137 | p-value | |
| Gender (% male) | 2014 (96) | 3714 (98) | N/A | 271 (88.1) | 11,883 (90.45) | N/A |
| Age mean [±SD] | 45.3 [10] 2080 | 43.0 [10.9] 3754 | <0.001 | 41.8 [11.2] n=324 | 40.1 [11.0] n=12,960 | 0.002 |
| BMI mean [±SD] | 36.3 [6.7] 2097 | 36.4 [6.2] 3799 | 0.0669 | 31.7 [7.1] n= 324 | 28.4 [5.2] n=13,085 | 0.001 |
| Neck size mean [±SD] | 17.9 [1.6] 1502 | 18.1 [1.6] 3061 | <0.001 | 16.7 [1.3] n= 155 | 16.4 [1.2] n= 8213 | <0.001 |
| EDS n (%)* | 485 (23) | 512 (13) | <0.001 | 110 (33.7) | 705 (5.4) | <0.001 |
| Witnessed apneas n (%)* | 523 (25) | 654 (17) | <0.001 | 0 | 0 | N/A |
| Hypertension n (%)* | 936 (45) | 1563 (41) | 0.011 | 51 (15.6) | 989 (7.5) | <0.001 |
| Diabetes n (%)* | 399 (19) | 624 (16) | 0.013 | 18 (5.5) | 260 (2.0) | <0.001 |
| Heart Disease n (%)* | 115 (5) | 168 (4) | 0.075 | 9 (2.8) | 123 (0.9) | 0.005 |
| GERD n (%)* | 309 (15) | 537 (14) | 0.561 | 44 (13.5) | 854 (6.5) | <0.001 |
BMI= body mass index; EDS= excessive daytime sleepiness; GERD= gastroesophageal reflux disease; N/A= not applicable; PSG= Polysomnogram
Number and % of participants that answered, “yes” on the SomniSage® questionnaire
Self-reported health conditions and anthropometric characteristics according to OSA diagnostic categories for drivers who received PSG testing are summarized in Tables 5 (males) and 6 (females). Both male and female drivers with definite OSA were on average older and more obese and the men had larger neck circumference. While obesity was highly prevalent in the definite OSA category (85%), 15% of the drivers in whom OSA was diagnosed were not obese. In the definite OSA category there were 231 participants with a BMI <30 kg/m2 (non-obese). Of those only 22 (9.5%) had a BMI <25 kg/m2 (normal) and 209 (90.5%) were in the overweight category.
Table 5. Comparison of self-reported health conditions and self-reported anthropometric characteristics on the Somni-Sage® questionnaire, according to OSA status categories for male participants, n=2,285.
(Negative OSA diagnosis: AHI < 5; Possible OSA diagnosis: ≥5 AHI ≤10; Definite OSA diagnosis: AHI > 10)
| Somni-Sage® parameter | Definite OSA diagnosis n (%) 1503 drivers | Possible OSA diagnosis n (%) 269 drivers | Negative OSA diagnosis n (%) 513 drivers | p-values | |
|---|---|---|---|---|---|
| EDS** | 341 (22.7) | 61 (22.7) | 139 (27.1) | 0.121 | |
| Witnessed Apneas** | 345 (23.0) | 40 (14.9) | 91 (17.7) | 0.001 | |
| Urinate at nigh** | 484 (32.0) | 75 (28.0) | 134 (26.0) | 0.023 | |
| Hypertension** | 660 (44.0) | 104 (38.7) | 170 (33) | <0.001 | |
| Diabetes** | 269 (17.9) | 51 (19.0) | 76 (14.8) | 0.207 | |
| Heart Problem** | 70 (4.7) | 21 (7.8) | 27 (5.3) | 0.105 | |
| Heart Operation** | 90 (6.0) | 24 (8.9) | 30 (5.9) | 0.184 | |
| Heart Burn | 205 (13.6) | 36 (13.4) | 77 (15) | 0.719 | |
| Age [mean ±SD] | 45.8 [10.0] 1491 drivers |
44.9 [10.3] 268 drivers |
42.0 [11.3] 503 drivers |
<0.001 | |
| BMI [mean ±SD] | 36.8 [6.9] 1499 drivers |
34.6 [6.6] 269 drivers |
32.6 [6.1] 512 drivers |
<0.001 | |
| AHI [mean ±SD] *** | 39 [28] 1503 drivers |
8 [2] 269 drivers |
2 [1] 513 drivers |
<0.001 | |
| ESS [mean ±SD] | 4.2 [3.5] 1468 drivers |
4.1 [3.2] 263 drivers |
3.8 [3.4] 220 drivers |
0.198 | |
| Neck size [mean ±SD] | 18.0 [1.6] 1080 drivers |
17.6 [1.5] 204 drivers |
17.3 [1.6] 371 drivers |
<0.001 | |
| Somni-Sage® Class | Higher Risk (Class 1*) | 1373 (91) | 239 (89) | 402 (78) | <0.001 |
| Lower Risk (Non-class 1) | 130 (9) | 30 (11) | 111 (22) | ||
AHI = apnea hypopnea index; BMI = body mass index; COPD = chronic obstructive pulmonary disease; EDS =excessive daytime sleepiness; ESS = Epworth sleepiness scale;
Class 1= high priority for further testing (PSG); Non-class 1= lower priority for further testing (PSG)
Number and (%) of participants that answered, “yes” on the Somni-Sage® questionnaire
AHI data are available only for those tested with polysomnography.
EDS was considered positive if a driver had an Epworth Sleepiness Scale (ESS) ≥10 and/or answered “often” to the following question: “Do you become drowsy while driving?”
With regard to symptoms and medical co-morbidities, no significant differences in prevalence were found in female drivers receiving a PSG based on AHI. For both men and women, there were no meaningful differences in the mean Epworth scores of drivers testing negative or positive for OSA as shown by non-parametric tests for trend. Similarly, there was also no evidence that daytime sleepiness as assessed by other questions on the OSA screening instrument was more prevalent in any OSA diagnostic category among those receiving diagnostic testing. Notably, the highest AHI recorded thus far in the program was 164/hour, yet that particular driver an Epworth score of 2. On the other hand, male drivers who tested positive for definite OSA were significantly more likely to report witnessed apneas, nocturia and hypertension.
Based on PSG confirmation, yield (number and proportion of all definite and definite plus possible OSA cases detected) and mean AHI observed in those with OSA cases identified using the OSA screening instrument categorization of High-risk (Class 1) and for various individual predictors are presented in Table 7. Notably, almost half (47.7%) of drivers diagnosed with OSA were found to have clinically severe OSA (AHI >30).
Table 7.
Yield and mean AHI for OSA cases identified using various individual predictors and Somni-Sage® class for OSA screening with polysomnography for confirmation.
| Yield** for definite OSA cases (AHI>10) n (%) [n of eligible cases] | AHI mean [± SD] For those with definite OSA diagnosis |
|
|---|---|---|
| Class 1* (High priority for further testing with PSG) | 1424 (90) | 40 [28] |
| Somni-Sage® parameters | ||
| EDS | 369 (23) [1580] | 37 [28] |
| Witnessed apneas | 369 (23) [1580] | 45 [30] |
| Urinate at night | 517 (33) [1580] | 41 [29] |
| Hypertension | 693 (44) [1580] | 39 [27] |
| Diabetes | 280 (18) [1580] | 37 [28] |
| ESS >10 | 99 (6) [1544] | 37 [28] |
| BMI ≥ 30 | 1342 (85) [1573] | 41 [29] |
| BMI ≥ 35 | 883 (56) [1573] | 45 [31] |
| BMI ≥ 40 | 446 (28) [1573] | 51 [32] |
| Neck size ≥17 | 854 (79) [1082] | 40 [28] |
| Neck size ≥18 | 596 (55) [1082] | 41 [28] |
| BMI ≥ 30 or Class 1 | 1519 (96) [1580] | 39 [28] |
AHI = apnea hypopnea index; BMI = body mass index; COPD = chronic obstructive pulmonary disease; EDS =excessive daytime sleepiness; ESS = Epworth sleepiness scale;
Class 1= high priority for further testing (PSG); Non-class 1= lower priority for further testing (PSG)
Yield is the n (%) of drivers with AHI >10 identified by each criterion or criteria
EDS was considered positive if a driver had an Epworth Sleepiness Scale (ESS) ≥10 and/or answered “often” to the following question: “Do you become drowsy while driving?”
The overall positive predictive value for definitive OSA among those deemed Class 1 (Higher Risk) by the OSA screening instrument was point-estimated at 68%, and point-estimated at 80% for AHI ≥5. This estimate assumes that the High-risk drivers who were not yet tested also have the same OSA prevalence as those who were tested. This is a reasonable assumption given that 90% of the High-risk drivers who were yet to be tested had a self-reported BMI >29 kg/m2 and 75% had a BMI > 32 kg/m2. Assuming the unlikely worst case scenario that none of the yet to be tested High-risk drivers would have OSA, the PPV estimates fall to 24% and 28%, respectively for AHI>10 and AHI≥5. Assuming another unlikely scenario where all yet to be tested High-risk drivers would have OSA, the PPV estimates reach 88% and 93%, respectively for AHI>10 and AHI≥5. Because Class 1 (Higher Risk) drivers who had PSGs were similar in terms of major OSA predictors than other Class 1 drivers (Table 7), the true PPV is likely close to the point estimates.
Because Class 1 (Higher Risk) drivers who had PSGs were similar in terms of major OSA predictors than other Class 1 drivers (Table 4), we derived conservative estimates of definite OSA (AHI >10) prevalence of 21% using the total number of Higher Risk drivers (n=5,908) (30%) and the best estimate for positive predictive value for Higher Risk status (68%).
Finally, we explored the question of whether drivers could learn over time to answer SomniSage in such a way as to avoid high risk classification by stratifying and examining the study data for each year of the program (see Table 8). These data suggest that while drivers may have “learned” to avoid a positive answer to excessive daytime sleepiness and witnessed apneas after the first two years of the program, nonetheless, the proportion of drivers being classified as high risk did not decrease over time. Moreover, the estimated positive predictive value of the instrument and the estimated OSA prevalence also did not decrease over time.
Table 8.
Number of CMV drivers screened as High-Risk on Somni-Sage, Excessive Daytime Sleepiness, Witnessed Apneass, estimated positive predictive values of Somni-Sage in the different years, estimated prevalence based on the assumption that High-risk drivers not tested with PSG would test positive in the same proportion as tested High-risk drivers.
| Year | High-risk drivers (Class 1) on Somni-Sage n (%) | Self-reported EDS of high-risk drivers on Somni-Sage n (%) | Self-reported witnessed apneas of high-risk drivers on Somn- Sage n (%) | Estimated Positive Predictive Value of Somni-Sage Class 1 | Estimated Prevalence of OSA (AHI>10) |
|---|---|---|---|---|---|
| 2006 | 2050 (29) | 591 (29) | 300 (14.6) | 70 % | 20.3 % |
| 2007 | 847 (25) | 124 (14.6) | 272 (32.1) | 63 % | 15.75 % |
| 2008 | 886 (30) | 98 (11.1) | 199 (22.5) | 67 % | 20.1 % |
| 2009 | 1589 (39) | 150 (9.4) | 314 (19.8) | 66 % | 25.7 % |
| 2010 | 536 (28) | 34 (6.3) | 92 (17.2) | 76 % | 21.3 % |
| Mean total | 5908 (30.5) | 997 (16.9) | 1177 (19.9) | 68 % | 21 % |
CMV= commercial motor vehicle; EDS= excessive daytime sleepiness; OSA= obstructive sleep apnea; PSG= Polysomnogram; WA= witnessed apneas
Class 1= high priority for further testing (PSG); Non-class 1= lower priority for further testing (PSG)
Discussion
This report demonstrates that a carefully constructed on-line screening instrument can have a high positive predictive value and lead to a large number of OSA diagnoses independent of commercial driver medical examinations. It also demonstrates that such programs are possible within the trucking industry despite high rates of driver turnover, as well as driver resistance to and suspicion of OSA screening. Another challenge is presented by business operational needs exact pressures when drivers are pulled out of service for testing. The model presented here allows drivers to complete screening on-line and have testing performed when indicated along their driving routes without service interruptions. Nonetheless, 64% of Higher Risk drivers had not received diagnostic confirmatory testing by the end of the study period, which is a significant limitation.
Major Results and Comparison to Previous Studies
The major results of our study regard the estimated positive predictive value of Somni-Sage® and its diagnostic yield and hence, estimated OSA prevalence. A Higher Risk categorization on Somni-Sage® was demonstrated to have an estimated 68% positive predictive value for definite OSA (AHI >10) and an 80% positive predictive value for an elevated AHI (for AHI ≥5). In comparison, the Israeli Traffic Ministry’s screening strategy reported by Dagan et al (35) of sending all drivers with a BMI >32 kg/m2 for a PSG, has a positive predictive value of 78%. That study did not report on drivers with a BMI ≤32 kg/m2 and estimates of OSA prevalence among all drivers were not reported. Studies reporting on the JTF screening criteria have demonstrated that screening tool to have a high positive predictive value ≥95% (1, 34); however, it has a considerably lower diagnostic yield with OSA prevalence estimates based on the screening yield of 12–13%. In the present study, conservative prevalence estimates of definite OSA (AHI >10) were 21% and 24% for AHI≥5, respectively. These findings are in agreement with two population-based OSA screening studies in drivers. Howard et al (2) estimated a 16% prevalence of OSA and Gurubhagavatula et al (5) a 28% prevalence for AHI ≥5, respectively. In further agreement with the latter study, which sampled both low and high-risk drivers, roughly 50% of all drivers were obese and few apnea cases (<4%) were identified among drivers with a normal BMI (<25 kg/m2) (5).
Another important finding relates to the limitations of the self-report with regard to the neck circumference, an important clinical OSA predictor. Many drivers either skipped this question or gave answers that were likely to be incorrect. Possible reasons for this weakness include the following. Most women do not know their collar size, because women’s clothes are not purchased using neck or collar size as a parameter for correct fit. Thus, few data were available for women. While a majority of male drivers reported collar sizes that were biologically plausible, a third provided no response or an implausible report. Because male drivers do not typically wear dress shirts to work, thus, many of them probably did not know their neck circumference.
An additional important finding with implications for attempts to implement more widespread screening among drivers was the employers’ limited ability to obtain PSGs on Higher Risk subjects. Specifically, among roughly 30% of drivers screened and categorized as Higher Risk for OSA, only 36% have received confirmatory diagnostic PSGs to date. We discuss this further below (see Limitations). With regard to loss to follow-up (defined here as failure to obtain diagnostic confirmation (PSGs) on drivers screened at High Risk), studies using the JTF screening guidelines(1, 34) also have documented that a large proportion of drivers refused or did not comply with PSG referrals.
Our data and those of previous studies (1, 34, 35) regarding the low yield of driver self-reported sleepiness should cast doubt on the reliability of using commercial driver reported daytime sleepiness as a stand-alone screen for sleep apnea risk or as a criterion for determining whether or not a commercial driver with OSA should be qualified to drive. The latter question will be best informed by future studies of commercial drivers analyzing crash risk as a function of AHI and other parameters in order to define which drivers are truly at elevated risk of crashes.
Limitations
Our study does have some limitations. The first is common to several previous smaller studies (1, 34, 35) in that we could not determine the true sensitivity and specificity of the screening instrument and individual predictors because we could not systematically evaluate the performance of the test in drivers with a lower probability of OSA against the gold standard PSG. In the present study, drivers who underwent PSG at the request of medical examiners, despite being categorized as Lower Risk on the screening instrument were not typical of the larger group of Lower Risk drivers and, in fact, were significantly older and more obese. Furthermore, because all High-risk drivers were not tested during the study period and some differences in symptom reporting existed between tested and non-tested High-risk drivers, some uncertainty regarding our estimates of positive predictive value exist. It is likely that the employers, CDME physicians and even drivers themselves could exert some differential selection that might result in earlier testing for some drivers who might be more likely to have OSA or are more symptomatic from their OSA, thus, biasing our PPV estimate towards an over-estimate. Nonetheless, given the very high mean BMI and high proportion of obesity among the untested High-risk drivers and other similarities between tested and un-tested High-risk drivers, we believe the true PPV to be close to our point estimates.
Second, the OSA screening instrument is a self-report instrument and we lacked medical examination data to corroborate drivers’ reports against objectively measured data and insurance claims for co-morbid conditions. Also many drivers, especially most female drivers, did not know their neck circumference and either skipped this item or entered a value likely to be invalid. Linking the OSA screening questionnaire to the medical history and the objectively measured components obtained at the time of the CDME would likely improve the accuracy of the OSA screening instrument and OSA screenings in general. However, given the high estimated positive predictive value of the screening instrument for confirmation of an elevated AHI (80% for AHI ≥5), and assuming we may have missed some drivers who under-reported weight or related conditions biases our results towards underestimates of the actual OSA prevalence. Future studies that are able to examine randomly selected lower risk drivers and those with other single predictors such as obesity will be able to refine more accurately screening instruments and strategies to balance sensitivity with predictive value.
Finally, another important limitation was that only 36% of Higher Risk drivers have received confirmatory diagnostic PSGs to date. It is important to put this issue in perspective and to understand the possible explanations for this finding. As discussed above, high loss to follow-up was also an issue in papers reporting on the JTF Consensus criteria in those cases, presumably due to drivers’ doctor-shopping. In the present study, multiple other challenges existed in addition to drivers seeking to avoid OSA diagnoses who may have quit driving with the index employer to avoid testing. One is the cost and availability of polysomnograms for employers, which limited the number of tests each trucking firm was able to obtain in any given year. Another likely contributing explanation derives from the high turnover rates in this industry. Depending on the strength of the economy, trucking firms can experience 40->100% turnover among drivers on an annual basis (38–40). Fortunately, Higher Risk drivers who were tested were similar on clinical OSA predictors to non-tested Higher Risk drivers allowing one to reasonably expect similar OSA prevalence between the two subgroups (see Table 4).
Strengths
Strengths of the present study relate primarily to the large sample size, which provided robust statistical power for most comparisons. Additionally, despite the limits of self-reports, the vast majority of subjects reported data conforming to medically expected ranges. For example, the total number of subjects who reported both their age and BMI within the designated ranges (age 21–75 years and BMI 17–65 kg/m2) was over 19,000 or 98% of the total number of drivers studied.
Table 3.
Comparison of self-reported health conditions on the Somni-Sage®questionnaire, according to drivers’ Somni-Sage® prioritization category (female participants, n= 1489)
| Somni-SageR Parameter | Somni-Sage® prioritization* | p-values | |
|---|---|---|---|
| Higher Risk (Class 1*) n (%) 180 drivers | Lower Risk (Non-class 1) n (%) 1309 drivers | ||
| EDS** | 53 (29) | 107 (8) | <0.001 |
| Witnessed Apneas** | 92 (51) | 0 (0) | <0.001 |
| Urinate at night** | 71 (39) | 157 (12) | <0.001 |
| Hypertension** | 76 (42) | 94 (7) | <0.001 |
| Diabetes** | 32 (18) | 41 (3) | <0.001 |
| Heart Problem** | 5 (3) | 22 (1.7) | 0.363 |
| Heart Operation** | 4 (2) | 21 (2) | 0.532 |
| Heart Burn** | 48 (27) | 141 (11) | <0.001 |
| COPD** | 5 (4.5) 111 drivers |
3 (0.2) 1272 drivers |
<0.001 |
| Family history of Heart Disease** | 20 (44) 45 drivers |
99 (22) 448 drivers |
0.002 |
| Family history of Hypertension** | 33 (73) 45 drivers |
172 (38) 448 drivers |
<0.001 |
| Family history of Diabetes** | 20 (44) 45 drivers |
163 (36) 448 drivers |
0.332 |
| Family history of Sleep Apnea | 9 (20) 45 drivers |
21 (5) 448 drivers |
0.001 |
| Family history of Cancer | 20 (44) 45 drivers |
136 (30) 448 drivers |
0.064 |
| Age mean [SD] | 46.6 [8.5] 178 drivers |
40.5 [9.6] 1299 drivers |
<0.001 |
| BMI mean [SD] | 36.2 [6.4] 180 drivers |
30.3 [7.2] 1300 drivers |
<0.001 |
| AHI mean [SD]*** | 16 [14] 89 drivers |
17 [26] 55 drivers |
0.143 |
| ESS mean [SD] | 4.8 [4.2] 160 drivers |
3.6 [3.3] 1287 drivers |
<0.002 |
|
ESS >10 n (%) mean [SD] |
16 (10) 160 drivers 14.0 [3.3] |
49 (4) 1287 drivers 13.8 [3.1] |
0.002 |
| Neck size mean [SD] | Insufficient data | ||
BMI = body mass index; COPD = chronic obstructive pulmonary disease; EDS =excessive daytime sleepiness; ESS = Epworth sleepiness scale;
Class 1= high priority for further testing (PSG); Non-class 1= lower priority for further testing (PSG)
Number and (%) of participants that answered, “yes” on the Somni-Sage® questionnaire
EDS was considered positive if a driver had an Epworth Sleepiness Scale (ESS) ≥10 and/or answered “often” to the following question: “Do you become drowsy while driving?”
Table 6. Comparison of self-reported health conditions and self-reported anthropometric characteristics on the Somni-Sage® questionnaire, according to OSA status categories for female participants, n=144.
(Negative OSA diagnosis: AHI < 5; Possible OSA diagnosis: ≥5 AHI ≤10; Definite OSA diagnosis: AHI > 10)
| Somni-Sage® parameter | Definite OSA diagnosis n (%) 77 drivers | Possible OSA diagnosis n (%) 22 drivers | Negative OSA diagnosis n (%) 45 drivers | p-value | |
|---|---|---|---|---|---|
| EDS** | 28 (36) | 8 (36) | 18 (40) | 0.944 | |
| Witnessed Apneas** | 24 (31) | 7 (32) | 16 (36) | 0.912 | |
| Urinate at night** | 33 (43) | 9 (41) | 11 (24) | 0.109 | |
| Hypertension** | 33 (43) | 9 (41) | 11 (24) | 0.109 | |
| Diabetes** | 11 (14) | 4 (18) | 6 (13) | 0.896 | |
| Heart Problem** | 4 (5) | 1 (4.5) | 1 (2) | 0.857 | |
| Heart Operation** | 3 (4) | 1 (4.5) | 1 (2) | 1.000 | |
| Heart Burn** | 15 (19.5) | 9 (41.0) | 11 (24.4) | 0.118 | |
| Age [mean ±SD] | 47.4 [8.2] 76 drivers |
44.8 [9.1] | 41.1 [10.4] 44 drivers |
0.008 | |
| BMI [mean ±SD] | 37.7 [7.0] 74 drivers |
36.5 [8.3] | 33.1 [6.4] | 0.004 | |
| AHI [mean ±SD]*** | 27 [21] | 8 [1] | 2 [1] | <0.001 | |
| ESS [mean ±SD] | 4.7 [3.9] 76 drivers |
4.1 [3.7] | 6 [3.6] 7 drivers |
0.361 | |
| Neck size [mean ±SD] | Insufficient data | ||||
| Somni-Sage® Class | Higher Risk (Class 1*) | 51 (66) | 14 (64) | 24 (53) | 0.352 |
| Lower Risk (Non-class 1) | 26 (34) | 8 (36) | 21 (47) | ||
AHI = apnea hypopnea index; BMI = body mass index; COPD = chronic obstructive pulmonary disease; EDS =excessive daytime sleepiness; ESS = Epworth sleepiness scale;
Class 1= high priority for further testing (PSG); Non-class 1= lower priority for further testing (PSG)
Number and (%) of participants that answered, “yes” on the Somni-Sage® questionnaire
AHI data are available only for those tested with polysomnography.
EDS was considered positive if a driver had an Epworth Sleepiness Scale (ESS) ≥10 and/or answered “often” to the following question: “Do you become drowsy while driving?”
Acknowledgments
This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (NIH Award #UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic health care centers, the National Center for Research Resources, or the National Institutes of Health. Dr. Berger is the President of Precision Pulmonary Diagnostics, LLC; PPD has received royalty payments for the use of the SomniSage questionnaire.
References
- 1.Talmage JB, Hudson TB, Hegmann KT, Thiese MS. Consensus criteria for screening commercial drivers for obstructive sleep apnea: evidence of efficacy. J Occup Environ Med. 2008;50:324–329. doi: 10.1097/JOM.0b013e3181617ab8. [DOI] [PubMed] [Google Scholar]
- 2.Howard ME, Desai AV, Grunstein RR, et al. Sleepiness, sleep-disordered breathing, and accident risk factors in commercial vehicle drivers. Am J Respir Crit Care Med. 2004;170:1014–1021. doi: 10.1164/rccm.200312-1782OC. [DOI] [PubMed] [Google Scholar]
- 3.Pack A, Dinges D, Maislin G. FMCSA, Publication No DOT-RT-02-030. 2002. A Study of Prevalence of Sleep Apnea Among Commercial Truck Drivers. [Google Scholar]
- 4.FMCSA; FMCSA. The FMCSA Medical Program: What’s New inCommercial Driver Medical Certification Professional Driver Meeting. U.S. Department of Transportation; Washington, D.C., USA: http://nrcmefmcsadotgov/documents/Professional_Driver_Meetingpdf. [Google Scholar]
- 5.Gurubhagavatula I, Maislin G, Nkwuo JE, Pack AI. Occupational screening for obstructive sleep apnea in commercial drivers. Am J Respir Crit Care Med. 2004;170:371–376. doi: 10.1164/rccm.200307-968OC. [DOI] [PubMed] [Google Scholar]
- 6.Moreno CR, Carvalho FA, Lorenzi C, et al. High risk for obstructive sleep apnea in truck drivers estimated by the Berlin questionnaire: prevalence and associated factors. Chronobiol Int. 2004;21:871–879. doi: 10.1081/cbi-200036880. [DOI] [PubMed] [Google Scholar]
- 7.Aldrich MS. Automobile accidents in patients with sleep disorders. Sleep. 1989;12:487–494. doi: 10.1093/sleep/12.6.487. [DOI] [PubMed] [Google Scholar]
- 8.Stoohs RA, Guilleminault C, Itoi A, Dement WC. Traffic accidents in commercial long-haul truck drivers: the influence of sleep-disordered breathing and obesity. Sleep. 1994;17:619–623. [PubMed] [Google Scholar]
- 9.George CF. Sleep. 5: Driving and automobile crashes in patients with obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2004;59:804–807. doi: 10.1136/thx.2003.007187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kingshott RN, Cowan JO, Jones DR, et al. The role of sleep-disordered breathing, daytime sleepiness, and impaired performance in motor vehicle crashes-a case control study. Sleep Breath. 2004;8:61–72. doi: 10.1007/s11325-004-0061-z. [DOI] [PubMed] [Google Scholar]
- 11.Mulgrew AT, Nasvadi G, Butt A, et al. Risk and severity of motor vehicle crashes in patients with obstructive sleep apnoea/hypopnoea. Thorax. 2008;63:536–541. doi: 10.1136/thx.2007.085464. [DOI] [PubMed] [Google Scholar]
- 12.Tregear S, Reston J, Schoelles K, Phillips B. Continuous positive airway pressure reduces risk of motor vehicle crash among drivers with obstructive sleep apnea: systematic review and meta-analysis. Sleep. 2010;33:1373–1380. doi: 10.1093/sleep/33.10.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellen RL, Marshall SC, Palayew M, Molnar FJ, Wilson KG, Man-Son-Hing M. Systematic review of motor vehicle crash risk in persons with sleep apnea. J Clin Sleep Med. 2006;2:193–200. [PubMed] [Google Scholar]
- 14.Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5:573–581. [PMC free article] [PubMed] [Google Scholar]
- 15.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. American journal of respiratory and critical care medicine. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 16.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. Bmj. 2000;320:479–482. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caples SM, Garcia-Touchard A, Somers VK. Sleep-disordered breathing and cardiovascular risk. Sleep. 2007;30:291–303. doi: 10.1093/sleep/30.3.291. [DOI] [PubMed] [Google Scholar]
- 18.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. American journal of respiratory and critical care medicine. 2005;172:1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. Jama. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 20.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 21.Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. American journal of respiratory and critical care medicine. 2002;166:159–165. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 22.Drager LF, Bortolotto LA, Lorenzi MC, Figueiredo AC, Krieger EM, Lorenzi-Filho G. Early signs of atherosclerosis in obstructive sleep apnea. American journal of respiratory and critical care medicine. 2005;172:613–618. doi: 10.1164/rccm.200503-340OC. [DOI] [PubMed] [Google Scholar]
- 23.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 24.Ronald J, Delaive K, Roos L, Manfreda J, Bahammam A, Kryger MH. Health care utilization in the 10 years prior to diagnosis in obstructive sleep apnea syndrome patients. Sleep. 1999;22:225–229. doi: 10.1093/sleep/22.2.225. [DOI] [PubMed] [Google Scholar]
- 25.Kapur V, Blough DK, Sandblom RE, et al. The medical cost of undiagnosed sleep apnea. Sleep. 1999;22:749–755. doi: 10.1093/sleep/22.6.749. [DOI] [PubMed] [Google Scholar]
- 26.Tarasiuk A, Greenberg-Dotan S, Brin YS, Simon T, Tal A, Reuveni H. Determinants affecting health-care utilization in obstructive sleep apnea syndrome patients. Chest. 2005;128:1310–1314. doi: 10.1378/chest.128.3.1310. [DOI] [PubMed] [Google Scholar]
- 27.Sjosten N, Vahtera J, Salo P, et al. Increased risk of lost workdays prior to the diagnosis of sleep apnea. Chest. 2009;136:130–136. doi: 10.1378/chest.08-2201. [DOI] [PubMed] [Google Scholar]
- 28.Bahammam A, Delaive K, Ronald J, Manfreda J, Roos L, Kryger MH. Health care utilization in males with obstructive sleep apnea syndrome two years after diagnosis and treatment. Sleep. 1999;22:740–747. doi: 10.1093/sleep/22.6.740. [DOI] [PubMed] [Google Scholar]
- 29.Hoffman B, Wingenbach DD, Kagey AN, Schaneman JL, Kasper D. The long-term health plan and disability cost benefit of obstructive sleep apnea treatment in a commercial motor vehicle driver population. J Occup Environ Med. 2010;52:473–477. doi: 10.1097/JOM.0b013e3181dbc8ab. [DOI] [PubMed] [Google Scholar]
- 30.George CF. Reduction in motor vehicle collisions following treatment of sleep apnoea with nasal CPAP. Thorax. 2001;56:508–512. doi: 10.1136/thorax.56.7.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.NTSB. Safety Recommendation. Washington, D.C. 20594: National Transportation Safety Board; 2009. http://wwwntsbgov/doclib/recletters/2009/H09_15_16pdf. [Google Scholar]
- 32.Hartenbaum N, Collop N, Rosen IM, et al. Sleep apnea and commercial motor vehicle operators: Statement from the joint task force of the American College of Chest Physicians, the American College of Occupational and Environmental Medicine, and the National Sleep Foundation. Chest. 2006;130:902–905. doi: 10.1378/chest.130.3.902. [DOI] [PubMed] [Google Scholar]
- 33.Durand G, Kales SN. Obstructive sleep apnea screening during commercial driver medical examinations: a survey of ACOEM members. J Occup Environ Med. 2009;51:1220–1226. doi: 10.1097/JOM.0b013e3181b8c16b. [DOI] [PubMed] [Google Scholar]
- 34.Parks P, Durand G, Tsismenakis AJ, Vela-Bueno A, Kales S. Screening for obstructive sleep apnea during commercial driver medical examinations. J Occup Environ Med. 2009;51:275–282. doi: 10.1097/jom.0b013e31819eaaa4. [DOI] [PubMed] [Google Scholar]
- 35.Dagan Y, Doljansky JT, Green A, Weiner A. Body Mass Index (BMI) as a first-line screening criterion for detection of excessive daytime sleepiness among professional drivers. Traffic Inj Prev. 2006;7:44–48. doi: 10.1080/15389580500412994. [DOI] [PubMed] [Google Scholar]
- 36.Xie W, Chakrabarty S, Levine R, Johnson R, Talmage JB. Factors associated with obstructive sleep apnea among commercial motor vehicle drivers. J Occup Environ Med. 2011;53:169–173. doi: 10.1097/JOM.0b013e3182068ceb. [DOI] [PubMed] [Google Scholar]
- 37.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 38.Burks SV, Belzer M, Kwan Q, Pratt S, Shackelford S. Transportation Research Circular Number E-C146. Transportation Research Board; Washington, DC: 2010. Trucking 101: An Industry Primer. [Google Scholar]
- 39.Burks SV, Carpenter J, Götte L, Rustichini A. Cognitive Skills Affect Economic Preferences, Social Awareness, and Job Attachment. Proceedings of the National Academy of Science. 2009;106:7745–7750. doi: 10.1073/pnas.0812360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watson R. TransportTopics. Alexandria, VA: American Trucking Associations; 2010. Driver Turnover Rate Rises. [Google Scholar]