Abstract
The vasoactive intestinal peptide (VIP) is a neuropeptide with wide distribution in both central and peripheral nervous systems, where it plays important regulatory role in many physiological processes. VIP displays a large biological functions including regulation of exocrine secretions, hormone release, fetal development, immune responses, etc. VIP appears to exert beneficial effect in neuro-degenerative and inflammatory diseases. The mechanism of action of VIP implicates two subtypes of receptors (VPAC1 and VPAC2), which are members of class B receptors belonging to the super-family of GPCR. This article reviews the current knowledge regarding the structure and molecular pharmacology of VPAC receptors. The structure–function relationship of VPAC1 receptor has been extensively studied, allowing to understand the molecular basis for receptor affinity, specificity, desensitization and coupling to adenylyl cyclase. Those studies have clearly demonstrated the crucial role of the N-terminal ectodomain (N-ted) of VPAC1 receptor in VIP recognition. By using different approaches including directed mutagenesis, photoaffinity labelling, NMR, molecular modelling and molecular dynamic simulation, it has been shown that the VIP molecule interacts with the N-ted of VPAC1 receptor, which is itself structured as a ‘Sushi’ domain. VPAC1 receptor also interacts with a few accessory proteins that play a role in cell signalling of receptors. Recent advances in the structural characterization of VPAC receptor and more generally of class B GPCRs will lead to the design of new molecules, which could have considerable interest for the treatment of inflammatory and neuro-degenerative diseases.
LINKED ARTICLES
This article is part of a themed section on Secretin Family (Class B) G Protein-Coupled Receptors. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.166.issue-1
Keywords: class B GPCR, GPCR, Inflammatory diseases, neurodegenerative diseases, PACAP, structure–function, VIP, VPAC receptors, accessory proteins
The vasoactive intestinal peptide (VIP), a ubiquitous neuropeptide
VIP is a ubiquitous neuropeptide of 28 amino acids, discovered in porcine duodenum by Said and Mutt (1970), which is present in central and peripheral nervous systems (Vaudry and Laburthe, 2006). VIP has been more recently identified in immune system where it plays the role of a ‘cytokine-like peptide’ (Delgado et al., 2001; Gomariz et al., 2001). In agreement with this widespread distribution, VIP is involved in many physiological and pathophysiological processes related to development, growth, cancers, immune responses, circadian rhythms, control of neuronal and endocrine cells and functions of the digestive, respiratory, reproductive and cardiovascular systems (Table 1). VIP belongs to a structural family of related peptides referred to as secretin/VIP family (Figure 1). This family encompasses VIP, pituitary adenylate cyclase activating peptide (PACAP), secretin, growth hormone-releasing factor (GRF), peptide having an histidine residue in N-terminal position and an isoleucine residue in C-terminal position (PHI and its human homolog PHM), helodermin, glucagon, gastric inhibitory polypeptide (GIP) and glucagon-like peptide 1 and 2 (GLP-1 and GLP-2). These natural ligands share some common properties: (1) they are all peptides with 27 to 44 amino acid residues; (2) they are synthesized and released by endocrine cells, neurons and/or immune cells; (3) they exhibit a marked propensity to form α-helices; (4) they contain a N-Cap structure in the N-terminal part (Neumann et al., 2008), which consists of an hydrophobic cluster between N-terminal hydrophobic residues and an hydrogen bond between two polar residues (Figure 2). All these peptides play an important role in physiological processes and strongly impact on human physiopathology (Table 2). The purpose of this review is to provide a selection of data regarding the current knowledge of the structure and molecular pharmacology of VIP receptors and also their ability to interact with accessory proteins.
Table 1.
Major physiological and pathophysiological actions of VIP
| Short-term | Exocrine secretions |
| Hormone release | |
| Muscle relaxation (vasodilator, bronchodilator) | |
| Metabolism | |
| Long-term | Circadian rhythms |
| Growth regulator of whole fetuses and embryonic brain | |
| Other effects | Neuroprotection |
| Suppression of inflammation | |
| Immunomodulation | |
| Psychiatric disorders | |
| Effects on cell proliferation in cancer |
Figure 1.

Sequence comparison of peptides of the VIP/secretin family. Sequence identity is represented by a black box, and sequence homologies are represented by grey boxes. Numbers indicate the length of the peptides.
Figure 2.
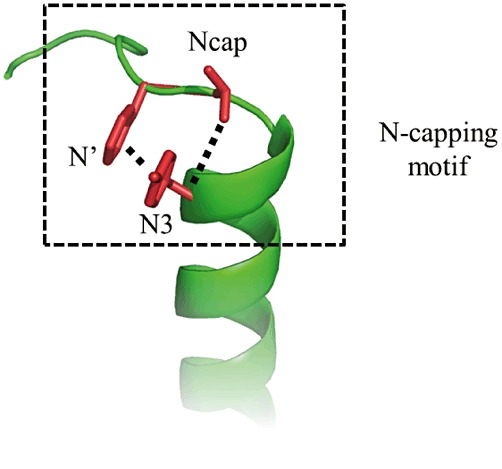
Generic representation of N-capping motif of class B GPCRs peptide ligands. The N-capping motif (type IA) is represented as (1) the hydrophobic interactions between side-chain groups of N′ and N3 residues (dashed lines); (2) the hydrogen bond between side chain of Ncap residue and backbone atom of N3 residue. See Neumann et al. (2008) for details.
Table 2.
Potential therapeutical interest of natural ligands of class B GPCRs in diseases
| Ligand | Diseases | References |
|---|---|---|
| VIP | Inflammation | Delgado et al., 2004 |
| Neurodegeneration | Gozes et al., 2003 | |
| PACAP | Neurodegeneration | Gozes et al., 2003 |
| Inflammation | Abad et al., 2006 | |
| GRF | Dwarfism | Campbell et al., 1995 |
| Glucagon | Diabetes | Brubaker, 2007 |
| GLP-1 | Diabetes | Brubaker, 2007 |
| GLP-2 | Short bowel syndrome | Jeppesen, 2006 |
| GIP | Diabetes | Inzucchi and McGuire, 2008 |
| PTH | Osteoporosis | Epstein, 2007 |
| Calcitonin | Osteoporosis | Mulder et al., 2006 |
| CRF | Stress | Gilligan and Li, 2004 |
The VPAC1 receptor, a class B GPCR prototype
VIP triggers biological responses through interaction with two subtypes of receptors, VPAC1 and VPAC2. VPAC1 and VPAC2 receptors bind with the same-affinity VIP and the other neuropeptide PACAP. These two receptors subtypes are mainly coupled to the G-protein Gs and stimulate cellular adenylyl cyclase activity (Laburthe et al., 2007). It should be noted that some groups have reported the ability of VIP to increase calcium levels in different cells (Dickson and Finlayson, 2009). This may be related to the fact that the VPAC1 receptor is able to interact with RAMPs (receptor activity-modifying proteins), in particular RAMP2, inducing a significant enhancement of agonist-mediated inositol trisphosphate production and subsequent effect on cellular calcium without affecting the coupling to adenylyl cyclase (Christopoulos et al., 2002). A previous report indicated that the VPAC1 receptor is able to homodimerize and heterodimerize with VPAC2 or secretin receptors (Harikumar et al., 2006). However, the relation between receptor oligomerization and the ability of VPAC receptor to trigger biological responses remains conjectural.
During the nineties, VPAC receptors were cloned (Lutz et al., 1993; Couvineau et al., 1994) as were many other receptors for peptides of the VIP/secretin family (Couvineau et al., 2010). These studies revealed the existence of a new GPCR subfamily named class B GPCR or class II GPCR or also ‘secretin-like’ receptors family. This small GPCR subfamily shares with the other GPCR classes the same general structural scheme with the presence of seven-transmembrane helices denoted as TM I to TM VII, which are interconnected by extracellular and intracellular loops (Fredrikson and Schiöth, 2006). The class B receptor family comprises 15 members including receptors for VIP, PACAP, secretin, glucagon, glucagon-like peptide-1, glucagon-like peptide-2, GRF, GIP and also includes receptors for peptides that have no sequence homology with VIP, including parathyroid hormone, calcitonin, calcitonin gene-related peptide and corticotropin-releasing factor (CRF) (Laburthe et al., 2007). The natural ligands of these class B GPCRs strongly influence human physiopathology and have been proposed as a promising candidates for the treatment of several diseases (Table 2).
Class B receptors have low sequence homologies with other members of the GPCR superfamily (Laburthe et al., 2007) and share several specific properties: (i) the presence of a large (>120 residues) and structured N-terminal ectodomain (N-ted), which is usually small in most class A GPCRs, the prototypes of which are rhodopsin and adrenergic receptors. The N-teds contain six highly conserved cysteine residues connected by three disulfide bridges, representing a signature of class B GPCRs. The N-ted of each class B receptor represents the major binding site for its cognate natural peptide ligand. (ii) The presence of a signal peptide probably involved in insertion of receptor in plasma membrane. (iii) The absence of archetypical class A GPCR motifs such as E/D-R-Y or NP-xx(x)-Y. (iv) A complex gene organization with many introns (Laburthe et al., 2007).
Cloning (Couvineau et al., 1994) of the human VPAC1 receptor allowed its extensive studies by site-directed mutagenesis and molecular chimerism (Laburthe et al., 2007), providing new insights into the molecular basis of interaction of VIP with its receptor in terms of (1) affinity (Couvineau et al., 1995); (2) specificity (Couvineau et al., 1996a,b; Du et al., 2002); (3) cellular addressing of the receptor to the plasma membrane (Couvineau et al., 1996a,b); (4) desensitization of the receptor (Marie et al., 2003); (5) association of the VPAC1 receptor with RAMP proteins (Christopoulos et al., 2002); (6) coupling to adenylyl cyclase (Couvineau et al., 2003). These studies revealed that the receptor N-ted plays a crucial role in peptide agonist binding (Laburthe et al., 2007). The structure–function relationship of VIP has been analysed in details by a complete alanine scan (Nicole et al., 2000), showing that the peptide has a diffuse pharmacophoric domain. The N-terminal 1–5 segment plays a crucial role in activation of adenylyl cyclase. Recently, we have identified a common structural motif that is encoded in all class B GPCR ligand N-terminal sequences (Neumann et al., 2008). This structural motif named N-cap was suggested to be involved in receptor activation and could serve as a template for rational design of drugs targeting VPAC receptors and more generally class B GPCRs (Neumann et al., 2008).
The physical sites of interaction between VIP and its VPAC1 receptor remained elusive until the development of photoaffinity experiments showing that the side chains of VIP in position 6, 22, 24 and 28 are in direct contact with different amino acids of the receptor N-ted, for example Asp107, Gly116, Cys122 and Lys127 (Figure 3) respectively (Tan et al., 2003; 2004; 2006; Ceraudo et al., 2008). Elucidation of VIP structure by NMR revealed that most of the 28 amino acid sequence has an α-helical structure (sequence 7–28) with the exception of the N-terminal 1–5 sequence, which has no defined structure in solution when unbound to the receptor (Figure 3). The development of a structural model of the VPAC1 receptor N-ted has made it possible to localize the binding site of VIP. The N-ted structure contains two anti-parallel β sheets and is stabilized by three disulphide bonds between residues Cys50 and Cys72, Cys63 and Cys105 and Cys86 and Cys122, and by a putative salt bridge involving Asp68-Arg103, sandwiched between the aromatic rings of Trp73 and Trp110 (Figure 3). The NMR structure of VIP has been docked in the VPAC1 receptor N-ted, giving rise to a valid model in which the N-ted nicely accommodates the VIP molecule, at least for the 6–28 sequence (Figure 3). This model has been submitted to molecular dynamic simulations over 14 ns in a box of water and appears to be highly stable (Ceraudo et al., 2008). As discussed above, the structure of the VIP N-terminal segment (1–5) is disordered in solution (Tan et al., 2004), and the VPAC1 receptor domain involved in the recognition of VIP N-terminus is still unknown. To address this issue, we have recently developed a new VIP photoaffinity probe in position 0 (Bpa°-VIP). Photoaffinity labelling experiment and peptide mapping analysis using this probe reveal that the VIP N-terminus physically interacts with N-ted corresponding to the receptor domain connecting the N-ted and the first transmembrane helix (Figure 3). This finding is in good agreement with a speculative, but largely accepted, mechanism for peptide–ligand interaction with class B GPCRs, which is referred to a ‘two-domain’ model (Hoare, 2005). In this model, the central and C-terminal parts of the peptide are trapped by the N-ted, which exposes the N-terminus of the peptide ligand in an appropriate orientation for interaction with the transmembrane region of the receptor (Hoare, 2005). It is clear that the N-capping signature in the VIP N-terminus may contribute to activation of adenylyl cyclase by peptide His1 into the receptor (Neumann et al., 2008).
Figure 3.
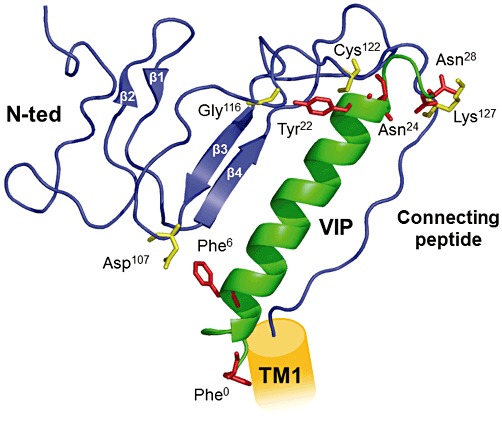
Structural model of VPAC1 receptor N-ted and docking of VIP. The figure shows a ribbon representation of receptor N-terminal ectodomain (N-ted; sequence 44–137) and docking of VIP. VPAC1 receptor N-ted: blue, main chain; VIP is shown in green. Photoaffinity experiments showed that Asp107, Gly116, Cys122, Lys127 (yellow) in the N-ted are in contact with the side chains of Phe6, Tyr22, Asn24, Asn28 and Phe° (see Laburthe et al., 2007 for details) in VIP (red) respectively. The connecting peptide is an 8 amino acid sequence connecting the receptor N-ted to the first transmembrane domain (TM1) of the receptor. The structure of the connecting peptide is currently unknown.
Structures of different recombinant N-teds of class B GPCRs such as gastric inhibitory polypeptide receptor (GIPR), parathyroid hormone receptor (PTHR), corticotropin-releasing hormone receptor 1 and 2 (CRF1R and CRF2R, respectively), glucagon-like peptide-1 receptor (GLP-1R) and pituitary adenylate cyclase-activating peptide receptor (PAC1R) have been obtained recently by X-ray crystallography or NMR spectroscopy (Parthier et al., 2009). These studies seem to indicate the existence of two different binding sites for ligands in N-teds of class B receptor (Couvineau et al., 2010). Analysis of these structure and/or molecular models revealed that N-teds of GIPR, PTHR, CRF1R, CRF2R and GLP-1R interact with ligands in regions encompassing the loop located between β1 and β2 sheets and the loop located between β3 and β4 sheets (Parthier et al., 2009). In contrast, the N-teds of PAC1R and VPAC1R bind peptides along β3 and β4 sheets of the sushi domain (Couvineau et al., 2010). However, a recent report based on the X-ray crystallography analysis of PAC1 receptor N-ted and the docking of PACAP indicates that PACAP could interact with its receptor as GIPR, PTHR, CRF1R, CRF2R and GLP-1R (Kumar et al., 2011). The real significance of these differences were unclear but may be tentatively related to the following interpretations: (1) Some structural determinations were carried-out in presence of ligands that have a low affinity (micromolar range) for the recombinant N-ted, whereas in other studies, ligand affinity is higher. It could be hypothesized that low and high affinity binding occur at different sites in the N-ted structure. (2) The determination of interaction between N-teds and ligands was mainly obtained in the presence of antagonist, but it some cases in the presence of an agonist. It could be hypothesized that agonists and antagonists bind to different domains in the N-teds. (3) Finally, we cannot exclude the possibility that ligands can bind by two different ways to N-ted of class B GPCR.
VPAC1 receptors and accessory proteins
VPAC receptors are able to increase intracellular concentration of cAMP by coupling to adenylyl cyclase through a Gs-protein, whereas some groups have reported the ability of VIP to increase calcium levels in different cells (Dickson and Finlayson, 2009). Besides their coupling with G-proteins, GPCRs can also interact with many non-G-proteins, which have been named ‘accessory proteins’, ‘receptor-interacting proteins’ or ‘GPCR interacting proteins (GIPs)’ (Bockaert et al., 2004). During the nineties, it became clear that GPCRs are also able to interact with non-G-proteins and to transduce signals independently of the G-proteins. A new concept of GPCR signalling was born, and various accessory proteins were found. A large new family of accessory proteins has been discovered displaying many biological functions such as modulation of receptor signalling, receptor targeting, receptor trafficking or compartmentalization, and even modulation of the pharmacological profile of receptors. Recruitment of accessory proteins by GPCRs is mainly ensured by specific sequence motifs in GPCRs, which cause recognition by accessory proteins (Bockaert et al., 2004). However, some accessory proteins interact with GPCRs through highly degenerate sequences or as yet unidentified motifs. The nature of accessory proteins is diverse. Indeed, the ‘accessory protein’ group encompasses various families of proteins (Brady and Limbird, 2002) such as receptor-activity-modifying proteins (RAMPs), PDZ (acronym of ‘PSD-95, Disc-large and ZO-1’) domain-containing proteins, cytoskeletal proteins, chaperone molecules or kinases (in particular the ‘kinase anchoring proteins (AKAPs)’).
As mentioned above, VPAC1 but not VPAC2 is able to interact with RAMPs. RAMPs are a family of three sub-types (RAMP 1, 2 and 3) of single transmembrane proteins that heterodimerize with GPCRs (Sexton et al., 2009). RAMPs are involved in regulation of glycosylation and trafficking of receptors and may drive the pharmacological profile of some GPCRs. The first observation indicating that RAMPs are able to modulate the cellular localization and the function of GPCRs was obtained from pharmacological studies of calcitonin-like receptor (CLR) where RAMPs are able to modify the pharmacological profile of CLR (Sexton et al., 2009). Overexpression of VPAC1 receptor and RAMPs in CHO cells induces the translocation of RAMP1, 2 and 3 to the plasma membrane, suggesting that VPAC1 receptor physically interact with RAMPs (Christopoulos et al., 2002). VPAC1 receptors are mainly coupled to the activation of adenylyl cyclase (Laburthe et al., 2007). However, in the presence of RAMP2, VPAC1 is able to induce a selective increase of phosphoinositide hydrolysis without altering cAMP production (Christopoulos et al., 2002). It should be noted that the functional role, if any, of VPAC1/RAMP1 and VPAC1/RAMP3 complexes remains unclear.
Sequence analysis of VPAC1 and VPAC2 receptors indicates the presence of a class I PDZ domain consensus sequence in the last residues (Figure 4) of the receptor C-tail (SLV for VPAC1 and SVI for VPAC2 receptor). PDZ domains are repeated sequences and represent ubiquitous protein–protein interaction domains comprising about 70 to 90 residues (Kurakin et al., 2007). These PDZ domains are present in multiple copies within proteins. Interaction of PDZ domains with receptors involves the three to four last residues of the C-terminal tails. Recognition consensus sequences are classed in two groups, that is class I PDZ (E/D-S/T-x-L/V/I) and class II PDZ (Φ-x- Φ), where Φ is any hydrophobic residue. About 400 different PDZ domains could be present in humans or mice (Beuming et al., 2005). Recently, a yeast two-hybrid assay using the carboxy terminus of VPAC1 has revealed that the PDZ domain of S-SCAM (synaptic scaffolding molecule), also named membrane-associated guanylate kinase inverted-2 (MAGI-2), is able to bind to the VPAC1 receptor C-tail (Gee et al., 2009). S-SCAM/MAGI-2 protein belongs to the membrane-associated guanylate kinase (MAGUKs) family of proteins present in junctional area where they are involved in attachment of adhesion molecules, receptors and intracellular signalling enzymes (Sheng and Sala, 2001). The guanylate kinase domain of mammalian MAGUKs is catalytically inactive but presents a Scr homology-3 (SH3) domain and a PDZ domain involved in protein–-protein interactions (Sheng and Sala, 2001). S-CAM recruits VPAC1 receptor to the junctional area near the apical end of lateral membranes of the colonic cancer cell line T84 (Gee et al., 2009). This recruitment results in inhibition of cAMP production induced by VIP, an inhibition of agonist-induced internalization of VPAC1 and a decrease of the VPAC1-mediated current through the cystic fibrosis transmembrane conductance regulator (CFTR) in Xenopus oocytes (Gee et al., 2009).
Figure 4.
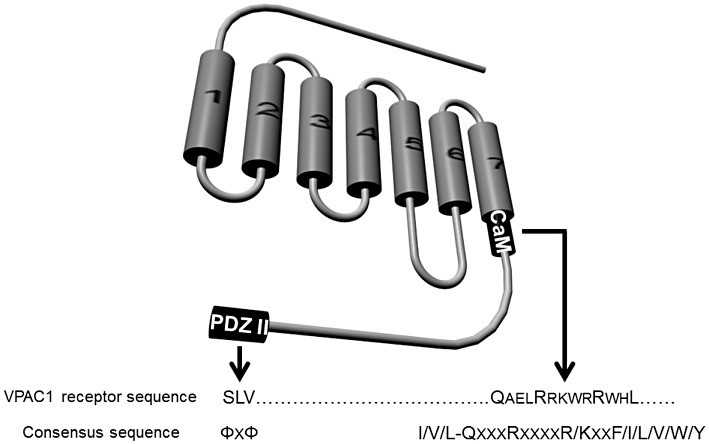
Schematic representation of the localization of interacting sites of accessory proteins in C-terminal tail of the VPAC1 receptor. PDZ II, class II PDZ (acronym of ‘PSD-95, Disc-large and ZO-1’) site; CaM, calmodulin site; Φ, represents a hydrophobic residue.
Besides the role of RAMPs and/or S-SCAM interaction with VPAC1 receptors, one report describes the possible interaction between calmodulin and this receptor (Mahon and Shimada, 2005). Calmodulin (CaM) is a ubiquitous calcium-sensing protein that regulates many intracellular proteins such as cytoskeletal elements, ion channels, kinases or phosphatases and various enzymes involved in GPCR signalling such as adenylyl cyclase, phosphodiesterase and phospholipases. CaM interacts with receptors through a degenerate motif (I/V/L-QxxxRxxxxR/Kxx-F/I/L/V/W/Y) in which hydrophobic and basic residues are crucial. An approach based on GST pull-down assay using GST-CaM, and recombinant C-tails of VPAC1 receptors (Figure 4) demonstrated a robust interaction between CaM and VPAC1 receptors (Mahon and Shimada, 2005). The functional role of this interaction still remains to be elucidated.
VIP, a promising therapeutic agent
A few years ago, VIP was identified as a potential therapeutic agent for various diseases including asthma (Groneberg et al., 2001), sexual impotence (Fahrenkrug et al., 1989), brain strokes (Dogrukol-Ak et al., 2004), chronic inflammation (Delgado et al., 2004), neuro-inflammation (Dejda et al., 2005), septic shock (Delgado et al., 2004) and cancers (Moody and Gozes, 2007). From these important physiopathological processes, anti-inflammatory and neuroprotective actions of VIP represent two major promising therapeutic uses of the peptide.
VIP appears to be a very potent anti-inflammatory peptide in animal models of various chronic inflammatory diseases (Table 3). This effect is mediated by modulation of T-helper balance by suppressing Th1 immune responses (Delgado et al., 2004). VIP inhibits leucocyte activation and migration, decreases NF-κB activation and expression of pro-inflammatory cytokines and chemokines (Gomariz et al., 2001). Although anti-inflammatory properties of VIP have been extensively reported in the literature (Delgado et al., 2004), new data using VIP-KO or VPAC1-KO indicate that VIP can also exert pro-inflammatory actions (Abad et al., 2010; Yadav et al., 2011). A very recent report reveals that VIP-deficient mice are resistant to the development of encephalomyelitis (EAE), indicating that in these conditions VIP plays unexpected permissive and/or pro-inflammatory actions (Abad et al., 2010). In the same way, VPAC1-KO mice are partially protected from DSS-induced colitis (Yadav et al., 2011). Clearly, a short-term administration of VIP ameliorates the clinical symptoms of chronic inflammation in animal models (Delgado et al., 2004), but conversely, it seems that genetic loss of VIP or VPAC1 receptor in mice result in a pro-inflammatory response. These recent results show that targeting specific VPAC receptors with agonist and/or antagonist could be considered in human therapy (Abad et al., 2010; Yadav et al., 2011). In spite of these recent findings, it is usually proposed that short-term administration of VIP and other VPAC receptor agonists may be beneficial in inflammatory disorders characterized by macrophage activation and Th1/Th2 misbalanced response.
Table 3.
Anti-inflammatory effects of short-term administration of VIP in animal models
| Disease | Organ | Animal model | Inductor |
|---|---|---|---|
| Lung Inflammation | Lung | Rat | carrageenan |
| Crohn disease | Intestine | Rat | TNBS, DSS |
| Rheumatoid arthritis | Joints | Rat | Collagen |
| Septic shock | Blood | Rat | LPS |
| Encephalomyelitis | Brain | Rat | MOG |
| Hepatitis | Liver | Mouse | Con-A |
Con-A, concanavalin A; DSS, dextran sulphate sodium; MOG, myelin oligodendrocyte glycoprotein; TNBS, 2,4,6-trinitrobenzene sulphonic acid.
A large body of study has associated VIP with neuroprotection. In the mid-eighties, a first report demonstrated that this peptide was able to prevent neuronal death associated with electrical blockade induced by the addition of tetrodotoxin (TTX) to primary spinal cord cultures (Brenneman and Eiden, 1986). Further studies have demonstrated that VIP plays a neuroprotective effects in various neurodegenerative diseases developed in animal models including Alzheimer's disease (Gozes et al., 1996), Parkinson's disease or encephalomyelitis (Gonzalez-Rey et al., 2005; 2006). These neuroprotective actions of VIP were associated with glial cells possessing VPAC receptors. Clearly, VIP induced from glial cells the secretion of various trophic molecules having neuroprotective properties (Dejda et al., 2005) such as IL-1, IL-6, protease nexin-1, the chemokine RANTES (Regulated upon Activation, Normal T-cell Expressed, and Secreted) and MIP (Macrophage Inflammatory Proteins). Moreover, VIP inhibits the production of pro-inflammatory cytokines such as TNF-α and/or IL-1β secreted by activated microglia, which is involved in neuro-inflammation observed in Parkinson's disease (Delgado and Ganea, 2003). VIP also induces neuroprotective effects by increasing the secretion of ADNF (activity-dependent neurotrophic factor) and/or by increasing the concentration of ADNP mRNA (activity-dependent neurotrophic protein) (Brenneman and Gozes, 1996; Gozes et al., 2000). These two protective proteins that belong to the heat shock protein family are able to prevent the neuronal death (Brenneman and Gozes, 1996) and represent some of most potent neuroprotective agents secreted by astroglia in response to VIP. Although the major neuroprotective effects of VIP can be explained by activation of adenylyl cyclase through VPAC receptors (Brenneman, 2007), some reports indicate that VIP-mediated effects on protection did not involve cAMP but rather a mobilization of intracellular calcium in astrocytes (Brenneman, 2007). Recently, it has been suggested that the VPAC2 receptor could be a potential target for the development of antipsychotic drugs related to duplications of VPAC2 receptor gene in schizophrenia (Vacic et al., 2011). However, a major drawback with the use of VIP in therapy is its high sensitivity to protease degradation. Indeed, removing of the first His1-Ser2 residues by peptidases, such as DPPIV (dipeptidyl peptidase IV), induces a drastic loss of affinity (Gourlet et al., 1997a,b). To circumvent this problem, VIP could be modified to increase its resistance to degradation by N-acylation of the peptide N-terminal end or by substitution of residues involved in proteolytic consensus sequences (dibasic doublets). Recent data indicated that N-terminal modifications of PACAP confer resistance to DPPIV (Bourgault et al., 2008). In the same way, acetylation of N-terminal end of VIP increases its stability in the presence of human serum (personal data). Other strategies have been developed to protect peptide against degradation by insertion of VIP into micelles or nanoparticles (Fernandez-Montesinos et al., 2009; Onyüksel and Mohanty, 2009). A second major obstacle that reduces the therapeutic use of VIP in humans is its ability to interact at high affinity with different receptors such as VPAC1 and VPAC2 subtypes but also, with lower affinity, with other class B GPCRs such as PACAP receptor, secretin receptor and/or GRF receptor (Laburthe et al., 2007). These cross-interactions may be responsible for the existence of strong side effects induced by VIP in humans including hypotension and diarrhoea (Laburthe et al., 2007). In this context, the development of specific ligands for VPAC1 and VPAC2 receptors with no affinity with other class B GPCRs is clearly crucial. It should be noted that only one non-peptide antagonist having low affinity for VPAC2 receptor is yet available (Chu et al., 2010). There is an abundant literature regarding the pharmacology of these receptors (Robberecht and Waelbroeck, 1998); moreover, drastic differences between species of VPAC receptor pharmacology have been described (Laburthe et al., 2002). Analysis of VIP structure–function relationships by a complete alanine scan (Nicole et al., 2000) allowed us to rationally design the most potent and specific peptide agonist for VPAC1 receptor currently available, that is [Ala11,22,28]-VIP (Nicole et al., 2000). This VIP derivative has 1000 times higher affinity for the VPAC1 receptor, which is mainly involved in anti-inflammatory action of VIP (Delgado et al., 2004), than for the VPAC2 receptor (Nicole et al., 2000). A selective high-affinity antagonist of the VPAC1 receptor, that is [Ac-His1,D-Phe2,K15, R16, L27]VIP(3–7)/GRF(8–27) named PG 97–269, was characterized (Gourlet et al., 1997a,b). The amino acid sequence of this antagonist has many similarities and identities with the sequence of VIP. However, one of the major differences is the presence of a d-phenylalanine in position 2 instead of a serine. This substitution probably plays an important role in antagonist properties of PG 97–269. Indeed, the presence of D-Phe, a hydrophobic residue, results in a perturbation in the formation of N-cap motif (Neumann et al., 2008) involving residues 2–6. Regarding the VPAC2 receptor, the cyclic peptide analogue of VIP [Ac-Glu8,OCH3-Tyr10,Lys12,Nle17,Ala19,Asp25,Leu26,Lys27,28-VIP(cyclo 21–25)] or Ro 25–1392 is a potent and selective agonist (Xia et al., 1997). In our opinion, there is still no satisfactory VPAC2 receptor antagonist since PG 99–465, a VIP analogue that antagonizes VIP action on VPAC2 receptor, has significant agonist activity on human VPAC1 receptor (Moreno et al., 2000).
Conclusions
The VPAC receptors, in particular VPAC1, are very promising targets for the development of therapeutic molecules. While new peptide derivatives specifically targeting VPAC receptor subtypes are now available, however, their very short half-life and the inconvenience related to their administration routes make them difficult to use in human therapy. It is to be hoped that recent advances of our knowledge of the structure of VPAC receptor site and more generally of class B GPCR binding sites will lead shortly to the design of non-peptide receptor agonists and/or antagonists. Such molecules would be of considerable interest in the therapy of many human diseases in particular inflammatory and neurodegenerative diseases.
Acknowledgments
This work was supported by Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique and the Université Paris 7.
Glossary
- Bpa
benzophenone
- KO
knock-out
- N-ted
N-terminal ectodomain
- TM
transmembrane domain
- VPAC
vasoactive Intestinal Peptide receptor
- VPAC1
vasoactive intestinal peptide receptor 1
- VPAC2
vasoactive intestinal peptide receptor 2
Conflicts of interest
None.
References
- Abad C, Gomariz RP, Waschek JA. Neuropeptide mimetics and antagonists in the treatment of inflammatory disease: focus on VIP and PACAP. Curr Top Med Chem. 2006;6:151–163. doi: 10.2174/156802606775270288. [DOI] [PubMed] [Google Scholar]
- Abad C, Tan YV, Lopez R, Nobuta H, Dong H, Phan P, et al. Vasoactive intestinal peptide loss leads to impaired CNS parenchymal T-cell infiltration and resistance to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2010;107:19555–19560. doi: 10.1073/pnas.1007622107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuming T, Skrabanek L, Niv MY, Mukherjee P, Weinstein H. PDZBase: a protein-protein interaction database for PDZ-domains. Bioinformatics. 2005;21:827–828. doi: 10.1093/bioinformatics/bti098. [DOI] [PubMed] [Google Scholar]
- Bockaert J, Fagni L, Dumuis A, Marin P. GPCR interacting proteins (GIP) Pharmacol Ther. 2004;103:203–221. doi: 10.1016/j.pharmthera.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Bourgault S, Vaudry D, Botia B, Couvineau A, Laburthe M, Vaudry H, et al. Novel stable PACAP analogs with potent activity towards the PAC1 receptor. Peptides. 2008;29:919–932. doi: 10.1016/j.peptides.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Brady AE, Limbird LE. G protein-coupled receptor interacting proteins: emerging role in localization and signal transduction. Cell Signal. 2002;14:297–309. doi: 10.1016/s0898-6568(01)00239-x. [DOI] [PubMed] [Google Scholar]
- Brenneman DE. Neuroprotection: a comparative view of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Peptides. 2007;28:1720–1726. doi: 10.1016/j.peptides.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Brenneman DE, Eiden LE. Vasoactive intestinal peptide and electrical activity influence neuronal survival. Proc Natl Acad Sci USA. 1986;83:1159–1162. doi: 10.1073/pnas.83.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenneman DE, Gozes I. A femtomolar-acting neuroprotective peptide. J Clin Invest. 1996;97:2299–2307. doi: 10.1172/JCI118672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker PL. Incretin-based therapies: mimetics versus protease inhibitors. Trends Endocrinol Metab. 2007;18:240–245. doi: 10.1016/j.tem.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Campbell RM, Bongers J, Felix AM. Rational design, synthesis, and biological evaluation of novel growth hormone releasing factor analogues. Biopolymers. 1995;37:67–88. doi: 10.1002/bip.360370204. [DOI] [PubMed] [Google Scholar]
- Ceraudo E, Murail S, Tan YV, Lacapère JJ, Neumann JM, Couvineau A, et al. The vasoactive intestinal peptide (VIP) alpha-Helix up to C terminus interacts with the N-terminal ectodomain of the human VIP/Pituitary adenylate cyclase-activating peptide 1 receptor: photoaffinity, molecular modeling, and dynamics. Mol Endocrinol. 2008;22:147–155. doi: 10.1210/me.2007-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos A, Christopoulos G, Morfis M, Adawela M, Laburthe M, Couvineau A, et al. Novel receptor partners and function of receptor activity-modifying proteins. J Biol Chem. 2002;278:3293–3297. doi: 10.1074/jbc.C200629200. [DOI] [PubMed] [Google Scholar]
- Chu A, Caldwell JS, Chen YA. Identification and characterization of a small molecule antagonist of human VPAC(2) receptor. Mol Pharmacol. 2010;77:95–101. doi: 10.1124/mol.109.060137. [DOI] [PubMed] [Google Scholar]
- Couvineau A, Rouyer-Fessard C, Darmoul D, Maoret JJ, Carrero I, Ogier-Denis E, et al. Human intestinal VIP receptor: cloning and functional expression of two cDNA encoding proteins with different N-terminal domains. Biochem Biophys Res Commun. 1994;200:769–776. doi: 10.1006/bbrc.1994.1517. [DOI] [PubMed] [Google Scholar]
- Couvineau A, Gaudin P, Maoret JJ, Rouyer-Fessard C, Nicole P, Laburthe M. Highly conserved aspartate 68, tryptophane 73 and glycine 109 in the N-terminal extracellular domain of the human VIP receptor are essential for its ability to bind VIP. Biochem Biophys Res Commun. 1995;206:246–252. doi: 10.1006/bbrc.1995.1034. [DOI] [PubMed] [Google Scholar]
- Couvineau A, Fabre C, Gaudin P, Maoret JJ, Laburthe M. Mutagenesis of N-glycosylation sites in the human vasoactive intestinal peptide 1 receptor. Evidence that asparagine 58 or 69 is crucial for correct delivery of the receptor to plasma membrane. Biochemistry. 1996a;35:1745–1752. doi: 10.1021/bi952022h. [DOI] [PubMed] [Google Scholar]
- Couvineau A, Rouyer-Fessard C, Maoret JJ, Gaudin P, Nicole P, Laburthe M. Vasoactive intestinal peptide (VIP)1 receptor. Three nonadjacent amino acids are responsible for species selectivity with respect to recognition of peptide histidine isoleucineamide. J Biol Chem. 1996b;271:12795–12800. doi: 10.1074/jbc.271.22.12795. [DOI] [PubMed] [Google Scholar]
- Couvineau A, Lacapère JJ, Tan YV, Rouyer-Fessard C, Nicole P, Laburthe M. Identification of cytoplasmic domains of hVPAC1 receptor required for activation of adenylyl cyclase. J Biol Chem. 2003;278:24759–24766. doi: 10.1074/jbc.M301916200. [DOI] [PubMed] [Google Scholar]
- Couvineau A, Ceraudo E, Tan YV, Laburthe M. VPAC1 receptor binding site: contribution of photoaffinity labeling approach. Neuropeptides. 2010;44:127–132. doi: 10.1016/j.npep.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Dejda A, Sokolowska P, Nowak JZ. Neuroprotective potential of three neuropeptides PACAP, VIP and PHI. Pharmacol Rep. 2005;57:307–320. [PubMed] [Google Scholar]
- Delgado M, Ganea D. Neuroprotective effect of vasoactive intestinal peptide(VIP) in a mouse model of Parkinson's disease by blocking microglial activation. FASEB J. 2003;17:944–946. doi: 10.1096/fj.02-0799fje. [DOI] [PubMed] [Google Scholar]
- Delgado M, Abad C, Martinez C, Leceta J, Gomariz RP. Vasoactive intestinal peptide prevents experimental arthritis by downregulating both autoimmune and inflammatory components of the disease. Nat Med. 2001;7:563–568. doi: 10.1038/87887. [DOI] [PubMed] [Google Scholar]
- Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev. 2004;56:249–290. doi: 10.1124/pr.56.2.7. [DOI] [PubMed] [Google Scholar]
- Dickson L, Finlayson K. VPAC and PAC receptors: from ligands to function. Pharmacol Ther. 2009;121:294–316. doi: 10.1016/j.pharmthera.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Dogrukol-Ak D, Tore F, Tuncel N. Passage of VIP/PACAP/secretin family across the blood-brain barrier: therapeutic effects. Curr Pharm Des. 2004;10:1325–1340. doi: 10.2174/1381612043384934. [DOI] [PubMed] [Google Scholar]
- Du K, Couvineau A, Rouyer-Fessard C, Nicole P, Laburthe M. Human VPAC1 receptor selectivity filter. Identification of a critical domain for restricting secretin binding. J Biol Chem. 2002;277:37016–37022. doi: 10.1074/jbc.M203049200. [DOI] [PubMed] [Google Scholar]
- Epstein S. Is cortical bone hip? What determines cortical bone properties? Bone. 2007;41:S3–S8. doi: 10.1016/j.bone.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J, Palle C, Jørgensen J, Ottesen B. Regulatory peptides in the mammalian urogenital system. Experientia Suppl. 1989;56:362–381. doi: 10.1007/978-3-0348-9136-3_19. [DOI] [PubMed] [Google Scholar]
- Fernandez-Montesinos R, Castillo PM, Klippstein R, Gonzalez-Rey E, Mejias JA, Zaderenko AP, et al. Chemical synthesis and characterization of silver-protected vasoactive intestinal peptide nanoparticles. Nanomedicine (Lond) 2009;4:919–930. doi: 10.2217/nnm.09.79. [DOI] [PubMed] [Google Scholar]
- Fredrikson R, Schiöth B. G Protein-coupled receptors in human genome. In: Rognan D, editor. Ligand Design for G Protein-Coupled Receptors. Vol. 30. Weinheim: Wiley-VCH; 2006. pp. 1–27. [Google Scholar]
- Gee HY, Kim YW, Jo MJ, Namkung W, Kim JY, Park HW, et al. Synaptic scaffolding molecule binds to and regulates vasoactive intestinal polypeptide type-1 receptor in epithelial cells. Gastroenterology. 2009;137:607–617. doi: 10.1053/j.gastro.2009.01.065. [DOI] [PubMed] [Google Scholar]
- Gilligan PJ, Li YW. Corticotropin-releasing factor antagonists: recent advances and exciting prospects for the treatment of human diseases. Curr Opin Drug Discov Devel. 2004;7:487–497. [PubMed] [Google Scholar]
- Gomariz RP, Martinez C, Abad C, Leceta J, Delgado M. Immunology of VIP: a review and therapeutical perspectives. Curr Pharm Des. 2001;7:89–111. doi: 10.2174/1381612013398374. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Chorny A, Fernandez-Martin A, Varela N, Delgado M. Vasoactive intestinal peptide family as a therapeutic target for Parkinson's disease. Expert Opin Ther Targets. 2005;9:923–929. doi: 10.1517/14728222.9.5.923. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rey E, Fernandez-Martin A, Chorny A, Martin J, Pozo D, Ganea D, et al. Therapeutic effect of vasoactive intestinal peptide on experimental autoimmune encephalomyelitis: down-regulation of inflammatory and autoimmune responses. Am J Pathol. 2006;168:1179–1188. doi: 10.2353/ajpath.2006.051081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlet P, De Neef P, Cnudde J, Waelbroeck M, Robberecht P. In vitro properties of a high affinity selective antagonist of the VIP1 receptor. Peptides. 1997a;18:1555–1560. doi: 10.1016/s0196-9781(97)00230-1. [DOI] [PubMed] [Google Scholar]
- Gourlet P, Vandermeers A, Robberecht P, Deschodt-Lanckman M. Vasoactive intestinal peptide (VIP) and pituitary adenylate cyclase-activating peptide (PACAP-27, but not PACAP-38) degradation by the neutral endopeptidase EC 3.4.24.11. Biochem Pharmacol. 1997b;54:509–515. doi: 10.1016/s0006-2952(97)00207-4. [DOI] [PubMed] [Google Scholar]
- Gozes I, Bardea A, Reshef A, Zamostiano R, Zhukovsky S, Rubinraut S, et al. Neuroprotective strategy for Alzheimer disease: intranasal administration of a fatty neuropeptide. Proc Natl Acad Sci USA. 1996;93:427–432. doi: 10.1073/pnas.93.1.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I, Zamostiano R, Pinhasov A, Bassan M, Giladi E, Steingart RA, et al. A novel VIP responsive gene. Activity dependent neuroprotective protein. Ann N Y Acad Sci. 2000;921:115–118. doi: 10.1111/j.1749-6632.2000.tb06957.x. [DOI] [PubMed] [Google Scholar]
- Gozes I, Divinsky I, Pilzer I, Fridkin M, Brenneman DE, Spier AD. From vasoactive intestinal peptide (VIP) through activity-dependent neuroprotective protein (ADNP) to NAP: a view of neuroprotection and cell division. J Mol Neurosci. 2003;20:315–322. doi: 10.1385/JMN:20:3:315. [DOI] [PubMed] [Google Scholar]
- Groneberg DA, Springer J, Fischer A. Vasoactive intestinal polypeptide as mediator of asthma. Pulm Pharmacol Ther. 2001;14:391–401. doi: 10.1006/pupt.2001.0306. [DOI] [PubMed] [Google Scholar]
- Harikumar KG, Morfis MM, Lisenbee CS, Sexton PM, Miller LJ. Constitutive formation of oligomeric complexes between family B G protein-coupled vasoactive intestinal polypeptide and secretin receptors. Mol Pharmacol. 2006;69:363–373. doi: 10.1124/mol.105.015776. [DOI] [PubMed] [Google Scholar]
- Hoare SRJ. Mechanism of peptide and nonpeptide ligand binding to class B G-protein-coupled receptors. Drug Discov Today. 2005;10:417–427. doi: 10.1016/S1359-6446(05)03370-2. [DOI] [PubMed] [Google Scholar]
- Inzucchi SE, McGuire DK. New drugs for the treatment of diabetes: part II: incretin-based therapy and beyond. Circulation. 2008;117:574–584. doi: 10.1161/CIRCULATIONAHA.107.735795. [DOI] [PubMed] [Google Scholar]
- Jeppesen PB. Glucagon-like peptide-2: update of the recent clinical trials. Gastroenterology. 2006;130:S127–S131. doi: 10.1053/j.gastro.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Kumar S, Pioszak A, Zhang C, Swaminathan K, Xu HE. Crystal Structure of the PAC1R Extracellular Domain Unifies a Consensus Fold for Hormone Recognition by Class B G-Protein Coupled Receptors. PLoS ONE. 2011;6:e19682. doi: 10.1371/journal.pone.0019682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurakin A, Swistowski A, Wu SC, Bredesen DE. The PDZ domain as a complex adaptive system. PLoS ONE. 2007;2:e953. doi: 10.1371/journal.pone.0000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laburthe M, Couvineau A, Marie JC. VPAC receptors for VIP and PACAP. Receptors Channels. 2002;8:137–153. [PubMed] [Google Scholar]
- Laburthe M, Couvineau A, Tan V. Class II G protein-coupled receptors for VIP and PACAP: structure, models of activation and pharmacology. Peptides. 2007;28:1631–1639. doi: 10.1016/j.peptides.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Lutz EM, Sheward WJ, West KM, Morrow JA, Fink G, Harmar AJ. The VIP2 receptor: molecular characterisation of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett. 1993;334:3–8. doi: 10.1016/0014-5793(93)81668-p. [DOI] [PubMed] [Google Scholar]
- Mahon MJ, Shimada M. Calmodulin interacts with the cytoplasmic tails of the parathyroid hormone 1 receptor and a sub-set of class b G-protein coupled receptors. FEBS Lett. 2005;579:803–807. doi: 10.1016/j.febslet.2004.12.056. [DOI] [PubMed] [Google Scholar]
- Marie JC, Rouyer-Fessard C, Couvineau A, Nicole P, Devaud H, El Benna J, et al. Serine 447 in the carboxyl tail of human VPAC1 receptor is crucial for agonist-induced desensitization but not internalization of the receptor. Mol Pharmacol. 2003;64:1565–1574. doi: 10.1124/mol.64.6.1565. [DOI] [PubMed] [Google Scholar]
- Moody TW, Gozes I. Vasoactive intestinal peptide receptors: a molecular target in breast and lung cancer. Curr Pharm Des. 2007;13:1099–1104. doi: 10.2174/138161207780619000. [DOI] [PubMed] [Google Scholar]
- Moreno D, Gourlet P, De Neef P, Cnudde J, Waelbroeck M, Robberecht P. Development of selective agonists and antagonists for the human vasoactive intestinal peptide VPAC2 receptor. Peptides. 2000;21:1543–1549. doi: 10.1016/s0196-9781(00)00309-0. [DOI] [PubMed] [Google Scholar]
- Mulder JE, Kolatkar NS, LeBoff MS. Drug insight: existing and emerging therapies for osteoporosis. Nat Clin Pract Endocrinol Metab. 2006;2:670–680. doi: 10.1038/ncpendmet0325. [DOI] [PubMed] [Google Scholar]
- Neumann JM, Couvineau A, Murail S, Lacapère JJ, Jamin N, Laburthe M. Class-B GPCR activation: is ligand helix-capping the key? Trends Biochem Sci. 2008;33:314–319. doi: 10.1016/j.tibs.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Nicole P, Lins L, Rouyer-Fessard C, Drouot C, Fulcrand P, Thomas A, et al. Identification of key residues for interaction of vasoactive intestinal peptide with human VPAC1 and VPAC2 receptors and development of a highly selective VPAC1 receptor agonist. J Biol Chem. 2000;275:24003–24012. doi: 10.1074/jbc.M002325200. [DOI] [PubMed] [Google Scholar]
- Onyüksel H, Mohanty PS. Rubinstein I. VIP-grafted sterically stabilized phospholipid nanomicellar 17-allylamino-17-demethoxy geldanamycin: a novel targeted nanomedicine for breast cancer. Int J Pharm. 2009;365:157–161. doi: 10.1016/j.ijpharm.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthier C, Reedtz-Runge S, Rudolph R, Stubbs MT. Passing the baton in class B GPCRs: peptide hormone activation via helix induction? Trends Biochem Sci. 2009;34:303–310. doi: 10.1016/j.tibs.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Robberecht P, Waelbroeck M. A critical view of the method for characterization of the VIP/PACAP receptor subclasses. Ann N Y Acad Sci. 1998;865:157–163. doi: 10.1111/j.1749-6632.1998.tb11174.x. [DOI] [PubMed] [Google Scholar]
- Said SI, Mutt V. Polypeptide with broad biological activity: isolation from small intestine. Science. 1970;169:1217–1218. doi: 10.1126/science.169.3951.1217. [DOI] [PubMed] [Google Scholar]
- Sexton PM, Poyner DR, Simms J, Christopoulos A, Hay DL. Modulating receptor function through RAMPs: can they represent drug targets in themselves? Drug Discov Today. 2009;14:413–419. doi: 10.1016/j.drudis.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Sheng M, Sala C. PDZ domains and the organization of supramolecular complexes. Annu Rev Neurosci. 2001;24:1–29. doi: 10.1146/annurev.neuro.24.1.1. [DOI] [PubMed] [Google Scholar]
- Tan YV, Couvineau A, Van Rampelbergh J, Laburthe M. Photoaffinity labeling demonstrates physical contact between vasoactive intestinal peptide and the N-terminal ectodomain of the human VPAC1 receptor. J Biol Chem. 2003;278:36531–36536. doi: 10.1074/jbc.M304770200. [DOI] [PubMed] [Google Scholar]
- Tan YV, Couvineau A, Laburthe M. Diffuse pharmacophoric domains of vasoactive intestinal peptide (VIP) and further insights into the interaction of VIP with the N-terminal ectodomain of human VPAC1 receptor by photoaffinity labeling with [Bpa6]-VIP. J Biol Chem. 2004;279:38889–38894. doi: 10.1074/jbc.M404460200. [DOI] [PubMed] [Google Scholar]
- Tan YV, Couvineau A, Murail S, Ceraudo E, Neumann JM, Lacapère JJ, et al. Peptide agonist docking in the N-terminal ectodomain of a class II G protein-coupled receptor, the VPAC1 receptor. Photoaffinity, NMR, and molecular modeling. J Biol Chem. 2006;281:12792–12798. doi: 10.1074/jbc.M513305200. [DOI] [PubMed] [Google Scholar]
- Vacic V, McCarthy S, Malhotra D, Murray F, Chou HH, Peoples A, et al. Duplications of the neuropeptide receptor gene VIPR2 confer significant risk for schizophrenia. Nature. 2011;471:499–503. doi: 10.1038/nature09884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudry H, Laburthe M. VIP, PACAP, and related peptides. From gene to therapy. Ann N Y Acad Sci. 2006;1070:1–633. [PubMed] [Google Scholar]
- Xia M, Sreedharan SP, Bolin DR, Gaufo GO, Goetzl EJ. Novel cyclic peptide agonist of high potency and selectivity for the type II vasoactive intestinal peptide receptor. J Pharmacol Exp Ther. 1997;281:629–633. [PubMed] [Google Scholar]
- Yadav M, Huang MC, Goetzl EJ. VPAC1 (vasoactive intestinal peptide (VIP) receptor type 1) G protein-coupled receptor mediation of VIP enhancement of murine experimental colitis. Cell Immunol. 2011;267:124–132. doi: 10.1016/j.cellimm.2011.01.001. [DOI] [PubMed] [Google Scholar]


