It is humbling and instructive that the most exquisitely specific group of insecticides known originates not from a laboratory, but instead from the common soil bacterium Bacillus thuringiensis (Bt). Insecticidal crystal proteins produced by Bt kill insects by binding to and disrupting their midgut membranes (1). Each of the numerous strains of Bt produces a characteristic set of crystal proteins (2). Each of these toxins is lethal to certain insects, yet does little or no harm to most other organisms, including people, wildlife, and even other insects (2).
Bt was first formally described from Thuringia, Germany, in 1911 and has been available in commercial formulations for insect control since the 1930s (3); yet until recently, it remained a minor component of pest management. Three factors set the stage for the emerging importance of Bt: evolution of resistance to insecticides in more than 500 species of insects and mites (4), rising concerns about environmental hazards of conventional insecticides, and breakthroughs in biotechnology. Genetic engineering has created transgenic varieties of many crops that express Bt toxins; such cultivars of transgenic corn, cotton, and potatoes were grown on a large scale in the United States for the first time during 1996. Transgenic plants armed with Bt toxins are defended against some of the most notorious pests, which reduces the need for insecticidal sprays. Because Bt is not toxic to arthropod natural enemies, opportunities for biological control are enhanced and the secondary pest outbreaks often caused by conventional insecticides are avoided. Thus, this new technology could yield enormous benefits for food production and environmental quality worldwide. Will the advent of Bt-expressing transgenic plants herald a new era of environmentally benign insect control? Or will the pests quickly adapt?
The paper by Gould et al. (5) in the current issue of the Proceedings brings us closer to answers by providing a direct estimate of the frequency of resistance to Bt in susceptible field populations of a major cotton pest, the tobacco budworm, Heliothis virescens. Because resistant individuals are rare initially, it is inherently difficult to estimate their frequency before populations are exposed to an insecticide. This frequency results from a balance between creation of resistant genotypes by mutation and selection against such mutants when the insecticide is not present (6, 7). The most widely cited estimate of the range of the initial frequency of resistance alleles (p) is 10−2 to 10−13 (6), which is a scientific-sounding way of saying “we don’t know.” Insect resistance to Bt is partially or completely recessive in most laboratory-selected strains and in field-selected strains of diamondback moth, the first insect to evolve resistance to Bt in open-field populations (8, 9). Recessive inheritance compounds the difficulty, because resistance is expressed only in homozygous individuals.
Gould et al. (5) attacked this vexing problem with an ingenious application of basic Mendelian genetics, pheromones, regional cooperation, laboratory-selected resistance, and sheer effort. The key is detection of heterozygotes as well as resistant homozygotes. Because many types of resistance are recessive, the ability to detect heterozygotes has broad applications. The frequency of resistant homozygotes is roughly p2, which means that if p is 10−3 and resistance is recessive, only one in a million insects will express resistance. In contrast, the Hardy-Weinberg principle predicts that the frequency of heterozygotes will be 2 × p × [1-p] or approximately 2p, which is two times the square root of the frequency of homozygotes. If p is 10−3, this computes to be about 1 in 500. Thus, in this example, the ability to identify heterozygotes provides a 2,000-fold increase in sensitivity.
Gould et al. (5) relied primarily on traps baited with female sex pheromone to obtain more than 2,000 wild male Heliothis from four states. Field collections were completed before commercialization of Bt cotton. Each wild male was allowed to mate individually with a female from a resistant laboratory strain (YHD2). The YHD2 strain had been selected for extremely high resistance to Cry1Ac, the Bt toxin that is expressed in transgenic cotton (10). Like field-evolved resistance to some Bt toxins in diamondback moth (8, 9, 11), resistance to Cry1Ac in the YHD2 strain of Heliothis appears to be recessive and associated with reduced binding of toxin to midgut membranes (10, 12). Because resistance in the YHD2 strain is controlled largely by a recessive gene at a single locus (BtR-4) (13), matings between resistant YHD2 females and homozygous susceptible wild males would produce F1 families composed entirely of susceptible heterozygotes. Matings between resistant YHD2 females and wild males heterozygous for resistance at the BtR-4 locus would produce F1 families with 50% resistant homozygotes and 50% susceptible heterozygotes. This procedure would also detect major resistance alleles at other loci if they had dominant expression.
Bioassays of the F1 progeny from 1,025 single-pair families revealed four families with 30% or more resistant larvae; tests of F2 progeny of three of these families confirmed the presence of the genes for resistance. Based on the four families, or more conservatively, the three confirmed families, estimates of the initial frequency of alleles for resistance are 2.0 × 10−3 or 1.5 × 10−3, respectively. These estimates can be viewed as lower limits because recessive genes for resistance at loci other than BtR-4 probably were not detected. Also, if reduced tendency to be attracted to pheromones, to mate, or to produce viable offspring is associated with resistance, genes for resistance might have been underrepresented. In addition, several families other than the four mentioned above had intermediate numbers of survivors, some of which might have been genetically resistant.
The direct estimates of initial resistance allele frequency from Gould
et al. (5) are consistent with an indirect estimate from
their laboratory selection experiment with the YHD2 strain (10).
Assuming that resistant mutants did not arise in the laboratory, one
can infer that at least one resistance allele was present originally in
the founders of the YHD2 strain. The indirect estimate of the frequency
of resistance alleles in the source population is at least
½n, where n is the number of diploid
individuals that started the strain (8). The YHD2 strain of
Heliothis was started with 490 field-collected diploid eggs,
which yields an estimated p of at least 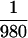 or
roughly 10−3. Analogous indirect estimates from more than
two dozen laboratory selection experiments with Bt and various species
of moths, beetles, and mosquitoes (8) show that the results with
Heliothis are not atypical.
or
roughly 10−3. Analogous indirect estimates from more than
two dozen laboratory selection experiments with Bt and various species
of moths, beetles, and mosquitoes (8) show that the results with
Heliothis are not atypical.
The data on resistance allele frequencies in susceptible populations imply that the success of the first generation of Bt transgenic plants could be short-lived. Predictions from genetic models suggest that with p equal to or greater than 10−3, resistance will evolve rapidly if pest populations receive prolonged and uniform exposure to Bt toxins (14–18). Initial increases in the frequency of resistance will depend on how quickly transgenic plants are adopted by growers. When transgenic varieties account for a small proportion of the total host plants used by a particular pest population, nontransgenic host plants that are nearby can provide a refuge from exposure to Bt toxin, which could delay resistance considerably. The 730,000 hectares of Bt cotton grown in the United States in 1996 was substantially more than the Bt corn and potatoes combined, yet it accounted for only about 13% of the total cotton crop (19). In some places, however, Bt cotton captured a much higher market share. Dramatic increases in availability of transgenic seed are also anticipated. Without measures to delay evolution of resistance, pests in some areas might adapt to Bt transgenic plants within a few years.
Among the many proposed resistance management tactics (20), refuges have received the most attention (14–18). The idea is that refuges from exposure to toxin enable survival of susceptible individuals, which decreases the intensity of selection (14–18). Ideally, relatively large numbers of susceptible individuals from refuges survive and mate with few resistant survivors from transgenic plants. Projections from computer simulations and data from small-scale experiments suggest that if resistance is recessive and mating is random, refuges can postpone resistance significantly (14–18, 21).
Cotton is the first transgenic crop for which the Environmental Protection Agency mandated resistance management tactics (22). Cotton growers have two options; both are based on the refuge concept. In the first option, for every 100 hectares of Bt cotton, they must plant at least 4 hectares of cotton without the Bt toxin gene. This slightly less than 4% refuge cannot be treated with insecticides that kill the major lepidopteran pests of cotton. In the second option, for every 100 hectares of Bt cotton, they must plant 25 hectares of cotton without the Bt toxin gene. These 25 hectares (20% of the total) can be treated with any insecticide except Bt subspecies kurstaki (which contains the same toxin as the transgenic plants). The first option might work well under ideal conditions, but if optimistic assumptions about inheritance or mating are violated, the number of susceptible individuals generated by a 4% refuge may not be sufficient to stem the tide of resistance. In the second option, supression of susceptibles by insecticides in the non-Bt cotton could essentially eliminate the refuge. The odds for delaying resistance could be improved by requiring larger refuges.
Excitement about the prospects for Bt-expressing transgenic plants and increasing knowledge about the genetics and mechanisms of resistance to Bt (2, 5, 8–13, 23) must be tempered with an admission of ignorance. Although many tactics have been proposed for delaying insect resistance to transgenic plants, none have been tested rigorously in the field. Nothing will be gained and much can be lost if we pretend to know more about resistance management than we really do. A lesson in the pitfalls of overzealous promotion occurred last summer when some growers found that Bt cotton did not adequately control the bollworm Helicoverpa zea. This raised false alarms about resistance, when, in fact, previously published data showed that the Cry1Ac toxin in Bt cotton is especially effective against H. virescens but less so against H. zea (24, 25).
We can and should refine models by incorporating the latest data, such as the timely findings of Gould et al. (5). Nonetheless, predictions from models and results from small-scale experiments are not a substitute for field experiments. Field tests of resistance management tactics are inherently difficult because they require monitoring of large, replicated plots for several years. The results from such experiments, however, will be extremely valuable not only for prolonging the efficacy of Bt, but also of other environmentally benign insecticides that will be delivered by transgenic plants or conventional means. The successful assault of Gould et al. (5) on the previously intractable problem of estimating initial resistance allele frequency provides inspiration to plunge ahead in this arena.
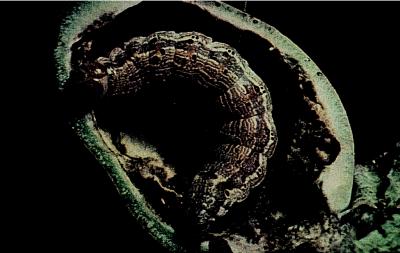
Figure 1.
A Heliothis larva devours a cotton boll. Transgenic cotton that expresses Bt toxin is protected from such attack, but pests may adapt quickly to this genetically engineered defense.
Figure 2.
Fed on a diet with a sublethal concentration of Bt toxin, a resistant Heliothis larva grows normally, but the growth of a susceptible Heliothis larva is stunted.
ABBREVIATION
- Bt
Bacillus thuringiensis
References
- 1.Gill S S, Cowles E A, Pietrantonio P V. Annu Rev Entomol. 1992;37:615–636. doi: 10.1146/annurev.en.37.010192.003151. [DOI] [PubMed] [Google Scholar]
- 2.Entwistle P, Bailey M J, Cory J, Higgs S, editors. Bacillus thuringiensis: An Environmental Biopesticide. New York: Wiley; 1993. [Google Scholar]
- 3.Beegle C C, Yamamoto T. Can Entomol. 1992;124:587–616. [Google Scholar]
- 4.Georghiou G P, Lagunes-Tejeda A. The Occurrence of Resistance to Pesticides in Arthropods. Rome: Food Agric. Org. U.N.; 1991. [Google Scholar]
- 5.Gould F, Anderson A, Jones A, Sumerford D, Heckel D, Lopez J, Micinski S, Leonard R, Laster M. Proc Natl Acad Sci USA. 1997;94:3519–3523. doi: 10.1073/pnas.94.8.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roush R T, McKenzie J A. Annu Rev Entomol. 1987;32:361–380. doi: 10.1146/annurev.en.32.010187.002045. [DOI] [PubMed] [Google Scholar]
- 7.Groeters F, Tabashnik B E, Finson N, Johnson M W. Evolution. 1994;48:197–201. doi: 10.1111/j.1558-5646.1994.tb01306.x. [DOI] [PubMed] [Google Scholar]
- 8.Tabashnik B E. Annu Rev Entomol. 1994;39:47–79. [Google Scholar]
- 9.Ferré J, Escriche B, Bel Y, Van Rie J. FEMS Microbiol Lett. 1995;132:1–7. [Google Scholar]
- 10.Gould F, Anderson A, Reynolds A, Bumgarner L, Moar W. J Econ Entomol. 1995;88:1545–1559. [Google Scholar]
- 11.Ferré J, Real M D, Van Rie J, Jansens S, Peferoen M. Proc Natl Acad Sci USA. 1991;88:5119–5123. doi: 10.1073/pnas.88.12.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee M K, Rajamohan F, Gould F, Dean D H. Appl Environ Microbiol. 1995;61:3836–3842. doi: 10.1128/aem.61.11.3836-3842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heckel D G, Gahan L C, Gould F, Anderson A. J Econ Entomol. 1997;90:75–86. [Google Scholar]
- 14.Georghiou G P, Taylor C E. J Econ Entomol. 1977;70:653–658. doi: 10.1093/jee/70.5.653. [DOI] [PubMed] [Google Scholar]
- 15.Gould F. Environ Entomol. 1986;15:11–23. [Google Scholar]
- 16.Mallet J, Porter P. Proc R Soc London Ser B. 1992;250:165–169. [Google Scholar]
- 17.Tabashnik B E. Proc R Soc London Ser B. 1994;255:7–12. [Google Scholar]
- 18.Alstad D N, Andow D A. Science. 1995;268:1894–1898. doi: 10.1126/science.268.5219.1894. [DOI] [PubMed] [Google Scholar]
- 19.Kaiser J. Science. 1997;273:423. [Google Scholar]
- 20.McGaughey W H, Whalon M E. Science. 1992;258:1451–1455. doi: 10.1126/science.258.5087.1451. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y B, Tabashnik B E. Proc R Soc London Ser B. 1997;264:605–610. [Google Scholar]
- 22.United States Environmental Protection Agency (1995) Pesticide Fact Sheet for Bacillus thuringiensis subsp. kurstaki Cry1Ac Delta-Endotoxin and Its Controlling Sequences as Expressed in Cotton.
- 23.Tabashnik, B. E., Liu, Y. B., Finson, N., Masson, L. & Heckel, D. G. (1997) Proc. Natl. Acad. Sci. USA 94, in press. [DOI] [PMC free article] [PubMed]
- 24.Stone T B, Sims S R. J Econ Entomol. 1993;86:989–994. [Google Scholar]
- 25.Mahaffey J S, Bacheler J S, Bradley J R, Jr, Van Duyn J W. Proc 1994 Beltwide Cotton Conf. 1994;2:1061–1063. [Google Scholar]