Abstract
Results from clinical trials have demonstrated that it is possible to prevent estrogen-responsive breast cancers by targeting the estrogen receptor with selective estrogen receptor modulators (SERMs) (tamoxifen, raloxifene, or lasofoxifene) or with aromatase inhibitors (AIs) (anastrozole, letrozole, or exemestene). Results from breast cancer treatment trials suggest that aromatase inhibitors may be even more effective in preventing breast cancer than SERMs. However, while SERMs and aromatase inhibitors do prevent the development of many ER-positive breast cancers, these drugs do not prevent ER-negative breast cancer. These results show that new approaches are needed for the prevention of this aggressive form of breast cancer. Our laboratory and clinical efforts have been focused on identifying critical molecular pathways in breast cells that can be targeted for the prevention of ER-negative breast cancer. Our preclinical studies have demonstrated that other nuclear receptors, such as RXR receptors, vitamin D receptors, as well as others are critical for the growth of ER-negative breast cells and for the transformation of these cells into ER-negative cancers. Other studies show that growth factor pathways including those activated by EGFR, Her2, and IGFR, which are activated in many ER-negative breast cancers, can be targeted for the prevention of ER-negative breast cancer in mice. Clinical studies have also shown that PARP inhibitors are effective for the treatment of breast cancers arising in BRCA-1 or -2 mutation carriers, suggesting that targeting PARP may also be useful for the prevention of breast cancers arising in these high-risk individuals. Most recently, we have demonstrated that ER-negative breast cancers can be subdivided into four distinct groups based on the kinases that they express. These groups include ER-negative/Her-2-positive groups (the MAPK and immunomodulatory groups) and ER-negative/Her2-negative groups (the S6K and the cell cycle checkpoint groups). These groups of ER-negative breast cancers can be targeted with kinase inhibitors specific for each subgroup. These preclinical studies have supported the development of several clinical trials testing targeted agents for the prevention of breast cancer. The results of a completed Phase II cancer prevention trial using the RXR ligand bexarotene in women at high risk of breast cancer will be reviewed, and the current status of an ongoing Phase II trial using the EGFR and Her2 kinase inhibitor lapatinib for the treatment of women with DCIS breast cancer will be presented. It is anticipated that in the future these molecularly targeted drugs will be combined with hormonal agents such as SERMs or aromatase inhibitors to prevent all forms of breast cancer.
13.1 Introduction
In the year 2009 over 190,000 new cases of breast cancer were diagnosed and approximately 40,000 deaths from breast cancer have occurred in the United States (Horner et al. 2009). Current strategies using endocrine agents have successfully prevented or treated estrogen receptor-positive breast cancers by interfering with estrogen signaling or production. The model-selective estrogen receptor modulator (SERM), tamoxifen, and another, less toxic antiestrogen drug, raloxifene, have been shown to prevent estrogen receptor-positive breast cancer in high-risk women (Fisher et al. 1998; Cummings et al. 1999; Cuzick et al. 2003). However, these drugs only reduced breast cancer incidence by 50%, and had no effect on preventing estrogen receptor-negative breast cancer, which accounts for 30% of all breast cancers (Fisher et al. 1998; Vogel et al. 2006). These factors make the compelling case that novel agents need to be discovered that will aid in the prevention and/or treatment of estrogen receptor-negative breast cancer.
While the action of antiestrogenic drugs used for cancer prevention is relatively well understood and improved pharmacologic agents are being developed, it is important to look for alternative molecular mechanisms by which biologically active chemical compounds can effectively reduce the incidence of breast cancer, regardless of the tissues' estrogen receptor status. This review will present the directions taken by current investigations to identify viable candidate drugs categorized by their mechanisms of action, and shed new insights into off-label applications of currently used therapeutics.
13.2 Endocrine Preventive Agents
In recent years, significant progress has been made in demonstrating that drugs targeted against the estrogen receptor, such as selective estrogen receptor modulators (SERMs) and aromatase inhibitors, are useful for the treatment and prevention of breast cancer.
13.3 Selective Estrogen Receptor Modulators
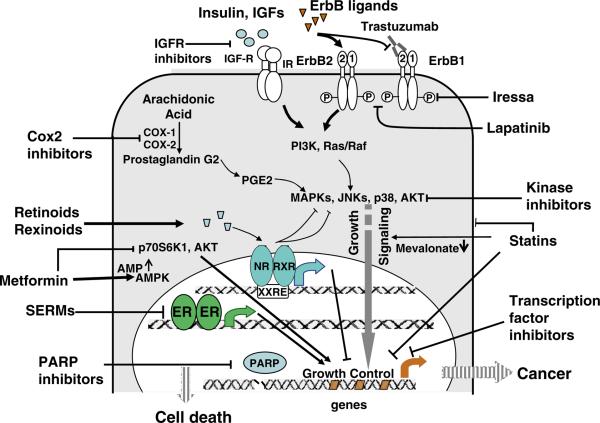
A host of preclinical models have been used over the years to demonstrate that estrogen is a key factor for the initiation and promotion of breast cancer, suggesting a potential therapeutic and preventive effect for antiestrogenic agents (Fig. 13.1). It has been established early on that pregnancy can promote, and bilateral oophorectomy is protective against breast cancer development. The accumulating knowledge of estrogen signaling and the identification of estrogen receptor ultimately led to the design of drugs targeting the estrogen receptor (Lerner et al. 1958). The first group of compounds that were used to suppress ER signaling are SERMs, compounds exhibiting selective agonistic or antagonistic properties depending on the target tissue. The first antiestrogenic agent to be approved for the treatment and ultimately for the prevention of breast cancer was tamoxifen (Honig 2001). Tamoxifen competitively inhibits binding of estrogen to ER and therefore is an antagonist of estrogenic signaling in breast tissue, but acts as an agonist in bone and the uterus. In several adjuvant studies, tamoxifen was found to significantly reduce the incidence of contralateral breast cancer as an endpoint (Cuzick and Baum 1985). This observation implicated the chemopreventive effect of tamoxifen in healthy women at high risk of breast cancer and led to a series of cancer prevention trials.
Fig. 13.1.
Pathways to target for the prevention of breast cancer. Estrogen-dependent (via the estrogen receptor alpha) and estrogen-independent pathways are shown
A comprehensive meta-analysis summarizing all tamoxifen prevention trials concluded that the overall reduction in breast cancer incidence by tamoxifen was 38% (95% CI, 28–46, p < 0.001, Table 13.1) (Cuzick et al. 2003). Tamoxifen over 5 years reduced the frequency of new ER-positive breast cancers by 48%, and long-term follow-up of the IBIS-I trial suggests that the risk-reducing effect of tamoxifen persists for at least 10 years, while most side effects of tamoxifen do not continue after the 5-year treatment period (Cuzick et al. 2007). In all these studies, tamoxifen had no effect on the development of ER-negative breast cancers (Fisher et al. 2005; Powles et al. 2007; Cummings et al. 1999; Cuzick et al. 2007; Veronesi et al. 2007). Apart from its benefits, long-term tamoxifen treatment was associated with side effects, including increased risk of endometrial cancer, venous thromboembolism, and hot flushes, which were observed in all studies. Concerns about tamoxifen-related toxicity, that limited its use as a preventive agent, became the driving force to identify novel SERMs with less toxicity and resulted in the development of raloxifene and lasofoxifene for clinical use.
Table 13.1.
Status of phase III breast cancer prevention trials
| Phase III breast cancer prevention trials of SERMs or aromatase inhibitors |
||||||
|---|---|---|---|---|---|---|
| Trial | Intervention | Sample size | Patient eligibility | Endpoints | Results | Reference |
| NSABP-P1 | Placebo vs tamoxifen, 20 mg | 13,388 | >1.6% 5-year risk | Incidence of IBC | 49% Reduction in breast cancer | Fisher et al. (2005) |
| Royal Marsden Hospital | Placebo vs tamoxifen, 20 mg | 2,494 | High-risk, family history | Incidence of IBC | Negative at 5 years; significantly lower risk of ER+ BC in the posttreatment (>8 years) period | Powles et al. (2007) |
| Italian breast cancer prevention trial with tamoxifen | Placebo vs tamoxifen, 20 mg | 5,408 | Normal-risk, hysterectomy | Incidence of IBC | Reduction of IBC in patients starting HRT after tamoxifen | Veronesi et al. (2007) |
| IBIS-I | Placebo vs tamoxifen, 20 mg | 7,152 | >2-fold relative risk, healthy | Frequency of IBC or DCIS | 31% Reduction in invasive ER-positive tumors | Cuzick et al. (2007) |
| MORE | Placebo vs raloxifen, 60 mg or 120 mg | 7,705 | Normal-risk, postmenopausal women with osteoporosis | Fracture risk, breast cancer | 76% Reduction in the risk of IBC during 3 years | Cummings et al. (1999) |
| NSABP STAR | Tamoxifen, 20 mg vs raloxifene 60 mg | 19,747 | ≥1.7% 5-year risk | Incidence of IBC | No difference (estimated a 50% reduction by both) | Vogel et al. (2006) |
| PEARL | Calcium + vitamin D, plus placebo vs 0.25–0.5 mg lasofoxifene | 8,556 | Women age 59–80, negative mammography, bone mineral density T score <−2.5 | Bone fractures, ER+ BC, coronary heart disease, stroke | Reduced risk of fractures, ER-positive breast cancer, coronary heart disease, stroke | Cummings (2010) |
| IBIS-II Prevention | Placebo vs anastrozole, 1 mg | 6,000 (planned) | Postmenopausal, high risk | Frequency of BC, both invasive and noninvasive | Earliest results in 2012 | Cuzick (2008) |
| IBIS-II DCIS | Placebo vs anastrozole or tamoxifen | 4,000 (planned) | Postmenopausal, prior ER+ DCIS | Incidence of new cancer in affected and contralateral breast | Earliest results in 2012 | Cuzick (2003, 2008) |
| MAP.3 | Placebo vs exemestane, 25 mg | 4,560 | Postmenopausal, 5-year risk > 1.67% based on Gail model | Frequency of invasive BC | Trial ongoing | Goss et al. (2007) |
The Study of Tamoxifen and Raloxifene (STAR) trial demonstrated that raloxifene was equivalent to tamoxifen in its ability to decrease the risk of breast cancer in high-risk postmenopausal women, but patients taking raloxifene had less toxicity, uterine cancers, and blood clots than those who received tamoxifen (Vogel et al. 2006). In 2007 in the United States, raloxifene received FDA approval for preventive use in postmenopausal women with osteoporosis or at high risk for invasive breast cancer. A more recently developed nonsteroidal SERM lasofoxifene exhibited a better toxicity profile in postmenopausal women with osteoporosis. As recently published results of the PEARL trial show, lasofoxifene (given at 0.5 mg/day for 5 years) reduced the incidence of ER-positive breast tumors (invasive and noninvasive) by 81% in postmenopausal women with osteoporosis. As primary end point the study also established that lasofoxifene reduced the risk of both vertebral and nonvertebral bone fractures and coronary disease, but increased the risk of venous thromboembolic events (Cummings et al. 2010).
13.4 Aromatase Inhibitors
Aromatase inhibitors present an alternative strategy to antagonize estrogen-dependent signaling by blocking the biosynthesis of estrogen through reversible (nonsteroidal agents such as letrozole and anastrozole) or irreversible (steroidal agents, i.e., exemestane) inhibition of the aromatase enzyme (Fig. 13.1). AIs have proven an effective approach to treatment of existing breast cancers in premenopausal women. Their chemopreventive potential was first identified in adjuvant studies, yet so far no AI has been fully evaluated for cancer-preventive use.
In animals and in humans AIs performed better than tamoxifen in potency and response duration to cause regression of breast tumors. Similarly, hypothesis-generating adjuvant trials involving AIs (ATAC, MA.17 and IES testing anastrazole, letrozole or exemestane, respectively) showed that the tested agents were more effective than tamoxifen to delay the recurrence of a prior breast cancer or prevent second primary cancers (Cuzick 2003; Goss et al. 2007). In addition, fewer thromboembolic events and endometrial cancers were reported following AI treatment, although the risk of fractures and arthralgias was increased with AI use (Chlebowski et al. 2009). Similarly, the MA.17 and MAP.3 trials have shown that switching from tamoxifen to letrozole or exemestane, respectively, reduced the risk of a contralateral formation of breast cancer in ER-positive patients. Studies on the long-term outcomes of the ATAC trial provided evidence of a larger carryover effect after 5 years of adjuvant treatment with anastrozole compared with tamoxifen (Forbes et al. 2008).
Meta-analyses of AI monotherapy and sequenced therapy (tamoxifen switched to AI) clinical trials showed that disease-free survival was significantly improved for both options and overall survival was prolonged for patients who switched from tamoxifen to AI therapy (Josefsson and Leinster 2010).
Based on the experience of the ATAC trial, the Breast International Group (BIG) proposed and is conducting the IBIS-II Prevention (high-risk portion of IBIS-II) trial, which will determine the cancer-preventive effect of anastrozole versus placebo in postmenopausal women at increased risk of breast cancer. The primary endpoint is the frequency of invasive and noninvasive breast cancer. A parallel randomized phase III trial termed IBIS-II – DCIS, is comparing anastrozole against tamoxifen in 4,000 women with locally excised DCIS (Cuzick 2008; Dunn and Ryan 2009). The primary endpoints are the comparison of the efficacy of adjuvant tamoxifen versus anastrozole in local control and prevention of contralateral disease, as well as the comparison of side effects.
In spite of promising results seen with AIs as chemopreventive agents, AIs can only be used in postmenopausal women and are not expected to suppress ER-negative breast cancer incidence. Thus, the prevention of receptor-negative breast cancer requires the identification of novel mechanisms that target nonestrogen signaling pathways.
13.5 New Strategies to Prevent Hormone-Independent Breast Cancer
13.5.1 PARP Inhibitors
The use of poly(ADP-ribose) polymerase (PARP) inhibitors constitutes a novel approach to targeted cancer therapy. Cells carrying heterozygous loss-of-function mutations of BRCA may lose the wild-type allele on the path of oncogenesis, making these cells deficient in their ability of homologous recombination. In the absence of PARP1, spontaneous single-strand breaks collapse DNA replication forks and trigger homologous recombination for repair, making any DNA damage lethal to the BRCA mutant cell. Bryant and collagues have elegantly shown that BRCA2-deficient cells, as a result of their deficiency in homologous recombination, are acutely sensitive to PARP inhibitors (Bryant et al. 2005). The requirement of a BRCA mutation to be present for a PARP inhibitor to be effective constitutes a “synthetic lethal” strategy selectively affecting BRCA1 or 2 mutant tumor cells (a compound targeting a particular pathway is selectively “lethal” to cells harboring a mutation in a complementary pathway).
Based on encouraging preclinical studies, Phase I clinical trials (NCI-08-C-0128, NCI-09-C-0048) were initiated, which assessed the safety, tolerability of the PARP inhibitors AZD2281, and ABT-888 in combination with DNA-damaging chemotherapeutic drugs cisplatin and gemcitabine. PARP inhibitors were scored based on PARP activity (formation of poly(ADP-ribose) moieties and γH2AX foci), DNA damage, cell proliferation, and cell death. The single agent Phase I clinical evaluation (NCT00516373) of the orally active PARP inhibitor olaparib (4-[(3-{[4-cyclopropylcarbonyl)piperazin-1-yl]carbonyl}-4-fluorophenyl)methyl] phthalazin-1(2H)-one), or AZD2281, has been recently completed and reported few of the adverse effects of conventional chemotherapy (Fong et al. 2009). A multicenter, single-arm phase II study of contiuous oral olaparib in BRCA1/BRCA2 carriers with recurrent, chemotherapy-refractory breast cancers provided a positive proof of concept, as olaparib at 400 mg bd was well tolerated and highly active (Tutt et al. 2009). Toxicity was reported as mainly mild, symptoms included fatigue (33%), nausea (27%), vomiting (15%), and anemia (4%). Given the relatively good tolerability profile of PARP inhibitors, these agents may be well suited for cancer-preventive use. Since the inhibition of a DNA repair enzyme in the absence of an exogenous DNA-damaging agent is sufficient to selectively kill tumor cells while posing no threat to normal cells, PARP inhibitors could present themselves as the ideal chemopreventive strategy. To further investigate their clinical applicability as chemopreventive agents, further investigations should be proposed in BRCA mutation carriers to assess the ability of PARP inhibitors of reducing the incidence of breast cancer.
13.6 Cell Growth Inhibitors
13.6.1 Statins
Statins potently inhibit cholesterol biosynthesis by blocking the production of mevalonate and therefore have been in clinical use as lipid-lowering drugs for over 30 years. In addition to reducing sterol biosynthesis, statins may interfere with microdomain formation, improve endothelial function by interfering with oxidative stress pathways (Mason et al. 2004). While their beneficial effects on mortality from cardiovascular disease have been well documented, the prospect of statins' utility as a cancer-preventive drug remains controversial. There is strong preclinical evidence to suggest that lipophilic statins, atorvastatin, lovastatin, simvastatin, and fluvastatin significantly inhibit the proliferation of breast cancer cell lines, and greater suppression was observed in ER-negative cell lines (Mueck et al. 2003). In addition, fluvastatin, simvastatin, and lovastatin, but not pravastatin, have been shown to possess growth inhibitory activities at therapeutic doses in ER-negative mouse breast cancer models (Campbell et al. 2006). However, epidemiologic studies have resulted in mixed conclusions. Kumar and coworkers have found a marked reduction of breast cancers associated with the administration of lipophilic statins (Kumar et al. 2006). In addition, breast cancer patients on long-term statin treatment have proportionately fewer ER/PR-negative tumors that are of lower grade and stage, than patients who never received statins (Kumar et al. 2008). On the other hand, Bonovas and coworkers concluded from a meta-analysis of seven randomized and nine observational breast cancer trials that statin use did not significantly affect breast cancer risk (Bonovas et al. 2005). Similar statistical evaluations were carried out by this group retrospectively to assess the cancer-preventive effects of statins against a number of other cancers, including prostate, colon, and skin, with similar negative results. However, such meta-analyses of randomized trials were criticized on the basis of including both lipophilic and nonlipophilic statin use. It was also argued that these trials were originally designed and the dosages set up with respect to cardiovascular endpoints, and so conclusions on breast cancer risk may be misleading.
Several phase II prevention trials are ongoing at the NCI using lipophilic statins (JHOC-J0485, V0407, BRSNSTU0010), to assess their cancer-preventive effects using biomarkers. Most recently a perioperative window trial was carried out focusing on the cancer-preventive effects of only fluvastatin in women with a diagnosis of DCIS or stage 1 breast cancer. The investigators reported measurable biologic changes with reduced tumor proliferation and increased apoptotic activity (Garwood et al. 2010). Since these effects were evident only in high-grade, very early-stage tumors, these results call for further evaluation of statins as chemopreventive agents for ER-negative high-grade breast cancers.
13.6.2 Metformin
Several studies have identified an increased risk of cancer in noninsulin-dependent diabetics (Xue and Michels 2007). A recent meta-analysis of 20 studies of breast cancer has confirmed the long-time notion that there is increased risk of breast cancer associated with diabetes (Larsson et al. 2007). It was suggested that insulin may activate various proliferative and antiapoptotic mechanisms leading to or promoting carcinogenesis (Papa and Belfiore 1996). Therefore, the question as to whether the lowering of insulin levels by antidiabetic therapy would decrease cancer incidence or cancer mortality merits investigation (Goodwin 2008).
Experimental studies have found that in carcinogen-treated mice or in tumor-prone transgenic mice exercise reduces the odds for the development of cancer. Studies in animal models have shown that AMP kinase activators such as phenformin, metformin, and AICAR inhibit tumor development. Furthermore, in vitro studies in epithelial cells have demonstrated that metformin activates the cellular energy sensor AMP kinase through the tumor suppressor LKB1, whose genetic loss is associated with Peutz-Jeghers syndrome and increased risk of epithelial cancers (Giardiello et al. 2000). Enzymes inhibited by AMPK include mammalian homolog of Target Of Rapamycin (mTOR), acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS), and glycerol phosphate acyltransferase (GPAT) – key regulators of protein, fatty acid, and glycerolipid syntheses. Multiple metabolic pathways indicating energy saturation (leptin, adiponectin, IL6, resveratrol) converge onto AMPK to induce downregulation of glycogen, fatty acid, and cholesterol synthesis. In addition, AMPK activation suppresses cell growth through the inhibition of protein synthesis and the upregulation of the p53 pathway resulting in the induction of p21, reduction of cyclin D1 levels, and cell cycle arrest (Zakikhani et al. 2006).
Metformin is a biguanide derivative approved for the treatment of type II diabetes mellitus, with antihyperglycemic properties. The prevailing view on the action of metformin is the reduction of hepatic glucose output by inhibition of gluconeogenesis, with resulting suppression of insulin levels (Shaw et al. 2005), but it is also thought to increase sensitivity of peripheral tissues to insulin (Bailey and Turner 1996). Whether a possible cancer-preventive effect can be attributed to this systemic effect or to a direct growth suppressive effect of metformin remains unclear. However, it has also been proposed that metformin by activating AMPK may be able to overcome breast cancer resistance to Her2 inhibitors while decreasing risk of cardiotoxicity (Vazquez-Martin et al. 2009).
The European Institute of Oncology in Milan is currently conducting a two-arm Phase II clinical trial testing metformin against placebo in women with early breast cancer. This presurgical randomized, double blind, placebo-controlled biomarker trial will enroll 100 histologically confirmed breast cancer patients and use metformin at 850 mg twice daily for 28 + 7 days before surgery, to assess drug activity. The primary endpoint of the study is the change in tumor and dysplastic/hyperplastic cell proliferation, as measured by the percentage of Ki67 positive cells (Cazzaniga et al. 2009). A successful outcome could pave the way for a subsequent chemoprevention trial with metformin (Goodwin et al. 2009).
The use of metformin for cancer prevention could be perceived as a paradigm shift in the drug development process. Because the same pathway may be involved in different tumors and in different diseases, molecularly targeted agents, which target pathways instead of diseases could present a better approach than the “one disease, one drug model.”
13.6.3 Retinoids
Retinoids, derivatives of vitamin A, are small, lipophilic molecules with pleiotropic effects on development, differentiation, and homeostatic regulation of most tissues (Szanto et al. 2004). Several lines of evidence from in vitro systems suggest that retinoids may control various mechanisms directly or indirectly suppressing cell growth, independent of the ER/PR status of the cell. We and others have shown that retinoids induce G1 cell cycle arrest, overexpression of the IGF binding proteins 3 and 6, RARβ, TGFβ, and down-regulate COX-2 and cyclin D1 expression, as well as inhibit the activity of the AP-1 transcription factor (Lee et al. 1996; Seewaldt et al. 1997; Yang et al. 2001; Kong et al. 2005; Wu et al. 2006; Uray et al. 2009).
Cancer prevention using specific compounds traces back to the early twentieth century when Wolbach first reported on his rat study with “tissue changes following deprivation of fat-soluble A vitamin” and the reverse changes that follow, when the rats are restored to an adequate diet (Wolbach and Howe 1925). Although there is little epidemiologic data supporting the use of vitamin A or carotenoids to reduce breast cancer risk, the ability of retinoids to prevent cancer was demonstrated later in various animal models. Retinyl acetate, and the synthetic retinoid N-(4-hydroxyphenyl) retinamide (4-HPR) have been used to prevent mammary carcinogenesis in rats exposed to chemical carcinogens (Moon et al. 1979). The naturally occurring 9-cis-RA was also found to suppress mammary tumors alone or in combination with low-dose tamoxifen (Wu et al. 2000; Anzano et al. 1994).
One of the first reports on molecular-targeted chemoprevention research demonstrated that an adjuvant high dose of isotretinoin (13-cis-retinoic acid) prevented second primary tumors in patients with curatively treated head and neck cancer (Hong et al. 1990). While this was a seminal discovery, which greatly helped establish translational retinoid research, the toxicity of this retinoid has prevented its general clinical use. Another naturally occurring retinoid 9-cis-retinoic acid (9cRA) reduces the incidence of mammary tumors, but it also induces liver toxicity, hair loss, and skin erythema (Wu et al. 2000).
While retinoids can only bind retinoic acid receptors, a special class of retinoids termed rexinoids activates hetero- or homodimers of the retinoid X receptor (RXR). The fact that RXRs are potential dimerization partners of a number of nuclear hormone receptors, such as RARs, VDR, TRs, PPARs, and others, yields a highly flexible and complex signaling system, able to induce gene expression characteristic of other partners. However, the composition of the nuclear receptor heterodimers that mediate the cancer-preventive effect of retinoids and rexinoids is unknown and is under investigation in our laboratory. Our laboratory and others have shown that retinoid X receptor-selective drugs (rexinoids) prevent breast cancer in mice more effectively and with less toxicity than retinoids. Specifically, the synthetic rexinoids bexarotene and LG100268 have been shown to prevent estrogen receptor-negative breast cancer in various mouse models including transgenic lines of MMTV-ErbB2 overexpressing, p53-null, and C3(1)-SV40 T-antigen (Tag) expressing mice and rats (Wu et al. 2002a, b; Liby et al. 2008; Medina et al. 2009; Woditschka et al. 2006). In addition, we have shown that LGD100268 singificantly prevents premalignant lesions including hyperplasia and ductal carcinoma in situ, suggesting that it affects mammary tumorigenesis at the early stages (Li et al. 2007).
The effectiveness of bexarotene to prevent breast cancer formation in humans may be accompanied by mild hyperlipidemia, skin rash, hypothyroidism, elevated liver enzymes, and neutropenia (at high doses only). The positive data from the preclinical studies provided the rationale to launch a human biomarker modulation trial using bexarotene in women at high risk of breast cancer in a 4-week period. This trial has determined that bexarotene significantly down-regulates cyclin D1 expression and decreases proliferation, as measured by Ki67 staining (this latter change did not reach statistical significance) (as presented in abstract form at the 2008 AACR Frontiers in Cancer Prevention Symposium). Interestingly, these responses occurred selectively in postmenopausal women. The results from this trial will provide a reference for other rexinoids to develop more effective and safer preventive drugs.
Combination treatment may represent a promising new strategy to suppress both ER-negative and ER-positive breast tumors, and the combination of rexinoids with antiestrogens may be particularly effective. Recent studies reported on the highly synergistic effect of the RXR agonist LGD100268 and the selective estrogen receptor modulator arzoxifene through the inhibition of cell growth and induction of apoptosis (Rendi et al. 2004; Liby et al. 2006). Early results from our ongoing animal experiments also indicate that tamoxifen used in combination with bexarotene or LG100268 is superior to either drug alone.
13.6.4 PPAR Agonists
Peroxisome proliferator-activated receptors (PPAR) are members of the nuclear hormone receptor superfamily selectively modulating the expression of their target genes forming heterodimers with RXRs. PPARγ plays an important role in adipocyte differentiation, insulin sensitivity, energy metabolism, and immune response. Recently, PPARγ has emerged as a promising target for cancer therapy based on the fact that its activation by synthetic ligands, thiazolidinediones (TZDs), was found to induce cell cycle arrest, apoptosis, and differentiation in human malignancies (Mueller et al. 1998; Yin et al. 2001). Troglitazone, a PPARγ ligand, induced apoptosis in both ER-positive and ER-negative human breast cancer cells (Clay et al. 1999; Yin et al. 2004). Activation of PPARγ by the antidiabetic drug rosiglitazone caused tumor-selective suppression of the NHE1 transporter and inhibition of cancer cell proliferation in vitro and in pathologic specimens from breast cancer patients (Kumar et al. 2009). The chemopreventive potential of PPARγ was demonstrated in experimental rodent mammary tumorigenesis models (Suh et al. 1999), as the PPARγ ligand GW7845 significantly reduced tumor incidence, multiplicity, and weight. Similarly, troglitazone prevented the development of DMBA-induced premalignant lesions in mouse mammary organ culture (Mehta et al. 2000). Natural occurring ligands such as conjugated linoleic acids may be regarded as a component of the diet that exert antineoplastic activity and its effect have also been found to inhibit progression of mammary tumorigenesis (Ip et al. 1999; Maggiora et al. 2004; Wendel and Heller 2009). These data suggested that PPAR ligands maybe useful in preventing the development of ER-negative breast cancers.
13.6.5 COX-2 Inhibitors
There is a wealth of epidemiological data to suggest that long-term use of aspirin or nonsteroidal antiinflammatory drugs (NSAIDs) is associated with a reduced risk of various cancers, in particular of the digestive system (Schreinemachers and Everson 1994). The biochemical effect of NSAIDs is the inhibition of cyclooxigenase (COX) enzymes, which play a crucial role in growth-promoting prostaglandin synthesis via the eicosanoid pathway (Fig. 13.1). Overexpression of the inducible isoform COX-2 has been demonstrated for several human cancers including, breast, lung, esophageal, hepatocellular, and most notably colorectal adenocarcinomas (Turini and DuBois 2002). It has also been suggested that there is cross talk between COX-2 and EGFR via MAPK signaling resulting in the induction of the COX-2 enzyme, which underscores the rationale for combination chemoprevention (Dannenberg et al. 2005). Meta-analysis on cohort and case–control studies has revealed that NSAID use may also be associated with a small decrease in the risk of breast cancer (Khuder and Mutgi 2001).
Multiple studies have tested the cancer-preventive effect of various NSAIDs and selective COX-2 inhibitors in animal models. Celecoxib, a selective COX-2 inhibitor, reduced the incidence and multiplicity of DMBA-induced mammary tumors in rat models by 68% and 86%, respectively (Harris et al. 2000). In MMTV-erbB2 transgenic mice, which mainly develop ER-negative cancers, celecoxib at 500 ppm delayed mammary tumor development and decreased the levels of PGE2 by 50%, suggesting that COX-2 inhibitors might be able to prevent ER-negative breast cancer (Howe et al. 2002). Conversely, other studies suggest that COX-2 might not be the only or ideal target in the eicosanoid pathway (Rigas and Kashfi 2005). This notion is supported by the facts that COX-2 is induced at a relatively later stage in carcinogenesis, NSAIDs may not require inhibition of COX-2 for their effect, and lastly, NSAIDs have a modest effect in the preventive setting.
There is a concern that inhibition of COX-2 in nonneoplastic cell might be harmful and cause unwanted toxicity. Indeed, phase III polyp prevention trials of COX-2 inhibitors uncovered rare, late, but serious cardiovascular toxicities of these drugs. In response to an increase in the risk of heart attacks observed in individuals taking celecoxib the FDA temporarily halted all ongoing celecoxib trials, including those for breast cancer prevention. Later the trials were allowed to continue, but these rare and serious side effects likely will limit the widespread use of COX-2 inhibitors as cancer prevention agents. However, use in populations at low risk for heart disease may be found to be safe and effective. Interfering with PGE2-induced signaling by targeting prostanoid (EP) receptors, such as EP4 or downstream targets, may also offer a safer alternative to COX-2 inhibition.
13.6.6 Tyrosine Kinase Inhibitors
13.6.6.1 The erbB System
Epithelial cells largely depend on extracellular growth signals transmitted by receptor tyrosine kinases (see Fig. 13.1). Epidermal and other growth factors trigger autophosphorylation of their receptors, which in turn initiates a cascade of signaling events involving the activation of the ras pathway kinases via Grb2/SOS and MAPK activation. These mitogenic signaling events result in the activation of cell cycle genes and inhibition of programmed cell death. Overexpression of the MAP kinases p44 Erk1 and p42 Erk2 have also been shown in human breast tumors (Salh et al. 1999).
Breast cancers often arise from aberrant expression or activation of the erbB family of growth factor receptors (Hynes and Lane 2005), consisting of four distinct members, epidermal growth factor receptor (EGFR, erbB1or Her-1), erbB2 (Her2 or neu), erbB3 (Her-3), and erbB4 (Her4). 20–30% of all human mammary tumors, particularly those that are ER-negative, overexpress the erbB2 oncogene or one of its homologues (Ariga et al. 2005). The EGFR mediates the actions of a family of growth factors, which includes EGF, transforming growth factor alpha, and the neuregulins.
The usefulness of TKIs for the treatment of cancer has been indicated by studies of imatinib mesylate (STI-571/Gleevec), a cAbl, and c-kit tyrosine kinase inhibitor that was used successfully for the treatment of chronic myelogenous leukemia and gastrointestinal stromal tumors (Savage and Antman 2002; Sawyers et al. 2002). Several different signaling inhibitors that block the activity of the ErbB family of receptors have been developed and approved for clinical use, including herceptin (Trastuzumab), gefitinib (Iressa), and erlotinib (Tarceva). Herceptin, a humanized monoclonal Her-2 antibody, is highly effective for the treatment Her-2-positive breast cancers, but antibody treatment may not be viable in the prevention setting. In preclinical models we have demonstrated that gefitinib prevents the development of ER-negative mammary tumors (Lu et al. 2003) and others have shown that it is superior to herceptin in suppressing growth of DCIS epithelial cells (Chan et al. 2001). Gefitinib was released for phase II and III testing for the treatment of lung, prostate, and breast cancer, and is now approved for the treatment of lung cancer. However, due to the onset of pulmonary fibrosis as a rare side effect attributed to the drug all cancer-preventive trials using gefitinib were put on hold.
Both in vitro and in vivo studies demonstrated that dual inhibition of EGFR and Her-2 more effectively suppresses cell growth and survival than the inhibition of either receptor alone (Moulder et al. 2001), prompting the development of multivalent erbB receptor inhibitors. A number of new EGFR-selective (PD153035, AG1478, AG1517, PD158780, PD165557, PKI 166) and multitarget (lapatinib, HKI-272, BIBW-2992, BMS-599626, targeting EGFR and Her-2; CI-1033 targeting EGFR, Her-2 and erbB4) inhibitors have been developed and are now in clinical testing for the treatment of various solid tumors.
Lapatinib (Tykerb®, GlaxoSmithKline) was first approved to use in combination with capecitabine (Xeloda), for women whose breast cancer had progressed on previous chemotherapies. Lapatinib had similar activity in HER2-positive breast tumors as a monotherapy or in combination with capecitabine. In early 2010, the US Food and Drug Administration granted approval to lapatinib for use in combination with the aromatase inhibitor letrozole for the treatment of postmenopausal women with hormone receptor positive metastatic breast cancer that overexpresses the Her-2 receptor and for whom hormonal therapy is indicated. Based on the results of the randomized phase III study EGF30008 the European Medicines Agency also issued its positive opinion for the authorization of lapatinib in combination with letrozole.
Our group has shown that lapatinib significantly delays breast cancer development in MMTV-erbB2 transgenic mice and prevents the development of premalignant mammary lesions (Strecker et al. 2009). These results suggest that lapatinib may be a good candidate as a cancer-preventive agent and the first neoadjuvant trial has begun in women with DCIS breast cancer in a multicenter clinical trial.
13.6.7 The IGF System
Insulin-like growth factors play a pertinent role in the growth regulation of mammary epithelial cells. IGF-I is bound with high affinity by type I insulin-like growth factor receptor (IGF-IR) and activates cellular proliferation in both normal growth and development and malignant transformation. IGF-I signals are mediated via phosphorylation of a family of insulin receptor substrate proteins (IRS), which may serve both complementary and overlapping functions to insulin receptor (IR) in the cell.
In addition to the important role of the IGF system in normal mammary development, it has been proposed that overproduction of GH or IGF-I can also cause the development of atypical hyperplasias or even carcinoma (Kleinberg et al. 2009). While it is not understood how early full-term pregnancy can provide natural protection against breast cancer, serum GH levels, and downstream signaling activity were shown to be decreased in parous animals compared to virgins (Dearth et al. 2010). Therefore, novel agents targeting the GH/IGF-I axis may provide a viable means to block formation and progression of neoplastic precursor lesions. As a proof of principle, the new IGF-IR tyrosine kinase inhibitor, BMS-536924 caused a blockade of cell proliferation in monolayer and 3D cell cultures, and reversed the ability of constitutively active IGF-IR to transform MCF10A cells (Litzenburger et al. 2009). Thus, agents known to block mammary gland development through inhibition of IGF-I action, may also present themselves as candidates for chemoprevention in women at high risk of breast cancer.
13.6.8 Conclusions and Future Directions
Since Michael Sporn introduced the term “chemoprevention” in the 1970s, the breast cancer prevention field has taken unexpected directions and is now facing new challenges. Firstly, while clinical trials have demonstrated that antiestrogen drug treatment of high-risk women can prevent breast cancer, there has not been widespread use of these drugs for cancer prevention. In addition, the testing of new preventive drugs has been slow and costly. To increase the success rate of future clinical cancer prevention research, it will be necessary to conduct many small-scale, phase 0, I, and II trials focused on high-risk women. Target populations should be carefully selected to identify high-risk individuals most likely to benefit from the cancer-preventive drug. In addition, the use of adaptive clinical trial designs may reduce the number of subjects needed for these studies and thus speed the pace of cancer prevention research.
Secondly, public awareness and sensitivity to possible rare adverse effects occurring from long-term preventive drug use have increased and have made the conduction of clinical cancer prevention trials much more difficult (as was the case with celecoxib and gefitinib). While many of these drugs are tolerated by cancer patients, the potential for even minor side effects becomes a major factor when considering chronic preventive therapy in healthy women. To overcome these problems, future cancer prevention drug development will need to identify effective drugs with minimal toxicity that may be used for short periods or in short pulses. Finally, it will be necessary to educate the public and medical community about the need for risk/benefit analysis when deciding upon cancer-preventive therapy. With a good understanding of the risk and benefits of future cancer-preventive agents, it will be possible to reduce cancer incidence in high-risk individuals who are likely to accept the risk of rare, minor toxicities from effective cancer-preventive drugs.
Acknowledgments
This work was supported by NCI/NIH Grants RO1 CA10121, RO1 CA78480, and a PROMISE grant from the Susan G. Komen for the Cure Foundation.
References
- Anzano MA, Byers SW, et al. Prevention of breast cancer in the rat with 9-cis-retinoic acid as a single agent and in combination with tamoxifen. Cancer Res. 1994;54(17):4614–4617. [PubMed] [Google Scholar]
- Ariga R, Zarif A, et al. Correlation of her-2/neu gene amplification with other prognostic and predictive factors in female breast carcinoma. Breast J. 2005;11(4):278–280. doi: 10.1111/j.1075-122x.2005.21463.x. [DOI] [PubMed] [Google Scholar]
- Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334(9):574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- Bonovas S, Filioussi K, et al. Use of statins and breast cancer: a meta-analysis of seven randomized clinical trials and nine observational studies. J Clin Oncol. 2005;23(34):8606–8612. doi: 10.1200/JCO.2005.02.7045. [DOI] [PubMed] [Google Scholar]
- Bryant HE, Schultz N, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434(7035):913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- Campbell MJ, Esserman LJ, et al. Breast cancer growth prevention by statins. Cancer Res. 2006;66(17):8707–8714. doi: 10.1158/0008-5472.CAN-05-4061. [DOI] [PubMed] [Google Scholar]
- Cazzaniga M, Bonanni B, et al. Is it time to test metformin in breast cancer clinical trials? Cancer Epidemiol Biomarkers Prev. 2009;18(3):701–705. doi: 10.1158/1055-9965.EPI-08-0871. [DOI] [PubMed] [Google Scholar]
- Chan KC, Knox WF, et al. Blockade of growth factor receptors in ductal carcinoma in situ inhibits epithelial proliferation. Br J Surg. 2001;88(3):412–418. doi: 10.1046/j.1365-2168.2001.01686.x. [DOI] [PubMed] [Google Scholar]
- Chlebowski R, Cuzick J, et al. Clinical perspectives on the utility of aromatase inhibitors for the adjuvant treatment of breast cancer. Breast. 2009;18(Suppl 2):S1–11. doi: 10.1016/S0960-9776(09)70002-5. [DOI] [PubMed] [Google Scholar]
- Clay CE, Namen AM, et al. Influence of J series prostaglandins on apoptosis and tumorigenesis of breast cancer cells. Carcinogenesis. 1999;20(10):1905–1911. doi: 10.1093/carcin/20.10.1905. [DOI] [PubMed] [Google Scholar]
- Cummings SR, Eckert S, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple outcomes of raloxifene evaluation. Jama. 1999;281(23):2189–2197. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- Cummings SR, Ensrud K, et al. Lasofoxifene in postmenopausal women with osteoporosis. N Engl J Med. 2010;362(8):686–96. doi: 10.1056/NEJMoa0808692. [DOI] [PubMed] [Google Scholar]
- Cuzick J. Aromatase inhibitors in prevention – data from the ATAC (arimidex, tamoxifen alone or in combination) trial and the design of IBIS-II (the second International Breast Cancer Intervention Study) Recent Results Cancer Res. 2003;163:96–103. doi: 10.1007/978-3-642-55647-0_9. discussion 264–266. [DOI] [PubMed] [Google Scholar]
- Cuzick J. IBIS II: a breast cancer prevention trial in postmenopausal women using the aromatase inhibitor anastrozole. Expert Rev Anticancer Ther. 2008;8(9):1377–1385. doi: 10.1586/14737140.8.9.1377. [DOI] [PubMed] [Google Scholar]
- Cuzick J, Baum M. Tamoxifen and contralateral breast cancer. Lancet. 1985;2(8449):282. doi: 10.1016/s0140-6736(85)90338-1. [DOI] [PubMed] [Google Scholar]
- Cuzick J, Forbes JF, et al. Long-term results of tamoxifen prophylaxis for breast cancer–96-month follow-up of the randomized IBIS-I trial. J Natl Cancer Inst. 2007;99(4):272–282. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- Cuzick J, Powles T, et al. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361(9354):296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- Dannenberg AJ, Lippman SM, et al. Cyclooxygenase-2 and epidermal growth factor receptor: pharmacologic targets for chemoprevention. J Clin Oncol. 2005;23(2):254–266. doi: 10.1200/JCO.2005.09.112. [DOI] [PubMed] [Google Scholar]
- Dearth RK, Delgado DA, et al. Parity-induced decrease in systemic growth hormone alters mammary gland signaling: A potential role in pregnancy protection from breast cancer. Cancer Preve Res (Phila Pa) 2010;3(3):312–321. doi: 10.1158/1940-6207.CAPR-09-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn BK, Ryan A. Phase 3 trials of aromatase inhibitors for breast cancer prevention: following in the path of the selective estrogen receptor modulators. Ann N Y Acad Sci. 2009;1155:141–161. doi: 10.1111/j.1749-6632.2009.03688.x. [DOI] [PubMed] [Google Scholar]
- Fisher B, Costantino JP, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- Fisher B, Costantino JP, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90(18):1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- Fong PC, Boss DS, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- Forbes JF, Cuzick J, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9(1):45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- Garwood ER, Kumar AS, et al. Fluvastatin reduces proliferation and increases apoptosis in women with high grade breast cancer. Breast Cancer Res Treat. 2010;119(1):137–44. doi: 10.1007/s10549-009-0507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardiello FM, Brensinger JD, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119(6):1447–1453. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- Goodwin PJ. Insulin in the adjuvant breast cancer setting: a novel therapeutic target for lifestyle and pharmacologic interventions? J Clin Oncol. 2008;26(6):833–834. doi: 10.1200/JCO.2007.14.7132. [DOI] [PubMed] [Google Scholar]
- Goodwin PJ, Ligibel JA, et al. Metformin in breast cancer: time for action. J Clin Oncol. 2009;27(20):3271–3273. doi: 10.1200/JCO.2009.22.1630. [DOI] [PubMed] [Google Scholar]
- Goss PE, Richardson H, et al. National Cancer Institute of Canada Clinical Trials Group MAP.3 Trial: evaluation of exemestane to prevent breast cancer in postmenopausal women. Clin Breast Cancer. 2007;7(11):895–900. doi: 10.3816/CBC.2007.n.057. [DOI] [PubMed] [Google Scholar]
- Harris RE, Alshafie GA, et al. Chemoprevention of breast cancer in rats by celecoxib, a cyclooxygenase 2 inhibitor. Cancer Res. 2000;60(8):2101–2103. [PubMed] [Google Scholar]
- Hong WK, Lippman SM, et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med. 1990;323(12):795–801. doi: 10.1056/NEJM199009203231205. [DOI] [PubMed] [Google Scholar]
- Honig SF. Tamoxifen for the reduction in the incidence of breast cancer in women at high risk for breast cancer. Ann N Y Acad Sci. 2001;949:345–348. doi: 10.1111/j.1749-6632.2001.tb04043.x. [DOI] [PubMed] [Google Scholar]
- Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A, Miller BA, Lewis DR, Eisner MP, Stinchcomb DG, Edwards BK, editors. SEER Cancer Statistics Review, 1975–2006. 2009 Retrieved July 15, 2009, 2009, from http://seer.cancer.gov/statfacts/html/breast.html, http://seer.cancer.gov/csr/1975_2006/
- Howe LR, Subbaramaiah K, et al. Celecoxib, a selective cyclooxygenase 2 inhibitor, protects against human epidermal growth factor receptor 2 (HER-2)/neu-induced breast cancer. Cancer Res. 2002;62(19):5405–5407. [PubMed] [Google Scholar]
- Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5(5):341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- Ip MM, Masso-Welch PA, et al. Conjugated linoleic acid inhibits proliferation and induces apoptosis of normal rat mammary epithelial cells in primary culture. Exp Cell Res. 1999;250(1):22–34. doi: 10.1006/excr.1999.4499. [DOI] [PubMed] [Google Scholar]
- Josefsson ML, Leinster SJ. Aromatase inhibitors versus tamoxifen as adjuvant hormonal therapy for oestrogen sensitive early breast cancer in postmenopausal women: Meta-analyese of monotherapy, sequenced therapy and extended therapy. Breast. 2010 doi: 10.1016/j.breast.2009.12.010. [DOI] [PubMed] [Google Scholar]
- Khuder SA, Mutgi AB. Breast cancer and NSAID use: a meta-analysis. Br J Cancer. 2001;84(9):1188–1192. doi: 10.1054/bjoc.2000.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinberg DL, Wood TL, et al. Growth hormone and insulin-like growth factor-I in the transition from normal mammary development to preneoplastic mammary lesions. Endocr Rev. 2009;30(1):51–74. doi: 10.1210/er.2008-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong G, Kim HT, et al. The retinoid X receptor-selective retinoid, LGD1069, down-regulates cyclooxygenase-2 expression in human breast cells through transcription factor crosstalk: implications for molecular-based chemoprevention. Cancer Res. 2005;65(8):3462–3469. doi: 10.1158/0008-5472.CAN-03-2912. [DOI] [PubMed] [Google Scholar]
- Kumar AP, Quake AL, et al. Repression of NHE1 expression by PPARgamma activation is a potential new approach for specific inhibition of the growth of tumor cells in vitro and in vivo. Cancer Res. 2009;69(22):8636–8644. doi: 10.1158/0008-5472.CAN-09-0219. [DOI] [PubMed] [Google Scholar]
- Kumar AS, Benz CC, et al. Estrogen receptor-negative breast cancer is less likely to arise among lipophilic statin users. Cancer Epidemiol Biomarkers Prev. 2008;17(5):1028–1033. doi: 10.1158/1055-9965.EPI-07-0726. [DOI] [PubMed] [Google Scholar]
- Kumar AS, Campbell M, et al. A call for clinical trials: lipophilic statins may prove effective in treatment and prevention of particular breast cancer subtypes. J Clin Oncol. 2006;24(13):2127. doi: 10.1200/JCO.2005.04.9882. author reply 2127–2128. [DOI] [PubMed] [Google Scholar]
- Larsson SC, Mantzoros CS, et al. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121(4):856–862. doi: 10.1002/ijc.22717. [DOI] [PubMed] [Google Scholar]
- Lee HY, Dawson MI, et al. Retinoic acid receptor- and retinoid X receptor-selective retinoids activate signaling pathways that converge on AP-1 and inhibit squamous differentiation in human bronchial epithelial cells. Cell Growth Differ. 1996;7(8):997–1004. [PubMed] [Google Scholar]
- Lerner LJ, Holthaus FJ, Jr, et al. A non-steroidal estrogen antiagonist 1-(p-2-diethylaminoethoxyphenyl)-1-phenyl-2-p-methoxyphenyl ethanol. Endocrinology. 1958;63(3):295–318. doi: 10.1210/endo-63-3-295. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang Y, et al. The Rexinoid LG100268 prevents the development of preinvasive and invasive estrogen receptor negative tumors in MMTV-erbB2 mice. Clin Cancer Res. 2007;13(20):6224–6231. doi: 10.1158/1078-0432.CCR-06-2681. [DOI] [PubMed] [Google Scholar]
- Liby K, Rendi M, et al. The combination of the rexinoid, LG100268, and a selective estrogen receptor modulator, either arzoxifene or acolbifene, synergizes in the prevention and treatment of mammary tumors in an estrogen receptor-negative model of breast cancer. Clin Cancer Res. 2006;12(19):5902–5909. doi: 10.1158/1078-0432.CCR-06-1119. [DOI] [PubMed] [Google Scholar]
- Liby K, Risingsong R, et al. Prevention and treatment of experimental estrogen receptor-negative mammary carcinogenesis by the synthetic triterpenoid CDDO-methyl Ester and the rexinoid LG100268. Clin Cancer Res. 2008;14(14):4556–4563. doi: 10.1158/1078-0432.CCR-08-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litzenburger BC, Kim HJ, et al. BMS-536924 reverses IGF-IR-induced transformation of mammary epithelial cells and causes growth inhibition and polarization of MCF7 cells. Clin Cancer Res. 2009;15(1):226–237. doi: 10.1158/1078-0432.CCR-08-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Speers C, et al. Effect of epidermal growth factor receptor inhibitor on development of estrogen receptor-negative mammary tumors. J Natl Cancer Inst. 2003;95(24):1825–1833. doi: 10.1093/jnci/djg117. [DOI] [PubMed] [Google Scholar]
- Maggiora M, Bologna M, et al. An overview of the effect of linoleic and conjugated-linoleic acids on the growth of several human tumor cell lines. Int J Cancer. 2004;112(6):909–919. doi: 10.1002/ijc.20519. [DOI] [PubMed] [Google Scholar]
- Mason RP, Walter MF, et al. Effects of HMG-CoA reductase inhibitors on endothelial function: role of microdomains and oxidative stress. Circulation. 2004;109(21 Suppl 1):II34–41. doi: 10.1161/01.CIR.0000129503.62747.03. [DOI] [PubMed] [Google Scholar]
- Medina D, Kittrell F, et al. Prevention of tumorigenesis in p53-null mammary epithelium by rexinoid bexarotene, tyrosine kinase inhibitor gefitinib, and celecoxib. Cancer Prev Res (Phila Pa) 2009;2(2):168–174. doi: 10.1158/1940-6207.CAPR-08-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta RG, Williamson E, et al. A ligand of peroxisome proliferator-activated receptor gamma, retinoids, and prevention of preneoplastic mammary lesions. J Natl Cancer Inst. 2000;92(5):418–423. doi: 10.1093/jnci/92.5.418. [DOI] [PubMed] [Google Scholar]
- Moon RC, Thompson HJ, et al. N-(4-Hydroxyphenyl)retinamide, a new retinoid for prevention of breast cancer in the rat. Cancer Res. 1979;39(4):1339–1346. [PubMed] [Google Scholar]
- Moulder SL, Yakes FM, et al. Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo. Cancer Res. 2001;61(24):8887–8895. [PubMed] [Google Scholar]
- Mueck AO, Seeger H, et al. Effect of statins combined with estradiol on the proliferation of human receptor-positive and receptor-negative breast cancer cells. Menopause. 2003;10(4):332–336. doi: 10.1097/01.GME.0000055485.06076.00. [DOI] [PubMed] [Google Scholar]
- Mueller E, Sarraf P, et al. Terminal differentiation of human breast cancer through PPAR gamma. Mol Cell. 1998;1(3):465–470. doi: 10.1016/s1097-2765(00)80047-7. [DOI] [PubMed] [Google Scholar]
- Papa V, Belfiore A. Insulin receptors in breast cancer: biological and clinical role. J Endocrinol Invest. 1996;19(5):324–333. doi: 10.1007/BF03347871. [DOI] [PubMed] [Google Scholar]
- Powles TJ, Ashley S, et al. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99(4):283–290. doi: 10.1093/jnci/djk050. [DOI] [PubMed] [Google Scholar]
- Rendi MH, Suh N, et al. The selective estrogen receptor modulator arzoxifene and the rexinoid LG100268 cooperate to promote transforming growth factor beta-dependent apoptosis in breast cancer. Cancer Res. 2004;64(10):3566–3571. doi: 10.1158/0008-5472.CAN-04-0234. [DOI] [PubMed] [Google Scholar]
- Rigas B, Kashfi K. Cancer prevention: a new era beyond cyclooxygenase-2. J Pharmacol Exp Ther. 2005;314(1):1–8. doi: 10.1124/jpet.104.080564. [DOI] [PubMed] [Google Scholar]
- Salh B, Marotta A, et al. Investigation of the Mek-MAP kinase-Rsk pathway in human breast cancer. Anticancer Res. 1999;19(1B):731–740. [PubMed] [Google Scholar]
- Savage DG, Antman KH. Imatinib mesylate–a new oral targeted therapy. N Engl J Med. 2002;346(9):683–693. doi: 10.1056/NEJMra013339. [DOI] [PubMed] [Google Scholar]
- Sawyers CL, Hochhaus A, et al. Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood. 2002;99(10):3530–3539. doi: 10.1182/blood.v99.10.3530. [DOI] [PubMed] [Google Scholar]
- Schreinemachers DM, Everson RB. Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology. 1994;5(2):138–146. doi: 10.1097/00001648-199403000-00003. [DOI] [PubMed] [Google Scholar]
- Seewaldt VL, Kim JH, et al. All-trans-retinoic acid mediates G1 arrest but not apoptosis of normal human mammary epithelial cells. Cell Growth Differ. 1997;8(6):631–641. [PubMed] [Google Scholar]
- Shaw RJ, Lamia KA, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310(5754):1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker TE, Shen Q, et al. Effect of lapatinib on the development of estrogen receptor-negative mammary tumors in mice. J Natl Cancer Inst. 2009;101(2):107–113. doi: 10.1093/jnci/djn436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh N, Wang Y, et al. A new ligand for the peroxisome proliferator-activated receptor-gamma (PPAR-gamma), GW7845, inhibits rat mammary carcinogenesis. Cancer Res. 1999;59(22):5671–5673. [PubMed] [Google Scholar]
- Szanto A, Narkar V, et al. Retinoid X receptors: X-ploring their (patho)physiological functions. Cell Death Differ. 2004;11(Suppl 2):S126–143. doi: 10.1038/sj.cdd.4401533. [DOI] [PubMed] [Google Scholar]
- Turini ME, DuBois RN. Cyclooxygenase-2: a therapeutic target. Annu Rev Med. 2002;53:35–57. doi: 10.1146/annurev.med.53.082901.103952. [DOI] [PubMed] [Google Scholar]
- Tutt A, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. J Clin Oncol (Suppl) 2009;27(18s) doi: 10.1056/NEJMoa0900212. Abstr. CRA501. [DOI] [PubMed] [Google Scholar]
- Uray IP, Shen Q, et al. Rexinoid-induced expression of IGFBP-6 requires RARbeta-dependent permissive cooperation of retinoid receptors and AP-1. J Biol Chem. 2009;284(1):345–353. doi: 10.1074/jbc.M804721200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Martin A, Oliveras-Ferraros C, et al. The antidiabetic drug metformin: a pharmaceutical AMPK activator to overcome breast cancer resistance to HER2 inhibitors while decreasing risk of cardiomyopathy. Ann Oncol. 2009;20(3):592–595. doi: 10.1093/annonc/mdn758. [DOI] [PubMed] [Google Scholar]
- Veronesi U, Maisonneuve P, et al. Tamoxifen for the prevention of breast cancer: late results of the Italian Randomized Tamoxifen Prevention Trial among women with hysterectomy. J Natl Cancer Inst. 2007;99(9):727–737. doi: 10.1093/jnci/djk154. [DOI] [PubMed] [Google Scholar]
- Vogel VG, Costantino JP, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295(23):2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- Wendel M, Heller AR. Anticancer actions of omega-3 fatty acids – current state and future perspectives. Anticancer Agents Med Chem. 2009;9(4):457–470. doi: 10.2174/1871520610909040457. [DOI] [PubMed] [Google Scholar]
- Woditschka S, Haag JD, et al. Neu-induced retroviral rat mammary carcinogenesis: a novel chemoprevention model for both hormonally responsive and nonresponsive mammary carcinomas. Cancer Res. 2006;66(13):6884–6891. doi: 10.1158/0008-5472.CAN-05-1823. [DOI] [PubMed] [Google Scholar]
- Wolbach SB, Howe PR. Tissue changes following deprivation of fat-soluble a vitamin. J Exp Med. 1925;42(6):753–777. doi: 10.1084/jem.42.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, DuPre E, et al. Receptor-selective retinoids inhibit the growth of normal and malignant breast cells by inducing G1 cell cycle blockade. Breast Cancer Res Treat. 2006;96(2):147–157. doi: 10.1007/s10549-005-9071-1. [DOI] [PubMed] [Google Scholar]
- Wu K, Kim HT, et al. Suppression of mammary tumorigenesis in transgenic mice by the RXR-selective retinoid, LGD1069. Cancer Epidemiol Biomarkers Prev. 2002a;11(5):467–474. [PubMed] [Google Scholar]
- Wu K, Kim HT, et al. 9-cis-Retinoic acid suppresses mammary tumorigenesis in C3(1)-simian virus 40 T antigen-transgenic mice. Clin Cancer Res. 2000;6(9):3696–3704. [PubMed] [Google Scholar]
- Wu K, Zhang Y, et al. The retinoid X receptor-selective retinoid, LGD1069, prevents the development of estrogen receptor-negative mammary tumors in transgenic mice. Cancer Res. 2002b;62(22):6376–6380. [PubMed] [Google Scholar]
- Xue F, Michels KB. Diabetes, metabolic syndrome, and breast cancer: a review of the current evidence. Am J Clin Nutr. 2007;86(3):s823–835. doi: 10.1093/ajcn/86.3.823S. [DOI] [PubMed] [Google Scholar]
- Yang L, Ostrowski J, et al. The retinoic acid receptor antagonist, BMS453, inhibits normal breast cell growth by inducing active TGFbeta and causing cell cycle arrest. Oncogene. 2001;20(55):8025–8035. doi: 10.1038/sj.onc.1204911. [DOI] [PubMed] [Google Scholar]
- Yin F, Bruemmer D, et al. Signaling pathways involved in induction of GADD45 gene expression and apoptosis by troglitazone in human MCF-7 breast carcinoma cells. Oncogene. 2004;23(26):4614–4623. doi: 10.1038/sj.onc.1207598. [DOI] [PubMed] [Google Scholar]
- Yin F, Wakino S, et al. Troglitazone inhibits growth of MCF-7 breast carcinoma cells by targeting G1 cell cycle regulators. Biochem Biophys Res Commun. 2001;286(5):916–922. doi: 10.1006/bbrc.2001.5491. [DOI] [PubMed] [Google Scholar]
- Zakikhani M, Dowling R, et al. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res. 2006;66(21):10269–10273. doi: 10.1158/0008-5472.CAN-06-1500. [DOI] [PubMed] [Google Scholar]