Abstract
BACKGROUND AND PURPOSE
Activation of brain α2-adrenoceptors in conscious rodents decreases heart rate (HR) and mean arterial blood pressure (MAP) and increases urine output and urinary sodium excretion. In vitro, α2-adrenoceptor stimulation activates Gαi(1–3), Gαo and Gαs-subunit protein-gated signal transduction pathways. Here we have investigated whether these same Gα-subunit protein-gated pathways mediate the cardiovascular and renal excretory responses to central α2-adrenoceptor activation in conscious Sprague-Dawley rats.
EXPERIMENTAL APPROACH
Rats were pre-treated by intracerebroventricular injection (i.c.v.) with an oligodeoxynucleotide (ODN) targeted to a Gαi1, Gαi2, Gαi3, Gαo, Gαs or a scrambled (SCR) ODN sequence (25 µg, 24 h). On the day of study, the α2-adrenoceptor agonist guanabenz (50 µg) or saline vehicle, was injected i.c.v. into ODN-pre-treated conscious rats. MAP and HR were recorded, and urine was collected for 150 min.
KEY RESULTS
In vehicle- and SCR ODN-pre-treated rats, i.c.v. guanabenz decreased MAP and HR, and produced marked diuretic and natriuretic responses. Selective ODN-mediated down-regulation of brain Gαi2-subunit proteins abolished the central guanabenz-induced hypotension and natriuresis. In contrast, following selective Gαs down-regulation, the characteristic hypotensive response to i.c.v. guanabenz was converted to an immediate increase in MAP. The bradycardic and diuretic responses to i.c.v. guanabenz were not blocked by pre-treatment with any ODN.
CONCLUSIONS AND IMPLICATIONS
There was functional selectivity of Gαi2 and Gαs subunit protein-gated signal transduction pathways in mediating the hypotensive and natriuretic, but not bradycardic or diuretic, responses evoked by central α2-adrenoceptor activation in vivo.
Keywords: Brain Gα-subunit proteins, GPCR, CNS, cardiovascular function, renal excretory function, α2-adrenoceptor, guanabenz
Introduction
Multiple GPCR systems located throughout the CNS modulate systemic cardiovascular and renal excretory function through complex downstream signalling pathways. However, the specific role(s) that brain Gα-subunit proteins (e.g. Gαi/o, Gαs) play in producing these central GPCR-mediated physiological responses in vivo remains essentially unknown.
The α2-adrenoceptor, a seven-transmembrane GPCR (Eason and Liggett, 1995; Nasman et al., 2001; receptor nomenclature follows Alexander et al., 2011), is highly expressed in brain regions involved in the central control of cardiovascular function and fluid and electrolyte homeostasis (Ruffolo et al., 1991). When activated, central α2-adrenoceptors decrease heart rate (HR), mean arterial blood pressure (MAP) and central sympathetic outflow to the kidneys (Grisk and DiBona, 1998; Huang and Leenen, 1998). Concurrent with these depressor responses, stimulation of central α2-adrenoceptors also increases urine output and urinary sodium excretion (UNaV) (Gellai and Edwards, 1988, Menegaz et al., 2001). The physiological importance that central α2-adrenoceptor systems play in the regulation of systemic cardiovascular function is highlighted by the therapeutic use of centrally acting α2-agonists for the treatment of hypertension (Degoute, 2007).
Following α2-adrenoceptor stimulation, intracellular GTP-binding regulatory protein coupling occurs, which triggers activation of multiple downstream signal transduction pathways. Immediately post-ligand binding, α2-adrenoceptors can signal via downstream Gαi(1–3), Gαo and Gαs subunit protein-gated pathways as shown in different in vitro model systems (Remaury et al., 1993; Eason and Liggett, 1995). Through these Gα-subunit protein pathways, activation of α2-adrenoceptors can lead to a range of cellular responses, including modulation of adenylate cyclase activity (inhibition via Gαi/o proteins vs. stimulation via Gαs proteins), inhibition of calcium channels, stimulation of potassium channels and mitogen-activated kinases (ERK1/2), all of which can modulate the activity of the CNS (Hein, 2006).
As shown in cell culture systems, the Gα-subunit selectivity and/or protein availability may play a critical role in determining the intracellular signalling response to GPCR activation following ligand binding (Nasman et al., 2001). Extending this further, in investigations performed in conscious rats, we demonstrated that the cardiovascular depressor (hypotension and bradycardia), but not diuretic, responses produced by activation of a central GPCR [the nociceptin/orphanin FQ (N/OFQ) peptide receptor] were completely abolished in rats that had been pre-treated (48 h) centrally with Pertussis toxin (PTX). These findings highlight the novel functionally selective central Gα-subunit protein-mediated control of cardiovascular versus renal excretory function in vivo (Wainford et al., 2008). However, as PTX, which was used in these studies, is an exotoxin that catalyses the ADP ribosylation of all Gαi/o subunit proteins, the specific Gαi(1–3) or Gαo subunit(s) involved in mediating central GPCR-evoked cardiovascular depressor responses were not elucidated. At present, it remains unknown as to which specific Gαi/o-protein subtype(s) is involved in producing the bradycardic and hypotensive responses to the activation of central GPCRs involved in the regulation of systemic cardiovascular haemodynamics (e.g. α2-adrenoceptors) in vivo.
Therefore, the aim of this study was to investigate the role(s) that individual brain Gαi(1–3), Gαo and Gαs subunit proteins play in the systemic cardiovascular depressor versus renal excretory responses to central α2-adrenoceptor activation in conscious Sprague-Dawley rats. Groups of rats were pre-treated (24 h) by intracerebroventricular (i.c.v.) injection with an oligodeoxynucleotide (ODN) sequence targeted to Gαi1, Gαi2, Gαi3, Gαo, Gαs or a scrambled (SCR) ODN sequence to acutely and selectively down-regulate the respective target protein throughout the CNS (Wainford et al., 2008; Wainford and Kapusta, 2010). Post-ODN treatment (24 h), we examined the cardiovascular and renal excretory responses evoked by central α2-adrenoceptor activation in response to i.c.v. guanabenz in conscious instrumented rats. The findings of these studies provide new insight into the intracellular mechanism of action of brain α2-adrenoceptors in vivo and demonstrate that brain Gαi2 and Gαs subunit protein-gated signalling pathways have critical functionally selective roles in producing the hypotensive and natriuretic, but not bradycardic or diuretic, responses elicited by activation of central α2-adrenoceptors in vivo.
Methods
Animals
All animal care and experimental procedures were in accordance with National Institutes of Health and Louisiana State University Health Sciences Center Institutional Animal Care and Use Committee guidelines for the Care and Use of Animals. Male Sprague-Dawley rats (Harlan, Indianapolis, IN, USA), 275–300 g, were housed individually under a 12 h light/dark cycle. Rats were fed standard rodent diet and allowed tap water ad libitum and were randomly assigned to experimental treatment groups.
Measurement of MAP, HR and renal excretory function
A stainless steel cannula was stereotaxically implanted into the right lateral cerebral ventricle of rats anaesthetized with ketamine (40 mg/kg,, i.m.) in combination with xylazine (5 mg/kg, i.m.) 5–7 days before experimentation as previously described (Wainford et al., 2008; Wainford and Kapusta, 2009; 2010). On the day of study, rats were anaesthetized with sodium methohexital (75 mg/kg i.p. and supplemented with 10 mg/kg given i.v., as needed) and instrumented with catheters in the left femoral artery, left femoral vein and bladder as described previously (Wainford et al., 2008; Wainford and Kapusta, 2009; 2010). Following surgical preparation, rats were placed in a rat holder and an i.v. infusion of isotonic saline (55 µL·min−1) was started and continued for the duration of the experiment. The experimental protocol commenced after the animal regained full consciousness and systemic cardiovascular (HR and MAP) and renal excretory functions (urine output and UNaV) stabilized (4–6 h). MAP and HR were continuously recorded from the arterial cannula, which was connected to an external pressure transducer (P23XL; Viggo Spectramed, Oxnard, CA, USA); the cardiovascular data were collected using computer-driven BIOPAC data acquisition software (MP100 and AcqKnowledge 3.8.2, BIOPAC Systems, Inc., Goleta, CA, USA). Urine was collected during a 20 min control period. Following this, guanabenz (50 µg in 5 µL) (Huang and Leenen, 1994; 1998) or saline vehicle (5 µL) was injected i.c.v. (n= 6 per group), urine was collected during consecutive 10 min experimental periods for 150 min. Urine volume was determined gravimetrically. Urine sodium concentration was measured by flame photometry (model 943; Instrumentation Laboratories, Lexington, MA, USA) and expressed as UNaV.
Gα-subunit protein ODN down-regulation studies
Rats (n= 6 per group) were pre-treated with a single i.c.v. injection of either a Gαi1 ODN (25 µg in 5 µL, 24 h; 5′-AGACCACTGCTTTGTA-3′), Gαi2 ODN (25 µg per 5 µL, 24 h; 5′-CTTGTCGATCATCTTAGA-3′), Gαi3 ODN (25 µg per 5 µL, 24 h; 5′-AAGTTGCGGTCGATCAT-3′), Gαo ODN (25 µg per 5 µL, 24 h; 5′-CGCCTTGCTCCGCTC-3′), Gαs ODN (25 µg per 5 µL, 24 h; 5′-TTGTTGGCCTCAGCGTG-3′) or a SCR ODN (25 µg per 5 µL, 24 h; 5′-GGGGGAAGTAGGTCTTGG-3′) (Rossi et al., 1995; Standifer et al., 1996; Hadjimarkou et al., 2002). Following a National Center for Biotechnology Information Basic Local Alignment Search Tool (BLAST) search of the Rattus norvegicus RefSeq protein database it was confirmed that (i) there was no similarity between the SCR ODN sequence and any rat protein gene sequence and (ii) that the targeted Gα-subunit ODN sequences administered in these studies were specific for their respective Gα-subunit protein sequences. On the day of the experiment, 24 h after i.c.v. ODN pre-treatment injections, all animals were instrumented for measurement of cardiovascular and renal parameters and subsequently, during the experimental protocol, received a single i.c.v. injection of guanabenz (50 µg in 5 µL) or isotonic saline vehicle (5 µL) (Huang and Leenen, 1994; 1998). In a subset of studies, animals received an i.c.v. injection of the α2-adrenoceptor antagonist yohimbine (5.9 µg; 15 nmol) (Kapusta et al., 2002) 10 min before the injection of guanabenz.
G-protein immunoblotting
Twenty-four hours following a single i.c.v. administration of saline vehicle (5 µL) or an ODN sequence (25 µg in 5 µL) and completion of the acute cardiovascular and renal protocol described above, animals were then killed by decapitation; whole brains were removed and frozen at −80°C (n = 6/group). Frontal brain cortex (BC), hypothalamic paraventricular nucleus (PVN) and ventrolateral medulla (VLM) samples were extracted from frozen brains cut on a cryostat using a brain punch tool (Stoelting, IL, USA). BC and PVN samples were taken using a punch diameter of 1.00 mm, VLM samples were taken using a punch diameter of 0.76 mm and were stored at −80°C. The location of the PVN and VLM was determined using visual landmarks (Paxinos and Watson, 1998; Wainford and Kapusta, 2009; 2010), and by identification of neuron populations in sections examined under a light microscope. Tissue lysates were prepared from frozen brain tissues, and protein levels were quantified. Lysates were resolved on SDS-PAGE gels and transferred to nitrocellulose membrane (GE Healthcare, Piscataway, NJ, USA). Gαi(1–3), Gαo and Gαs levels were determined using antibodies purchased from Santa Cruz Biotechnologies (Santa Cruz, CA, USA), directed against Gαi1 (1:100, sc-391) (Olianas et al., 2007), Gαi2 (1:200, sc-13534), Gαi3 (1:1000, sc-262) (Bensimon et al., 2004), Gαo (1:200, sc-382) and Gαs (1:1000, sc-823) (Miggin et al., 2003); protein levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (anti-GAPDH 1:1000, ab-9483, Abcam, MA, USA) (Wainford et al., 2008; Wainford and Kapusta, 2009; 2010). Chemiluminescent immunoreactive bands were detected by horseradish peroxidase-conjugated secondary antibody; data were imaged and quantified using Bio-Rad Quantity One image analysis software (Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
All data are expressed as mean ± SEM. The magnitude of the changes in cardiovascular and renal excretory parameters at different time points after i.c.v. injection of guanabenz were compared with respective group control values by a one-way repeated measures anova with subsequent Dunnett's test. Differences occurring between treatment groups (e.g. i.c.v. SCR ODN vs. Gαi2 ODN) were assessed by a two-way repeated measure anova with pre-treatment group (e.g. saline vehicle) being one fixed effect and time the other, with the interaction included. The time (minutes) was then the repeated factor. Post hoc analysis was performed using Bonferroni's test. Where appropriate, a Student's t-test was also used to compare means between two groups. In each case, statistical significance was defined as P < 0.05.
Materials
Guanabenz and yohimbine were supplied by Sigma Aldrich, St. Louis, MO; ketamine by Vedco Inc., St. Joseph, MO; xylazine by Butler, Columbus, OH; methohexital by JHP Pharmaceuticals, LLC, Rochester, MI.
Results
SCR ODN pre-treatment does not alter the cardiorenal responses to i.c.v. guanabenz
In conscious Sprague-Dawley rats pre-treated with isotonic saline vehicle (5 µL, 24-h), i.c.v. guanabenz (50 µg) produced characteristic concurrent reductions in HR and MAP followed by a delayed onset increase in urine flow rate and UNaV (Figure 1). Peak bradycardia and hypotension were observed 20 min and 30 min following i.c.v. guanabenz administration respectively. HR and MAP remained significantly depressed for 40 min, returning to baseline control levels by 60 min after guanabenz injection. The maximal diuretic response was observed 80–90 min after guanabenz injection, with urine flow rate returning to pre-drug control levels by the end of the experimental period (150 min). No change in UNaV was detected until 80 min after guanabenz administration; from which time point thereafter, a significant natriuretic response was observed for the duration of the experimental protocol. Central administration of the α2-adrenoceptor antagonist yohimbine (5.9 µg; 15 nmol) 10 min before the i.c.v. injection of guanabenz (50 µg) completely blocked the cardiovascular and renal excretory responses to i.c.v. guanabenz (Figure 1).
Figure 1.
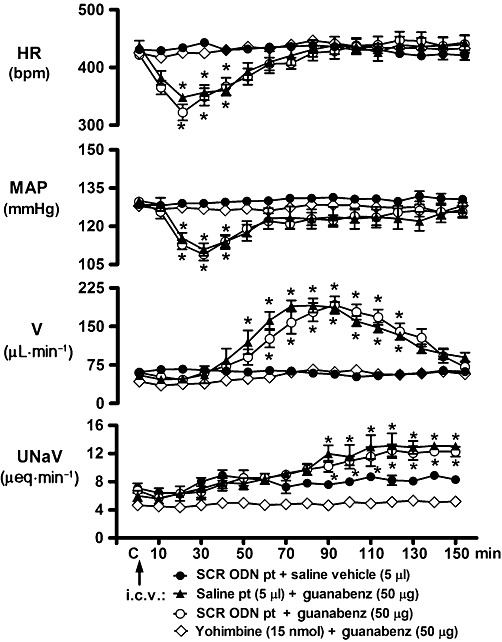
Effect of i.c.v. SCR ODN pre-treatment (pt) on the cardiovascular and renal excretory responses to central guanabenz administration in conscious male Sprague-Dawley rats. The values are means ± SEM and illustrate the cardiovascular and renal effects of i.c.v. guanabenz (50 µg) in six conscious rats per group that were pre-treated for 24 h with i.c.v. SCR ODN (25 µg in 5 µL) or isotonic saline vehicle (5 µL) or received an i.c.v. pre-treatment of the α2-adrenoceptor antagonist yohimbine (15 nmol) 10 min prior to the administration of i.c.v. guanabenz. V, urine flow rate. *P < 0.05, compared with respective group control value (designated C); one-way repeated measures anova.
Pre-treatment with SCR ODN (25 µg in 5 µL, i.c.v., for 24 h) did not alter baseline control cardiovascular or renal excretory parameters (shown as C; Figure 1) or animal body weight (SCR ODN, ▵+3 ± 2 g). As illustrated (Figure 1; open circles), SCR ODN pre-treatment did not alter either the duration or magnitude of the cardiovascular depressor or renal excretory responses to the central administration of guanabenz. Further, i.c.v. administration of isotonic saline (5 µL) did not alter any cardiovascular or renal parameter under investigation over the duration of the experimental protocol in SCR ODN-pre-treated animals (Figure 1).
Selective central ODN-mediated down-regulation of brain Gα-subunit protein expression
Immunoblotting studies revealed regional differences in the endogenous expression of Gα-subunits within the brain of male Sprague-Dawley rats. Gαo protein was expressed at equivalent levels in the BC and PVN, with lower levels detected in the VLM (Figure 2A). The expression of Gαi1 protein was greatest in BC with lower but comparable levels present in PVN and VLM tissue. Gαi3 protein was present at a low level in all three brain regions examined. BC tissue had low expression levels of both Gαi2 and Gαs subunit proteins; in contrast both the PVN and VLM exhibited high expression levels of these subunit proteins. SCR ODN pre-treatment did not alter brain Gα-subunit protein expression levels in any tissue examined (Figure 2A and B).
Figure 2.
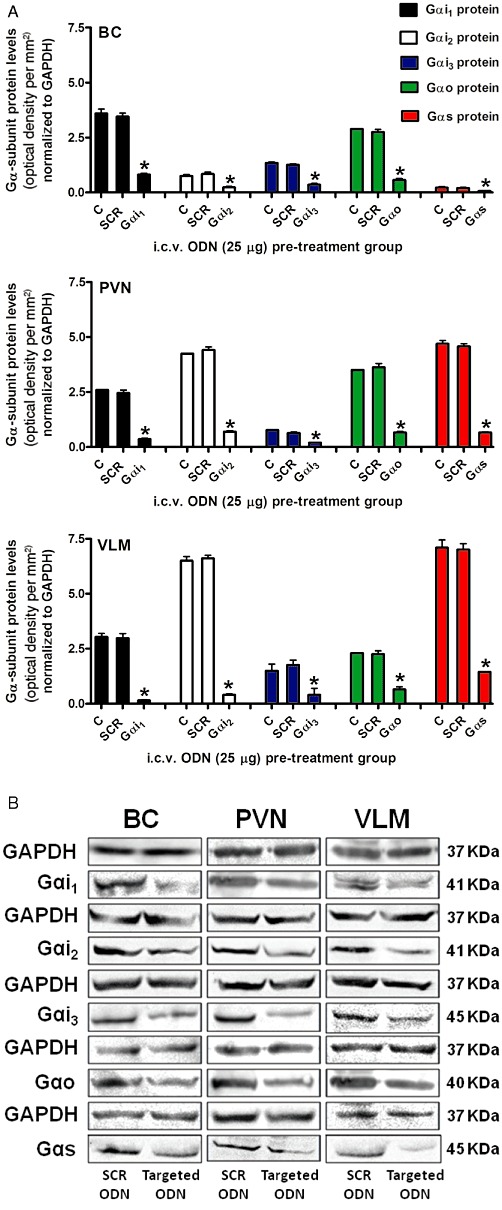
(A) Effect of central targeted Gα-subunit ODN pre-treatment on Gα-subunit protein levels normalized to GAPDH and expressed as optical density units per mm2 (mean ± SEM) in the BC, hypothalamic PVN and VLM of male Sprague-Dawley rats pre-treated (24 h) with i.c.v. either saline (5 µL) or a SCR (SCR), Gαi1, Gαi2, Gαi3, Gαo or Gαs ODN (25 µg in 5 µL each) (n = 6 per group), and (B) Representative immunoblots illustrating GAPDH, Gαi1, Gαi2, Gαi3, Gαo and Gαs subunit protein levels in the BC, PVN and VLM from male Sprague-Dawley rats pre-treated (24 h; i.c.v.) with either a selective Gα-subunit protein-targeted ODN sequence or a SCR ODN sequence (25 µg in 5 µL each). *P < 0.05, compared with i.c.v. SCR ODN-pre-treated animal group value; unpaired Student's t test.
As observed for SCR ODN pre-treatment, i.c.v. Gαi1, Gαi2, Gαi3, Gαo, or Gαs ODN pre-treatment (each 25 µg in 5 µL for 24 h) did not significantly alter animal body weight (Gαi1 ODN, ▵−3 ± 1 g; Gαi2 ODN, ▵+4 ± 3 g, Gαi3 ODN, ▵−3 ± 2 g, Gαo ODN, ▵−5 ± 3 g, Gαs ODN, ▵+ 2 ± 1 g) or baseline control levels for cardiovascular and renal excretory function (corresponding group values denoted ‘C’ in Figures 5 and 6, data not shown). Following 24 h central ODN pre-treatment, Gαi1, Gαi2, Gαi3, Gαo and Gαs ODN sequences reduced respective target Gα protein expression levels in the BC, PVN and VLM (Figure 2A and B). Gα subunit protein levels were reduced significantly, compared with both naïve animals and animals pre-treated i.c.v. with a non-specific control SCR ODN sequence, by Gα-subunit protein targeted ODN's in a sequence specific manner. ODN-mediated protein down-regulation resulted in at least 70% reduction in the respective target protein in the BC compared with naïve rats or SCR ODN-pre-treated animals. Down-regulation of target proteins was greater in PVN and VLM tissue with a reduction in protein expression of at least 80 and 85% in PVN and VLM tissue respectively. These findings demonstrate successful ODN dispersal throughout the brain and efficacy in reducing the target protein following a single i.c.v. injection at a dose of 25 µg following a pre-treatment time period of 24 h.
Figure 5.
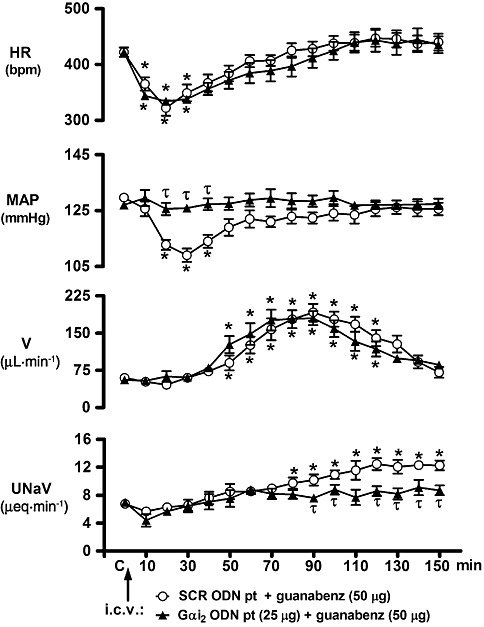
Effect of i.c.v. Gαi2 ODN pre-treatment on the cardiovascular and renal excretory responses to central guanabenz administration in conscious male Sprague-Dawley rats. The values are means ± SEM and illustrate the cardiovascular and renal effects of i.c.v. guanabenz (50 µg) in six conscious rats per group that were pre-treated (24 h) with i.c.v. SCR ODN (25 µg per 5 µL) or a Gαi2 ODN (25 µg in 5 µL). Data for SCR ODN pre-treatment (pt) are transposed from Figure 1 for comparison and clarity. Abbreviations as in Figure 1. *P < 0.05, compared with respective group control value (designated C); one-way repeated measures anova. τP < 0.05, compared with SCR ODN pt group value at respective time point; two-way repeated measures anova.
Figure 6.
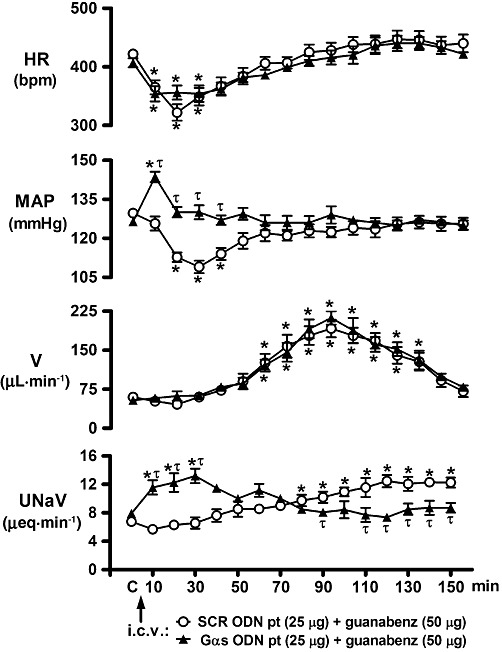
Effect of i.c.v. Gαs ODN pre-treatment on the cardiovascular and renal excretory responses to central guanabenz administration in conscious male Sprague-Dawley rats. The values are means ± SEM and illustrate the cardiovascular and renal effects of i.c.v. guanabenz (50 µg) in six conscious rats per group that were pre-treated (24 h) with i.c.v. SCR ODN (25 µg in 5 µL) or a Gαs ODN (25 µg in 5 µL). Data for SCR ODN pre-treatment (pt) are transposed from Figure 1 for comparison and clarity. Abbreviations as in Figure 1. *P < 0.05, compared with respective group control value (designated C);one-way repeated measures anova. τP < 0.05, compared with SCR ODN pt group value at respective time point; two-way repeated measures anova.
Additional immunoblotting studies were performed to confirm the in vivo selectivity of Gα-subunit targeted ODN sequences (Figure 3A and B). In these studies, brain tissue samples from all ODN-treated groups for which physiological data are presented in Figures 4–6 were examined for the expression of all non-targeted Gα-subunit proteins under investigation (i.e. Gαi1, Gαi2, Gαi3 and Gαo expression in a Gαs ODN-pre-treated group). As illustrated (Figure 3A and B), the central pre-treatment (25 µg, 24 h) of male Sprague-Dawley rats with a targeted Gα-subunit ODN sequence resulted in highly significant and selective target protein down-regulation.
Figure 3.
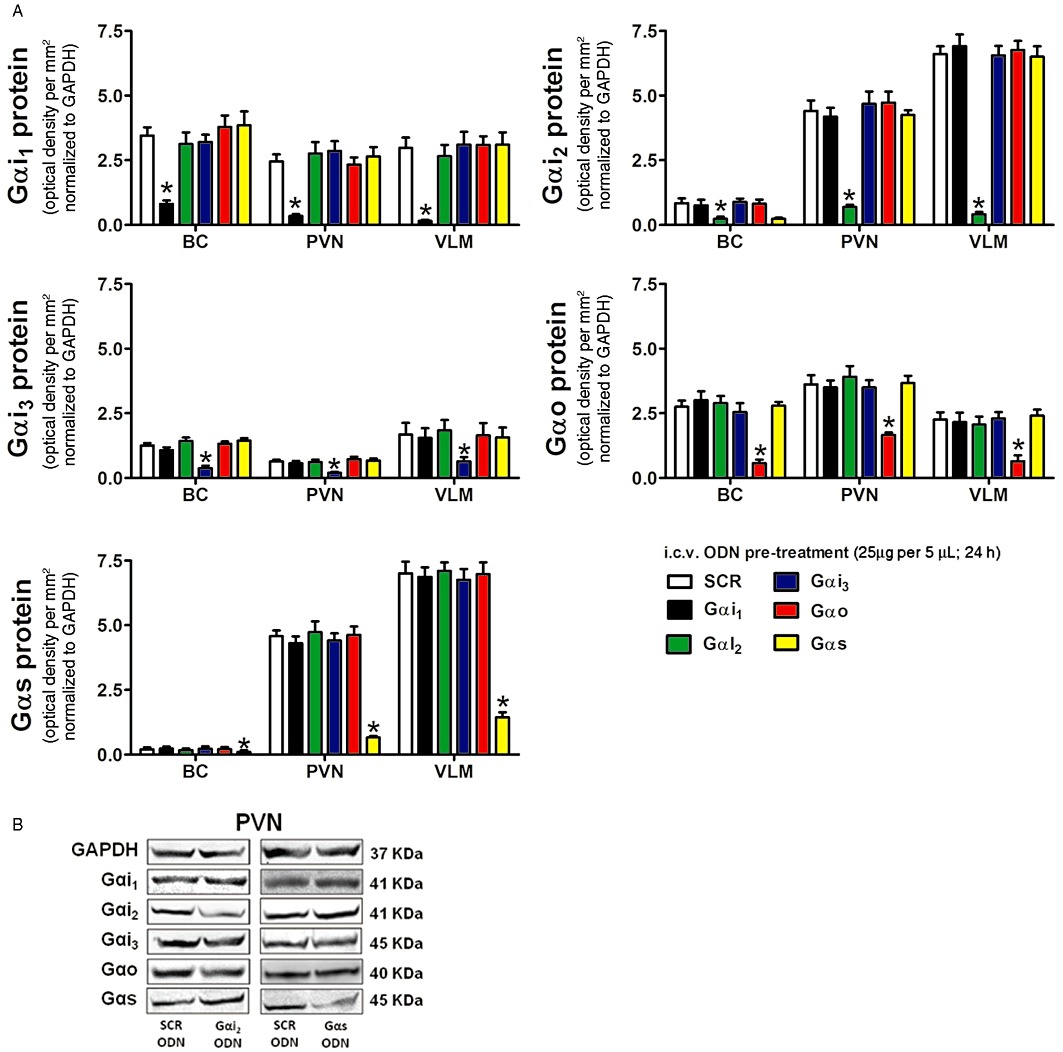
(A) Selectivity of central Gαi1, Gαi2, Gαi3, Gαo and Gαs targeted ODN pre-treatment on Gα-subunit protein levels normalized to GAPDH and expressed as optical density units per mm2 (mean ± SEM) in the BC, hypothalamic PVN and VLM of male Sprague-Dawley rats pre-treated (24 h; i.c.v.) with a SCR, Gαi1, Gαi2, Gαi3, Gαo or Gαs ODN (25 µg in 5 µL each) (n= 6 per group) *P < 0.05, compared with i.c.v. SCR ODN-pre-treated animal group value; one-way repeated measures anova and (B) representative immunoblots illustrating GAPDH, Gαi1, Gαi2, Gαi3, Gαo and Gαs subunit protein levels in the PVN from male Sprague-Dawley rats pre-treated (24 h; i.c.v.) with a Gαi2 or Gαs ODN sequence.
Figure 4.

(A) Peak HR depressor response in bpm and (B) peak MAP depressor response in mmHg produced by i.c.v. guanabenz (50 µg in 5 µL) in conscious male Sprague-Dawley rats pre-treated i.c.v. with either saline (24 h; 5 µL), yohimbine (10 min; 5.9 µg; 15 nmol) or a SCR, Gαi1, Gαi2, Gαi3, Gαo or Gαs ODN (24 h; 25 µg in 5 µL each) (n= 6 per group). The values are the mean ± SEM. *P < 0.05, compared with i.c.v. SCR ODN-pre-treated animal group value. **P < 0.01, compared with i.c.v. SCR ODN-pre-treated animal group value; one-way anova. Note that these data were obtained in the same animals for which protein expression data is presented in Figure 2.
Functionally selective effects of central Gα-subunit proteins in the cardiovascular depressor responses to central guanabenz
Following i.c.v. guanabenz injection, the peak changes in HR and MAP were typically observed 20 min and 30 min post injection, respectively, and were completely blocked by i.c.v. pre-treatment with the α2-adrenoceptor antagonist yohimbine (Figure 1, time course responses; Figure 4A and B, peak magnitudes). Pre-treatment of animals with a SCR ODN sequence did not alter the magnitude or timing of peak guanabenz-induced bradycardia or hypotension (Figure 4A and B). I.c.v. pre-treatment of rats with an ODN selective for Gαo was the only ODN pre-treatment that significantly reduced the magnitude of the central guanabenz-evoked bradycardia. I.c.v. pre-treatment with Gαi3 and Gαs ODN sequences resulted in an observable, but non-statistically significant, reduction (approximately 30%) in the magnitude of the bradycardic response (Figures 4A and 6). In contrast, central Gαi1 and Gαi2 ODN pre-treatments had no effect on guanabenz-evoked bradycardia.
I.c.v. pre-treatment with a Gαi1, Gαi3 or a Gαo ODN did not alter the peak hypotensive response to the central administration of guanabenz (Figure 4B). Further, although not shown, these ODN sequences did not alter the time course of the hypotensive response observed following central guanabenz administration, (illustrated in Figure 1) in saline and vehicle pre-treated animals. In contrast to these findings, central Gαi2 ODN pre-treatment completely abolished the hypotensive response to i.c.v. guanabenz. However, as demonstrated in Figures 4A and 5 (time course studies), central down-regulation of Gαi2 subunit proteins failed to prevent central guanabenz-evoked bradycardia. In contrast, in central Gαs ODN-pre-treated rats (Figures 4A and 6), i.c.v. guanabenz produced an immediate increase in MAP resulting in a significant hypertensive response. This was the opposite response to the characteristic hypotension produced by i.c.v. injection of guanabenz. As shown in Figure 6 (time course studies), the pressor response to i.c.v. guanabenz in Gαs pre-treated rats was of short duration with MAP returning to control levels by 20 min after agonist administration.
Functionally selective effects of central Gα-subunit proteins in the diuretic and natriuretic responses to central guanabenz
In both saline vehicle and SCR ODN-pre-treated groups, i.c.v. guanabenz produced a profound, but with delayed onset, diuresis, which in both groups featured an identical peak increase in urine output, was of a comparable magnitude and time course between the two experimental groups (Table 1 and Figure 1). Central pre-treatment with a Gαi1, Gαi2, Gαi3, Gαo, or a Gαs ODN did not alter the diuretic response to i.c.v. guanabenz. There were no significant differences observed between these experimental groups in cumulative urine output, peak change in urine output or, as illustrated for Gαi2 and Gαs pre-treatments (data from other groups not shown), the duration of the diuretic response (Table 1, Figures 5 and 6).
Table 1.
Effects of pre-treatment (i.c.v.) with ODNs (for Gαi1, Gαi2, Gαi3, Gαo, Gαs or SCR) on the diuretic and natriuretic responses to i.c.v. guanabenz in conscious Sprague-Dawley rats
| i.c.v. ODN pre-treatment (24 h; 25 µg) | Cumulative urine output (µL per 150 min) | Peak Δ urine output (µL·min−1) | Peak Δ UNaV (µeq·min−1) |
|---|---|---|---|
| Saline vehicle | 10 569 ± 619 | 136 ± 9 | 6.2 ± 0.8 |
| Scrambled (SCR) | 9 998 ± 496 | 132 ± 11 | 5.9 ± 0.6 |
| Gαi1 | 10 246 ± 513 | 153 ± 16 | 6.1 ± 0.8 |
| Gαi2 | 9 933 ± 361 | 125 ± 11 | 0.6 ± 0.3** |
| Gαi3 | 10 960 ± 691 | 143 ± 16 | 6.2 ± 0.9 |
| Gαo | 10 171 ± 514 | 127 ± 10 | 6.2 ± 0.5 |
| Gαs | 10 654 ± 461 | 150 ± 13 | 5.8 ± 0.6 |
The values are means ± SEM and illustrate the diuretic (expressed as cumulative urine output in µL per 150 min and peak change in urine output in µL·min−1) and natriuretic [expressed as peak change in urinary sodium excretion (UNaV) in µeq·min−1] effects of i.c.v. guanabenz (50 µg) in six conscious rats per group that were pre-treated (24 h) with an ODN (25 µg in 5 µL) or isotonic saline vehicle (5 µL).
P < 0.01, compared with SCR ODN pre-treatment group value.
Following central saline vehicle or SCR ODN pre-treatment, i.c.v. guanabenz evoked a significant and prolonged increase in UNaV (Figure 1, Table 1). Prior down-regulation of central Gαi1, Gαi3 or Gαo subunit proteins did not alter the magnitude (Table 1) or time course (data not shown) of the natriuretic response evoked by i.c.v. guanabenz. The natriuretic response to central administration of guanabenz was completely abolished in rats pre-treated with a Gαi2 ODN (Table 1). Although the peak natriuretic response to i.c.v. guanabenz was not altered in Gαs ODN-pre-treated rats (Table 1), the time course of the natriuresis was significantly altered in this treatment group (Figure 6). More specifically, in Gαs ODN-pre-treated rats the natriuresis produced by i.c.v. guanabenz was observed immediately following drug administration with the peak increase in UNaV observed at 30 min after drug administration (Figure 6). In this group, UNaV returned to pre-drug control levels 50 min after guanabenz injection and remained at basal levels for the remainder of the 150 min experimental protocol. As previously noted, this pattern of natriuresis is in contrast to the renal excretory response produced by central guanabenz in SCR ODN-pre-treated rats, in which the natriuresis was delayed in onset with a peak response at 120 min after agonist administration (Figures 1 and 6).
Discussion
Through the central administration of selective Gα-subunit protein-targeted ODNs, we have demonstrated that brain Gαi2 and Gαs subunit protein-gated signalling pathways are critical for mediating the hypotensive and natriuretic, but not bradycardic and diuretic, responses produced by the central administration of the α2-adrenoceptor agonist guanabenz in conscious rats. These observations provide compelling in vivo support for the pharmacological paradigm of functional selectivity (Patel et al., 2010). These findings also establish that in vivo, the physiological responses produced by the activation of a CNS GPCR, in this case, the α2-adrenoceptor, is greatly influenced by the availability and/or brain protein expression levels of individual downstream Gα-subunit proteins. Thus, following a single ligand–receptor interaction, multiple downstream signalling pathways are differentially activated to evoke multiple integrated cardiovascular versus renal excretory responses.
In vivo,α2-adrenoceptor agonists inhibit sympathetic outflow to many organs to facilitate the reported cardiovascular depressor and renal excretory responses produced by these GPCR ligands. Particularly well established is the relationship between the decrease in renal sympathetic nerve activity and the natriuretic response observed following central administration of different α2-adrenoceptor agonists (Grisk and DiBona, 1998; Huang and Leenen, 1998). Our observation that both the hypotensive and natriuretic responses to i.c.v. guanabenz are abolished in animals pre-treated centrally with Gαi2 ODN is of high physiological importance and highlights a key role for Gαi2 protein pathways in regulating MAP and UNaV post CNS α2-adrenoceptor activation. Further, this finding validates existing in vitro data (Remaury et al., 1993; Eason and Liggett, 1995) and confirms a role of Gαi2 -subunit proteins in mediating α2-adrenoceptor-evoked responses. The selectivity of this response profile to Gαi2 and not Gαi1, Gαi3 or Gαo-subunit proteins also suggests that this response is not conserved across Gαi/o subunit proteins. This selective response profile compares favourably with a previous in vivo study conducted in mice in which the antinoceptive effects of three different α2-adrenoceptor agonists administered i.c.v. were selectively mediated by Gαi3 -subunit proteins (Raffa et al., 1996). Although not examined in these studies, we speculate that prior down-regulation of central Gαi2 proteins in rats caused a failure of i.c.v. guanabenz to inhibit renal sympathetic nerve activity, the action of which is likely to be responsible for preventing the natriuretic response to this ligand. However, as shown by the concurrent blockade of the hypotensive response to i.c.v. guanabenz, it appears that down-regulation of central Gαi2 proteins may have also prevented the reduction in sympathetic nerve traffic to additional vascular beds (e.g. splanchnic) involved in the maintenance of systemic arterial MAP. In contrast, these data suggest that suppression of central sympathetic outflow to the heart is not impaired as shown by the classical bradycardic response that was still produced by i.c.v. gaunabenz in Gαi2-ODN-treated animals. In these studies, the bradycardia to i.c.v. guanabenz was significantly attenuated by Gαo subunit protein down-regulation, data that suggest a predominant role of Gαo-subunits in the guanabenz-evoked signalling pathways that mediate bradycardia. Taken in conjunction with our previous finding that the bradycardia evoked by i.c.v. administration of the opioid-like neuropeptide N/OFQ was abolished by pre-treatment with PTX (Wainford et al., 2008), which inhibits the activity of all Gαi/o subunit proteins, these data suggest that there is potential functional redundancy across Gα-subunit protein-gated pathways to elicit bradycardia following guanabenz administration. Alternatively, guanabenz evoked bradycardia may be mediated via a mechanism independent of GPCR Gα-subunit signalling (e.g. βγ or β-arrestin).
As discussed above, selective down-regulation of brain Gαi2 proteins completely prevented the hypotensive response to central α2-adrenoceptor stimulation in conscious rats. In the present investigation, we also observed the highly unexpected finding that the selective ODN-mediated down-regulation of brain Gαs-subunit proteins converted the classical hypotensive response to central α2-adrenoceptor activation into an immediate and profound hypertensive response. With regards to the immediate natriuresis observed in Gαs ODN-pre-treated animals, we believe that this physiological response was a result of the rapid elevation in MAP (i.e. a pressure natriuresis), which occurred immediately following i.c.v. guanabenz. Collectively, these findings provide strong in vivo evidence that Gα-subunit protein availability can significantly modulate the physiologically important cardiovascular and renal excretory responses to ligand binding, as has been predicted by in vitro studies and by mathematical modelling (Nasman et al., 2001; Hein, 2006).
In the present studies the cellular mechanism(s) by which prior down-regulation of brain Gαi2 or Gαs proteins altered the MAP responses to central guanabenz was not determined. However, in vitro experiments have demonstrated that the predominant cellular action of α2-adrenoceptors, which is mediated principally via Gαi2-subunit protein signal transduction, is the inhibition of adenylate cyclase activity (Remaury et al., 1993; Nasman et al., 2001; Hein, 2006). Therefore, we hypothesize that following stimulation of brain α2-adrenoceptors, activation of a downstream Gαi2-subunit protein-gated pathway triggers the suppression of central sympathetic outflow by a signalling pathway involving, at least in part, inhibition of adenylate cyclase activity. Based on this premise, down-regulation of Gαi2 proteins in the brain may abolish the central sympathoinhibitory and thus the hypotensive and natriuretic responses to central guanabenz by preventing the α2-adrenoceptor-coupled inhibition of cAMP. A functional coupling to Gαs has also been reported for α2-adrenoceptors, which is apparent at high agonist concentration or after inhibition of Gαi (Eason and Liggett, 1995; Wade et al., 1999). While this sequence of events is possible and has been suggested to participate in mediating the phenomenon of adenylate cyclase supersensitvitiy (Watts and Neve, 2005), it remains to be explained why i.c.v. guanabenz produced an immediate and profound hypertensive response in rats that had undergone prior down-regulation of brain Gαs proteins. It is also possible that the observed functional selectivity of Gαi2 and Gαs subunit proteins to mediate the hypotensive and natriuretic effects evoked by activation of brain α2-adrenoceptors reflects an alteration of the inhibition of Ca2+ channels in sympathetic neurons. As has been demonstrated in vitro, altered inhibition of Ca2+ channels in sympathetic neurones, mediated by adrenoceptors, can occur via PTX-sensitive (i.e. Gαi/o pathways) and non-PTX-sensitive (e.g. Gαs) pathways, or via modulation of the composition of the βγ protein dimer associated with the α-subunit (Delmas et al., 1999).
In contrast to our current findings is a recent report that whole body deletions of the genes encoding the Gαi(1–3) subunit proteins in mice did not alter the cardiovascular depressor effects (i.e. bradycardia and hypotension) evoked by peripheral administration of the α2-adrenoceptor agonist medetomidine (Albarran-Juarez et al., 2009). While the reasons for these contrasting findings have yet to be determined, the divergence may be partially explained by differences in species of animals tested, the mode by which removal/down-regulation of specific Gα-subunit proteins was achieved [e.g. transgenic whole body deletions of target genes vs. CNS delivery of targeted Gα-subunit ODNs), presence/absence of anaesthesia and route of administration (i.v. bolus vs. i.c.v. injection) of different α2-adrenoceptor agonists (medetomidine vs. guanabenz].
Following i.c.v. injection and circulation throughout the brain, it is likely that guanabenz activates Gαi2 / Gαs-subunit protein-gated pathways located within several brain sites involved in the regulation of cardiovascular and fluid/electrolyte homeostasis. This may include, but is not limited to, the PVN of the hypothalamus, the rostral VLM (RVLM) and the nucleus reticularis gigantocellularis (NRGC) (Chen and Chan, 1989; Menegaz et al., 2001). The neurons located within these established renal and cardiovascular control centres possess significant quantities of α2-adrenoceptors (Tavares et al., 1996), which may potentially be coupled to different Gα-subunit proteins as a mechanism to elicit differential physiological responses (i.e. functional selectivity). However, the current studies did not determine the locus of the reported Gαi2 and Gαs-subunit protein interactions within the CNS that occur downstream of the α2-adrenoceptor. Site-specific microinjection experiments (e.g. into discrete brain sites), which are beyond the scope of the current manuscript, will be conducted in subsequent experiments to establish the CNS sites in which individual Gα-subunit protein pathways act to produce the observed responses to central α2-adrenoceptor stimulation. An additional issue not addressed by the current studies is the subtype of α2-adrenoceptor (i.e. α2A, α2B or α2C) that is activated by i.c.v. guanabenz administration. The α2A receptor is widely distributed throughout the CNS, particularly in key cardiovascular control centres such as PVN, VLM and locus coeruleus (Scheinin et al., 1994), and has an established role in modulating neurotransmitter release (Lahdesmaki et al., 2004). Therefore, it is likely this subtype may play a predominant role in the observed responses. However, due to the expression of the α2B receptor in the thalamus and expression of the α2C-subtype, which can also modulate neurotransmitter release, in many brain sites (Scheinin et al., 1994), a role of these subtypes in the observed responses cannot be excluded.
In contrast to effects on MAP and UNaV, the prior ODN-mediated down-regulation of central Gαi/o or Gαs-subunit proteins did not alter the diuretic responses to i.c.v. guanabenz. This observation indicates that other signal transduction pathways are involved in mediating the effects of central α2-adrenoceptors on the renal handling of water. Our laboratory has previously established that exogenous (Wainford et al., 2008) or endogenous (Wainford and Kapusta, 2010) activation of central GPCR pathways can influence urine output via differentially altering the secretion of arginine vasopressin (AVP) through a pathway involving downstream central Gαz (inhibitory) and Gαq (stimulatory) subunit proteins. A significant component of the diuretic response elicited by central α2-adrenoceptor stimulation is mediated by the suppression of AVP secretion (Brooks et al., 1986; Cabral et al., 1998). Thus, the pattern of diuresis produced by central α2-adrenoceptors may also involve alterations in AVP secretion mediated via Gαz/Gαq-subunit protein-gated pathways.
In conclusion, these studies significantly advance our understanding of the functional selectivity of brain Gα-subunit protein-gated pathways in the physiological responses evoked by central α2-adrenoceptor activation. Using a targeted ODN approach in vivo, we demonstrated that the hypotensive and natriuretic responses to selective central α2-adrenoceptor activation are abolished by Gαi2 down-regulation or converted to a pressor response by Gαs down-regulation. In contrast, the profound α2-agonist-stimulated diuresis was unaltered by prior down-regulation of individual brain Gαi(1–3), Gαs or Gαo proteins. Collectively, these findings demonstrate that central Gα-subunit protein-gated pathways play a functionally selective role in producing differential cardiovascular versus renal excretory responses post-ligand binding at α2-adrenoceptors. In response to integrated physiological stimuli, selective activation of individual central Gα-subunit pathways may potentially play an important role in governing the endogenous regulation of systemic cardiovascular function and fluid and electrolyte homeostasis. Therefore, we suggest modulation of either the expression and/or activity of individual endogenous Gα-subunit proteins represents a target area for the future development of pharmacological therapies designed to alter systemic cardiovascular parameters (e.g. antihypertensive medications) versus renal excretory function (water diuretics, natriuretic compounds).
Acknowledgments
The research presented in this manuscript was funded to RDW by the American Heart Association Grant 09BGIA2250585 and the National Institute of Health Grant NHLBI HL 107330 to DRK by the National Institute of Health grants NIDDK DK43337, NHLBI HL 071212 and the American Heart Association grant 08552930 and to RDW/DRK by the NIH NCRR COBRE P20 RR018766 grant.
Glossary
- AVP
arginine vasopressin
- BC
brain cortex
- bpm
beats per minute
- HR
heart rate
- i.c.v.
intracerebroventricular
- MAP
mean arterial blood pressure
- N/OFQ
nociceptin/orphanin FQ
- NRGC
nucleus reticularis gigantocellularis
- ODN
oligodeoxynucleotide
- PTX
Pertussis toxin
- PVN
paraventricular nucleus
- SCR
scrambled
- UNaV
urinary sodium excretion
- V
urine flow rate
- VLM
ventrolateral medulla
Conflicts of interest
None.
References
- Albarran-Juarez J, Gilsbach R, Piekorz RP, Pexa K, Beetz N, Schneider J, et al. Modulation of alpha2-adrenoceptor functions by heterotrimeric Galphai protein isoforms. J Pharmacol Exp Ther. 2009;331:35–44. doi: 10.1124/jpet.109.157230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th Edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensimon M, Chang AI, de Bold ML, Ponce A, Carreras D, De Bold AJ. Participation of G proteins in natriuretic peptide hormone secretion from heart atria. Endocrinology. 2004;145:5313–5321. doi: 10.1210/en.2004-0698. [DOI] [PubMed] [Google Scholar]
- Brooks DP, Share L, Crofton JT. Central adrenergic control of vasopressin release. Neuroendocrinology. 1986;42:416–420. doi: 10.1159/000124480. [DOI] [PubMed] [Google Scholar]
- Cabral AD, Kapusta DR, Kenigs VA, Varner KJ. Central alpha2-receptor mechanisms contribute to enhanced renal responses during ketamine-xylazine anesthesia. Am J Physiol. 1998;275:R1867–R1874. doi: 10.1152/ajpregu.1998.275.6.R1867. [DOI] [PubMed] [Google Scholar]
- Chen CH, Chan SH. Involvement of postsynaptic alpha 2-adrenoceptors and guanine nucleotide-binding protein in guanabenz-induced cardiovascular suppressant effects in the rat. Neurosci Lett. 1989;105:183–188. doi: 10.1016/0304-3940(89)90034-7. [DOI] [PubMed] [Google Scholar]
- Degoute CS. Controlled hypotension: a guide to drug choice. Drugs. 2007;67:1053–1076. doi: 10.2165/00003495-200767070-00007. [DOI] [PubMed] [Google Scholar]
- Delmas P, Abogadie FC, Milligan G, Buckley NJ, Brown DA. βγ dimmers derived from Go and Gi proteins contribute different components of adrenergic inhibition of Ca2+ channels in rat sympathetic neurons. J Physiol. 1999;518:23–36. doi: 10.1111/j.1469-7793.1999.0023r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eason MG, Liggett SB. Identification of a Gs coupling domain in the amino terminus of the third intracellular loop of the α2A-adrenergic receptor. Evidence for distinct structural determinants that confer Gs versus Gi coupling. J Biol Chem. 1995;270:24753–24760. doi: 10.1074/jbc.270.42.24753. [DOI] [PubMed] [Google Scholar]
- Gellai M, Edwards RM. Mechanism of alpha 2-adrenoceptor agonist-induced diuresis. Am J Physiol. 1988;255:F317–F323. doi: 10.1152/ajprenal.1988.255.2.F317. [DOI] [PubMed] [Google Scholar]
- Grisk O, DiBona GF. Influence of arterial baroreceptors and intracerebroventricular guanabenz on synchronized renal nerve activity. Acta Physiol Scand. 1998;163:209–218. doi: 10.1046/j.1365-201x.1998.00357.x. [DOI] [PubMed] [Google Scholar]
- Hadjimarkou MM, Silva RM, Rossi GC, Pasternak GW, Bodnar RJ. Feeding induced by food deprivation is differentially reduced by G-protein alpha-subunit antisense probes in rats. Brain Res. 2002;955:45–54. doi: 10.1016/s0006-8993(02)03361-9. [DOI] [PubMed] [Google Scholar]
- Hein L. Adrenoceptors and signal transduction in neurons. Cell Tissue Res. 2006;326:541–551. doi: 10.1007/s00441-006-0285-2. [DOI] [PubMed] [Google Scholar]
- Huang BS, Leenen FH. Brain ‘ouabain’ mediates the sympathoexcitatory and hypertensive effects of high sodium intake in Dahl salt-sensitive rats. Circ Res. 1994;74:586–595. doi: 10.1161/01.res.74.4.586. [DOI] [PubMed] [Google Scholar]
- Huang BS, Leenen FH. Both brain angiotensin II and ‘ouabain’ contribute to sympathoexcitation and hypertension in Dahl S rats on high salt intake. Hypertension. 1998;32:1028–1033. doi: 10.1161/01.hyp.32.6.1028. [DOI] [PubMed] [Google Scholar]
- Kapusta DR, Dayan LA, Kenigs VA. Nociceptin/orphanin FQ modulates the cardiovascular, but not renal, responses to stress in spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 2002;29:254–259. doi: 10.1046/j.1440-1681.2002.03639.x. [DOI] [PubMed] [Google Scholar]
- Lahdesmaki J, Sallinen J, MacDonald E, Scheinin M. Alpha2A-Adrenoceptors are important modulators of the effects of d-amphetamine on startle reactivity and brain monoamines. Neuropsychopharmacology. 2004;29:1282–1293. doi: 10.1038/sj.npp.1300428. [DOI] [PubMed] [Google Scholar]
- Menegaz RG, Kapusta DR, Mauad H, Cabral AM. Activation of alpha(2)-receptors in the rostral ventrolateral medulla evokes natriuresis by a renal nerve mechanism. Am J Physiol. 2001;281:R98–R107. doi: 10.1152/ajpregu.2001.281.1.R98. [DOI] [PubMed] [Google Scholar]
- Miggin SM, Lawler OA, Kinsella BT. Palmitoylation of the human prostacyclin receptor. Functional implications of palmitoylation and isoprenylation. J Biol Chem. 2003;278:6947–6958. doi: 10.1074/jbc.M210637200. [DOI] [PubMed] [Google Scholar]
- Nasman J, Kukkonen JP, Ammoun S, Akerman KE. Role of G-protein availability in differential signaling by alpha 2-adrenoceptors. Biochem Pharmacol. 2001;62:913–922. doi: 10.1016/s0006-2952(01)00730-4. [DOI] [PubMed] [Google Scholar]
- Olianas MC, Dedoni S, Onali P. Proteinase-activated receptors 1 and 2 in rat olfactory system: layer-specific regulation of multiple signaling pathways in the main olfactory bulb and induction of neurite retraction in olfactory sensory neurons. Neuroscience. 2007;146:1289–1301. doi: 10.1016/j.neuroscience.2007.02.059. [DOI] [PubMed] [Google Scholar]
- Patel CB, Noor N, Rockman HA. Functional selectivity in adrenergic and angiotensin signalling systems. Mol Pharmacol. 2010;78:983–992. doi: 10.1124/mol.110.067066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th edn. New York: Academic Press; 1998. [Google Scholar]
- Raffa RB, Connelly CD, Chambers JR, Stone DJ. α-subunit G-protein antisense oligodeoxynucleotide effects on supraspinal (i.c.v.) α2-adrenoceptor antinocieption in mice. Life Sci. 1996;58:PL77–PL80. doi: 10.1016/0024-3205(95)02301-1. [DOI] [PubMed] [Google Scholar]
- Remaury A, Larrouy D, Daviaud D, Rouot B, Paris H. Coupling of the alpha 2-adrenergic receptor to the inhibitory G-protein Gi and adenylate cyclase in HT29 cells. Biochem J. 1993;292:283–288. doi: 10.1042/bj2920283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi GC, Standifer KM, Pasternak GW. Differential blockade of morphine and morphine-6 beta-glucuronide analgesia by antisense oligodeoxynucleotides directed against MOR-1 and G-protein alpha subunits in rats. Neurosci Lett. 1995;198:99–102. doi: 10.1016/0304-3940(95)11977-5. [DOI] [PubMed] [Google Scholar]
- Ruffolo RR, Jr, Nichols AJ, Stadel JM, Hieble JP. Structure and function of alpha-adrenoceptors. Pharmacol Rev. 1991;43:475–505. [PubMed] [Google Scholar]
- Scheinin M, Lomasney JW, Hayden-Hixson DM, Schambra UB, Caron MG, Lefkowitz RJ, et al. Distribution of alpha 2-adrenergic receptor subtype gene expression in rat brain. Brain Res Mol Brain Res. 1994;21:133–149. doi: 10.1016/0169-328x(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Standifer KM, Rossi GC, Pasternak GW. Differential blockade of opioid analgesia by antisense oligodeoxynucleotides directed against various G protein alpha subunits. Mol Pharmacol. 1996;50:293–298. [PubMed] [Google Scholar]
- Tavares A, Handy DE, Bogdanova NN, Rosene DL, Gavras H. Localization of alpha 2A- and alpha 2B-adrenergic receptor subtypes in brain. Hypertension. 1996;27:449–455. doi: 10.1161/01.hyp.27.3.449. [DOI] [PubMed] [Google Scholar]
- Wade SM, Lim WK, Lan KL, Chung DA, Namamori M, Neubig RR. G(i) activator region of alpha(2A)-adrenergic receptors: distinct basic residues mediate G(i) versus G(s) activation. Mol Pharmacol. 1999;56:1005–1013. doi: 10.1124/mol.56.5.1005. [DOI] [PubMed] [Google Scholar]
- Wainford RD, Kapusta DR. Chronic high-NaCl intake prolongs the cardiorenal responses to central N/OFQ and produces regional changes in the endogenous brain NOP receptor system. Am J Physiol. 2009;296:R280–R288. doi: 10.1152/ajpregu.00096.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainford RD, Kapusta DR. Hypothalamic paraventricular nucleus G alpha q subunit protein pathways mediate vasopressin dysregulation and fluid retention in salt-sensitive rats. Endocrinology. 2010;151:5403–5414. doi: 10.1210/en.2010-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainford RD, Kurtz K, Kapusta DR. Central G-alpha subunit protein-mediated control of cardiovascular function, urine output, and vasopressin secretion in conscious Sprague-Dawley rats. Am J Physiol. 2008;295:R535–R542. doi: 10.1152/ajpregu.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts VJ, Neve KA. Sensitization of adenylate cyclase by Galpha i/o-coupled receptors. Pharmacol Ther. 2005;106:405–421. doi: 10.1016/j.pharmthera.2004.12.005. [DOI] [PubMed] [Google Scholar]


