Abstract
We examined the effect of concanamycin A and bafilomycin A1, inhibitors of the vacuolar proton-ATPase, on maturation and expression of Ret, a tyrosine kinase receptor for glial cell line-derived neurotrophic factor. Ret appeared as 150- and 170-kDa bands on sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels and both forms were sensitive to peptide-N-glycosidase F. Western and immunocytochemical analyses revealed that the 150-kDa immature form of Ret accumulated in the Golgi apparatus upon treatment with vacuolar proton-ATPase inhibitors, whereas, the 170-kDa mature form of Ret was dramatically decreased. The result suggests that glycosylation of Ret during the conversion from immature forms to mature forms is pH sensitive, and is likely initiated in the acidic trans-Golgi apparatus. In contrast, glycosylation of nascent receptors to become immature receptors appeared to be pH insensitive, and are likely to take place in the endoplasmic reticulum. The immature form of Ret was present in the plasma membrane when the cells were treated with the vacuolar proton-ATPase inhibitors. In conclusion, the acidification of the Golgi apparatus is crucial for maturation of Ret but not indispensable for trafficking of receptors to the membrane.
Keywords: bafilomycin A1, concanamycin A, EGFR, PC12 cells, Ret
Ret, originally identified as an oncogene activated by DNA rearrangement (Takahashi et al. 1985) encodes the Ret tyrosine kinase expressed primarily on neural crest-derived and urogenital cells (Pachnis et al. 1993; Avantaggiato et al. 1994). Mutations in the Ret gene are associated with several human diseases such as multiple endocrine neoplasia type 2A and 2B, familial medullary thyroid carcinoma, and Hirschsprung’s disease (Edery et al. 1994; Hofstra et al. 1994; Romeo et al. 1994). Ret is a functional receptor for the glial cell line-derived neurotrophic factor (GDNF) family of ligands including GDNF, neuturin, artemin and persephin, which are required for the survival of various neurons in the central nervous system and peripheral nervous system. GDNF family ligands bind to and activate Ret when bound to the GDNF family receptors 1–4 (Airaksinen and Saarma 2002). Like other receptor tyrosine kinases (RTKs), Ret can activate various signaling pathways including RAS/extracellular signal-regulated kinase, phosphatidylinositol 3-kinase/Akt, p38 mitogen activated protein kinase and c-Jun N-terminal kinase (Ichihara et al. 2004). The Ret proteins are derived from a single polypeptide core of 120 kDa and are modified by post-translational glycosylation to be 150 kDa and 170 kDa (Takahashi et al. 1991). It is suggested that the 170 kDa Ret protein represents the mature glycosylated form present on the cell surface, while the 150 kDa protein represents the immature glycosylated form present in the cytoplasmic membrane component (Takahashi et al. 1993). The expression of Ret is dynamically regulated by various extracellular stimuli (Hirata and Kiuchi 2007; Pierchala et al. 2007). Although the newly synthesized 120 kDa Ret in the endoplasmic reticulum (ER) need to be glycosylated and transported to the plasma membrane along the exocytotic pathway, the maturation and trafficking of Ret protein are not well known.
Acidification of organelles is crucial for many biological processes, including receptor-ligand dissociation in early endosomes, the selective activation of lysosomal hydrolases, antigen processing by immune cells, efficient infection by viruses and other pathogens, and sorting of newly synthesized proteins in the trans-Golgi network (Schoonderwoert et al. 2000; Weisz 2003). Proton transport into intracellular organelles is mainly mediated by vacuolar proton-ATPase (V-ATPase) (Nelson and Taiz 1989; Saroussi and Nelson 2009). V-ATPase is a multimeric complex that functions as a proton pumping rotary nano-motor and is found in the Golgi complex, secretory vesicles, synaptic vesicles, endosomes, lysosomes, and the plasma membrane (Marshansky and Futai 2008). V-ATPase-driven proton pumping and organellar acidification is essential for vesicular trafficking along both the exocytotic and endocytotic pathways. Concanamycin A and bafilomycin A1 are well-known inhibitors of the V-ATPase (Drose and Altendorf 1997).
To investigate the involvement of acidification in the maturation and trafficking of newly synthesized Ret, we examined the effect of concanamycin A and bafilomycin A1 on the expression of Ret and compared with that of epidermal growth factor receptor (EGFR), known as a highly glycosylated RTK in cultured cells. EGFR protein is similarly synthesized from a single polypeptide core of 130 kDa, and is modified by post-translational glycosylation to yield a 170 kDa-mature glycoprotein in the plasma membrane (Soderquist and Carpenter 1984; Slieker et al. 1986). We found that intracellular immature forms of Ret and EGFR accumulated in the Golgi apparatus, and that mature forms of Ret and EGFR disappeared by the treatment of cells with V-ATPase inhibitors. This suggested that the acidification of the Golgi apparatus is crucial for the maturation of Ret and EGFR. The immature 150 kDa forms of Ret and EGFR could be detected in the plasma membrane of cells treated with V-ATPase inhibitors, suggesting that the immature forms were transported to the cell surface under the inhibition of V-ATPase. Similar results were obtained for Trk, a tyrosine kinase receptor for nerve growth factor.
Materials and methods
Materials
Concanamycin A was obtained from Wako Pure Chemicals (Osaka, Japan). Bafilomycin A1, brefeldin A and tunicamycin were obtained from Sigma-Aldrich (St. Louis, MO, USA). Primary antibodies (Abs) used were as follows: rabbit polyclonal anti-Ret (C-19), rabbit polyclonal anti-EGFR (1005), mouse monoclonal anti-Trk (B-3), mouse monoclonal anti-α-tubulin (TU-02) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit polyclonal anti-p75 nerve growth factor receptor (NGFR) (Promega Corporation, Madison, WI, USA), rabbit polyclonal anti-microtubule-associated protein 1 light chain 3 (LC3) (Medical & Biological Laboratories, Nagoya, Japan), and mouse monoclonal anti-58K Golgi protein (58K-9) (Abcam plc, Cambridge, UK). Specificity of antibodies has been confirmed by the western blotting. Fluorescein-conjugated AffiniPure goat anti-rabbit IgG (H + L) and rhodamine-conjugated AffiniPure goat anti-mouse IgG (H + L) were obtained from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA, USA). Peptide-N-glycosidase F (PNGase F) was obtained from New England Biolabs (NEB) (Beverly, MA, USA).
Cell culture
PC12 cells were grown in Dulbecco’s modified Eagle’s medium (Wako Pure Chemicals) supplemented with 7% horse serum (BioWhittaker, Walkersville, MD, USA) and 4% fetal bovine serum (Equitech-Bio, Inc., Kerrville, TX, USA) at 37°C in 5% CO2.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotting
Samples were homogenized in sodium dodecyl sulfate (SDS) sample buffer [62.5 mM Tris–HCl (pH 6.8), 2% SDS, 10% glycerol, 0.1% bromophenol blue] by sonication. The protein concentration in each preparation was measured using a DC Protein Assay kit (Bio-Rad Laboratories, Hercules, CA, USA) using γ-globulin as a standard. β-Mercaptoethanol was added (5%), and the mixture was boiled for 5 min. Aliquots containing 40 µg of protein were subjected to SDS–polyacrylamide gel electrophoresis (PAGE) and then transferred to nitrocellulose membranes [Hybond-enhanced chemiluminescence (ECL), GE Healthcare UK Ltd., Buckinghamshire, UK]. Membranes were blocked with blocking buffer [5% nonfat dry milk in PBST (phosphate-buffered saline, 0.05% Tween 20)] for 1 h, then incubated with the blocking buffer containing anti-Ret (1 : 500), anti-EGFR (1 : 500), anti-p75 NGFR (1 : 2000), anti-LC3 (1 : 1000) and anti-α-tubulin (1 : 1000). Blots were washed and incubated with the secondary antibodies conjugated to horseradish peroxidase (1 : 2000, Cell Signaling Technology, Beverly, MA, USA). Immunoreactive bands were visualized by ECL (GE Healthcare UK Ltd.) or SuperSignal West Dura (Thermo Scientific, Yokohama, Japan). In some cases, blots were reprobed with different Abs after stripping in 62.5 mM Tris–HCl (pH 6.7), 100 mM β-mercaptoethanol, and 2% SDS at 55°C for 30 min.
Biotinylation of cell surface proteins and pull-down assay using streptavidin beads
Subconfluent cultures grown in 10 cm dishes were exposed to sulfo-NHS-S-S-biotin for 30 min at 4°C, rinsed three times with glycine quenching buffer (192 mM glycine, 25 mM Tris, pH 8.3) and solubilized in 12 volumes of lysis buffer [150 mM NaCl, 50 mM Tris–HCl, 0.5% deoxycholic acid (w/v), 1% Nonidet P-40 (Wako Pure Chemicals), and protease inhibitors] for 30 min on ice. The resulting lysate was centrifuged at 10 000 g for 2 min at 4°C. The supernatant was incubated with streptavidin beads for 60 min at 4°C (10–50 µg protein/µL beads pre-equilibrated with PBS). After a brief centrifugation the supernatant was removed, and the beads were washed three times with lysis buffer. Absorbed proteins were eluted with 2× SDS sample buffer. Approximately quarter of the recovered sample prepared from cells grown in 10 cm dishes per condition was analyzed by SDS–PAGE and immunoblotting.
Immunocytochemistry
PC12 cells were grown on collagen-coated coverslips. Cells were fixed in 4% formaldehyde in PBS for 15 min, washed in several changes of PBS for 30 min, and permeabilized in 0.2% Triton X-100 in PBS for 3 min. The specimens were then blocked in 10% goat serum in PBS for 30 min. Cells were incubated in a mixture of two primary antibodies: (i) a rabbit polyclonal Ret antibody at a 1 : 100 dilution and (ii) a mouse monoclonal 58K-9 antibody at a 1 : 200 dilution for 24 h at 4°C. Cells on the coverslips were rinsed with PBS three times. The cells were then incubated for 1 h at 25°C in a secondary antibody cocktail containing the species-specific-FITC and rhodamine-conjugated secondary antibodies. Cells on the coverslips were rinsed with PBS, mounted onto glass microscope slides using ProLong Gold Antifade Reagent with 4′, 6-diamino-2-phenylindole (DAPI; Invitrogen, Carlsbad, CA, USA). The stained cells were subjected to fluorescence microscopy using a Zeiss laser scanning confocal microscope (Thornwood, NY, USA).
Results
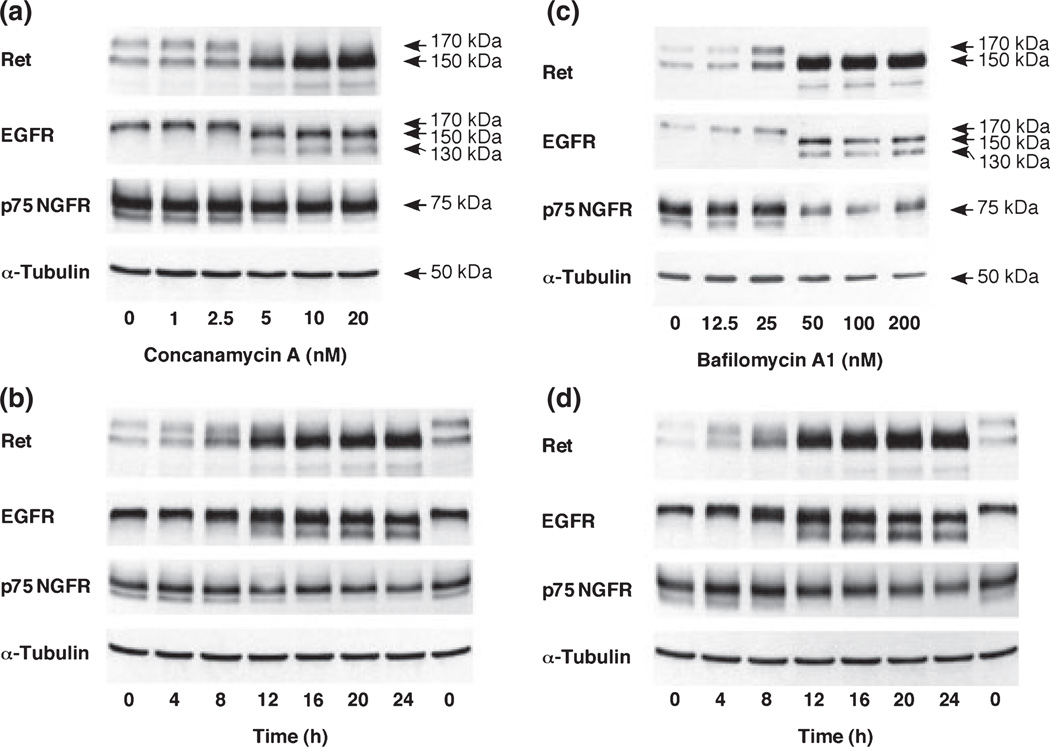
To examine the role of acidification in the expression of Ret and EGFR, PC12 cells were treated with concanamycin A and bafilomycin A1, specific inhibitors of V-ATPase (Fig. 1a–d). Ret in PC12 cells appeared as 150 kDa and 170 kDa bands on SDS–PAGE gels as reported in neuroblastoma cells (Takahashi et al. 1991). The 170 kDa Ret protein species was down-regulated within 4 h by 5 nM concanamycin A and 50 nM bafilomycin A1 treatment, respectively. In contrast, an increase in 150 kDa species was observed at 8 h, reaching a maximum at 20 h and at 12 h by concanamycin A and bafilomycin A1, respectively. Similarly, a 170 kDa EGFR protein composed of a 130 kDa single polypeptide and N-linked oligosaccharides was down-regulated with treatment with 5 nM concanamycin A and 50 nM bafilomycin A1. Furthermore, the 150 kDa immature type EGFR and 130 kDa non-glycosylated EGFR accumulated in a concentration- and time-dependent manner. These findings suggest that glycosylation of immature Ret and EGFR to mature types are inhibited, but glycosylation of single polypeptides to immature types normally occur when the activity of V-ATPase is inhibited. In contrast to Ret and EGFR, the expression pattern of p75 NGFR was not affected by increasing concentration of V-ATPase inhibitors. The down-regulation of mature growth factor receptors and the accumulation of immature growth factor receptors may be accompanied with apoptosis because the concentrations of concanamycin A and bafilomycin A1 that affect receptor glycosylation are similar to those that cause caspase 7 activation and DNA fragmentation (Appendix S1, Figure S1a and b). V-ATPase inhibitor-induced apoptosis is not mediated by the ER stress pathway. Both concanamycin A and bafilomycin A1 did not increase the expression of a growth arrest and DNA damage-inducible 158 protein and a 78-kDa glucose-regulated protein, the ER stress-responsive proteins (data not shown), and did not cause activating transcription factor 6 activation (Figure S2).
Fig. 1.
Effect of concanamycin A and bafilomycin A1 on various growth factor receptors in PC12 cells. (a,c) Cells were treated with various concentrations of concanamycin A or bafilomycin A1 for 24 h. (b,d) Cells were treated with 5 nM concanamycin A or 50 nM bafilomycin A1 for the indicated periods. Whole cell lysates (40 µg protein) were subjected to SDS–PAGE and immunoblotted with anti-Ret, anti-p75 NGFR, anti-EGFR, and anti-α-tubulin antibodies.
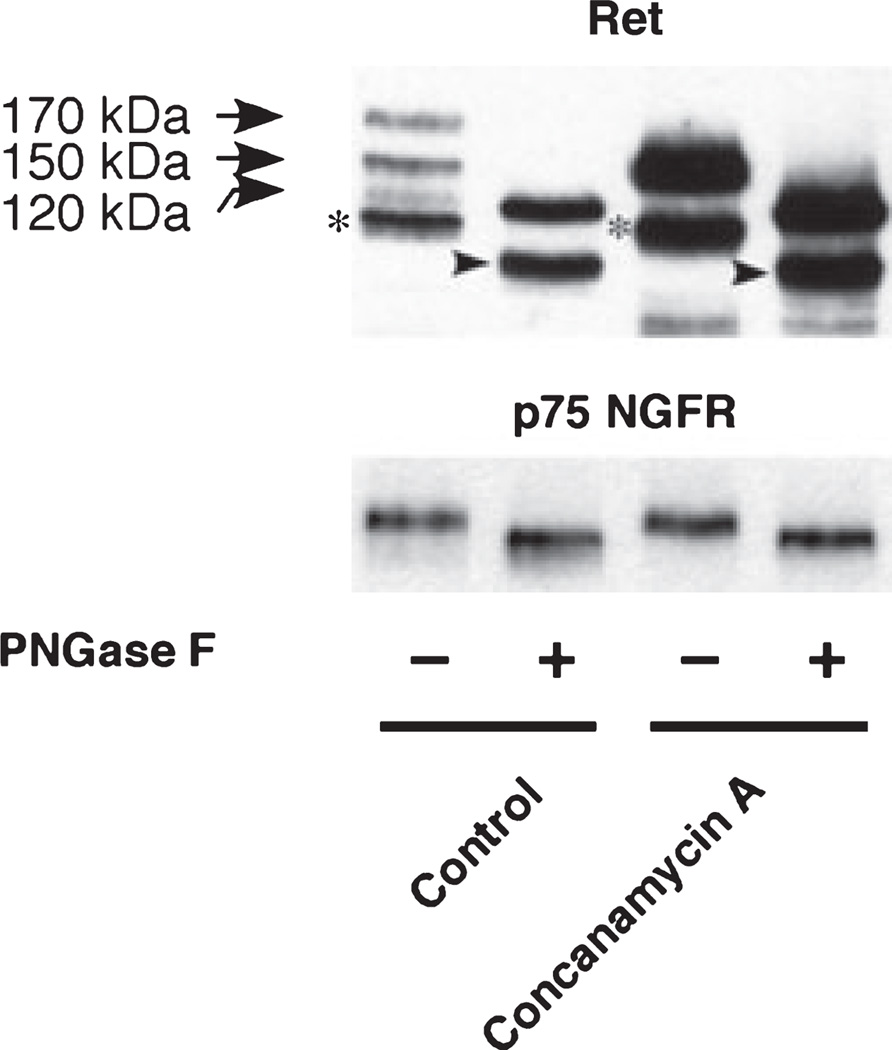
To demonstrate the nature of glycosylation and thus underline the maturation state of these glycoforms, the mobility of the Ret species of PC12 cells was determined after digestion with PNGase F, which hydrolyzes nearly all types of N-glycan chains from glycoproteins. As shown in Fig. 2, both p170 and p150 forms in the control and p150 form in concanamycin A-treated cell extracts resulted in a single p120 form after treating with PNGase F. The result suggests that both p170 and p150 forms are highly glycosylated. The signals that appeared just under p120 form in the PNGase F-non-treated samples (denoted by an asterisk), and its deglycosylated forms (denoted by an arrow head) that appeared in the PNGase F-treated samples are probably degradation products during the experimental procedures because such intense bands were not observed in other blots. The deglycosylation experiments allowed a differentiation between the glycoforms on the blots. Similar results were obtained using bafilomycin A1 (data not shown). p75 NGFR also shifted to lower molecular weight by the treatment with PNGase F.
Fig. 2.
Effect of PNGase F treatment of detergent extracts from PC12 treated with V-ATPase inhibitors. Representative extracts were incubated with (+) or without (−) PNGase F, followed by western immunoblot analysis with the Ret or p75 NGFR antibodies. PC12 cells were treated with 5 nM concanamycin A for 20 h. The cell pellets were lysed with 1× Glycoprotein Denaturing Buffer (0.5% SDS, 40 mM DTT, New England Biolabs) and stored at −80°C until use. Protein concentration was determined by the Bradford protein assay (Bio-Rad Laboratories). Cell lysates (200 µg protein) were denatured by heating at 100°C for 10 min and aliquot of cell lysates (20 µg protein) were treated with PNGase F at 37°C for 60 min according to the manufacturer’s protocol. Asterisk: the signals that appeared just under p120 form in the PNGase F-non-treated samples; arrow head: deglycosylated forms of the signals denoted by an asterisk in the PNGase F-treated samples.
To determine whether the increase in 150 kDa Ret and EGFR proteins induced by V-ATPase inhibitors was accompanied by a corresponding increase in the transcription of the mRNA encoding these proteins, semi-quantitative RT-PCR analysis was carried out. The levels of Ret mRNA and EGFR mRNA were not increased at 8-h post-treatment of concanamycin A (Appendix S1 and Figure S3). These results indicate that p150 Ret and EGFR accumulations caused by the V-ATPase inhibitors are not regulated at the transcriptional level.
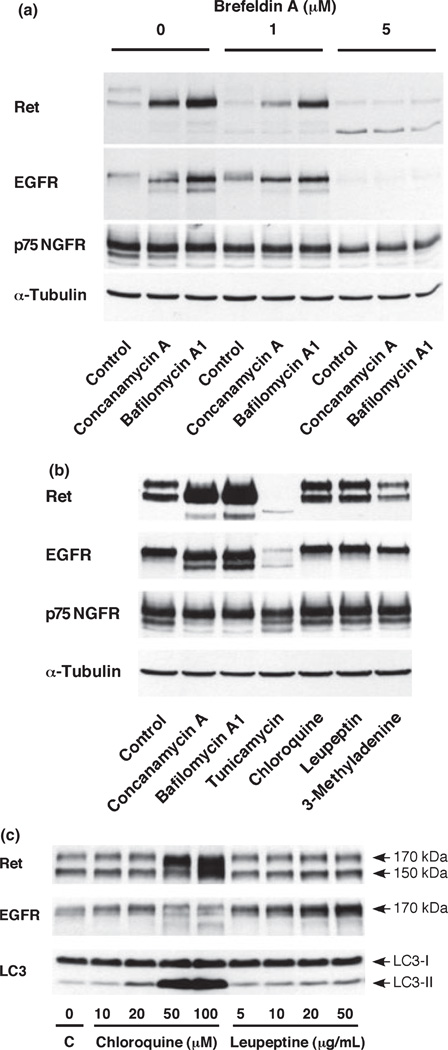
We next used pharmacological approaches to further investigate associations of Ret and EGFR with the organelle. The fungal metabolite brefeldin A has been widely used to disrupt the Golgi structure and block protein secretion by its ability to perturb the activity of guanine nucleotide exchange factors in membrane traffic (Klausner et al. 1992; Chardin and McCormick 1999). Addition of brefeldin A to PC12 cells treated with concanamycin A or bafilomycin A1 blocked accumulation of immature Ret and EGFR, suggesting that the accumulation of immature Ret and EGFR occur in the Golgi apparatus (Fig. 3a). V-ATPase inhibitors are often used as inhibitors of the acidification of lysosomes and endosomes. Therefore, we examined chloroquine, leupeptine and 3-methyladenine, used as lyososomal inhibitors, to clarify the involvement of lysosomes. As shown in Fig. 3(b), these lysosomal inhibitors did not affect the expression pattern of Ret and EGFR. Chloroquine and leupeptin increased the overall levels of Ret and EGFR (Fig. 3c). Chloroquine at 50 µM or higher affected the maturation of Ret and EGFR differently than V-ATPase inhibitors. The effect of chloroquine or leupeptin was confirmed by an increase in LC3-II, the 16 kDa form of LC3 specific for autophagosomal membranes (Fig. 3c) as previously reported (Suzuki et al. 2002). These results indicate that accumulation of immature Ret and EGFR was not because of the impairment of lysosomal degradation. Finally, the effects of tunicamycin, a widely used inhibitor of protein glycosylation, were examined (Fig. 3b). Levels of both mature and immature types of Ret and EGFR were almost completely diminished. Instead, unglycosylated 120 kDa Ret and 130 kDa EGFR were observed. Taken together, these findings suggest that 120 kDa Ret and 130 kDa EGFR are glycosylated to 150 kDa in the ER, and transported to the Golgi apparatus where these proteins are further glycosylated to the 170 kDa mature forms.
Fig. 3.
Effects of various chemicals on the expression of Ret, EGFR and p75 NGFR. (a) Effect of brefeldin A on the expression of Ret, EGFR and p75 NGFR in PC12 cells treated with V-ATPase inhibitors. Cells were treated with 5 nM concanamycin A or 50 nM bafilomycin A1 for 16 h in the absence or presence of brefeldin A. (b) Effects of tunicamycin and lysosomal inhibitors on the expression of Ret, EGFR, and p75 NGFR in PC12 cells. Cells were cultured for 20 h with either no supplement (control) or in the presence of one of the following: 5 nM concanamycin, 50 nM bafilomycin A1, 1 µg/mL tunicamycin, 10 µM chloroquine, 5 µg/mL leupeptin, and 5 mM 3-methyladenine. (c) Chloroquine and leupeptin induce biochemical markers of autophagy. Whole cell lysates (40 µg protein) were subjected to SDS–PAGE and immunoblotted with anti-Ret, anti-EGFR, anti-p75 NGFR, anti-LC3, and anti-α-tubulin antibodies.
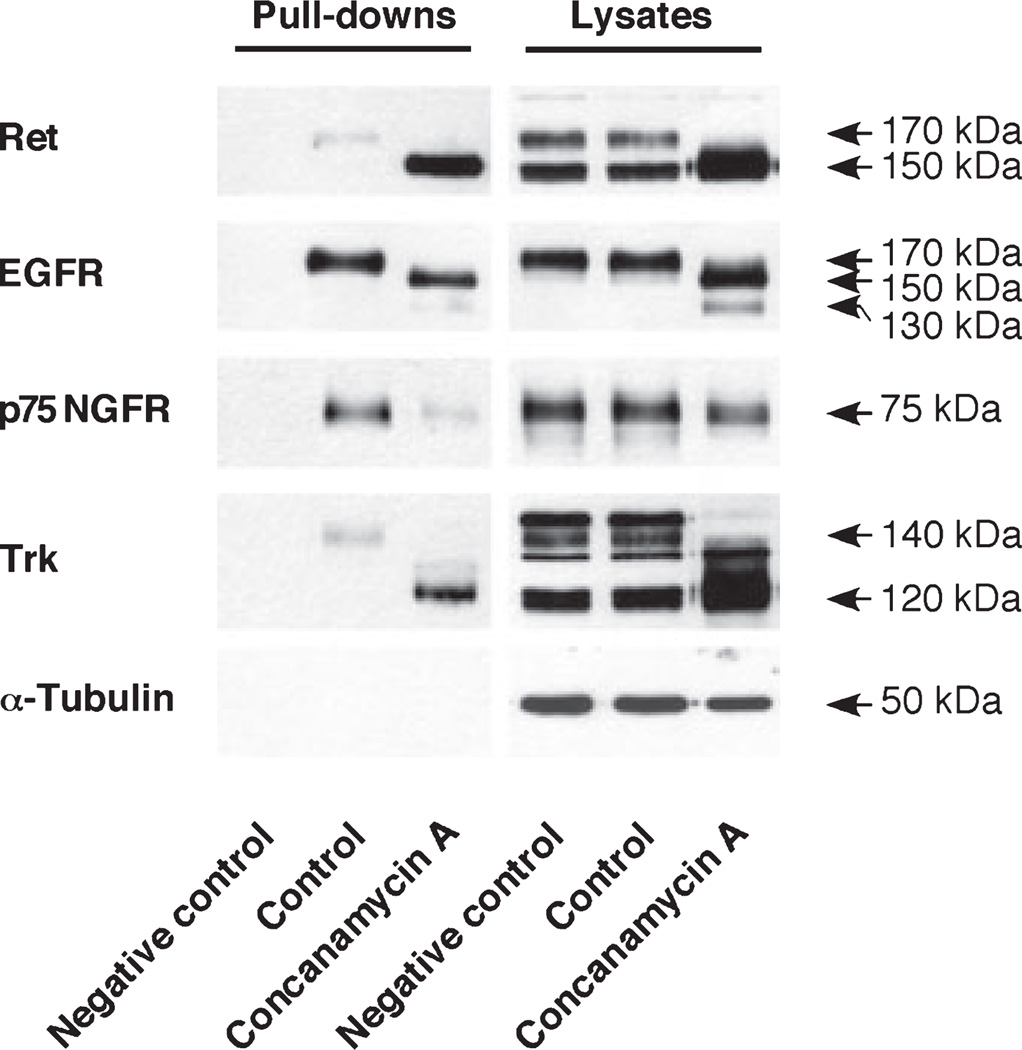
In general, mature forms of growth factor receptors are localized at the cell surface, and immature forms are present intracellularly under physiological conditions. To examine the localization of immature forms of Ret and EGFR in cells treated with concanamycin A, the cell surface proteins were biotinylated and pulled-down by streptavidin (Fig. 4). When these immunoprecipitates were subjected to SDS–PAGE/western blot analysis, 170 kDa Ret and 170 kDa EGFR were found on the cell surface in the control cells as we expected. Surprisingly, 150 kDa Ret and 150 kDa EGFR, immature species of both receptors, were clearly detected in the cell surface fraction of cells treated with concanamycin A. The high affinity nerve growth factor receptor, Trk, showed similar results. These results suggest that the trafficking of tyrosine kinase receptors from the Golgi apparatus to the cell membrane occurs in the presence of a V-ATPase inhibitor. Although cells were undergoing apoptosis (Figure S1a and b), the membrane integrity was maintained under the condition based on lactate dehydrogenase release (data not shown). Therefore, it is not likely that compartmental breakdown have led to a false positive result in the biotinylation experiment. We next examined whether the 150 kDa immature forms of Ret localized to the plasma membrane respond to GDNF using PC12 cells over-expressing GDNF family receptor α1. Our preliminary results demonstrated that GDNF increased the phosphorylation of Akt and p44/42 MAPK in concanamycin A-treated cells (Appendix S1 and Figure S4).
Fig. 4.
Ret and EGFR immature species were found in the cell surface. PC12 cells were treated with 5 nM concanamycin A for 20 h. Ret and EGFR in cell lysates pull-downs by streptavidin after cell surface biotinylation as follows. Cells were exposed to 0.5 µg/mL sulfo-NHS-S-S-biotin in PBS buffer for 30 min at 4°C, lysed, and incubated with streptavidin-agarose beads (10 µg protein/µL beads) for 1 h at 4°C. The beads were washed and proteins were eluted using 2× sample buffer and run on 7% SDS–PAGE gels. Pull-downed Ret was detected using a SuperSignal® West Dura Extended Duration Substrate (Thermo Scientific). As a negative control, no sulfo-NHS-S-S-biotin was added.
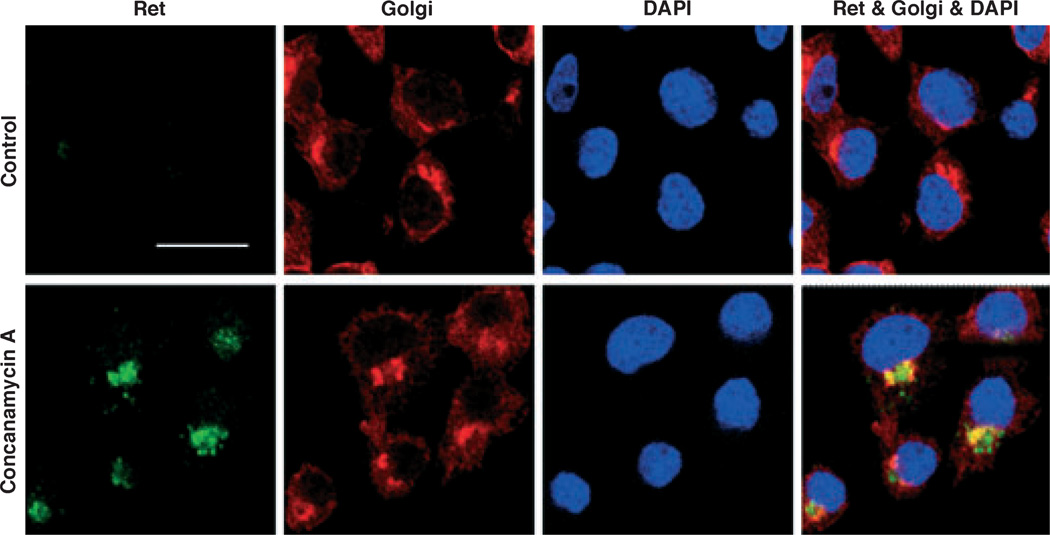
Finally, to confirm the intracellular localization of immature 150 kDa Ret, we carried out the immunocytochemical analysis shown in Fig. 5. The co-localized Golgi marker and Ret protein are depicted in yellow color. The results demonstrated the localization of the immature p150 kDa Ret in the Golgi apparatus. The presence of Ret protein in non-Golgi areas (green spots in bottom right corner of Fig. 5) suggest that Ret is present in compartments besides Golgi. The treatment of PC12 cells with bafilomycin A1 gave similar results (data not shown). Immunofluorescence failed to confirm the cell surface localization of the immature Ret in inhibited cells. This might be because of the large difference in Ret levels between the Golgi apparatus and the cell surface. It is also conceivable that pull-down assay plus western blotting with ECL detection using a SuperSignal® West Dura Extended Duration Substrate is much more sensitive than immunocytochemistry with fluorescence detection.
Fig. 5.
Co-localization of immature Ret and 58K Golgi-specific protein. PC12 cells were treated with 5 nM concanamycin A for 20 h and immunostained with Ret (green) and 58K Golgi protein (58K-9) (red) antibodies with DAPI (blue) nuclear staining. Scale bar is 8 µm.
Discussion
Acidification of intracellular organelles is important for the sorting of lysosomally destined enzymes, for the formation of regulated secretory granules, and for the efficient constitutive cell surface delivery or secretion of proteins (Seksek et al. 1995; Demaurex et al. 1998; Schoonderwoert and Martens 2001; Weisz 2003). Growth factor receptors elicit proliferation, migration, and the differentiation of a variety of cell types through various signal transduction pathways. Ligand-dependent receptor internalization, intracellular trafficking through endosomes, and the degradation processes in lysosomes are extensively investigated (Kurten 2003). However, the role of acidification in receptor maturation and intracellular delivery of newly synthesized RTKs is not well understood. In this paper, we showed that acidification is required for glycosylation of Ret and EGFR in the Golgi apparatus but not in the ER, whereas it is not indispensable for cell surface delivery of immature growth factor receptors.
In the presence of the V-ATPase inhibitors, concanamycin A and bafilomycin A1, glycosylation of Ret and EGFR in the conversion from immature types to mature types was inhibited in a concentration- and time-dependent manner. As a result of V-ATPase inhibition, immature types of Ret and EGFR accumulated. Our immunocytochemical study revealed that the immature Ret was co-localized to the Golgi marker, a 58 kDa Golgi protein (Fig. 5). The trans-Golgi network has been demonstrated to be slightly acidified (pH ~ 6.0) by several groups (Schoonderwoert and Martens 2001; Weisz 2003). It is unclear exactly why the perturbation of pH inhibits the terminal glycosylation of Ret and EGFR. In fact, the glycosylation in rat hepatic Golgi fractions was optimal at approximately pH 6.5, but retained more than 50% activity at neutral pH (Bergeron et al. 1985). Although the influence of increasing pH on the enzyme activities for glycosylation may be limited in vitro, actual effects of differences in pH in the narrow range contained in the cells is not known. The mechanism caused the inhibition of glycosylation in the Golgi apparatus by the V-ATPase inhibitors needs to be further clarified. In contrast, glycosylation of nascent p120 Ret and p130 EGFR to immature Ret and EGFR appeared to be V-ATPase insensitive and appeared to take place in the ER (which is thought to have a neutral pH similar to the cytosol) (Schoonderwoert and Martens 2001). Consistently, V-ATPase inhibitors did not cause ER stress, as judged by the western analyses of a growth arrest and DNA damage-inducible 158 protein and a 78-kDa glucose-regulated protein, or by activating transcription factor 6 activation (Figure S2). Additionally, our experiments show that the expression of p75 NGFR was simply decreased in the presence of V-ATPase inhibitors and brefeldin A. Although p75 NGFR is a glycoprotein (Yeaman et al. 1997), the results indicate that the modification occurs in the ER.
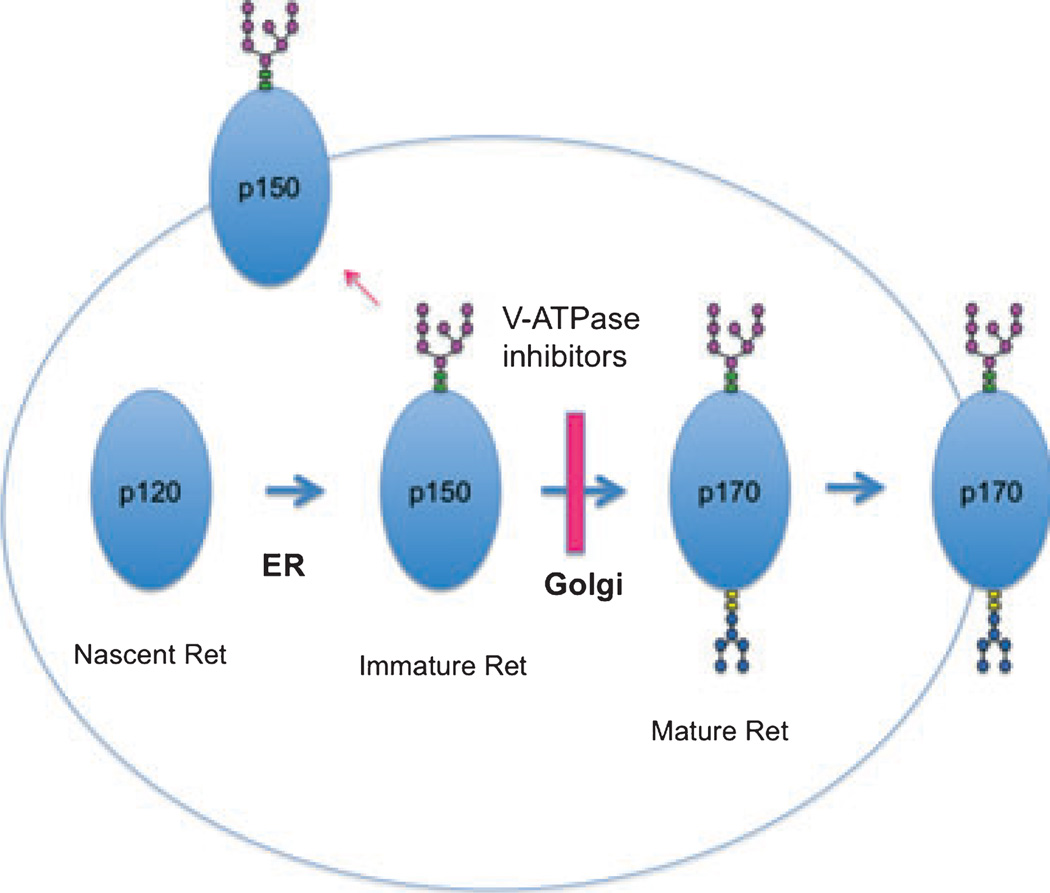
The accumulation of immature types in the Golgi apparatus was confirmed by observations using brefeldin A, which disassembles the Golgi apparatus and blocks the protein transport from the ER to the Golgi complex (Klausner et al. 1992). When the cells were treated with brefeldin A, the effects of the V-ATPase inhibitors were reduced, suggesting that immature types are transported to the Golgi apparatus after nascent proteins are glycosylated to immature types in the ER (Fig. 6). Although little is known about the process of Ret receptor maturation, a similar process was proposed by Gamou and Shimizu for EGFR biosynthesis in an oral squamous cell carcinoma cell line (NA cells) (Gamou and Shimizu 1988). The accumulation of immature Ret and EGFR may not only be because of the inhibition of glycosylation in the Golgi apparatus, but also to the disturbance of the efficient constitutive cell surface delivery of receptors. Biotin-labeling experiments showed that immature Ret, EGFR and Trk receptors all appeared on the cell surface. Although this experiment is not quantitative enough to allow calculation of how much of the intracellular accumulated pool reaches the plasma membrane, it could be a few percentage of the intracellular pool. Soderquist and Carpenter also showed that 160 kDa immature forms of EGFR resulting from treatment of cells with glycosylation inhibitors, swainsonine or monensin, were present on the cell surface (Soderquist and Carpenter 1984). In addition, they suggested that 160 kDa immature forms from the swainsonine-treated cells retain kinase activity, as well as EGF binding activity and phosphorylation substrate capacity. Our preliminary data demonstrated that the 150 kDa immature forms of Ret localized to the plasma membrane are also functional at least in part for signaling transductions (Figure S4). It may be highly-favorable for cells if immature forms of tyrosine kinase receptors still respond to growth factors under pH stressed condition. Accumulating evidence suggests that abnormal regulation of organelle pH is related to disease processes. Because the expression of Ret and EGFR appear to be involved in the control of cell survival and proliferation in various cell types, the details of the effects of acidification on the expression of these proteins may further our knowledge of several diseases.
Fig. 6.
Proposed working model for the maturation and trafficking of Ret.
Supplementary Material
Acknowledgements
This work was supported in part by the Grants-in-Aid for Scientific Research (C) from Japan Society for the Promotion of Science.
Abbreviations used
- ECL
enhanced chemiluminescence
- EGFR
epidermal growth factor receptor
- ER
endoplasmic reticulum
- GDNF
glial cell line-derived neurotrophic factor
- LC3
light chain 3
- NGFR
nerve growth factor receptor
- PAGE
polyacrylamide gel electrophoresis
- PBS
phosphate-buffered saline
- PNGase F
peptide-N-glycosidase F
- RTK
receptor tyrosine kinase
- SDS
sodium dodecyl sulfate
- V-ATPase
vacuolar proton-ATPase
Footnotes
Supporting information
Additional Supporting Information may be found in the online version of this article:
Appendix S1. Supplementary Materials and Methods.
Figure S1. V-ATPase inhibitors caused apoptosis in PC12 cells.
Figure S2. Concanamycin A and bafilomycin A1 did not cause the ER stress.
Figure S3. Effects of concanamycin A on Ret and EGFR mRNA expressions in PC12 cells.
Figure S4. Effects of GDNF on the phosphorylation of Akt and p44/42 MAPK in PC12 cells over-expressing GFRα1.
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer-reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
References
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat. Rev. Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Avantaggiato V, Dathan NA, Grieco M, Fabien N, Lazzaro D, Fusco A, Simeone A, Santoro M. Developmental expression of the RET protooncogene. Cell Growth Differ. 1994;5:305–311. [PubMed] [Google Scholar]
- Bergeron JJ, Paiement J, Khan MN, Smith CE. Terminal glycosylation in rat hepatic Golgi fractions: heterogeneous locations for sialic acid and galactose acceptors and their transferases. Biochim. Biophys. Acta. 1985;821:393–403. doi: 10.1016/0005-2736(85)90043-4. [DOI] [PubMed] [Google Scholar]
- Chardin P, McCormick F. Brefeldin A: the advantage of being uncompetitive. Cell. 1999;97:153–155. doi: 10.1016/s0092-8674(00)80724-2. [DOI] [PubMed] [Google Scholar]
- Demaurex N, Furuya W, D’Souza S, Bonifacino JS, Grinstein S. Mechanism of acidification of the trans-Golgi network (TGN) In situ measurements of pH using retrieval of TGN38 and furin from the cell surface. J. Biol. Chem. 1998;273:2044–2051. doi: 10.1074/jbc.273.4.2044. [DOI] [PubMed] [Google Scholar]
- Drose S, Altendorf K. Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J. Exp. Biol. 1997;200:1–8. doi: 10.1242/jeb.200.1.1. [DOI] [PubMed] [Google Scholar]
- Edery P, Lyonnet S, Mulligan LM, Pelet A, Dow E, Abel L, Holder S, Nihoul-Fekete C, Ponder BA, Munnich A. Mutations of the RET proto-oncogene in Hirschsprung’s disease. Nature. 1994;367:378–380. doi: 10.1038/367378a0. [DOI] [PubMed] [Google Scholar]
- Gamou S, Shimizu N. Glycosylation of the epidermal growth factor receptor and its relationship to membrane transport and ligand binding. J. Biochem. 1988;104:388–396. doi: 10.1093/oxfordjournals.jbchem.a122478. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Kiuchi K. Rapid down-regulation of Ret following exposure of dopaminergic neurons to neurotoxins. J. Neurochem. 2007;102:1606–1613. doi: 10.1111/j.1471-4159.2007.04695.x. [DOI] [PubMed] [Google Scholar]
- Hofstra RM, Landsvater RM, Ceccherini I, et al. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature. 1994;367:375–376. doi: 10.1038/367375a0. [DOI] [PubMed] [Google Scholar]
- Ichihara M, Murakumo Y, Takahashi M. RET and neuroendocrine tumors. Cancer Lett. 2004;204:197–211. doi: 10.1016/S0304-3835(03)00456-7. [DOI] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J. Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurten RC. Sorting motifs in receptor trafficking. Adv. Drug Deliv. Rev. 2003;55:1405–1419. doi: 10.1016/j.addr.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Marshansky V, Futai M. The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function. Curr. Opin. Cell Biol. 2008;20:415–426. doi: 10.1016/j.ceb.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson N, Taiz L. The evolution of H+-ATPases. Trends Biochem. Sci. 1989;14:113–116. doi: 10.1016/0968-0004(89)90134-5. [DOI] [PubMed] [Google Scholar]
- Pachnis V, Mankoo B, Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development. 1993;119:1005–1017. doi: 10.1242/dev.119.4.1005. [DOI] [PubMed] [Google Scholar]
- Pierchala BA, Tsui CC, Milbrandt J, Johnson EM. NGF augments the autophosphorylation of Ret via inhibition of ubiquitin-dependent degradation. J. Neurochem. 2007;100:1169–1176. doi: 10.1111/j.1471-4159.2006.04292.x. [DOI] [PubMed] [Google Scholar]
- Romeo G, Ronchetto P, Luo Y, et al. Point mutations affecting the tyrosine kinase domain of the RET proto-oncogene in Hirschsprung’s disease. Nature. 1994;367:377–378. doi: 10.1038/367377a0. [DOI] [PubMed] [Google Scholar]
- Saroussi S, Nelson N. The little we know on the structure and machinery of V-ATPase. J. Exp. Biol. 2009;212:1604–1610. doi: 10.1242/jeb.025866. [DOI] [PubMed] [Google Scholar]
- Schoonderwoert VT, Martens GJ. Proton pumping in the secretory pathway. J. Membr. Biol. 2001;182:159–169. doi: 10.1007/s00232-001-0040-2. [DOI] [PubMed] [Google Scholar]
- Schoonderwoert VT, Holthuis JC, Tanaka S, Tooze SA, Martens GJ. Inhibition of the vacuolar H+-ATPase perturbs the transport, sorting, processing and release of regulated secretory proteins. Eur. J. Biochem. 2000;267:5646–5654. doi: 10.1046/j.1432-1327.2000.01648.x. [DOI] [PubMed] [Google Scholar]
- Seksek O, Biwersi J, Verkman AS. Direct measurement of trans-Golgi pH in living cells and regulation by second messengers. J. Biol. Chem. 1995;270:4967–4970. doi: 10.1074/jbc.270.10.4967. [DOI] [PubMed] [Google Scholar]
- Slieker LJ, Martensen TM, Lane MD. Synthesis of epidermal growth factor receptor in human A431 cells Glycosylation-dependent acquisition of ligand binding activity occurs post-translationally in the endoplasmic reticulum. J. Biol. Chem. 1986;261:15233–15241. [PubMed] [Google Scholar]
- Soderquist AM, Carpenter G. Glycosylation of the epidermal growth factor receptor in A-431 cells. The contribution of carbohydrate to receptor function. J. Biol. Chem. 1984;259:12586–12594. [PubMed] [Google Scholar]
- Suzuki T, Nakagawa M, Yoshikawa A, Sasagawa N, Yoshimori T, Ohsumi Y, Nishino I, Ishiura S, Nonaka I. The first molecular evidence that autophagy relates rimmed vacuole formation in chloroquine myopathy. J. Biochem. 2002;131:647–651. doi: 10.1093/oxfordjournals.jbchem.a003147. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Ritz J, Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. 1985;42:581–588. doi: 10.1016/0092-8674(85)90115-1. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Buma Y, Taniguchi M. Identification of the ret proto-oncogene products in neuroblastoma and leukemia cells. Oncogene. 1991;6:297–301. [PubMed] [Google Scholar]
- Takahashi M, Asai N, Iwashita T, Isomura T, Miyazaki K, Matsuyama M. Characterization of the ret proto-oncogene products expressed in mouse L cells. Oncogene. 1993;8:2925–2929. [PubMed] [Google Scholar]
- Weisz OA. Acidification and protein traffic. Int. Rev. Cytol. 2003;226:259–319. doi: 10.1016/s0074-7696(03)01005-2. [DOI] [PubMed] [Google Scholar]
- Yeaman C, Le Gall AH, Baldwin AN, Monlauzeur L, Le Bivic A, Rodriguez-Boulan E. The O-glycosylated stalk domain is required for apical sorting of neurotrophin receptors in polarized MDCK cells. J. Cell Biol. 1997;139:929–940. doi: 10.1083/jcb.139.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.