Abstract
BACKGROUND AND PURPOSE
Amylin (Amy) is an important glucoregulatory peptide and AMY receptors are clinical targets for diabetes and obesity. Human (h) AMY receptor subtypes are complexes of the calcitonin (CT) receptor with receptor activity-modifying proteins (RAMPs); their rodent counterparts have not been characterized. To allow identification of the most clinically relevant receptor subtype, the elucidation of rat (r) AMY receptor pharmacology is necessary.
EXPERIMENTAL APPROACH
Receptors were transiently transfected into COS-7 cells and cAMP responses measured in response to different agonists, with or without antagonists. Competition binding experiments were performed to determine rAmy affinity.
KEY RESULTS
rCT was the most potent agonist of rCT(a) receptors, whereas rAmy was most potent at rAMY1(a) and rAMY3(a) receptors. rAmy bound to these receptors with high affinity. Rat α-calcitonin gene-related peptide (CGRP) was equipotent to rAmy at both AMY receptors. Rat adrenomedullin (AM) and rAM2/intermedin activated all three receptors but were most effective at rAMY3(a). AC187, AC413 and sCT8-32 were potent antagonists at all three receptors. rαCGRP8-37 displayed selectivity for rAMY receptors over rCT(a) receptors. rAMY8-37 was a weak antagonist but was more effective at rAMY1(a) than rAMY3(a).
CONCLUSIONS AND IMPLICATIONS
AMY receptors were generated by co-expression of rCT(a) with rRAMP1 or 3, forming rAMY1(a) and rAMY3(a) receptors, respectively. CGRP was more potent at rAMY than at hAMY receptors. No antagonist tested was able to differentiate the rAMY receptor subtypes. The data emphasize the need for and provide a useful resource for developing new CT or AMY receptor ligands as pharmacological tools or potential clinical candidates.
LINKED ARTICLES
This article is part of a themed section on Secretin Family (Class B) G Protein-Coupled Receptors. To view the other articles in this section visit http://dx.doi.org/10.1111/bph.2012.166.issue-1
Keywords: adrenomedullin, adrenomedullin 2, amylin, area postrema, calcitonin, CGRP, IAPP, intermedin, receptor activity-modifying protein, RAMP
Introduction
The calcitonin (CT) peptide family is a group of therapeutically important peptides, which includes CT, amylin (Amy), α- and β-calcitonin gene-related peptide (CGRP), adrenomedullin (AM) and AM2 (also known as intermedin). Despite sharing only limited amino acid sequence identity, key structural features are shared, including an N-terminal ring structure formed by a disulphide bond and an amidated C-terminus (Poyner et al., 2002). Amy (also known as islet amyloid polypeptide; IAPP) is a 37-amino-acid peptide hormone, which plays an important role in regulating glucose homeostasis. Following nutrient influx, Amy is co-secreted with insulin from pancreatic islet beta cells (Ogawa et al., 1990; Young, 2005a). This peptide may regulate blood glucose levels by inhibiting insulin secretion from the pancreas (Wang et al., 1993; Young, 2005b). However, the primary sites of action for Amy appear to be brain nuclei that are devoid of the blood–brain barrier, such as the area postrema (AP) (Lutz, 2009; Potes and Lutz, 2010; Trevaskis et al., 2010b). Here, Amy may act to regulate food intake by contributing to meal ending satiation (Lutz et al., 2001; Roth et al., 2012) and slow the rate by which nutrients are delivered into the circulation from the gastrointestinal tract (Clementi et al., 1996). Amy may also act as an adiposity signal and a regulator of energy expenditure (Wielinga et al., 2007; 2010; Zhang et al., 2011).
In type I and the late stages of type II diabetes, pancreatic islet beta-cell mass is substantially reduced and there is correspondingly less insulin and Amy to be released into the circulation (Ogawa et al., 1990; Makimattila et al., 2000). Logically, dual treatment with both Amy and insulin offers promise for the treatment of diabetes. Therefore, Amy replacement therapy, in the form of a synthetic human Amy peptide analogue, Pramlintide ([Pro25,28,29]-human Amy), together with insulin is approved for assisting with the control of blood glucose in these patients (Ryan et al., 2005). Further potential for Amy as a therapy is actively being explored, with recent data suggesting that combined Amy and leptin therapy may have profound effects on controlling body weight (Roth et al., 2008; Ravussin et al., 2009; Trevaskis et al., 2010b).
There appears to be substantial complexity in the Amy receptor system. Amy binds with low affinity to the CT receptor, a Gαs-coupled, family B GPCR. However, in the presence of receptor activity-modifying proteins (RAMPs), Amy affinity is substantially enhanced (Christopoulos et al., 1999; Muff et al., 1999; Leuthauser et al., 2000; Kuwasako et al., 2004; Hay et al., 2005). Accordingly, co-expression of the CT receptor with the three RAMPs yields AMY1, AMY2 and AMY3 receptors, respectively (Poyner et al., 2002). RAMPs can also interact with other receptors, such as the interaction of RAMP1 with the CT receptor-like receptor (CLR) to form the CGRP receptor. The Amy peptide and receptor (AMY) are distinguished by the use of lower- and uppercase letters, respectively. The human (h) CT receptor has two major splice variants; hCT(a) and hCT(b), with the latter containing a 16-amino-acid insert in the first intracellular loop (Egerton et al., 1995; Poyner et al., 2002). Therefore, taking into account only the two major hCT receptor splice variants and their interactions with RAMPs, there are at least six potential hAMY receptor subtypes (Tilakaratne et al., 2000; Poyner et al., 2002). The pharmacology and signalling of these receptors differ and are strongly influenced by cellular background; this effect is most evident for the hAMY2 receptor (Tilakaratne et al., 2000; Morfis et al., 2008).
Despite the clear clinical relevance of Amy, it is not known which AMY receptor subtype mediates the therapeutically beneficial actions of Amy/pramlintide and whether there are different receptors at work in the CNS and peripheral tissues. For this reason, it is important to understand the pharmacology of Amy receptors and determine which subtype may be most usefully exploited in the clinic. In particular, non-injectable subtype-specific small-molecule agonists or positive allosteric modulators would be valuable additions to the pharmaceutical armoury used to treat diabetes.
Rodent models represent major systems for the physiological investigation of hormone action and pre-clinical validation of drugs. Substantial data for Amy were obtained in such systems, leading to the development of pramlintide. However, in accordance with the clinical situation, it is unknown which receptor Amy/pramlintide acts through in rodents. Receptor antagonists could be used in rodents to define the clinically relevant AMY receptor subtype(s) to enhance drug discovery efforts. Understanding rat (r) AMY receptors is particularly relevant as rat models display higher predictive value for therapies targeting AMY receptors on weight loss in humans than mouse models (Trevaskis et al., 2010a). It has been assumed that rodent receptors for Amy are complexes of CT receptors and RAMPs, and rodent AMY receptors are named according to the human convention (Poyner et al., 2002; Alexander et al., 2011). However, this has never been experimentally tested and the only published pharmacological study of non-human CT receptor/RAMP combinations indicated that porcine (p) RAMPs did not enhance Amy affinity at pCT receptors (Kikumoto et al., 2003). Therefore, it is vital to validate this nomenclature and determine the pharmacology of defined CT receptor/RAMP combinations in other species. Furthermore, there is reliance in the literature on the ‘selective’ Amy receptor antagonist, AC187, but there are little data at cloned receptors to support this. At hAMY receptors, the discrimination of this peptide between the AMY1(a) and CT(a) receptor was less than 10-fold. Therefore, the aim of this study was to characterize the pharmacology of rCT receptor/RAMP combinations. This will provide key information regarding the pharmacological discrimination of putative AMY receptor subtypes in rodents and a key resource for the further development of Amy agonists for the treatment of diabetes and obesity.
In rats, rCT(a) is equivalent to hCT(a) (Poyner et al., 2002). We expressed rCT(a) with or without rRAMPs in COS-7 cells, which display a null phenotype for Amy and related receptor components (Hay et al., 2006; Bailey and Hay, 2006), and screened a series of species-matched peptide agonists and antagonists. As CT(a), RAMP1 and RAMP3 appear to be expressed in the rat AP, potentially a major site of Amy action, we have focussed on characterizing these combinations (Ueda et al., 2001; Becskei et al., 2004). This study has shown for the first time that the co-expression of rCT(a) with rRAMP1 or 3 produces functional AMY receptors in rats. Nevertheless, these receptors displayed distinct differences in pharmacology compared with their human counterparts. This work has important implications for the discrimination of CT, AMY and CGRP receptors in rodents and highlights the need for selective ligands for these receptors.
Methods
DNA constructs
rCT(a) was kindly provided by Prof Patrick M Sexton (Monash Institute of Pharmaceutical Sciences, Australia). rRAMP3 and rCLR were previously described (Njuki et al., 1993; Hay et al., 2003). Mouse CLR was kindly provided by Prof Walter Born (University of Zurick, Switzerland). rRAMP1 was kindly provided by Dr Christopher Salvatore (Merck Research Laboratories, PA, USA). All constructs were untagged.
Cloning of mouse RAMP3
Mouse RAMP3 (GenBank accession number AF146524) was cloned by reverse transcriptase- RT-PCR from total RNA (1 µg) extracted from mouse soleus muscle. RNA was heated at 65°C for 10 min and primed using random hexamers. Reverse transcription was performed using Expand™-RT (Roche, Auckland, New Zealand) for 10 min at 30°C, followed by 45 min at 42°C. Reaction conditions were as follows: Tris–HCl (50 mM); KCl (40 mM); MgCl2 (5 mM); Tween-20 (0.5%); dithiothreitol (10 mM); RNase inhibitor (20 U); Expand-RT (50 U) (Boehringer Mannheim, Mannheim, Germany); dNTPs (1 mM); pH 8.3. Oligonucleotide primers for RAMP3 were 5-TAGGATCCTGCATCTTAGTTGGCCATGA-3 (sense)/5-ATAGAATTCATCCAGCAGATCCTCAAGC-3 (antisense). Underlined nucleotides, respectively, indicate BamHI and EcoRI restriction sites introduced to facilitate cloning. PCR amplification was performed in an Eppendorf Mastercycler R gradient Thermal cycler for 40 cycles of 94°C (1 min), 55°C (2 min) and 72°C (3 min). Reaction conditions were: Tris/HCl (10 mM), KCl (50 mM), MgCl2 (1.1 mM), oligonucleotides (0.5 µM), gelatin (0.01% w/v), dNTP's (200 µM), 2.5 U REDTaq™ DNA polymerase (Sigma, Saint Louis, MO), cDNA template (10 ng), at pH 8.3. PCR products were isolated and subcloned into pcDNA3.1 + (Invitrogen, Auckland, New Zealand) through the BamHI and EcoRI restriction sites. Sequence analysis at the DNA sequencing facility, School of Biological Sciences, University of Auckland, confirmed the sequence.
Cell culture and transfection
Culture of Cos 7 cells was performed as previously described (Bailey and Hay, 2006). Briefly, cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 8% heat inactivated fetal bovine serum and 5% v/v penicillin/streptomycin and kept in a 37°C humidified 95% air/5% CO2 incubator. For cAMP assays, cells were plated into 96-well plates at a density of approximately 30 000 cells per well (or 10 000 for AlphaScreen), and for radioligand binding studies, cells were plated at a density of approximately 100 000 cells per well in 24-well plates. One day after plating, cells were transfected using polyethylenimine (PEI) as previously described (Bailey and Hay, 2006). Briefly, plasmid DNA (250 ng per well of a 96-well plate or 1 µg per well of a 24-well plate) at a 1:1 ratio of RAMP or vector to receptor DNA was incubated with PEI, in 5% glucose for approximately 10 min before being added to complete growth medium and then to the wells. Cells were used for experimentation 36–48 h later.
cAMP assay
Most cAMP assays were performed as described previously (Bailey and Hay, 2006). Briefly, transfected cells were serum-deprived in DMEM containing 1 mM IBMX and 0.1% BSA for 30 min. Agonists were then either added alone, or antagonists were added first followed immediately by agonists and incubated at 37°C for 15 min. cAMP was extracted with ice-cold ethanol and measured by competition between 3H-cAMP and PKA as described previously (Bailey and Hay, 2006). For studies characterizing rAM2 potency at rodent AM2 and CGRP receptors, cAMP was measured as per a modification to the standard AlphaScreen method (AlphaScreen cAMP assay kit; Perkin-Elmer Life and Analytical Sciences, Waltham, MA, USA) to permit use in adherent cells as described previously (Gingell et al., 2010). The plates were then read using an Envision plate reader (Perkin-Elmer Life and Analytical Sciences).
Radioligand binding
Cells were washed once with PBS (at room temperature) and then incubated for 2 h at room temperature in DMEM containing 0.1% BSA and ∼100 pM [125I]-rAmy (Perkin-Elmer Life and Analytical Sciences) in the absence or presence of different concentrations of unlabelled rAmy or rαCGRP to a total volume of 500 µL. rAmy at a concentration of 1 µM was used to define non-specific binding. Following this incubation, the cells were washed once with ice-cold PBS before being lysed with 500 µL 0.2 M NaOH. The cell lysate was transferred to Eppendorf tubes and counted for 1 min per tube.
Tissue collection
Two tissues that have been reported to express the CGRP2 receptor phenotype were used (Hay et al., 2008). Vas deferens (VD) was carefully dissected from four male Wistar rats, and internal anal sphincters (IAS) were carefully dissected from three male Wistar rats and immediately frozen at −80°C. All animal care and experimental procedures were conducted in accordance with the New Zealand animal welfare act (1999) and approved by the University of Auckland Animal Ethics Committee.
RNA extraction
The VD and IAS tissues were individually ground under liquid nitrogen to a fine powder. Total RNA was extracted from the ground tissue using TRIZOL® Reagent (Invitrogen) according to the manufacturer's protocol, resuspended in 50 µL DEPC treated water and stored at −80°C. The concentration of total RNA was determined by a NanoDrop® ND-1000 Spectrophotometer (Thermo Scientific, Wilmington, DE, USA), and RNA integrity was determined by gel electrophoresis.
RT-PCR and primers
First strand cDNA was synthesized by using a SuperScript™ first-strand synthesis system (Invitrogen). Total RNA (5 µg) was incubated with or without SuperScript™ II reverse transcriptase in the presence of random hexamer primers. Primers were developed for their specificity to rCLR, rCT receptors and rRAMP1. The sequences of the forward and reverse primers designed to target rCLR were CCAACGGATTACATTGCATA and CAGTAAAGCAGCACAAATGG, rCT receptors were ATGAGGTTCCTTCTCCTGAACAGG and CGGTCATAGCACTTGTACTGAGCA and to target rRAMP1 were GAGGACATGGAGACCATAGG and CAGTCATGAGCAGTGTGACC, respectively. The product sizes were 397 base pairs for the rCLR primers, 267 base pairs for the rRAMP1 primers and 173 base pairs for rCT receptor. The annealing temperature used for all sets of primers was 57°C. PCR reactions were performed in a total volume of 20 µL per reaction, using Pfu polymerase (Promega, Madison, WI, USA) and containing 2 µL of the appropriate cDNA template. Reactions containing no template were also set up as controls. PCR cycling comprised a single step of 95°C for 2 min followed by 35 cycles of 95°C for 45 s, 56°C for 45 s, 72°C for 90 s and a single final extension step of 72°C for 7 min. PCR products (10 µL) were electrophoresed with a size marker for 60 min in a 2% (w/v) agarose gel containing SYBR Safe™ DNA gel stain (Invitrogen) and visualized on a Biorad™ imaging system under UV transillumination.
Drugs chemicals and other materials
rAM, rCT, rαCGRP, rβCGRP, hαCGRP8-37 and rαCGRP8-37 were purchased from American Peptide (Sunnyvale, CA, USA). rAM2 (47 amino acids), sCT (Cys(Et)2,7)hαCGRP (Cys(ACM)2,7)hαCGRP, sCT8-32 and AC187 were purchased from Bachem (Bubendorf, Switzerland). rAmy and rAmy8-37 were purchased from both American Peptide and Bachem. AC413 was kindly provided by Amylin Pharmaceuticals, Inc. (San Diego, CA) AC187 and AC413 are N-terminally acetylated and C-terminally amidated peptides; their sequences are shown in Figure 1. All peptides were dissolved in water to make 1 mM stock solutions and stored as aliquots in siliconized microcentrifuge tubes at −30°C. When making up these solutions, the peptide content was taken into account, but where no data sheet was supplied, content was assumed to be 80%. BSA, IBMX, PKA and activated charcoal were purchased from Sigma-Aldrich (St. Louis, MO, USA). DMEM and TrypLE were purchased from Invitrogen, and forskolin was purchased from Tocris (Bristol, UK). All other reagents were of analytical grade.
Figure 1.

Amino acid sequences of rAmy8-37, sCT8-32, AC413 and AC187. Alignments were performed with ClustalW. Identical residues are underlined.
Data analysis
Data were analysed using GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA, USA). In each assay, cAMP data were first normalized to the maximal (100%) response obtained to 50 µM forskolin, and the minimum (no agonist/basal) that was present as a control on each plate or alternatively cAMP concentrations were determined from cAMP standard curves. For agonist responses, pEC50 values were obtained by fitting a four-parameter logistic equation to the concentration–response curve data. To determine if the Hill slope was significantly different from one for agonist potency curves, F-tests were performed. For the majority of experiments, the Hill slope was not significantly different from unity. The agonist potency curves were therefore re-fitted with a Hill slope constrained to 1 and pEC50s obtained. For calculation of antagonist potency values (pA2), agonist concentration–response curves were fitted in the absence or presence of antagonist and analysed by Global Schild analysis as previously described (Hay et al., 2005). To determine if Schild slopes were significantly different from unity within each experiment, F-tests were performed. In the majority of experiments, Schild slopes were not significantly different from one and were therefore constrained to 1; under these conditions the resulting estimate of pA2 represents the pKB.
In radioligand binding experiments, the specific binding was first calculated for each receptor, and then data were normalized to this value before being fitted to a four-parameter logistic equation. As the Hill slopes were not significantly different from one by F-test, they were constrained to one for calculation of pIC50 values.
For statistical analysis, pEC50, pIC50 and pKB values from individual experiments were combined and compared using unpaired t-tests or one-way anova followed by Tukey's post hoc test where appropriate. Unless stated otherwise, all potency and affinity values are expressed as logarithms, and all data are expressed as mean ± SEM. Significance was achieved at P < 0.05. n refers to the number of independent experiments (i.e. individual transient transfections and subsequent manipulations).
Results
Pharmacology of rat calcitonin receptors – cAMP assay
In order to determine the impact of RAMP co-transfection with CT receptors, it was important to characterize the pharmacology of the rat CT receptor in the absence of RAMPs. rCT potently stimulated cAMP production via rCT(a) and was significantly more potent than any other agonist tested (Figures 2 and 3A, Table 1). rAmy and rαCGRP were approximately 20-fold less potent than rCT with rβCGRP, rAM and rAM2 acting as weaker agonists. The pEC50 values are displayed in Table 1, with corresponding curves in Figure 2 and summary graph with statistical analysis in Figure 3. (Cys(Et)2,7)hαCGRP and (Cys(ACM)2,7)hαCGRP failed to produce any elevation in cAMP over the baseline (data not shown). To provide further information from the agonist data, additional comparisons between agonists at this receptor are presented as supplementary data (Table S1). This revealed that rAmy was significantly more potent than rAM or rAM2. There were no other significant differences.
Figure 2.
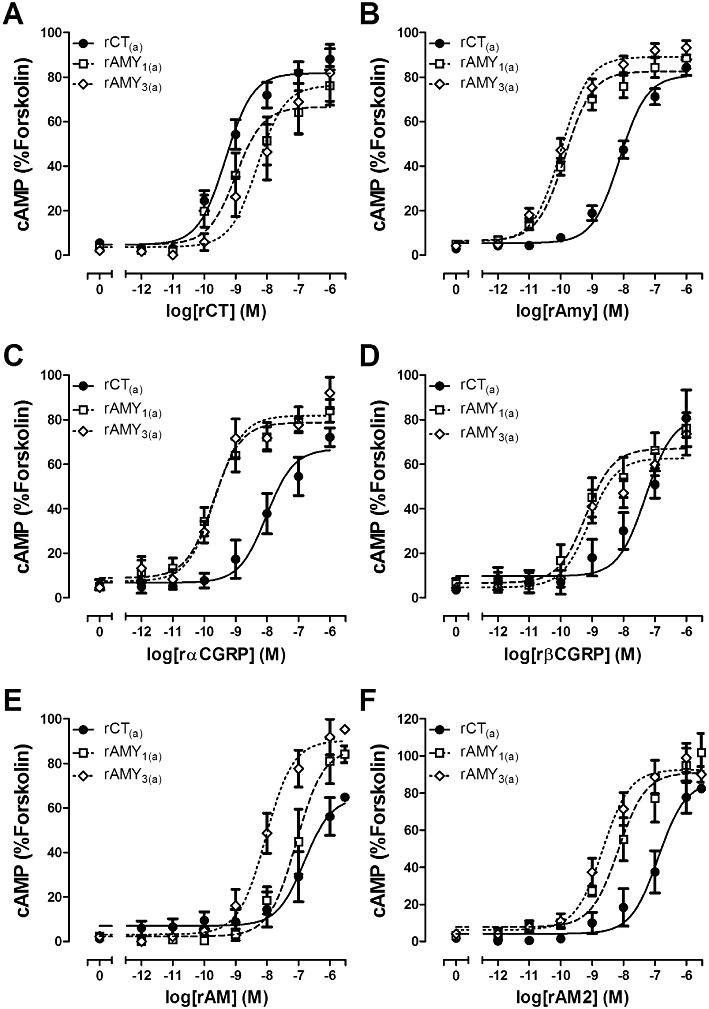
Stimulation of cAMP production by (A) rat (r) CT, (B) rAmy, (C) rαCGRP, (D) rβCGRP, (E) rAM and (F) rAM2 in COS-7 cells transfected to express either rCT(a) receptors, rAMY1(a) receptors or rAMY3(a) receptors as indicated. Data are expressed as a percentage of the cAMP response generated by 50 µM forskolin and are mean ± SEM of four to 23 combined experiments, performed in duplicate or triplicate.
Figure 3.
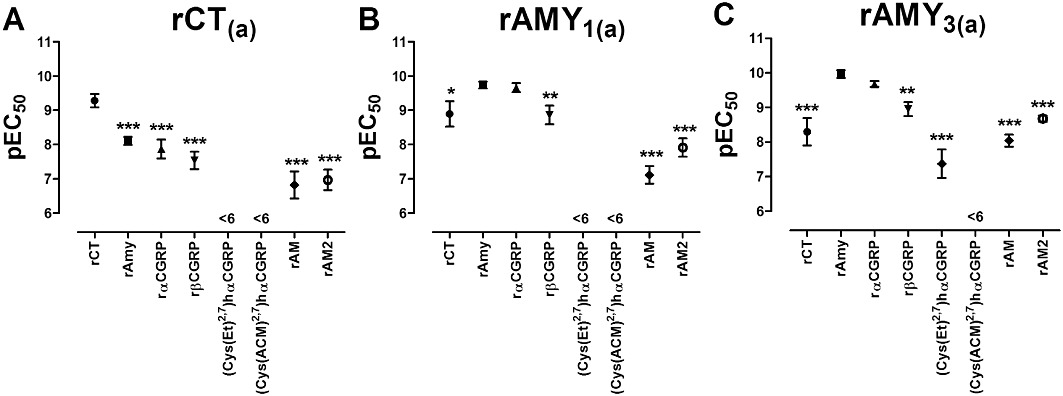
Distribution of pEC50 values for the agonists rCT, rAmy, rαCGRP, rβCGRP, (Cys(Et)2,7)hαCGRP, (Cys(ACM)2,7)hαCGRP, rAM and rAM2 in COS-7 cells transfected to express either (A) rCT(a) receptors, (B) rAMY1(a) receptors or (C) rAMY3(a) receptors. Data are mean ± SEM of four to 23 combined experiments. *P < 0.05, **P < 0.01, ***P < 0.001 by one-way anova followed by Tukey's test. For simplification, only comparisons against the cognate agonist for each receptor are shown (rCT for CT(a), rAmy for rAMY1(a) and rAMY3(a)); other comparisons are shown in supplementary Tables S1–3. (Cys(Et)2,7)hαCGRP and (Cys(ACM)2,7)hαCGRP were excluded from the analysis except in the rAMY3(a) receptor, where (Cys(Et)2,7)hαCGRP produced a sufficient response to be included.
Table 1.
Agonist potencies (pEC50 values) for cAMP production at rCT(a), rAMY1(a) and rAMY3(a) receptors
| Agonist | rCT(a) receptor | rAMY1(a) receptor | rAMY3(a) receptor |
|---|---|---|---|
| rAmy | 8.11 ± 0.12 (15) | 9.74 ± 0.10 (23)*** | 9.97 ± 0.11 (18)*** |
| rCT | 9.28 ± 0.20 (11) | 8.90 ± 0.37 (6) | 8.30 ± 0.40 (4)* |
| rαCGRP | 7.87 ± 0.28 (7) | 9.66 ± 0.13 (13)*** | 9.68 ± 0.08 (6)*** |
| rβCGRP | 7.54 ± 0.25 (7) | 8.87 ± 0.27 (9)* | 8.95 ± 0.20 (6) |
| (Cys(ACM)2,7)hαCGRP | <6 (4) | <6 (4) | <6 (4) |
| (Cys(Et)2,7)hαCGRP | <6 (4) | <6 (4) | 7.70 ± 0.39 (4)a |
| rAM | 6.82 ± 0.39 (6) | 7.12 ± 0.26 (4) | 8.04 ± 0.17 (5)*,+ |
| rAM2 | 6.97 ± 0.30 (6) | 7.92 ± 0.27 (4) | 8.68 ± 0.09 (5)*** |
Data are mean ± SEM. Values in parentheses represent the number of individual experiments analysed.
Weak partial agonist. Comparisons between receptors were performed by one-way anova followed by Tukey's test (vs. rCT(a),
P < 0.05;
P < 0.01;
P < 0.001. vs. rAMY1(a),
P < 0.05). Comparisons of agonists within each column can be found in Figure 3 and Tables S1–S3.
A series of peptide fragments, reported to be antagonists of CT, Amy and CGRP receptors were then profiled at rCT(a). sCT8-32, AC187 and AC413 were similarly effective antagonists of rCT(a) receptors (Table 2, Figures 4A, 5A and 6A). In contrast, 10 µM rAmy8-37 and 10 µM rαCGRP8-37 were unable to produce a significant shift in the agonist curve at rCT(a) receptors (Table 2, Figures 7A and 8A). Statistical analysis is shown in Figure 9A.
Table 2.
Summary of pKB values for antagonism of rAmy-induced cAMP production at rCT(a), rAMY1(a) and rAMY3(a) receptors
| Antagonist | rCT(a) receptor | rAMY1(a) receptor | rAMY3(a) receptor |
|---|---|---|---|
| sCT8-32 | 8.13 ± 0.23 (4) | 7.42 ± 0.28 (4) | 8.17 ± 0.16 (4) |
| AC187 | 7.78 ± 0.11 (4) | 8.24 ± 0.36 (4) | 8.11 ± 0.23 (3) |
| AC413 | 8.09 ± 0.15 (4)** | 8.97 ± 0.14 (4) | 8.60 ± 0.15 (4) |
| rAMY8-37 | <5 (4)a | 6.16 ± 0.14 (5) | 5.53 ± 0.11 (4)* |
| rαCGRP8-37 | <5 (3) | 7.07 ± 0.15 (4) | 7.00 ± 0.08 (3) |
| hαCGRP8-37 | – | 7.62 ± 0.14 (4) | – |
Data are mean ± SEM. Values in parentheses represent the number of individual experiments analysed.
rCT was used as agonist. Comparisons between receptors were performed using unpaired t-tests or one-way anova followed by Tukey's tests where appropriate (vs. rAMY1(a),
P < 0.05,
P < 0.01). Comparisons of antagonists within each column can be found in Figure 9.
Figure 4.
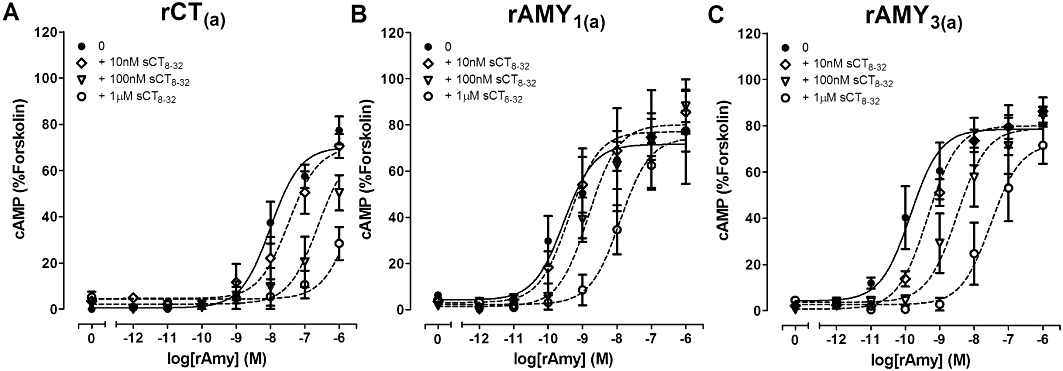
Antagonism of rAmy-induced cAMP production by the indicated concentrations of sCT8-32 in COS-7 cells transfected to express either (A) rCT(a) receptors, (B) rAMY1(a) receptors or (C) rAMY3(a) receptors. Data are expressed as a percentage of the cAMP response generated by 50 µM forskolin. Data are mean ± SEM of four combined experiments, performed in triplicate or quadruplicate.
Figure 5.
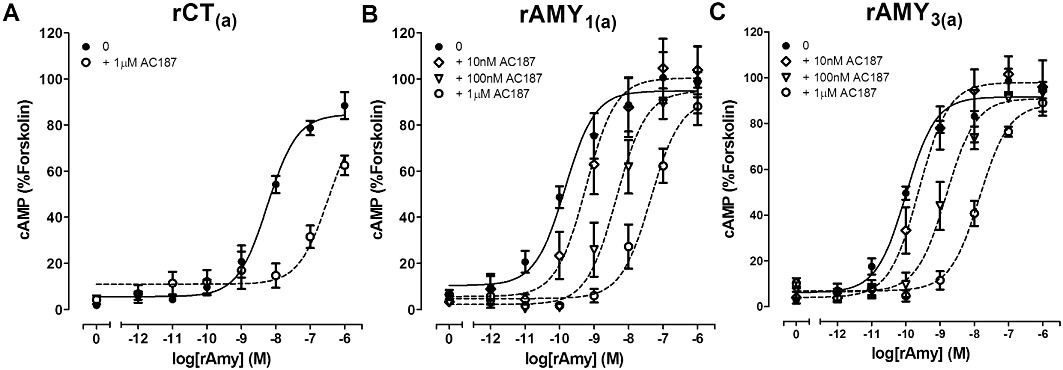
Antagonism of rAmy-induced cAMP production by the indicated concentrations of AC187 in COS-7 cells transfected to express either (A) rCT(a) receptors, (B) rAMY1(a) receptors or (C) rAMY3(a) receptors. Data are expressed as a percentage of the cAMP response generated by 50 µM forskolin. Data are mean ± SEM of three to four combined experiments, performed in triplicate or quadruplicate.
Figure 6.
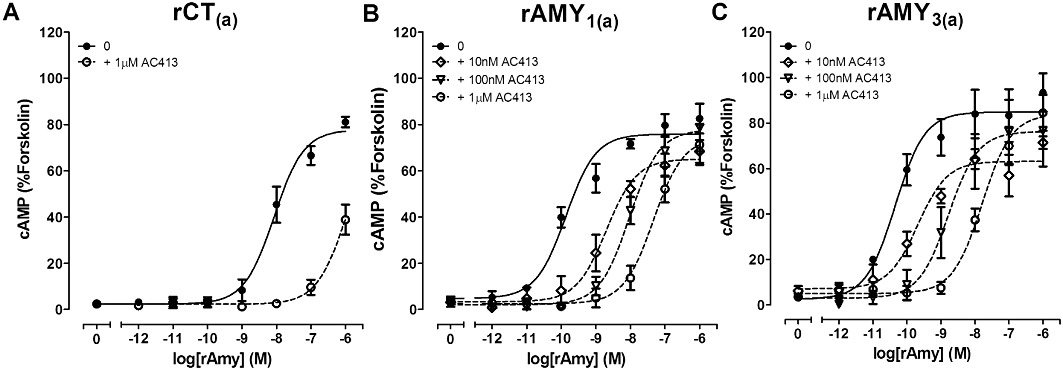
Antagonism of rAmy-induced cAMP production by the indicated concentrations of AC413 in COS-7 cells transfected to express either (A) rCT(a) receptors, (B) rAMY1(a) receptors or (C) rAMY3(a) receptors. Data are expressed as a percentage of the cAMP response generated by 50 µM forskolin. Data are mean ± SEM of four combined experiments, performed in triplicate or quadruplicate.
Figure 7.
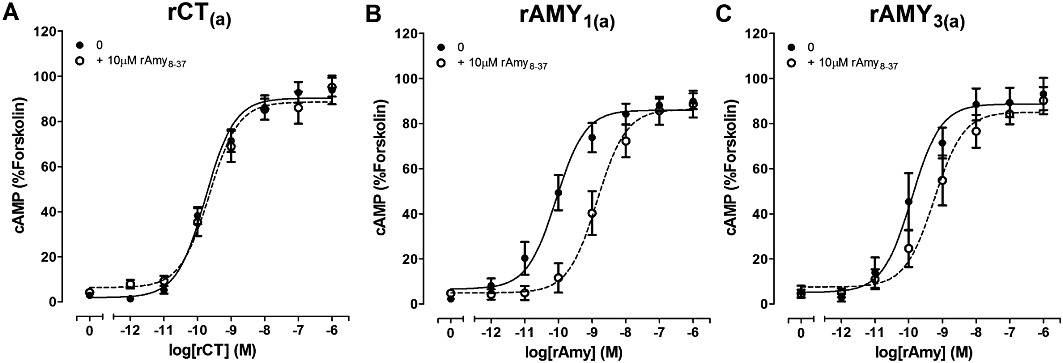
Antagonism of rCT or rAmy-induced cAMP production by 10 µM rAmy8-37 in COS-7 cells transfected to express either (A) rCT(a) receptors, (B) rAMY1(a) receptors or (C) rAMY3(a) receptors. Data are expressed as a percentage of the cAMP response generated by 50 µM forskolin. Data are mean ± SEM of four to five combined experiments, performed in triplicate or quadruplicate.
Figure 8.
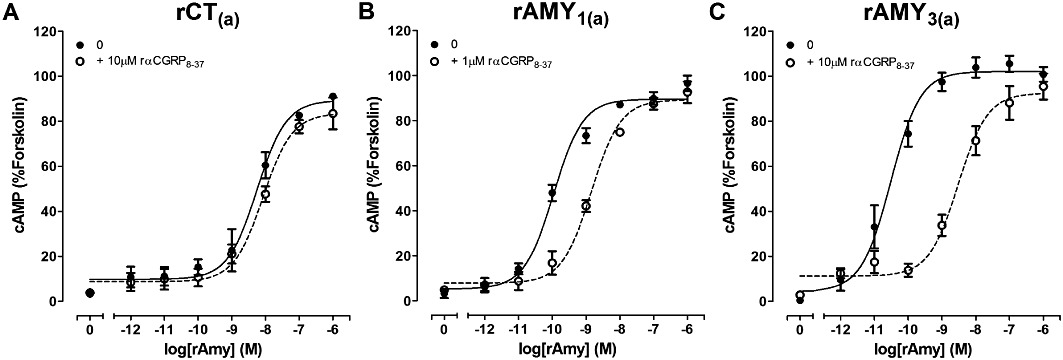
Antagonism of rAmy-induced cAMP production by the indicated concentrations of rαCGRP8-37 in COS-7 cells transfected to express either (A) rCT(a) receptors, (B) rAMY1(a) receptors or (C) rAMY3(a) receptors. Data are expressed as a percentage of the cAMP response generated by 50 µM forskolin. Data are mean ± SEM of three to four combined experiments, performed in triplicate or quadruplicate.
Figure 9.
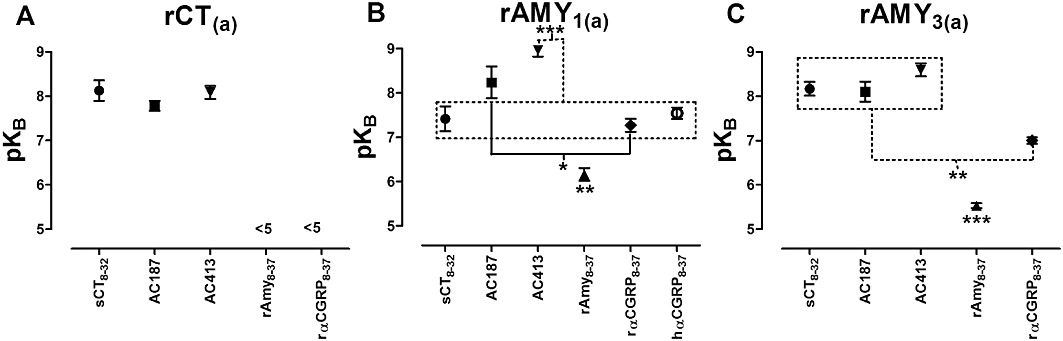
Distribution of pKB values for the antagonists sCT8-32, AC187, rAmy8-37, rαCGRP8-37 and hαCGRP8-37 in COS-7 cells transfected to express either (A) rCT(a) receptors, (B) rAMY1(a) receptors or (C) rAMY3(a) receptors. Data are mean ± SEM of three to five combined experiments. *P < 0.05, **P < 0.01, ***P < 0.001 by one-way anova followed by Tukey's tests. Data points in boxes are significantly different, as indicated, from the comparator.
Pharmacology at rAMY1 receptors – cAMP assay
rCT(a) and rRAMP1 were co-transfected to form putative rAMY1(a) receptors. rAmy and rαCGRP were effective agonists, which displayed almost identical potencies; there was no significant difference between them (Table 1, Figures 2B and C, 3B). All other agonists were significantly weaker compared with these two agonists; rCT and rβCGRP were approximately 10-fold weaker as agonists in cells transfected with these receptor components (Table 1, Figure 2A and D). rAM2 and rAM were approximately 100-fold and 1000-fold weaker than rAmy (Figures 2E and F, 3B) but were not significantly different from one-another (Table S2). Other comparisons are shown in Table S2. (Cys(Et)2,7)hαCGRP and (Cys(ACM)2,7)hαCGRP failed to produce any elevation in cAMP over the baseline.
AC413 was the most effective antagonist of rAMY1(a) receptors, followed by AC187 and sCT8-32. AC413 was significantly more effective than sCT8-32 but not AC187 (Figures 4B, 5B, 6B and 9B, Table 2). rAmy8-37 was also able to antagonize this receptor but more weakly than the other antagonists (Table 2, Figures 7B and 9B). rαCGRP8-37 was as effective as sCT8-32 but weaker than AC187 and AC413 (Table 2, Figures 8B and 9B). hαCGRP8-37 was also an effective antagonist (Table 2). Figure 9B shows all statistical comparisons between these antagonists.
Pharmacology at rAMY3 receptors – cAMP assay
To examine the pharmacology of putative rAMY3(a) receptor phenotype, cells were transfected with rCT(a) and rRAMP3. rAmy potently stimulated cAMP production in these cells (Table 1, Figures 2B and 3C); rαCGRP was similarly potent (Table 1, Figure 2C). All other agonists were significantly weaker at this receptor (Figure 3C). Whilst (Cys(ACM)2,7)hαCGRP was without measurable effect (Cys(Et)2,7)hαCGRP behaved as a weak partial agonist compared with rAmy and the other agonists tested (Emax, 18.8% ± 2.6 forskolin, n= 4). The statistical comparison between agonists is shown in Table S3.
AC187, AC413 and sCT8-32 were equally effective antagonists of rAMY3(a) receptors, followed by rαCGRP8-37 (Table 2, Figures 4C, 5C, 6C and 8C). rAmy8-37 acted as a weak antagonist of this receptor and was significantly less effective than the other antagonists tested (Table 2, Figure 7C). Figure 9C shows all statistical comparisons between these antagonists.
Comparison of pharmacology across rat CT(a), AMY1(a) and AMY3(a) receptor subtypes – cAMP assay
Comparison of agonist potencies between receptors by one-way anova followed by Tukey's tests (Table 1) revealed that rCT potency was significantly reduced at rAMY3(a) compared with rCT(a). It was also reduced at rAMY1(a), but this did not reach statistical significance. rAmy potency was significantly increased at both rAMY1(a) and rAMY3(a) compared with rCT(a), although there was no difference between rAMY1(a) and rAMY3(a). rαCGRP and rβCGRP behaved similarly to rAmy, although significance was not reached for rAMY3(a) with rβCGRP. rAM potency was not significantly enhanced at the rAMY1(a) receptor compared with the rCT(a) receptor but was enhanced at the rAMY3(a) receptor. This was reflected by significant differences between rAMY1(a) and rAMY3(a) receptors, with rAM exhibiting higher potency at rAMY3(a) than rAMY1(a). For rAM2, the potency of this peptide was also enhanced at rAMY3(a) relative to rCT(a), but in this case, the difference in potency between rAMY1(a) and rAMY3(a) did not reach statistical significance.
Comparisons of the antagonist potency data across the three receptors showed that rAmy8-37 was a selective antagonist of the rAMY1(a) receptor, compared with rAMY3(a) and rCT(a) where no measurable shift in the concentration–response curve to rCT was detected (Table 2). rαCGRP8-37 was without effect at rCT(a) but was able to antagonize rAmy responses at rAMY1(a) and rAMY3(a), although there was no significant difference between these receptors. There were no significant differences in sCT8-32 or AC187 potency among the three receptors, although there was a trend towards a decrease in sCT8-32 potency at rAMY1(a) compared with the other two receptors and a trend towards increased AC187 potency at rAMY1(a) and rAMY3(a) versus rCT(a). AC413 had higher potency at the rAMY1(a) and rAMY3(a) receptors compared with rCT(a), but this only reached statistical significance for rAMY1(a).
Pharmacology of rat CT(a), AMY1(a) and AMY3(a) receptor subtypes – radioligand binding
The co-transfection of rCT(a) with either rRAMP1 and rRAMP3 resulted in significant enhancement in rAmy potency. To confirm the induction of Amy receptor phenotype, radioligand binding studies were conducted. There was negligible specific binding of [125I]-rAmy to cells transfected with rCT(a) alone under these experimental conditions (Figure 10A). However, the specific binding of [125I]-rAmy was increased when rCT(a) was co-transfected with rRAMP1 (P < 0.05) or rRAMP3 (P < 0.01) (Figure 10A). In competition binding assays, rAmy displaced [125I]-rAmy from rAMY1(a) and rAMY3(a) receptors with pIC50s of 8.62 ± 0.01 and 8.64 ± 0.02 (n= 3), respectively (Figure 10B). Similarly, rαCGRP displaced [125I]-rAmy from rAMY1(a) receptors with a pIC50 of 8.43 ± 0.09 (n= 3) (Figure 10C). At rAMY3(a) receptors, rαCGRP displaced [125I]-rAmy with a pIC50 of 7.92 ± 0.03 (n= 2).
Figure 10.
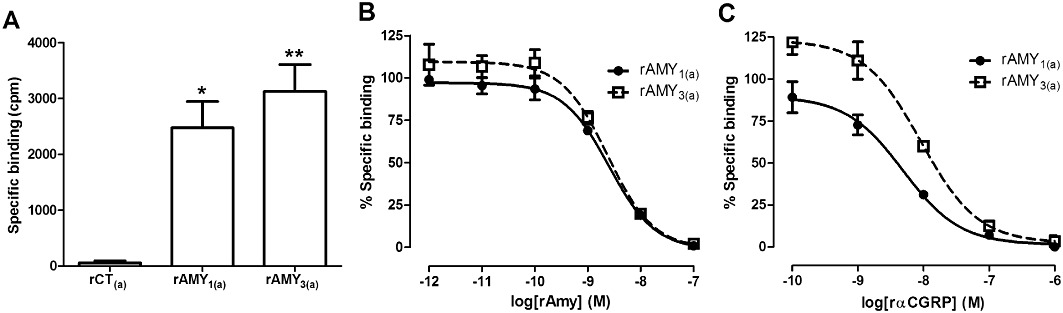
Specific binding of 100 pM [125I]-rAmy in (A) Cos-7 cells transfected to express either rCT(a) receptors, rAMY1(a) receptors or rAMY3(a) receptors and competitive displacement of 100 pM [125I]-rAmy by (B) rAmy or (C) rαCGRP in COS-7 cells transfected to express either rat AMY1(a) receptors or rat AMY3(a) receptors expressed as a percentage maximum of 100 pM [125I]-rAmy specific binding. Data are mean ± SEM of three to five (A) or two to three (B and C) combined experiments, performed in duplicate or triplicate.
Pharmacology of CGRP-responsive receptors – cAMP assay
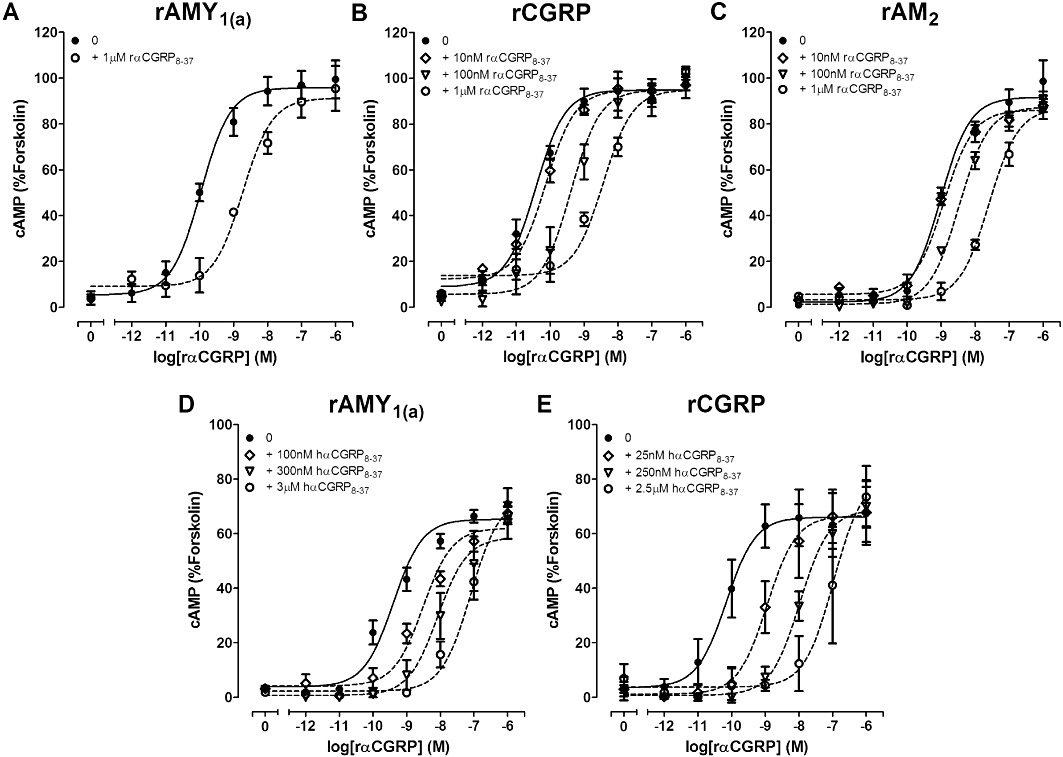
The AMY1 receptor has been suggested to represent a major molecular correlate responsible for observations of relatively CGRP8-37-insensitive CGRP-responsive receptors in the literature, known formerly as ‘CGRP2 receptors’ (Hay et al., 2008). This explanation is based on studies with human receptors whilst the reported heterogeneity is from rodents or other species. Therefore, we compared the antagonist potency of αCGRP8-37 at rAMY1(a) and rCGRP receptors stimulated by rαCGRP. rαCGRP potently stimulated cAMP production in cells transfected with rCGRP receptors (pEC50; 10.50 ± 0.16, n= 7) and rAMY1(a) receptors (pEC50; 9.66 ± 0.13, n= 13). rαCGRP displayed higher potency at rCGRP receptors, compared with the rAMY1(a) receptor (P < 0.01 by one-way anova followed by Tukey's test). Antagonism of rαCGRP-mediated cAMP stimulation by rαCGRP8-37 was significantly greater (P < 0.01 by one-way anova followed by a Tukey's test) at the rCGRP receptor (pKB; 8.12 ± 0.27, n= 3) than at the rAMY1(a) receptor (pKB; 7.20 ± 0.16, n= 3) (Figures 11A and B, 12A). The AM2 receptor has also been suggested as a molecular correlate for the ‘CGRP2 receptor’ and so rαCGRP8-37 was also compared at this receptor. rAM2 receptors were effectively activated by rαCGRP with a pEC50 of 8.96 ± 0.13, n= 3 (P < 0.001 vs. rCGRP and rAMY1(a) receptors by one-way anova followed by a Tukey's test). This receptor was also antagonized by rαCGRP8-37 (pKB; 7.32 ± 0.19, n= 3) but less potently than the rCGRP receptor, although this difference did not reach statistical significance (Figures 11C and 12A).
Figure 11.
Antagonism of rαCGRP-induced cAMP production by the indicated concentrations of rαCGRP8-37 or hαCGRP8-37 in COS-7 cells transfected to express either (A) and (D) rAMY1(a) receptors or (B) and (E) rCGRP receptors or (C) rAM2 receptors. Data are expressed as a percentage of the cAMP response generated by 50 µM forskolin. Data are mean ± SEM of three to four combined experiments, performed in triplicate or quadruplicate.
Figure 12.
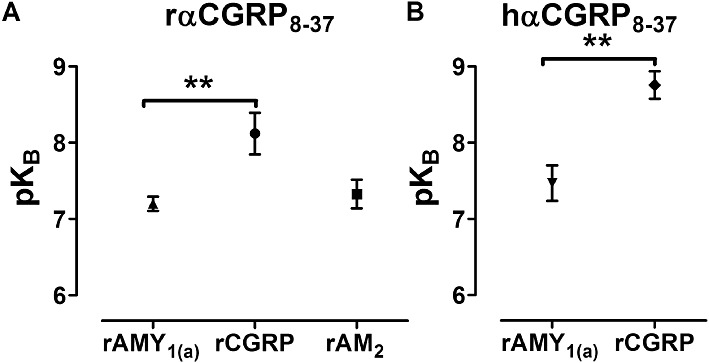
Distribution of pKB values for (A) rαCGRP8-37 or (B) hαCGRP8-37 in COS-7 cells transfected to express either rAMY1(a) receptors, rCGRP receptors or rAM2 receptors using rαCGRP as the agonist. Data are mean ± SEM of three to five combined experiments. Comparisons were performed by (A) one-way anova followed by Tukey's tests or (B) unpaired t-test (**P < 0.01).
As many researchers use hαCGRP8-37, we also compared antagonism by this peptide at rCGRP and rAMY1(a) receptors; a similar pattern to rαCGRP8-37 was observed (Figure 11D and E, with hαCGRP8-37 being significantly less effective (P < 0.01 by Student's t-test) at the rAMY1(a) (pKB; 7.47 ± 0.23, n= 4) than CGRP receptor (pKB; 8.76 ± 0.18, n= 4) (Figure 12B). Both antagonists behaved in an equivalent manner using rAmy or rαCGRP as agonists (Table 2, Figure 12).
Expression of rCTR, rCLR and rRAMP1
Observations of CGRP8-37-insensitive receptors have been made in tissues such as the internal anal sphincter (IAS) and vas deferens (VD). In order to assess the potential physiological contribution of rAMY1(a) receptors to this pharmacology, the expression of rAMY1(a) receptor components was examined in these two tissues. We found that IAS and VD from Wistar rats both expressed mRNA for rCLR, rCT receptors and rRAMP1, although the band intensity of rCT receptors in IAS in our example was weaker than the others (Figure 13). However, as this is not a quantitative technique and the band intensities of each product varied between each individual preparation (data not shown), we cannot estimate relative abundance. A further study would be required to do this.
Figure 13.
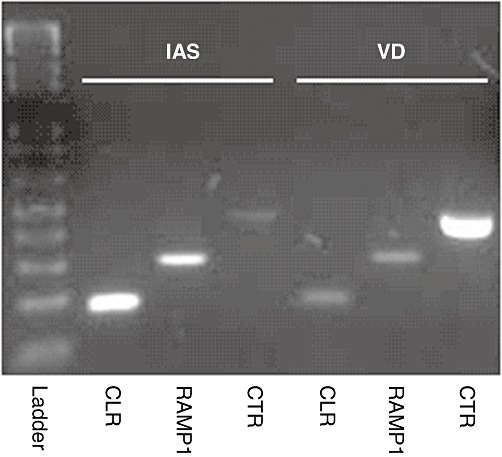
Representative gel electrophoresis image showing the expression of mRNA encoding rCLR, rRAMP1 and rCT receptor in rat IAS and VD. Similar results were obtained from tissue derived from three to four adult male Wistar rats.
The pharmacology of AM2 at CGRP and AM2 receptors, compared with AMY and CT receptors
The pharmacology of AM2 is ill-defined and has not been reported at rodent CT family receptors. Therefore, it was important to compare our observations of rAM2 potency at rAMY and rCT(a) receptors with rCGRP and rAM2 receptors under similar conditions. To examine the pharmacology of the rAM2 peptide at rCGRP and rAM2 receptors, the ability of cells transfected with rCLR and rRAMP1 or rRAMP3 to stimulate cAMP production with rAM, rαCGRP or rAM2 was determined. All potently stimulated cAMP production at rCGRP and rAM2 receptors (Table 3, Figure 14). rαCGRP was more potent at rCGRP compared to rAM2 receptors and rAM was more potent at rAM2 compared with rCGRP receptors. Interestingly, rAM2 displayed highest potency at rAM2 compared with rCGRP receptors. At rCGRP receptors rαCGRP displayed higher potency than rAM or rAM2; rAM and rAM2 were equipotent (Figure 14A). The converse was observed at rAM2 receptors with rαCGRP displaying lower potency than rAM or rAM2; rAM and rAM2 were equipotent (Figure 14B). Similar pharmacology to rAM2 receptors was observed for mouse AM2 receptors (Figure 14C).
Table 3.
Agonist potencies (pEC50 values) for cAMP production at rCGRP, rAM2 and mouse (m) AM2 receptors
| Agonist | rCGRP receptor | rAM2 receptor | mAM2 receptor |
|---|---|---|---|
| rαCGRP | 10.53 ± 0.04 (5) | 8.59 ± 0.13 (8)++ | 8.65 ± 0.10 (4) |
| rAM | 8.50 ± 0.43 (5)** | 10.15 ± 0.26 (7)***,+++ | 10.24 ± 0.08 (3)*** |
| rAM2 | 8.79 ± 0.02 (3)** | 9.93 ± 0.26 (3)***,+ | 10.05 ± 0.21 (3)*** |
Data are mean ± SEM. Values in parentheses represent the number of individual experiments analysed. In these experiments, cAMP concentrations were determined by AlphaScreen. Comparisons between rCGRP and rAM2 receptors were performed by Student's t-test (
P < 0.05;
P < 0.01;
P < 0.001). Comparisons between peptides within each receptor were performed by one-way anova followed by Tukey's tests. Comparisons shown are against rαCGRP (
P < 0.01;
P < 0.001). No significant differences between rAM and rAM2 were observed.
Figure 14.
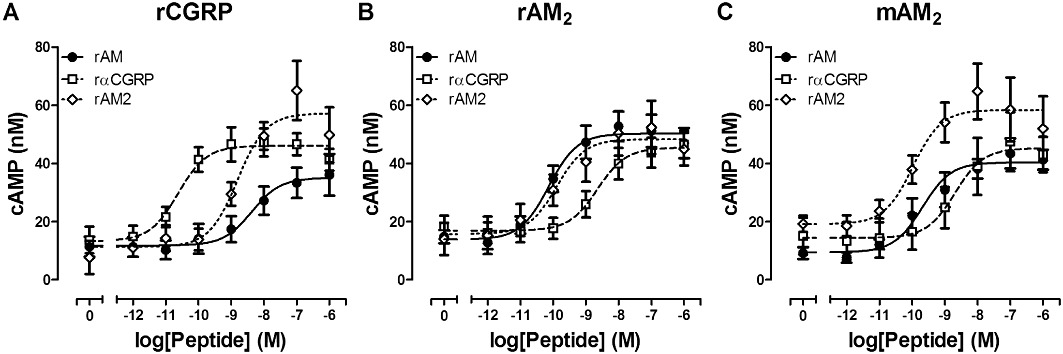
Stimulation of cAMP production by rAM, rAM2 or rαCGRP in COS-7 cells transfected to express either (A) rCGRP receptors, (B) rAM2 receptors or (C) mouse (m) AM2 receptors. Data are expressed as the concentration of cAMP generated and are mean ± SEM of three to eight combined experiments, performed in triplicate.
Comparison of rAM2 potencies by one-way anova followed by Tukey's test revealed that rAM2 was more potent at rAM2 receptors compared with rCGRP (P < 0.01), rCT(a) rAMY1(a) (P < 0.001) and rAMY3(a) (P < 0.05) receptors. The rank order of potency for rAM2 across these receptors was rAM2 > rCGRP = rAMY3(a) > rAMY1(a) > rCT(a) (Table 1, Table 3).
Discussion and conclusions
Additional Amy receptor modulators could be valuable medicines. However, their development depends on determination of the Amy receptor subtypes involved in the specific biological processes of this peptide. This is not known in humans or rodent models of disease. To complement molecular methods of identifying AMY receptor subtypes, we herein report the pharmacological characterization of rAMY receptors. We provide a valuable resource; allowing the complex overlapping pharmacology previously observed in many rodent tissues to be better understood (Sexton et al., 1994; Riediger et al., 1999).
As observed for recombinant human receptors (Christopoulos et al., 1999; Muff et al., 1999; Hay et al., 2005), rCT(a) alone produced a rCT receptor, whereas the co-expression of rCT(a) with either rRAMP1 or rRAMP3 produced AMY receptors. This is defined by significant enhancement in rAmy potency and induction of high affinity rAmy binding (Poyner et al., 2002). This contrasts with pCT(a) also transfected into COS-7 cells, where hAmy potency in cAMP assays was not increased by the presence of any pRAMP (Kikumoto et al., 2003). Enhancement in pCGRP potency in the presence of pRAMP1 was observed, consistent with our data and earlier studies (Christopoulos et al., 1999; Kuwasako et al., 2004; Hay et al., 2005). The apparent disparity in pharmacology between human, rat and pCT(a)/RAMP complexes are not obviously explained by methodological differences. A contributing factor could be the species of Amy used. Alternatively, Amy potency might have been enhanced if an alternative signalling molecule to cAMP was measured (Morfis et al., 2008).
rαCGRP potency at rCT(a), rAMY1(a) and rAMY3(a) receptors mirrored that of rAmy, displaying equivalent potency to rAmy at all receptors. This differs from hAMY receptors, where there was greater discrimination between hαCGRP and rAmy at hAMY3(a) receptors (Hay et al., 2005). rβCGRP tended to be less potent than rαCGRP at rCT(a), rAMY1(a) and rAMY3(a) receptors. This also contrasts with human receptors, which displayed a reversal of this trend at hCT(a) and hAMY1(a) and similar potency for hαCGRP and hβCGRP at hAMY3(a) (Hay et al., 2005). Some of these differences may be explained by sequence differences between peptides and/or receptor subunits. However, no clear pattern is apparent, and further work is needed to understand these differences.
The linear CGRP analogues (Cys(Et)2,7)hαCGRP and (Cys(ACM)2,7)hαCGRP, both failed to significantly enhance cAMP production at either rCT(a) or rAMY1(a) receptors. (Cys(ACM)2,7)hαCGRP was also without effect at rAMY3(a), although (Cys(Et)2,7)hαCGRP behaved as a weak partial agonist. This may reflect their nature as partial agonists. However, it is again interesting that there was a reversal in trend at the rat versus human receptors. Both linear analogues were capable of activating hAMY1(a) but not hAMY3(a) receptors under similar experimental conditions. However, the use of transient transfections and slightly different transfection ratios of RAMP to CT(a) may have resulted in differences in expression and therefore receptor number between the studies.
rCT potency was reduced with rRAMP co-expression, consistent with earlier observations at human receptors and was most noticeable with AMY3(a). This is consistent with the suggestion that less ‘free’ CT(a) is available to be expressed in the presence of RAMP3 and indicates that RAMP3 may interact more strongly with rCT(a) than RAMP1 (Hay et al., 2005). ‘Free’ CT(a) may occur as it can be expressed independent of RAMP at the cell surface, but it is difficult to quantify the potential different populations of each receptor. It is interesting that we still observed this with equal transfection of RAMP and CT(a), whereas previously RAMP was in slight excess in the transfection protocol (Hay et al., 2005). The potencies of most other peptides were enhanced with RAMP expression, suggesting that RAMP is either directly involved in their binding or generates favourable conformations of CT(a) for binding. In contrast, the presence of RAMP does not influence CT interactions in the same way. hCT has relatively low affinity and weakly competes with [125I]-rAmy binding in cells transfected with CT(a) and RAMP1 or RAMP3 (Tilakaratne et al., 2000).
rAM potency was substantially enhanced at rAMY3(a) receptors over rCT(a). This has previously been observed for human receptors in HEK293 cells but not in COS-7 cells (Kuwasako et al., 2003; Hay et al., 2005). It is unclear how important the AMY3(a) receptor might be to AM physiology or pathophysiology, but our observation, together with the earlier work, does emphasize the need to use multiple probes wherever possible to assist with receptor identification.
We also observed enhanced rAM2 potency at rAMY3(a), compared with rCT(a). AM2 was most potent at rAMY3(a) versus rAMY1(a) and rCT(a). This contrasts with earlier work for human receptors where either hAM2 (47 amino acids as used here) or the shorter form (40 amino acids) were both more potent at hAMY1(a) (Takei et al., 2004; Hay et al., 2005). To investigate this further, we compared rAM2 potency at rCGRP and rAM2 receptors. These data support a RAMP3 bias, with rAM2 showing highest potency at the rAM2 versus the rCGRP receptor. Several studies in the literature with AM2 support this (Chang et al., 2004; Takei et al., 2004; Qi et al., 2008; Hong et al., 2012; Kuwasako et al., 2011). Unfortunately, there is little quantification of potency in many studies, and so it is difficult to draw firm conclusions. Nevertheless, accumulating data seem to suggest that AM2 is a potent agonist of the AM2 receptor. This is also supported by our observations at the mouse AM2 receptor.
N-terminally truncated CT-family peptides act as antagonists (Alexander et al., 2011). Therefore, we compared the antagonist potencies of sCT8-32, rAmy8-37 and rαCGRP8-37 at rCT(a), rAMY1(a) and rAMY3(a). In addition, we used AC187 and AC413, which are modified forms of sCT8-32 and rAmy8-37. AC187 is predominantly sCT8-32 but has two substitutions with the equivalent rAmy residues at positions 30 and 32 (Figure 1). On the other hand, AC413 is mostly rAmy8-37 but has the central portion replaced with a sequence of sCT residues. AC413 also has an additional serine to alanine substitution towards the peptide C-terminus.
sCT8-32 was an effective but non-selective antagonist across these three receptors, though it trended towards being weaker at rAMY1(a) receptors. This pattern is consistent with studies in human receptors. sCT8-32 is therefore unlikely to be a useful tool for discriminating between rat CT and AMY receptor subtypes.
AC187 is commonly used as an antagonist of Amy and has been reported to be a selective AMY receptor antagonist (Young et al., 1994). For example, AC187 administration has been shown to reduce glucagon secretion (Gedulin et al., 2006) and increase food intake (Rushing et al., 2001). However, where tested at cloned receptors its ability to distinguish between hAMY1(a) and hAMY3(a) over hCT(a) receptors was limited (∼10-fold) (Hay et al., 2005). We now report that AC187 was an effective antagonist across all three rat receptors examined, but it did not display significantly selective antagonism between AMY and CT receptors. A trend towards greater effectiveness at rAMY1(a) was observed, but the effect was very small. This may relate to the two amino acids of rAmy that have been incorporated (Figure 1). Therefore, AC187 displays significant antagonism at rCT(a), rAMY1(a) and rAMY3(a) but is unable to discriminate between them. Thus, AC187 displays similar limitations to sCT8-32 and should also be used with caution. Its usefulness is likely to depend upon the concentration of agonist present: antagonism of a low Amy concentration would suggest AMY receptor involvement but would not distinguish between AMY1(a) and AMY3(a) receptors. Conversely, this antagonist may be a useful tool for discriminating between CT(a)-based and CLR-based receptors, particularly with respect to agonism by CGRP, which can effectively activate both CGRP and AMY receptors (Muff et al., 1999; Leuthauser et al., 2000; Kuwasako et al., 2004; Bailey and Hay, 2006). AC187 is a weak antagonist of CGRP receptors (Bailey and Hay, 2006).
Amy8-37 is generally considered a weak antagonist of rAmy and rαCGRP responses (Poyner et al., 1998; Hay et al., 2005; Bailey and Hay, 2006), with some notable exceptions (Cornish et al., 1999; Danaher et al., 2008). Here, rAmy8-37 was also a weak antagonist, with no measurable affinity at rCT(a), but measurable antagonism at rAMY1(a) and rAMY3(a) receptors. The pKB we observed at rAMY1(a) is higher than previous observations at hAMY1(a); 6.16 versus 5.59, respectively (both using rAMY8-37) (Hay et al., 2005). Similarly, rAmy8-37 was more effective at rAMY3(a) than hAMY3(a); pKB 5.53 versus <5, respectively. This difference between human and rat receptor systems could explain why in some rodent studies effective antagonism by Amy8-37 is observed (Wang et al., 1993; Ye et al., 2001), despite reports of negligible potencies or affinities (Aiyar et al., 1995; Hay et al., 2005). However, we acknowledge that the antagonist is still very weak, even at the rat receptors. Importantly, rAmy8-37 exhibited selective antagonism for rAMY1(a) over rAMY3(a) receptors. Thus, rAmy8-37 displays some characteristics necessary to effectively discriminate rAMY receptor subtypes. Therefore, whilst rAmy8-37 is unlikely to be useful as a practical tool for distinguishing AMY receptor subtypes in its own right, it could serve as a basis for developing other peptides.
AC413 has also been reported as an amylin receptor antagonist (Young, 2005c). This peptide was the most effective antagonist of rAMY1(a) receptors, but the discrimination against rCT(a) receptors was less than 10-fold. This peptide was also slightly more effective at inhibiting rAmy responses at hAMY1(a) receptors than hCT(a) or hAMY3(a). This suggests that AC413 does have an AMY1(a) receptor bias, but it does not have sufficient selectivity to be a useful tool. AC413 shares most sequence identity with rAmy8-37 and also shows a preference for rat and hAMY1(a) receptors over AMY3(a) and CT(a) receptors. Therefore, the incorporation of the sCT sequence appears to confer increased affinity with the limited degree of AMY1(a) receptor selectivity being retained in the remaining Amy sequence. There may be scope for further modifications to the AC413 sequence that improve the separation between receptors; such tools are urgently needed.
Of the antagonists tested, rαCGRP8-37 displayed the greatest selectivity for rAMY receptors over rCT(a) (>100-fold) but was unable to distinguish between AMY receptor subtypes. However, αCGRP8-37 antagonizes rαCGRP activity at other CGRP-responsive receptors; it displays significantly higher potency at CGRP receptors and similar antagonism at AM2 receptors to AMY receptors. This indicates that rαCGRP8-37 would need to be used in concert with other antagonists to effectively distinguish CGRP-responsive receptor subtypes. These studies were expanded to include hαCGRP8-37 for compatibility with our earlier work and because it is used interchangeably with rαCGRP8-37 in rodent studies. hαCGRP8-37 antagonized rAmy and rαCGRP-mediated rAMY1(a) receptor activation with similar potency to rαCGRP8-37.
Historically, two distinct subtypes of the CGRP receptor were described; CGRP1 and CGRP2. These receptor subtypes were defined based on relative sensitivity to CGRP8-37. This classification has been rendered obsolete by the molecular and pharmacological characterization of the CGRP and related receptors (Hay et al., 2008). The CGRP receptor (formerly CGRP1) is a complex of CLR and RAMP1, whereas the molecular composition of the CGRP2 receptor phenotype most likely comprises other CGRP-responsive receptors such as AMY1 and AM2 receptors (Hay et al., 2008). Nevertheless, this explanation has been based on pharmacological characterization of human receptors. Thus, it was important to confirm this in rodents, which is principally where the heterogeneity is reported. Both hαCGRP8-37 and rαCGRP8-37 displayed weaker antagonism of rαCGRP-stimulated cAMP accumulation at rAMY1(a) compared with rCGRP receptors. For completeness, the rAM2 receptor was also included and behaved in a similar manner to the rAMY1(a) receptor. The potential physiological contribution of rAMY1(a) receptors to CGRP receptor heterogeneity was also examined by determining the expression of rAMY1(a) receptor subunits in two rat tissues (VD and IAS), which have been historically reported to contain ‘CGRP2’ receptors (Hay, 2007). Rat vas deferens RAMP3 expression has been reported to be extremely low, suggesting that AM2 receptors are unlikely to contribute to the ‘CGRP2’ receptor phenotype in this tissue (Hay and Smith, 2001). From our observation of PCR products relating to the different subunits, we conclude that it is plausible that these tissues have the capacity to express CGRP and AMY1 receptors, although further quantitative and co-localization studies are needed to confirm this. Furthermore, rat VD has been reported to express an Amy-sensitive receptor that is antagonized by CGRP8-37 (Wisskirchen et al., 1998; Poyner et al., 1999). The data confirm that the ‘CGRP2’ receptor phenotype is most likely to be explained by CGRP-responsive receptors such as AMY1 or AM2 receptors.
In conclusion, this study has shown for the first time that the co-expression of rCT(a) with rRAMP1 or 3 produces functional AMY receptor subtypes in rats, which display equivalent potency for rAmy and rαCGRP. Some differences in pharmacology were noted between the rodent receptors and previous work with human receptors, and so it is important not to assume that there will always be a direct translation between species. The work has reinforced the urgent need for new tools with which to distinguish CT from AMY receptors and AMY receptor subtypes from one-another. The most commonly used tools at present are not adequate and caution should be applied when interpreting data that use them. Nevertheless, with careful use of combinations of existing agonists and antagonists, it should be possible to distinguish receptors, but this may not always be practical. The data presented provide a useful resource that could be employed in developing new CT or AMY receptor ligands. These would be valuable pharmacological tools or leads for developing new treatments for diabetes, obesity and bone disorders.
Acknowledgments
This work was supported by a University of Auckland Early Career Research Excellence Award to DLH, the Maurice and Phyllis Paykel Trust and New Zealand Lotteries Commission (Health). The authors thank Dr David R Poyner (Aston University, UK) for helpful discussions.
Glossary
- AM
adrenomedullin
- AM2
adrenomedullin 2/intermedin
- Amy
amylin
- AMY
amylin receptor
- AP
area postrema
- CT
calcitonin
- CGRP
calcitonin gene-related peptide
- CLR
calcitonin receptor-like receptor
- IAPP
islet amyloid polypeptide
- IAS
internal anal sphincter
- RAMP
receptor activity-modifying protein
- VD
vas deferens
Conflicts of interest
The authors have no conflicts of interest to declare.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Table S1 Statistical comparison of agonist potencies (pEC50 values) for cAMP production at rCT(a) receptors. Comparisons between agonists were performed using one-way anova followed by Tukey's tests (N.S. P > 0.05; *P < 0.05; **P < 0.01;***P < 0.001). pEC50 values could not be determined for (Cys(ACM)2,7)hαCGRP or (Cys(Et)2,7)hαCGRP
Table S2 Statistical comparison of agonist potencies (pEC50 values) for cAMP production at rAMY1(a) receptors. Comparisons between agonists were performed using one-way anova followed by Tukey's tests (N.S. P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001). pEC50 values could not be determined for (Cys(ACM)2,7)hαCGRP or (Cys(Et)2,7)hαCGRP
Table S3 Statistical comparison of agonist potencies (pEC50 values) for cAMP production at rAMY3(a) receptors. Comparisons between agonists were performed using one-way anova followed by Tukey's tests (N.S. P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001). pEC50 values could not be determined for (Cys(ACM)2,7)hαCGRP
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Aiyar N, Baker E, Martin J, Patel A, Stadel JM, Willette RN, et al. Differential calcitonin gene-related peptide (CGRP) and amylin binding sites in nucleus accumbens and lung: potential models for studying CGRP/amylin receptor subtypes. J Neurochem. 1995;65:1131–1138. doi: 10.1046/j.1471-4159.1995.65031131.x. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey RJ, Hay DL. Pharmacology of the human CGRP1 receptor in Cos 7 cells. Peptides. 2006;27:1367–1375. doi: 10.1016/j.peptides.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Becskei C, Riediger T, Zund D, Wookey P, Lutz TA. Immunohistochemical mapping of calcitonin receptors in the adult rat brain. Brain Res. 2004;1030:221–233. doi: 10.1016/j.brainres.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Chang CL, Roh J, Hsu SY. Intermedin, a novel calcitonin family peptide that exists in teleosts as well as in mammals: a comparison with other calcitonin/intermedin family peptides in vertebrates. Peptides. 2004;25:1633–1642. doi: 10.1016/j.peptides.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Christopoulos G, Perry KJ, Morfis M, Tilakaratne N, Gao Y, Fraser NJ, et al. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol Pharmacol. 1999;56:235–242. doi: 10.1124/mol.56.1.235. [DOI] [PubMed] [Google Scholar]
- Clementi G, Caruso A, Cutuli VM, de Bernardis E, Prato A, Amico-Roxas M. Amylin given by central or peripheral routes decreases gastric emptying and intestinal transit in the rat. Experientia. 1996;52:677–679. doi: 10.1007/BF01925572. [DOI] [PubMed] [Google Scholar]
- Cornish J, Callon KE, Lin CQ, Xiao CL, Gamble GD, Cooper GJ, et al. Comparison of the effects of calcitonin gene-related peptide and amylin on osteoblasts. J Bone Miner Res. 1999;14:1302–1309. doi: 10.1359/jbmr.1999.14.8.1302. [DOI] [PubMed] [Google Scholar]
- Danaher RN, Loomes KM, Leonard BL, Whiting L, Hay DL, Xu LY, et al. Evidence that alpha-calcitonin gene-related peptide is a neurohormone that controls systemic lipid availability and utilization. Endocrinology. 2008;149:154–160. doi: 10.1210/en.2007-0583. [DOI] [PubMed] [Google Scholar]
- Egerton M, Needham M, Evans S, Millest A, Cerillo G, McPheat J, et al. Identification of multiple human calcitonin receptor isoforms: heterologous expression and pharmacological characterization. J Mol Endocrinol. 1995;14:179–189. doi: 10.1677/jme.0.0140179. [DOI] [PubMed] [Google Scholar]
- Gedulin BR, Jodka CM, Herrmann K, Young AA. Role of endogenous amylin in glucagon secretion and gastric emptying in rats demonstrated with the selective antagonist, AC187. Regul Pept. 2006;137:121–127. doi: 10.1016/j.regpep.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Gingell JJ, Qi T, Bailey RJ, Hay DL. A key role for tryptophan 84 in receptor activity-modifying protein 1 in the amylin 1 receptor. Peptides. 2010;31:1400–1404. doi: 10.1016/j.peptides.2010.03.027. [DOI] [PubMed] [Google Scholar]
- Hay DL. What makes a CGRP2 receptor? Clin Exp Pharmacol Physiol. 2007;34:963–971. doi: 10.1111/j.1440-1681.2007.04703.x. [DOI] [PubMed] [Google Scholar]
- Hay DL, Smith DM. Adrenomedullin receptors: molecular identity and function. Peptides. 2001;22:1753–1763. doi: 10.1016/s0196-9781(01)00532-0. [DOI] [PubMed] [Google Scholar]
- Hay DL, Howitt SG, Conner AC, Schindler M, Smith DM, Poyner DR. CL/RAMP2 and CL/RAMP3 produce pharmacologically distinct adrenomedullin receptors: a comparison of effects of adrenomedullin22-52, CGRP8-37 and BIBN4096BS. Br J Pharmacol. 2003;140:477–486. doi: 10.1038/sj.bjp.0705472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DL, Christopoulos G, Christopoulos A, Poyner DR, Sexton PM. Pharmacological discrimination of calcitonin receptor: receptor activity-modifying protein complexes. Mol Pharmacol. 2005;67:1655–1665. doi: 10.1124/mol.104.008615. [DOI] [PubMed] [Google Scholar]
- Hay DL, Christopoulos G, Christopoulos A, Sexton PM. Determinants of 1-piperidinecarboxamide, N-[2-[[5-amino-l-[[4-(4-pyridinyl)-l-piperazinyl]carbonyl]pentyl]amino]-1- [(3,5-dibromo-4-hydroxyphenyl)methyl]-2-oxoethyl]-4-(1,4-dihydro-2-oxo-3(2 H)-quinazolinyl) (BIBN4096BS) affinity for calcitonin gene-related peptide and amylin receptors–the role of receptor activity modifying protein 1. Mol Pharmacol. 2006;70:1984–1991. doi: 10.1124/mol.106.027953. [DOI] [PubMed] [Google Scholar]
- Hay DL, Poyner DR, Quirion R. International Union of Pharmacology. LXIX. Status of the calcitonin gene-related peptide subtype 2 receptor. Pharmacol Rev. 2008;60:143–145. doi: 10.1124/pr.108.00372. [DOI] [PubMed] [Google Scholar]
- Hong Y, Hay DL, Quirion R, Poyner DR. The pharmacology of Adrenomedullin 2/Intermedin. Br J Pharmacol. 2012;166:110–120. doi: 10.1111/j.1476-5381.2011.01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikumoto K, Katafuchi T, Minamino N. Specificity of porcine calcitonin receptor and calcitonin receptor-like receptor in the presence of receptor-activity-modifying proteins. Hypertens Res. 2003;26(Suppl.):S15–S23. doi: 10.1291/hypres.26.s15. [DOI] [PubMed] [Google Scholar]
- Kuwasako K, Kitamura K, Nagoshi Y, Eto T. Novel calcitonin-(8-32)-sensitive adrenomedullin receptors derived from co-expression of calcitonin receptor with receptor activity-modifying proteins. Biochem Biophys Res Commun. 2003;301:460–464. doi: 10.1016/s0006-291x(02)03072-3. [DOI] [PubMed] [Google Scholar]
- Kuwasako K, Cao YN, Nagoshi Y, Tsuruda T, Kitamura K, Eto T. Characterization of the human calcitonin gene-related peptide receptor subtypes associated with receptor activity-modifying proteins. Mol Pharmacol. 2004;65:207–213. doi: 10.1124/mol.65.1.207. [DOI] [PubMed] [Google Scholar]
- Kuwasako K, Kitamura K, Nagata S, Hikosaka T, Takei Y, Kato J. Shared and separate functions of the RAMP-based adrenomedullin receptors. Peptides. 2011;32:1540–1550. doi: 10.1016/j.peptides.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Leuthauser K, Gujer R, Aldecoa A, McKinney RA, Muff R, Fischer JA, et al. Receptor-activity-modifying protein 1 forms heterodimers with two G-protein-coupled receptors to define ligand recognition. Biochem J. 2000;351(Pt 2):347–351. [PMC free article] [PubMed] [Google Scholar]
- Lutz TA. Control of food intake and energy expenditure by amylin-therapeutic implications. Int J Obes (Lond) 2009;33(Suppl. 1):S24–S27. doi: 10.1038/ijo.2009.13. [DOI] [PubMed] [Google Scholar]
- Lutz TA, Mollet A, Rushing PA, Riediger T, Scharrer E. The anorectic effect of a chronic peripheral infusion of amylin is abolished in area postrema/nucleus of the solitary tract (AP/NTS) lesioned rats. Int J Obes Relat Metab Disord. 2001;25:1005–1011. doi: 10.1038/sj.ijo.0801664. [DOI] [PubMed] [Google Scholar]
- Makimattila S, Fineman MS, Yki-Jarvinen H. Deficiency of total and nonglycosylated amylin in plasma characterizes subjects with impaired glucose tolerance and type 2 diabetes. J Clin Endocrinol Metab. 2000;85:2822–2827. doi: 10.1210/jcem.85.8.6721. [DOI] [PubMed] [Google Scholar]
- Morfis M, Tilakaratne N, Furness SG, Christopoulos G, Werry TD, Christopoulos A, et al. Receptor activity-modifying proteins differentially modulate the G protein-coupling efficiency of amylin receptors. Endocrinology. 2008;149:5423–5431. doi: 10.1210/en.2007-1735. [DOI] [PubMed] [Google Scholar]
- Muff R, Buhlmann N, Fischer JA, Born W. An amylin receptor is revealed following co-transfection of a calcitonin receptor with receptor activity modifying proteins-1 or -3. Endocrinology. 1999;140:2924–2927. doi: 10.1210/endo.140.6.6930. [DOI] [PubMed] [Google Scholar]
- Njuki F, Nicholl CG, Howard A, Mak JC, Barnes PJ, Girgis SI, et al. A new calcitonin-receptor-like sequence in rat pulmonary blood vessels. Clin Sci (Lond) 1993;85:385–388. doi: 10.1042/cs0850385. [DOI] [PubMed] [Google Scholar]
- Ogawa A, Harris V, McCorkle SK, Unger RH, Luskey KL. Amylin secretion from the rat pancreas and its selective loss after streptozotocin treatment. J Clin Invest. 1990;85:973–976. doi: 10.1172/JCI114528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potes CS, Lutz TA. Brainstem mechanisms of amylin-induced anorexia. Physiol Behav. 2010;100:511–518. doi: 10.1016/j.physbeh.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Poyner DR, Soomets U, Howitt SG, Langel U. Structural determinants for binding to CGRP receptors expressed by human SK-N-MC and Col 29 cells: studies with chimeric and other peptides. Br J Pharmacol. 1998;124:1659–1666. doi: 10.1038/sj.bjp.0702032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyner DR, Taylor GM, Tomlinson AE, Richardson AG, Smith DM. Characterization of receptors for calcitonin gene-related peptide and adrenomedullin on the guinea-pig vas deferens. Br J Pharmacol. 1999;126:1276–1282. doi: 10.1038/sj.bjp.0702437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W, et al. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev. 2002;54:233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- Qi T, Christopoulos G, Bailey RJ, Christopoulos A, Sexton PM, Hay DL. Identification of N-terminal receptor activity-modifying protein residues important for calcitonin gene-related peptide, adrenomedullin, and amylin receptor function. Mol Pharmacol. 2008;74:1059–1071. doi: 10.1124/mol.108.047142. [DOI] [PubMed] [Google Scholar]
- Ravussin E, Smith SR, Mitchell JA, Shringarpure R, Shan K, Maier H, et al. Enhanced weight loss with pramlintide/metreleptin: an integrated neurohormonal approach to obesity pharmacotherapy. Obesity (Silver Spring) 2009;17:1736–1743. doi: 10.1038/oby.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riediger T, Schmid HA, Young AA, Simon E. Pharmacological characterisation of amylin-related peptides activating subfornical organ neurones. Brain Res. 1999;837:161–168. doi: 10.1016/s0006-8993(99)01697-2. [DOI] [PubMed] [Google Scholar]
- Roth JD, Roland BL, Cole RL, Trevaskis JL, Weyer C, Koda JE, et al. Leptin responsiveness restored by amylin agonism in diet-induced obesity: evidence from nonclinical and clinical studies. Proc Natl Acad Sci USA. 2008;105:7257–7262. doi: 10.1073/pnas.0706473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth JD, Erickson MR, Parkes DG. GLP-1R and amylin agonism in metabolic disease: complementary mechanisms and future opportunities. Br J Pharmacol. 2012;166:121–136. doi: 10.1111/j.1476-5381.2011.01537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushing PA, Hagan MM, Seeley RJ, Lutz TA, D'Alessio DA, Air EL, et al. Inhibition of central amylin signaling increases food intake and body adiposity in rats. Endocrinology. 2001;142:5035–5038. doi: 10.1210/endo.142.11.8593. [DOI] [PubMed] [Google Scholar]
- Ryan GJ, Jobe LJ, Martin R. Pramlintide in the treatment of type 1 and type 2 diabetes mellitus. Clin Ther. 2005;27:1500–1512. doi: 10.1016/j.clinthera.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Sexton PM, Paxinos G, Kenney MA, Wookey PJ, Beaumont K. In vitro autoradiographic localization of amylin binding sites in rat brain. Neuroscience. 1994;62:553–567. doi: 10.1016/0306-4522(94)90388-3. [DOI] [PubMed] [Google Scholar]
- Takei Y, Hyodo S, Katafuchi T, Minamino N. Novel fish-derived adrenomedullin in mammals: structure and possible function. Peptides. 2004;25:1643–1656. doi: 10.1016/j.peptides.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Tilakaratne N, Christopoulos G, Zumpe ET, Foord SM, Sexton PM. Amylin receptor phenotypes derived from human calcitonin receptor/RAMP coexpression exhibit pharmacological differences dependent on receptor isoform and host cell environment. J Pharmacol Exp Ther. 2000;294:61–72. [PubMed] [Google Scholar]
- Trevaskis JL, Lei C, Koda JE, Weyer C, Parkes DG, Roth JD. Interaction of leptin and amylin in the long-term maintenance of weight loss in diet-induced obese rats. Obesity (Silver Spring) 2010a;18:21–26. doi: 10.1038/oby.2009.187. [DOI] [PubMed] [Google Scholar]
- Trevaskis JL, Parkes DG, Roth JD. Insights into amylin-leptin synergy. Trends Endocrinol Metab. 2010b;21:473–479. doi: 10.1016/j.tem.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Ueda T, Ugawa S, Saishin Y, Shimada S. Expression of receptor-activity modifying protein (RAMP) mRNAs in the mouse brain. Brain Res Mol Brain Res. 2001;93:36–45. doi: 10.1016/s0169-328x(01)00179-6. [DOI] [PubMed] [Google Scholar]
- Wang ZL, Bennet WM, Ghatei MA, Byfield PG, Smith DM, Bloom SR. Influence of islet amyloid polypeptide and the 8-37 fragment of islet amyloid polypeptide on insulin release from perifused rat islets. Diabetes. 1993;42:330–335. doi: 10.2337/diab.42.2.330. [DOI] [PubMed] [Google Scholar]
- Wielinga PY, Alder B, Lutz TA. The acute effect of amylin and salmon calcitonin on energy expenditure. Physiol Behav. 2007;91:212–217. doi: 10.1016/j.physbeh.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Wielinga PY, Lowenstein C, Muff S, Munz M, Woods SC, Lutz TA. Central amylin acts as an adiposity signal to control body weight and energy expenditure. Physiol Behav. 2010;101:45–52. doi: 10.1016/j.physbeh.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisskirchen FM, Burt RP, Marshall I. Pharmacological characterization of CGRP receptors mediating relaxation of the rat pulmonary artery and inhibition of twitch responses of the rat vas deferens. Br J Pharmacol. 1998;123:1673–1683. doi: 10.1038/sj.bjp.0701783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye JM, Lim-Fraser M, Cooney GJ, Cooper GJ, Iglesias MA, Watson DG, et al. Evidence that amylin stimulates lipolysis in vivo: a possible mediator of induced insulin resistance. Am J Physiol Endocrinol Metab. 2001;280:E562–E569. doi: 10.1152/ajpendo.2001.280.4.E562. [DOI] [PubMed] [Google Scholar]
- Young A. Amylin and the integrated control of nutrient influx. In: Young AA, editor. Advances in Pharmacology. 1st edn. Vol. 52. Maryland Heights: Academic Press; 2005a. pp. 67–77. [DOI] [PubMed] [Google Scholar]
- Young A. Inhibition of insulin secretion. In: Young AA, editor. Advances in Pharmacology. 1st edn. Vol. 52. Maryland Heights: Academic Press; 2005b. pp. 173–192. [DOI] [PubMed] [Google Scholar]
- Young A. Receptor pharmacology. In: Young AA, editor. Advances in Pharmacology. 1st edn. Vol. 52. Maryland Heights: Academic Press; 2005c. pp. 47–65. [DOI] [PubMed] [Google Scholar]
- Young AA, Gedulin B, Gaeta LS, Prickett KS, Beaumont K, Larson E, et al. Selective amylin antagonist suppresses rise in plasma lactate after intravenous glucose in the rat. Evidence for a metabolic role of endogenous amylin. FEBS Lett. 1994;343:237–241. doi: 10.1016/0014-5793(94)80563-6. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Liu X, Morgan DA, Kuburas A, Thedens DR, Russo AF, et al. Neuronal receptor activity modifying protein-1 promotes energy expenditure in mice. Diabetes. 2011;60:1063–1071. doi: 10.2337/db10-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


