Abstract
BACKGROUND AND PURPOSE
Transient receptor potential vanilloid 4 (TRPV4) is a Ca2+-permeable channel with multiple modes of activation. Apigenin is a plant-derived flavone, which has potential preventive effects on the development of cardiovascular disease. We set out to explore the effects of apigenin on TRPV4 channel activity and its role in vasodilatation.
EXPERIMENTAL APPROACH
The effects of apigenin (0.01–30 µM) on TPRV4 channels were investigated in HEK293 cells over-expressing TRPV4, rat primary cultured mesenteric artery endothelial cells (MAECs) and isolated small mesenteric arterial segments using whole-cell patch clamp, fluorescent Ca2+ imaging, intracellular recording and pressure myography.
KEY RESULTS
Whole-cell patch clamp and fluorescent Ca2+ imaging in HEK cells over-expressing TRPV4 showed that apigenin concentration-dependently stimulated the TRPV4-mediated cation current and Ca2+ influx. In MAECs, apigenin stimulated Ca2+ influx in a concentration-dependent manner. These increases in cation current and Ca2+ influx were markedly inhibited by TRPV4-specific blockers and siRNAs. Furthermore, pressure myography and intracellular recording in small third-order mesenteric arteries showed that apigenin dose-dependently evoked smooth muscle cell membrane hyperpolarization and subsequent vascular dilatation, which were significantly inhibited by TRPV4-specific blockers. TRPV4 blocker or charybdotoxin (200 nM) plus apamin (100 nM) diminished the apigenin-induced dilatation.
CONCLUSION AND IMPLICATIONS
This is the first study to demonstrate the selective stimulation of TRPV4 by apigenin. Apigenin was found to activate TRPV4 channels in a dose-dependent manner in HEK cells over-expressing TRPV4 and in native endothelial cells. In rat small mesenteric arteries, apigenin acts on TRPV4 in endothelial cells to induce EDHF-mediated vascular dilatation.
Keywords: apigenin, TRPV4, endothelial cell, vasodilatation
Introduction
TRPV4, a member of the transient receptor potential (TRP) superfamily of non-selective cation channels, has six transmembrane-spanning domains with a putative pore region (Nilius et al., 2003). The channel is widely expressed in a variety of tissues, including brain, skin, smooth muscle, kidney, liver and vascular endothelium, where it has diverse functional roles (Nilius et al., 2004; Plant and Strotmann, 2007; Pan et al., 2008b).
TRPV4 is characterized by multimodal activation properties. It can be activated by hypotonic cell swelling (Arniges et al., 2004), moderate heat (Vriens et al., 2004), the synthetic phorbol ester 4α-phorbol 12,13-didecanoate (Watanabe et al., 2002; 2003; Kottgen et al., 2008), arachidonic acid and its metabolite epoxyeicosatrienoic acid (Vriens et al., 2005). It is also involved in sensing flow (Kohler et al., 2006; Loot et al., 2008). TRPV4 is activated by flow shear stress and is involved in flow sensing in vascular endothelial cells and renal epithelial cells (Kohler et al., 2006; Wu et al., 2007; Ma et al., 2010a,b).
Flavonoids are a group of small molecules derived from plant-based compounds with the common flavone (2-phenyl-g-benzopyrone) structure. They have received considerable attention for their potential properties in protecting against cancer, heart disease and other pathological conditions. Apigenin (4′,5,7-trihydroxyflavone) is one of the most widely studied flavones. Evidence shows that it is a potent inhibitor of cell proliferation and angiogenesis (Fotsis et al., 1997). Besides these anti-tumour properties, apigenin also improves endothelial function by increasing endothelial NOS activity (Olszanecki et al., 2002). The increase in NO bioavailability, together with its anti-inflammatory properties, makes apigenin an interesting potential therapeutic option for the prevention of cardiovascular disease. Moreover, apigenin has a stimulating effect on the cystic fibrosis transmembrane conductance regulator (Illek et al., 2000) and the Na+/K+/2Cl- co-transporter (Niisato et al., 1999). However, to our knowledge, there is no report about the effect of apigenin on a TRP cation channel.
In the present study, we used live-cell fluorescent calcium imaging, patch clamp, intracellular microelectrode recording and pressure myography to study the effects of apigenin on TRPV4 channels and vascular tension. We found that apigenin activated TRPV4 channels in HEK cells over-expressing TRPV4 and in native endothelial cells. Furthermore, apigenin dose-dependently acted through TRPV4 to relax rat small mesenteric arteries.
Methods
Cell preparation and culture
Primary mesenteric arterial endothelial cells (MAECs) were isolated from male Sprague–Dawley rats of 250–300 g as described elsewhere (Ma et al., 2010b). Briefly, rats were placed in a chamber and killed by asphyxiation in carbon dioxide. The abdomen was opened, and the heart was perfused with PBS to remove circulating blood from the vessels. The small intestine was dissected out, and all the vein branches of the mesenteric bed were rapidly excised. The remaining arterial branches were digested with 0.02% collagenase in endothelial cell basal medium for 45 min at 37°C. After centrifugation at 1600×g for 5 min, the pelleted cells were re-suspended in endothelial cell growth medium supplemented with 1% bovine brain extract and plated in a flask. Non-adherent cells were removed 1 h later. The adherent endothelial cells were cultured at 37°C with 5% CO2 for 3–5 days. These cells were used for experiments without further passage. The identity of endothelial cells was verified by immunostaining with an antibody against von Willebrand's factor.
HEK293 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 µg·mL−1 penicillin and 100 U·mL−1 streptomycin.
Cloning and transfection
The mouse TRPV4 gene (NM_022017) was a gift from Dr Bernd Nilius, Belgium. HEK cells were transfected using Lipofectamine 2000 (Invitrogen, Grand Island, NY, USA). MAECs were transfected with TRPV4 siRNAs or a scrambled siRNA as negative control or GAPDH siRNA as positive control by electroporation using Nucleofector II following the procedure in the manufacturer's instruction manual. The sequences for rat TRPV4-siRNA were AGUCCUUGAACUUGCGAGACAGGUG (sense strand) and CACCUGUCUCGCAAGUUCAAGGACU (antisense strand) of siTRPV4-1, which are a 100% match to the rat but not to the mouse TRPV4 gene (22/25nt) or AUCUCAUGGCGGUUCUCGAUCUUGC (sense strand) and GCAAGAUCGAGAACCGCCAUGAGAU (antisense strand) of siTRPV4-2 from Invitrogen. siRNA rescue experiments were performed by co-transfection of mouse TRPV4 plasmid with rat siTRPV4-1 into rat endothelial cells. About 80% of HEK293 cells and ∼70% of MAECs were successfully transfected by these protocols as indicated by control transfection using a GFP-expressing pCAGGS vector. Functional studies were performed 2–3 days post transfection. The effect of siRNAs on cell viability was checked by 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
Immunoblots
Immunoblots were performed as described elsewhere (Kwan et al., 2004). The whole-cell lysates from MAECs and TRPV4 over-expressing HEK cells were extracted with detergent extraction buffer, which contained 1% (v/v) Nonidet P-40, 150 mM NaCl, 20 mM Tris–HCl, pH 8.0, with addition of protease inhibitor cocktail tablets. Proteins were resolved on 8% SDS/PAGE gels and then transferred to PVDF membranes. The membranes were incubated at 4°C overnight with primary anti-TRPV4 (1:200) or anti-GAPDH (1:200) or anti-β-tubulin (1:200) in PBST containing 0.1% Tween 20 and 5% non-fat dry milk. Immunodetection was accomplished using horseradish peroxidase-conjugated secondary antibody, followed by electrochemiluminescence detection.
[Ca2+]i measurement
HEK cells and rat MAECs were loaded with 10 µM Fura-2/AM (Ma et al., 2010a). Normal physiological saline solution (NPSS) containing (in mM) 140 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 glucose, 5 HEPES, pH 7.4. Fura-2 fluorescence signals were measured at room temperature (∼23°C) using dual excitation at 340 and 380 nm using an Olympus fluorescence imaging system (Olympus, Tokyo, Japan). Fura-2 ratio change (340/380) was then converted to [Ca2+]i based on the calibration using a calibration kit (Invitrogen).
Pressure myography
For the pressure myographic studies, phenylephrine (0.1–4 µM, concentration varied to achieve similar constriction in different arteries) was used to preconstrict the artery to 55–70% of its initial diameter (Liu et al., 2006). The artery was transferred to a pressure myograph (Model 110P, Danish Myotechnology Aarhus, Denmark) filled with oxygenated Krebs solution at 37°C. The external diameter of the artery was recorded continuously with a CCD camera using MyoView software (Danish Myotechnology). The endothelium was mechanically removed by rubbing the luminal surface of the ring several times with a small stainless steel wire (40 µm diameter). To confirm that the responses were mediated by EDHF, ACh-induced relaxation were tested in the presence of charybdotoxin (ChTX, 200 nM) and apamin (100 nM), which are known to inhibit the intermediate- and small-conductance calcium activated potassium channels (IKCa and SKCa, respectively) localized in the endothelium. These inhibitors were added to the bath medium 30 min before the second application of ACh. The functional removal of the endothelium was verified if the ring failed to relax in response to 1 µM ACh.
Membrane potential measurement of smooth muscle cells in arterial segments using microelectrodes
Briefly, segments of rat mesenterial arteries were dissected into ∼3 mm rings. The arterial segments were opened longitudinally and equilibrated for 60 min in Krebs solution oxygenated with a gas mixture of 95% O2 and 5% CO2. Membrane potential was measured using glass microelectrodes filled with 3 M KCl (resistance: 40–60 MΩ) in the presence of Nw-nitro-l-arginine methyl ester (l-NAME; 100 µM) plus indomethacin (Indo; 10 µM). Electrodes were inserted into smooth muscle cells from the outside surface. Successful impalement of a cell was indicated by an abrupt drop in voltage, followed by a sharp return to baseline on exit. Electrical signals were monitored continuously by an EPC9 amplifier (HEKA) with Pulse software (HEKA Electronic Inc., Lambrecht, Germany).
Whole-cell patch clamp
Whole-cell current was measured with an EPC-9 patch clamp amplifier as described elsewhere (Voets et al., 2002). The pipette solution contained (in mM) 20 CsCl, 100 Cs+-aspartate, 1 MgCl2, 4 ATP, 0.08 CaCl2, 10 BAPTA, 10 HEPES, pH 7.2. The bath solution contained (in mM) 150 NaCl, 6 CsCl, 1 MgCl2, 1.5 CaCl2, 10 glucose, 10 HEPES, pH 7.4.
Cells were clamped at 0 mV. Whole-cell current density (pA/pF) was recorded in response to successive voltage pulses of +80 and −80 mV for 100 ms. These current values were then plotted against time. The recordings were made before and after apigenin application. All currents were sampled at 50 kHz and filtered at 5 kHz, and the data were analysed with PulseFit (HEKA Electronics Inc.). The electrophysiological experiments were performed at room temperature.
Materials
HEK293 was from ATCC (Manassas, VA, USA). Apigenin (≥99%) was from Sigma (St. Louis, MO, USA); endothelial cell growth medium, endothelial cell basal medium and bovine brain extract were from Lonza, Inc. (Basel, Switzerland) Fura-2/AM and pluronic F127 were from Molecular Probes Inc. (Eugene, OR, USA) Scrambled siRNA, TRPV4 siRNAs, GAPDH siRNA, GAPDH and β-tubulin antibodies were from Invitrogen. RN1734 was from Menai Organics Ltd. (Bangor, Gwynedd, UK), and HC067047 was from Tocris (Bristol, UK). 4α-Phorbol 12,13-didecanoate (4α-PDD) was from Calbiochem (Darmstadt, Germany). Anti-TRPV4 (ACC-034) was from Alomone Labs Ltd. (Jerusalem, Israel). Luteolin (≥98%), tangeritin (≥98%), chrysin (≥98%) and baicalein (≥98%) were from Wingkinco Ltd. (Yongjian, China). Fura-2 was from Molecular Probes Inc. Endothelial cell growth medium and bovine brain extract were from Lonza Inc.
Data analysis
Data are presented as mean ± SEM or the mean and 95% confidence limits (CI) in parentheses. The concentration of the vasorelaxant producing a half-maximal response (EC50) was determined by curve fitting with Hill's equation using Prism GraphPad 4.0 software (GraphPad Software Inc., San Diego, CA). Statistical comparisons were made using one-way anova followed by the Newman–Keuls test. P < 0.05 was taken as significant.
Results
Apigenin stimulates TRPV4-mediated cation current in HEK cells over-expressing TRPV4
TRPV4 cDNA was transiently transfected into HEK cells, and they had a higher TRPV4 protein level on Western blots than vector-transfected cells (Figure 1A). We tested a range of apigenin concentrations (0.01–30 µM) on TRPV4 channel activity. In whole-cell patch clamp recordings, bath application of apigenin dose-dependently induced an outward current at +80 mV and an inward current at −80 mV with EC50 value of 4.32 µM (3.62–5.15, 95% CI) in cells over-expressing TRPV4 (Figure 1B,C). TRPV4 blockers, either ruthenium red (5 µM), RN1734 (6 µM) (Vincent et al., 2009) or HC067047 (0.5 µM) (Everaerts et al., 2010), abolished the currents (Figure 1D). In control experiments, apigenin failed to increase the whole-cell current in vector-transfected HEK cells (Figure 1E). Addition of vehicle (0.1% DMSO) to HEK cells over-expressing TRPV4 also had no effect on the whole-cell current (Figure 1D).
Figure 1.
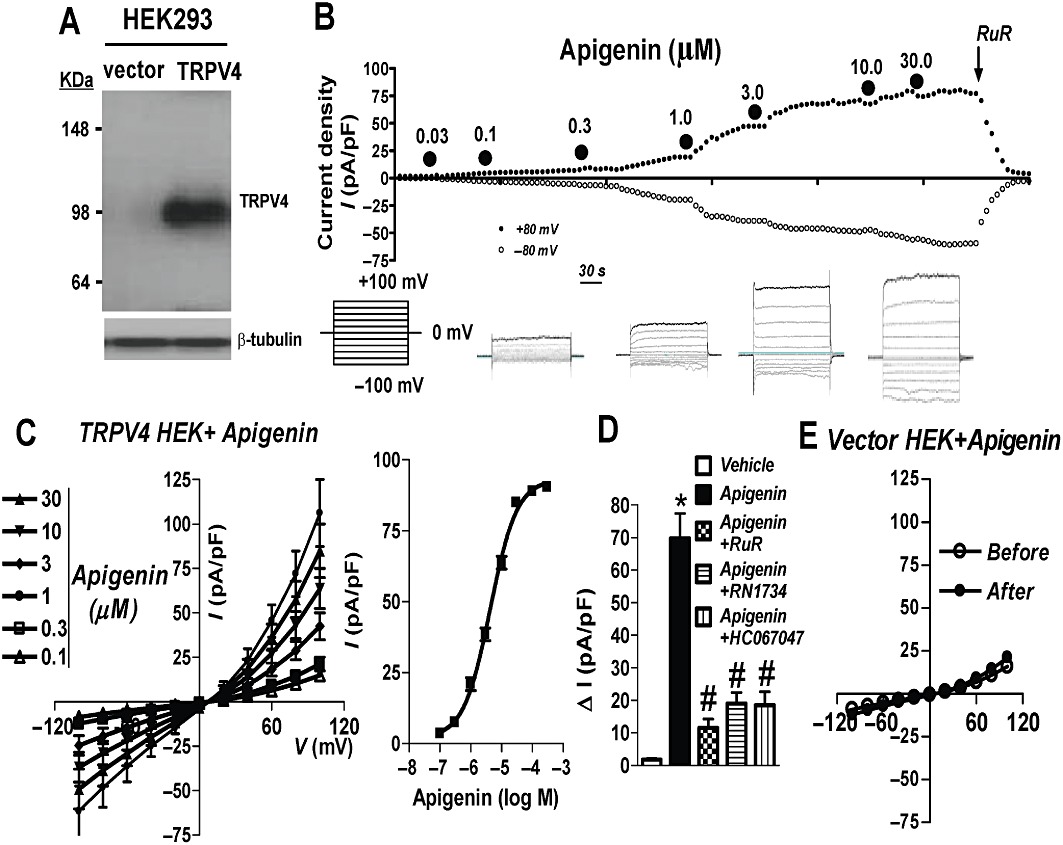
Apigenin stimulates TRPV4-mediated cation current in TRPV4-over-expressing HEK cells. (A) Representative immunoblot probed with anti-TRPV4 and anti-β-tubulin (n = 3). Left, vector-transfected HEK cells; right, TRPV4-transfected HEK cells. (B) upper panel, representative time course of whole-cell current in a TRPV4-over-expressing HEK cell. Current was sampled every 10 s at +80 and −80 mV. Apigenin and ruthenium red (RuR) were applied to the bath at the time points indicated by the filled circles and the arrow. Lower panel, representative traces showing voltage protocol and corresponding whole-cell currents of apigenin in HEK cells over-expressing TRPV4. (C) Left panel, current–voltage relationships as in (B). Right panel, plot of concentration–response with curve fitting to determine the apigenin EC50. (D) Summary data showing the whole-cell current in response to apigenin in the presence of TRPV4 blockers RuR (5 µM) or RN1734 (6 µM) or HC067047 (0.5 µM) (15–30 min incubation before apigenin application). Vehicle, 0.1% DMSO. (E) Current–voltage relationships in vector-transfected HEK cells. Values are presented as mean ± SEM (n = 5–8). #P < 0.05 compared with apigenin. *P < 0.05 compared with vehicle.
Apigenin stimulates a TRPV4-mediated increase in [Ca2+]i in HEK cells over-expressing TRPV4
Fluorescence Ca2+ imaging was used to test the effect of apigenin on TRPV4-mediated Ca2+ influx. Bath application of apigenin (0.01–30 µM) elicited an increase in [Ca2+]i in cells over-expressing TRPV4 in a concentration-dependent manner (Figure 2A,B). Analysis of the concentration–response curve yielded an EC50 value of 4.55 µM (3.56–5.81, 95% CI) (Figure 2C). Extracellular TRPV4 blockers, either ruthenium red (5 µM), or RN1734 (6 µM) or HC067047 (0.5 µM), abolished the apigenin-induced [Ca2+]i rises (Figure 2C). In control experiments, apigenin failed to increase [Ca2+]i in vector-transfected HEK cells (Figure 2E). Addition of vehicle (0.1% DMSO) to HEK cells over-expressing TRPV4 also had no effect on [Ca2+]i level (Figure 2C).
Figure 2.
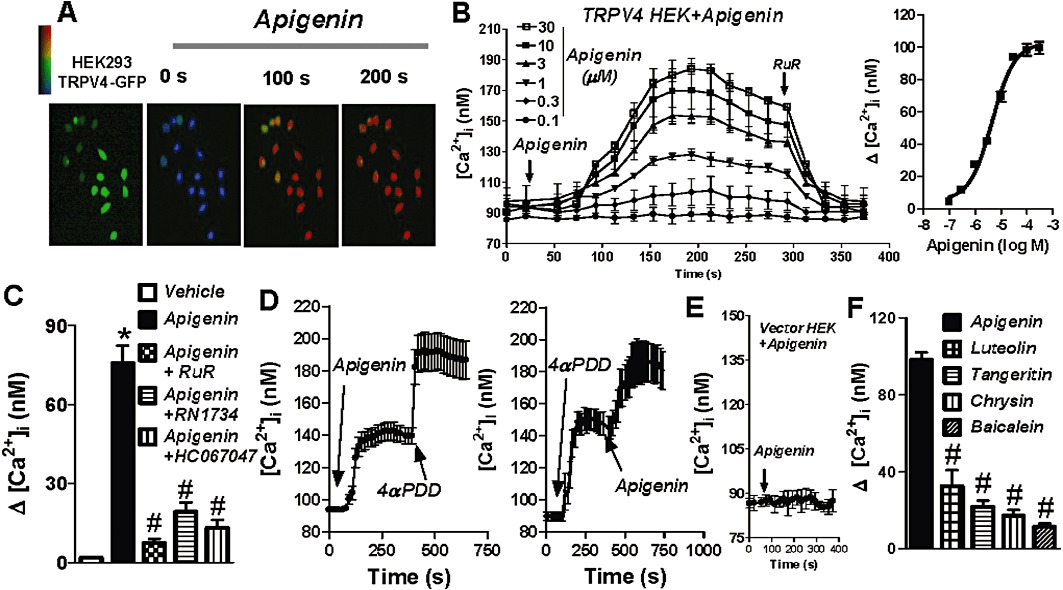
Apigenin stimulates a TRPV4-mediated [Ca2+]i rise in HEK cells over-expressing TRPV4. Representative images (A) and time course of [Ca2+]i change (B) in TRPV4-transfected HEK cells in response to different concentrations of apigenin. Cells were bathed in NPSS. Apigenin and RuR were applied to the bath at the time points indicated by the arrow. Right panel in (B), plot of concentration–response with curve fitting to determine the apigenin EC50. (C) Summary data showing the maximal [Ca2+]i rise in response to apigenin in the presence of the TRPV4 blockers, RuR (5 µM) or RN1734 (6 µM) or HC067047 (0.5 µM) (15–30 min incubation before apigenin application). Vehicle, 0.1% DMSO. (D) Representative time course of [Ca2+]i change in TRPV4-transfected HEK cells. Apigenin (4.5 µM) and 4α-PDD (1 µM) were applied to the bath at the time points indicated by arrows. (E) [Ca2+]i changes in vector-transfected HEK cells. (F) Summary data showing the maximal [Ca2+]i rise in response to the series of flavones apigenin, luteolin, tangeritin, chrysin and baicalein. Values are presented as mean ± SEM (n = 4–5, 8–20 cells per experiment). #P < 0.05 compared with apigenin. *P < 0.05 compared with vehicle.
Furthermore, if HEK cells over-expressing TRPV4 were pretreated with the TRPV4 agonist 4α-PDD (1 µM), which increased [Ca2+]i, subsequent addition of apigenin (4.5 µM) evoked a further increase in [Ca2+]i (Figure 2D). Conversely, if the cells were pretreated with apigenin (4.5 µM), 4α-PDD (1 µM) still elicited a further increase in [Ca2+]i (Figure 2D). These results suggested that apigenin and 4α-PDD share different activation sites on TRPV4.
Four other flavones, luteolin, baicalein, tangeritin and chrysin, were selected to examine their effects on the TRPV4 channel in HEK cells over-expressing TRPV4 by use of fluorescent Ca2+ imaging experiments. The results show that all four of them stimulated the TRPV4 channel inducing a significantly smaller maximal increase in [Ca2+]i than apigenin (Figure 2F).
Apigenin activates TRPV4 channels in native endothelial cells
TRPV4 is expressed in primary cultures of MAECs (Ma et al., 2010b). This was confirmed in the present study by Western blot of cultured MAECs using a TRPV4-specific antibody (Figure 3A). In functional studies, bath application of apigenin (0.01–30 µM) elevated [Ca2+]i in these cells in a concentration-dependent manner with an EC50 value of 4.30 µM (3.01–6.15, 95% CI) (Figure 3B–D). To further explore the role of TRPV4 in the apigenin-induced [Ca2+]i rises in native endothelial cells, firstly, extracellular TRPV4 activator and blockers were used. Bath application of TRPV4-specific agonist, 4αPDD (1 µM), increased [Ca2+]i. Either ruthenium red (5 µM), RN1734 (6 µM) or HC067047 (0.5 µM) abolished the apigenin-induced increase in [Ca2+]i (Figure 3E).
Figure 3.
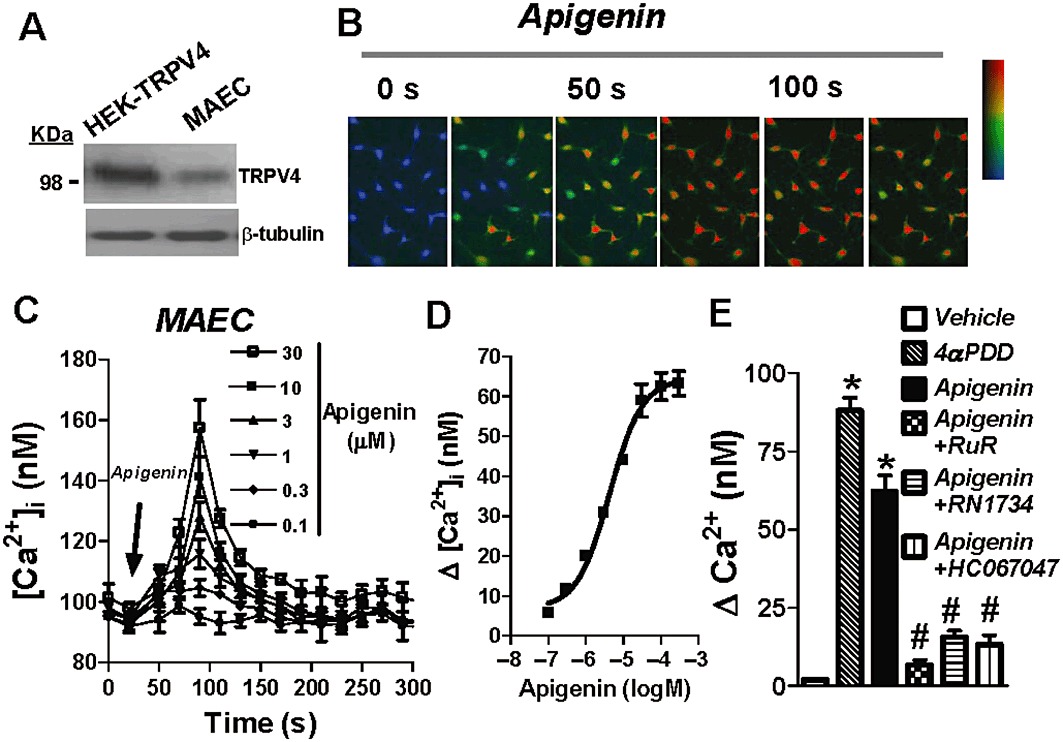
Concentration-dependent stimulation of [Ca2+]i rise in native endothelial cells by apigenin. (A) Representative immunoblots probed with anti-TRPV4 and anti-β-tubulin (n = 3). Left, TRPV4-transfected HEK cells; right, rat primary cultured MAECs. Representative images (B) and time course (C) of [Ca2+]i change in rat MAECs in response to different concentrations of apigenin. Cells were bathed in NPSS. (D) Plot of concentration–response with curve fitting to determine the apigenin EC50. (E) Summary data showing the maximal [Ca2+]i rise in response to 4αPDD (1 µM) or apigenin in the presence of the TRPV4 blockers, RuR (5 µM) or RN1734 (6 µM) or HC067047 (0.5 µM) (15–30 min incubation before apigenin application). Vehicle, 0.1% DMSO. Values are presented as mean ± SEM (n = 4–6, 8–20 cells per experiment). #P < 0.05 compared with apigenin. *P < 0.05 compared with vehicle.
Next, the cells were treated with two TRPV4-specific siRNAs, which both effectively knocked down the expression of TRPV4 proteins by 82 ± 4% (n = 3) and 71 ± 5% (n = 3) (Figure 4A) but had no effect on the cell viability compared with scrambled siRNA., the -induced The effect of apigenin, at its EC50 value, on [Ca2+]i was significantly reduced in cells that had been treated with TRPV4-specific siRNAs (Figure 4B,C).
Figure 4.
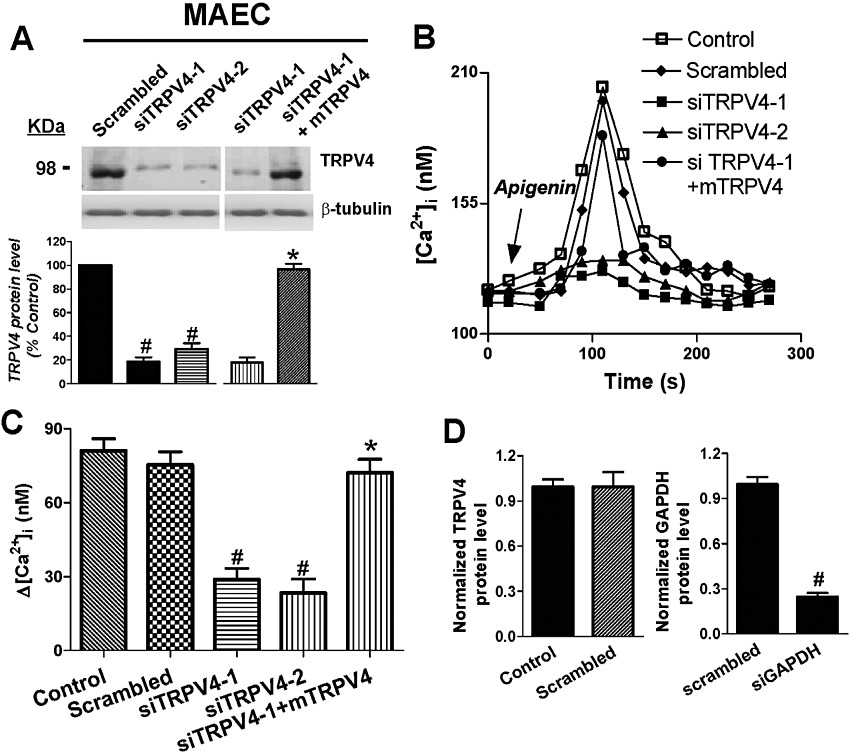
Apigenin acts through TRPV4 to mediate the [Ca2+]i rise in native endothelial cells. (A) Effectiveness of TRPV4-siRNAs against their target in rat primary cultured MAECs. Shown are representative images (upper) and summary (lower) of immunoblot experiments. Control siRNA with scrambled sequence had no effect. Mouse TRPV4 plasmid (mTRPV4) was introduced into rat endothelial cells with or without rat TRPV4-siRNAs. Immunoblots with anti-β-tubulin antibody showed that equal amounts of protein were loaded onto each lane. (B) Time course of [Ca2+]i change in rat MAECs in response to apigenin. (C) Summary showing the maximal [Ca2+]i rise in (B). Cells were bathed in NPSS. (D) Confirmation of the effect of negative (scrambled) and positive siRNA (GAPDH siRNA) in endothelial cells. Values are presented as mean ± SEM (n = 4–6, 8–20 cells per experiment). #P < 0.05 compared with scrambled siRNA or control. *P < 0.05 compared with siTRPV4-1.
Furthermore, when mouse TRPV4 plasmid was introduced as a rescue agent against the rat TRPV4-siRNA in rat endothelial cells, the knockdown effects of rat siTRPV4-1 on both TRPV4 expression level and increases in [Ca2+]i were reversed (Figure 4A,B,C). GAPDH-specific siRNA was chosen as an endogenous positive control, which simultaneously knocked down the expression of GAPDH, but scrambled siRNA had no effect (Figure 4D). The results clearly indicate that the apigenin-activated increase in [Ca2+]i in native endothelial cells was mainly through the TRPV4 channel.
Apigenin dose-dependently acts through endothelial TRPV4 to modulate smooth muscle cell membrane potential and vascular tone in rat small mesenteric arteries
The effect of apigenin on smooth muscle cell membrane potential and vascular tone was then studied. Third-order mesenteric arteries were isolated from Sprague–Dawley rats, and intracellular recordings were performed to measure the membrane potential changes in smooth muscle cells in arterial segments before and after the application of apigenin. Apigenin (0.03–30 µM) dose-dependently induced membrane hyperpolarization, which was significantly inhibited by the TRPV4 blockers ruthenium red (5 µM), RN1734 (6 µM) and HC067047 (0.5 µM) (Figure 5A). Functional removal of the endothelium also significantly attenuated the membrane hyperpolarization to apigenin (Figure 5A).
Figure 5.
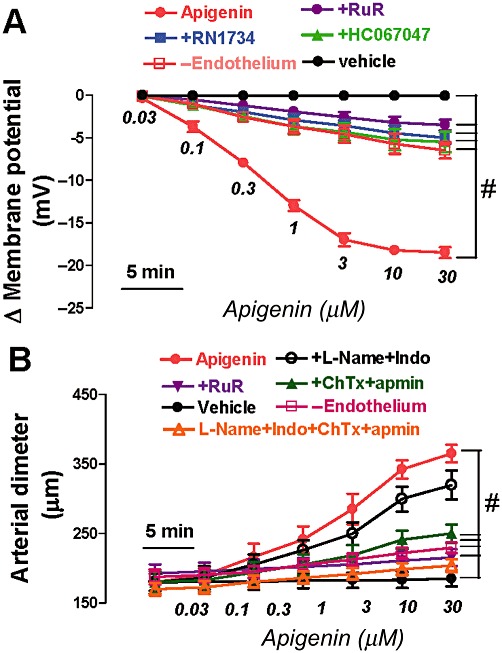
Apigenin dose-dependently acts through TRPV4 to modulate smooth muscle cell membrane potential and vascular tone in rat third-order small mesenteric arteries. (A) Summary data showing apigenin-induced smooth muscle cell hyperpolarization in endothelium-intact mesenteric artery. (B) Summary data showing the concentration–response curves for apigenin-induced dilatation in endothelium-intact mesenteric artery. Apigenin was added cumulatively at 5 min intervals. Incubation for 10–30 min with RuR (5 µM), or RN1734 (6 µM) or HC067047 (0.5 µM) before application of apigenin. Incubation for 30 min with ChTx (200 nM) plus apamin (100 nM) or l-NAME (100 µM) plus Indo (10 µM). In (B), the arteries were preconstricted with phenylephrine (Phe, 0.1–4 µM). Vehicle, 0.1% DMSO; -Endothelium, removal of endothelium. Values are presented as mean ± SEM (n = 4–6). #P < 0.05 compared with apigenin.
Furthermore, third-order mesenteric arteries were mounted in a pressure myograph for measurement of changes in vessel diameter. Bath application of apigenin (0.03–30 µM) induced concentration-dependent dilatation, which was significantly attenuated by functional removal of the endothelium (Figure 5B). To further assess the possible involvement of endothelium-derived hyperpolarizing factor (EDHF) in apigenin-induced vasodilatation, combined treatment with ChTx (200 nM) plus apamin (100 nM), which inhibits the intermediate- and small-conductance calcium-activated potassium channels in endothelia, diminished the apigenin-induced dilatation (Figure 5B). However, pretreatment with l-NAME (100 µM) plus indomethacin (Indo, 10 µM), which are NOS and COX inhibitors, only slightly suppressed the apigenin-induced vasodilatation. ChTX (200 nM) and apamin (100 nM) in the presence of l-NAME (100 µM) plus Indo (10 µM) abolished the vasodilatation (Figure 5B). Furthermore, the TRPV4 blocker, ruthenium red (5 µM) significantly suppressed the vasodilatation induced by apigenin, suggesting the involvement of TRPV4 channels and EDHF (Figure 5B). Addition of vehicle (0.1% DMSO) had no effect on either the membrane hyperpolarization or vasodilatation (Figure 5A,B).
Discussion and conclusions
In the present study, we found that apigenin was capable of stimulating TRPV4-mediated cation currents and increase in [Ca2+]i in HEK cells over-expressing TRPV4. In primary cultures of MAECs, apigenin elevated [Ca2+]i in a concentration-dependent manner; this effect was markedly reduced by TRPV4-specific siRNAs and blockers. Furthermore, in small mesenteric arteries, apigenin dose-dependently acted through TRPV4 to induce EDHF release and subsequent vasodilatation.
Many lines of evidence show that flavonoids modulate vascular calcium-permeable channels. Phloretin, biochanin A (Figtree et al., 2000), genistein (Wijetunge et al., 1992), scutellarin (Pan et al., 2008a) and catechin (Ghayur et al., 2007) inhibit vascular calcium currents. Quercetin and its rutinoside rutin activate calcium channels (Saponara et al., 2002; 2008; Fusi et al., 2003b). The main green tea flavonoid epigallocatechin has biphasic effects on calcium-permeable channels resulting in an initial vasoconstriction and a late vasorelaxation (Huang et al., 1998; Alvarez-Castro et al., 2004; Kim et al., 2004; Campos-Toimil and Orallo, 2007). Myricetin has similar complex effects on calcium channels (Fusi et al., 2003a; 2005). Recently, it has been shown that genistein stimulates the TRPC5 channel in bovine aortic endothelial cells (Wong et al., 2010). In the present study, whole-cell patch clamp and fluorescent Ca2+ imaging were used to determine whether apigenin indeed activates TRPV4 channels. In HEK cells over-expressing TRPV4, we found that apigenin concentration-dependently stimulated the TRPV4-mediated cation current and Ca2+ influx. In rat primary cultured MAECs, apigenin stimulated Ca2+ influx in a concentration-dependent manner, and this was markedly inhibited by TRPV4-specific siRNAs. These data confirmed an activating action of apigenin on the TRPV4 channel, which functions as a non-selective Ca2+-permeable cation channel. Moreover, apigenin-stimulated currents displayed relatively linear current–voltage relationships with a slight outward rectification at positive voltage when the bath solution contained physiological concentrations of mono- and divalent cations. Its reversal potential was about 3 ± 1 mV. This characteristic is consistent with the biophysics of TRPV4 from previous reports (Vriens et al., 2005; Vincent et al., 2009). Thus, the present study is the first to demonstrate activation of a TRP channel by flavones. In the TRPV subfamily, TRPV1, V2, V3 and V4, which share quite similar structure and gating properties, are activated by high temperatures from warm to noxious heat, shear stress and painful stimuli. Thus, it is possible that other TRPV isoforms can be activated by apigenin or other flavones, and this needs to be addressed in further experiments.
Apigenin has a variety of pharmacological activities, including anti-inflammatory (Gerritsen et al., 1995), antispasmodic (Capasso et al., 1991) and hypotensive (Loizzo et al., 2007). Moreover, intake of apigenin may contribute to the prevention of hypertension in adults (Cassidy et al., 2011) and reduce the risk of death from coronary heart disease (Hertog et al., 1993) and the incidence of stroke (Keli et al., 1996), suggesting that apigenin is beneficial to the cardiovascular system. In the present study, the actions and potential targets of apigenin on rat endothelial cells and small mesenteric arteries were investigated. Endothelial cells form a unique signal-transducing surface in the vascular system and express various membrane ion channels, which underpin a variety of functional roles, such as control of Ca2+ influx and modulation of the membrane potential. Ca2+ can enter vascular endothelial cells through several different groups of non-selective cation channels (Nilius and Droogmans, 2001). Ca2+ influx may elevate the cytosolic Ca2+ level globally throughout the cell or may increase the Ca2+ level within defined subcellular microdomains. The increase in cytosolic Ca2+ concentration then stimulates the endothelial cells to generate EDHF and other vasoactive agents (Feletou and Vanhoutte, 2006). In rat primary cultured MAECs, we found that apigenin stimulated TRPV4-mediated Ca2+ influx in a concentration-dependent manner in vascular endothelial cells. Pressure myograph studies using small third-order mesenteric arteries showed that apigenin evoked a dose-dependent vascular dilatation, the effect of which was inhibited by TRPV4 blockers. Taken together, the results strongly suggest that apigenin acts through TRPV4 to modulate endothelial Ca2+ influx and subsequent vascular dilatation. Furthermore, combined treatment with ChTx plus apamin also diminished the apigenin-induced dilatation and TRPV4-specific blockers significantly inhibited the apigenin-induced membrane hyperpolarization, suggesting that the dilatation is mediated through EDHF. It is likely that apigenin opens endothelial TRPV4 channels, and the subsequent rise in cytosolic Ca2+ stimulates EDHF release, which then induces vascular dilatation.
In conclusion, apigenin activates TRPV4 channels in a dose-dependent manner in HEK cells over-expressing TRPV4 and in native endothelial cells. In rat small mesenteric arteries, apigenin acts on TRPV4 in endothelial cells to induce EDHF-mediated vascular dilatation.
Acknowledgments
This study was supported by the China National Natural Science Foundation (81100185) and the Fundamental Research Funds for the Central Universities (JUSRP21134) for Dr Xin MA and Strategic Action Plan for Science and Technology Innovation from Chinese Academy of Sciences (XDA01040200) for Dr Jian JIN.
Glossary
- [Ca2+]i
intracellular calcium concentration
- ChTx
charybdotoxin
- EDHF
endothelium-derived hyperpolarizing factor
- GFP
green fluorescence protein
- Indo
indomethacin
- l-NAME
Nw-nitro-l-arginine methyl ester
- MAECs
primary cultured rat mesenteric arterial endothelial cells
- siRNA
small interfering RNA
- TRPV4
transient receptor potential vanilloid 4
Conflicts of interest
The authors state no conflict of interest.
References
- Alvarez-Castro E, Campos-Toimil M, Orallo F. (-)-Epigallocatechin-3-gallate induces contraction of the rat aorta by a calcium influx-dependent mechanism. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:496–506. doi: 10.1007/s00210-004-0923-8. [DOI] [PubMed] [Google Scholar]
- Arniges M, Vazquez E, Fernandez-Fernandez JM, Valverde MA. Swelling-activated Ca2+ entry via TRPV4 channel is defective in cystic fibrosis airway epithelia. J Biol Chem. 2004;279:54062–54068. doi: 10.1074/jbc.M409708200. [DOI] [PubMed] [Google Scholar]
- Campos-Toimil M, Orallo F. Effects of (-)-epigallocatechin-3-gallate in Ca2+ -permeable non-selective cation channels and voltage-operated Ca2+ channels in vascular smooth muscle cells. Life Sci. 2007;80:2147–2153. doi: 10.1016/j.lfs.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Capasso A, Pinto A, Sorrentino R, Capasso F. Inhibitory effects of quercetin and other flavonoids on electrically-induced contractions of guinea pig isolated ileum. J Ethnopharmacol. 1991;34:279–281. doi: 10.1016/0378-8741(91)90048-i. [DOI] [PubMed] [Google Scholar]
- Cassidy A, O'Reilly EJ, Kay C, Sampson L, Franz M, Forman JP, et al. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am J Clin Nutr. 2011;93:338–347. doi: 10.3945/ajcn.110.006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaerts W, Zhen X, Ghosh D, Vriens J, Gevaert T, Gilbert JP, et al. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc Natl Acad Sci U S A. 2010;107:19084–19089. doi: 10.1073/pnas.1005333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol. 2006;26:1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- Figtree GA, Griffiths H, Lu YQ, Webb CM, MacLeod K, Collins P. Plant-derived estrogens relax coronary arteries in vitro by a calcium antagonistic mechanism. J Am Coll Cardiol. 2000;35:1977–1985. doi: 10.1016/s0735-1097(00)00645-8. [DOI] [PubMed] [Google Scholar]
- Fotsis T, Pepper MS, Aktas E, Breit S, Rasku S, Adlercreutz H, et al. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997;57:2916–2921. [PubMed] [Google Scholar]
- Fusi F, Saponara S, Frosini M, Gorelli B, Sgaragli G. L-type Ca2+ channels activation and contraction elicited by myricetin on vascular smooth muscles. Naunyn Schmiedebergs Arch Pharmacol. 2003a;368:470–478. doi: 10.1007/s00210-003-0836-y. [DOI] [PubMed] [Google Scholar]
- Fusi F, Saponara S, Pessina F, Gorelli B, Sgaragli G. Effects of quercetin and rutin on vascular preparations: a comparison between mechanical and electrophysiological phenomena. Eur J Nutr. 2003b;42:10–17. doi: 10.1007/s00394-003-0395-5. [DOI] [PubMed] [Google Scholar]
- Fusi F, Sgaragli G, Saponara S. Mechanism of myricetin stimulation of vascular L-type Ca2+ current. J Pharmacol Exp Ther. 2005;313:790–797. doi: 10.1124/jpet.104.080135. [DOI] [PubMed] [Google Scholar]
- Gerritsen ME, Carley WW, Ranges GE, Shen CP, Phan SA, Ligon GF, et al. Flavonoids inhibit cytokine-induced endothelial cell adhesion protein gene expression. Am J Pathol. 1995;147:278–292. [PMC free article] [PubMed] [Google Scholar]
- Ghayur MN, Khan H, Gilani AH. Antispasmodic, bronchodilator and vasodilator activities of (+)-catechin, a naturally occurring flavonoid. Arch Pharm Res. 2007;30:970–975. doi: 10.1007/BF02993965. [DOI] [PubMed] [Google Scholar]
- Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zhang A, Lau CW, Chen ZY. Vasorelaxant effects of purified green tea epicatechin derivatives in rat mesenteric artery. Life Sci. 1998;63:275–283. doi: 10.1016/s0024-3205(98)00273-2. [DOI] [PubMed] [Google Scholar]
- Illek B, Lizarzaburu ME, Lee V, Nantz MH, Kurth MJ, Fischer H. Structural determinants for activation and block of CFTR-mediated chloride currents by apigenin. Am J Physiol Cell Physiol. 2000;279:C1838–C1846. doi: 10.1152/ajpcell.2000.279.6.C1838. [DOI] [PubMed] [Google Scholar]
- Keli SO, Hertog MG, Feskens EJ, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch Intern Med. 1996;156:637–642. [PubMed] [Google Scholar]
- Kim HJ, Yum KS, Sung JH, Rhie DJ, Kim MJ, Min DS, et al. Epigallocatechin-3-gallate increases intracellular [Ca2+] in U87 cells mainly by influx of extracellular Ca2+ and partly by release of intracellular stores. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:260–267. doi: 10.1007/s00210-003-0852-y. [DOI] [PubMed] [Google Scholar]
- Kohler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, et al. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol. 2006;26:1495–1502. doi: 10.1161/01.ATV.0000225698.36212.6a. [DOI] [PubMed] [Google Scholar]
- Kottgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, et al. TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol. 2008;182:437–447. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan HY, Huang Y, Yao X. Regulation of canonical transient receptor potential isoform 3 (TRPC3) channel by protein kinase G. Proc Natl Acad Sci U S A. 2004;101:2625–2630. doi: 10.1073/pnas.0304471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Ngai CY, Huang Y, Ko WH, Wu M, He GW, et al. Depletion of intracellular Ca2+ stores enhances flow-induced vascular dilatation in rat small mesenteric artery. Br J Pharmacol. 2006;147:506–515. doi: 10.1038/sj.bjp.0706639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loizzo MR, Said A, Tundis R, Rashed K, Statti GA, Hufner A, et al. Inhibition of angiotensin converting enzyme (ACE) by flavonoids isolated from Ailanthus excelsa (Roxb) (Simaroubaceae) Phytother Res. 2007;21:32–36. doi: 10.1002/ptr.2008. [DOI] [PubMed] [Google Scholar]
- Loot AE, Popp R, Fisslthaler B, Vriens J, Nilius B, Fleming I. Role of cytochrome P450-dependent transient receptor potential V4 activation in flow-induced vasodilatation. Cardiovasc Res. 2008;80:445–452. doi: 10.1093/cvr/cvn207. [DOI] [PubMed] [Google Scholar]
- Ma X, Cao J, Luo J, Nilius B, Huang Y, Ambudkar IS, et al. Depletion of intracellular Ca2+ stores stimulates the translocation of vanilloid transient receptor potential 4-c1 heteromeric channels to the plasma membrane. Arterioscler Thromb Vasc Biol. 2010a;30:2249–2255. doi: 10.1161/ATVBAHA.110.212084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Qiu S, Luo J, Ma Y, Ngai CY, Shen B, et al. Functional role of vanilloid transient receptor potential 4-canonical transient receptor potential 1 complex in flow-induced Ca2+ influx. Arterioscler Thromb Vasc Biol. 2010b;30:851–858. doi: 10.1161/ATVBAHA.109.196584. [DOI] [PubMed] [Google Scholar]
- Niisato N, Ito Y, Marunaka Y. Activation of Cl channel and Na+/K+/2Cl cotransporter in renal epithelial A6 cells by flavonoids: genistein, daidzein, and apigenin. Biochem Biophys Res Commun. 1999;254:368–371. doi: 10.1006/bbrc.1998.9952. [DOI] [PubMed] [Google Scholar]
- Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev. 2001;81:1415–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- Nilius B, Watanabe H, Vriens J. The TRPV4 channel: structure-function relationship and promiscuous gating behaviour. Pflugers Arch. 2003;446:298–303. doi: 10.1007/s00424-003-1028-9. [DOI] [PubMed] [Google Scholar]
- Nilius B, Vriens J, Prenen J, Droogmans G, Voets T. TRPV4 calcium entry channel: a paradigm for gating diversity. Am J Physiol Cell Physiol. 2004;286:C195–C205. doi: 10.1152/ajpcell.00365.2003. [DOI] [PubMed] [Google Scholar]
- Olszanecki R, Gebska A, Kozlovski VI, Gryglewski RJ. Flavonoids and nitric oxide synthase. J Physiol Pharmacol. 2002;53:571–584. [PubMed] [Google Scholar]
- Pan Z, Feng T, Shan L, Cai B, Chu W, Niu H, et al. Scutellarin-induced endothelium-independent relaxation in rat aorta. Phytother Res. 2008a;22:1428–1433. doi: 10.1002/ptr.2364. [DOI] [PubMed] [Google Scholar]
- Pan Z, Yang H, Mergler S, Liu H, Tachado SD, Zhang F, et al. Dependence of regulatory volume decrease on transient receptor potential vanilloid 4 (TRPV4) expression in human corneal epithelial cells. Cell Calcium. 2008b;44:374–385. doi: 10.1016/j.ceca.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant TD, Strotmann R. TRPV4. Handb Exp Pharmacol. 2007;179:189–205. doi: 10.1007/978-3-540-34891-7_11. [DOI] [PubMed] [Google Scholar]
- Saponara S, Sgaragli G, Fusi F. Quercetin as a novel activator of L-type Ca(2+) channels in rat tail artery smooth muscle cells. Br J Pharmacol. 2002;135:1819–1827. doi: 10.1038/sj.bjp.0704631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saponara S, Sgaragli G, Fusi F. Quercetin antagonism of Bay K 8644 effects on rat tail artery L-type Ca(2+) channels. Eur J Pharmacol. 2008;598:75–80. doi: 10.1016/j.ejphar.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Vincent F, Acevedo A, Nguyen MT, Dourado M, DeFalco J, Gustafson A, et al. Identification and characterization of novel TRPV4 modulators. Biochem Biophys Res Commun. 2009;389:490–494. doi: 10.1016/j.bbrc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Voets T, Prenen J, Vriens J, Watanabe H, Janssens A, Wissenbach U, et al. Molecular determinants of permeation through the cation channel TRPV4. J Biol Chem. 2002;277:33704–33710. doi: 10.1074/jbc.M204828200. [DOI] [PubMed] [Google Scholar]
- Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci U S A. 2004;101:396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, et al. Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res. 2005;97:908–915. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, et al. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Janssens A, Wondergem R, Droogmans G, Nilius B. Modulation of TRPV4 gating by intra- and extracellular Ca2+ Cell Calcium. 2003;33:489–495. doi: 10.1016/s0143-4160(03)00064-2. [DOI] [PubMed] [Google Scholar]
- Wijetunge S, Aalkjaer C, Schachter M, Hughes AD. Tyrosine kinase inhibitors block calcium channel currents in vascular smooth muscle cells. Biochem Biophys Res Commun. 1992;189:1620–1623. doi: 10.1016/0006-291x(92)90262-j. [DOI] [PubMed] [Google Scholar]
- Wong CO, Huang Y, Yao X. Genistein potentiates activity of the cation channel TRPC5 independently of tyrosine kinases. Br J Pharmacol. 2010;159:1486–1496. doi: 10.1111/j.1476-5381.2010.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Gao X, Brown RC, Heller S, O'Neil RG. Dual role of the TRPV4 channel as a sensor of flow and osmolality in renal epithelial cells. Am J Physiol Renal Physiol. 2007;293:F1699–F1713. doi: 10.1152/ajprenal.00462.2006. [DOI] [PubMed] [Google Scholar]


