Abstract
Trichuris muris is a natural pathogen of mice and is biologically and antigenically similar to species of Trichuris that infect humans and livestock1. Infective eggs are given by oral gavage, hatch in the distal small intestine, invade the intestinal epithelial cells (IECs) that line the crypts of the cecum and proximal colon and upon maturation the worms release eggs into the environment1. This model is a powerful tool to examine factors that control CD4+ T helper (Th) cell activation as well as changes in the intestinal epithelium. The immune response that occurs in resistant inbred strains, such as C57BL/6 and BALB/c, is characterized by Th2 polarized cytokines (IL-4, IL-5 and IL-13) and expulsion of worms while Th1-associated cytokines (IL-12, IL-18, IFN-γ) promote chronic infections in genetically susceptible AKR/J mice2-6. Th2 cytokines promote physiological changes in the intestinal microenvironment including rapid turnover of IECs, goblet cell differentiation, recruitment and changes in epithelial permeability and smooth muscle contraction, all of which have been implicated in worm expulsion7-15. Here we detail a protocol for propagating Trichuris muris eggs which can be used in subsequent experiments. We also provide a sample experimental harvest with suggestions for post-infection analysis. Overall, this protocol will provide researchers with the basic tools to perform a Trichuris muris mouse infection model which can be used to address questions pertaining to Th proclivity in the gastrointestinal tract as well as immune effector functions of IECs.
Keywords: Infection, Issue 51, Trichuris muris, mouse, Th2, intestine, inflammation
Protocol
1. Propagating Trichuris muris eggs
To generate new batches of Trichuris muris eggs, infect 20-30 immunodeficient mice (eg. NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG) or 129S6/SvEvTac-Rag2tm1Fwa (RAG2-/-)) or genetically susceptible mice (eg. AKR/J) that are 6-8 weeks old with approximately 300 Trichuris muris eggs by oral gavage.
After 32–35 days sacrifice the mice by CO2 asphyxiation.
Expose the ventral side of the mouse and wet the abdomen with 70% ethanol.
Using forceps grasp the abdominal skin and make a small incision using scissors. Cut the skin to expose the abdominal contents.
Identify the cecum and the proximal large intestine, and remove them.
Place the cecum and proximal large intestine in a 10 cm petri dish containing Dulbecco's Modified Eagle Medium (DMEM) with 500 U/ml penicillin and 500 μg/ml streptomycin (P/S). Cut the cecum open to expose the lumen and the worms. Using forceps place cecum in a beaker of phosphate buffered saline (PBS) and shake gently to remove feces.
Return cecum to petri dish and using smooth curved forceps (Roboz RS-5047) gently pull out worms and place them in a well of a 6 well plate containing 5 mls of DMEM + P/S.
Repeat this process for all of the mice.
Put 6 well plate in a Tupperware container with a damp paper towel to maintain humidity and incubate at 37°C for 4 hrs.
After 4 hrs remove worms using smooth curved forceps and split them into 2 new wells of the 6 well plate containing 5mL DMEM + P/S. Harvest the contents of the original well and place in a 15 ml falcon tube. Spin tube at 3000 rpm and remove and reserve supernatant as 4 hr antigen (can be used for cell restimulation after PBS dialysis, where typical protein yields are 10-20 mg per 20 mice) and resuspend egg-containing pellet in autoclaved tap water.
Put the 6 well plate back at 37°C overnight. Remove and dispose of the worms. Harvest the contents of the wells into a falcon tube and spin at 3000 rpm for 5 min. Remove supernatant and reserve as overnight antigen (can be used for Trichuris Ag specific antibody isotype ELISAs after PBS dialysis, where typical protein yields are 10-20 mg per 20 mice) and resuspend egg-containing pellet in autoclaved tap water.
Combine 4 hr and overnight eggs and filter the eggs through the 70μm strainer to remove any leftover worms then transfer into a 75 cm2 flask. Keep flask in a dark place covered in foil for 6 weeks at room temperature (RT).
Wash eggs in autoclaved tap water and resuspend at 50 viable eggs in 50 μl. To do this, centrifuge eggs at 3000 rpm for 5 min and resuspend in ˜50ml sterile tap water. Remove 50 μl eggs and place on a glass slide. Using the 40x magnification count the number of viable embryonated eggs in the 50 μl. Dilute eggs to give 50 viable eggs in 50 μl. For images of viable eggs please see the study by Kopper and Mansfield16.
2. Acute infection of experimental mice with Trichuris muris eggs (200 eggs/mouse)
Thoroughly resuspend eggs and remove enough volume to treat the number of mice X 2 (eg. for 10 mice take 200 μl x 10 mice x 2 = 4 mls of eggs) and place them in a 14 ml snap cap tube.
Invert the 14 ml snap cap tube to mix. Bring up 200 μl of eggs into 1 ml syringe on an appropriate size feeding needle (for mice weighing 25 grams we used 18 gauge 1 1/2" needles). In one hand scruff the mouse and using the other hand administer eggs by oral gavage .
Wait 21 days.
3. Experimental harvest
On day 21 sacrifice the mice using CO2.
Collect blood from mice by cardiac puncture, in a non-heparinized syringe, and store at 4°C. The following day isolate serum and freeze at -20°C. Serum can be used for detection of Trichuris-specific IgGs and IgE by ELISA.
Expose the ventral side of the mouse and wet the abdomen with 70% ethanol.
Using forceps grasp the abdominal skin and make a small incision using scissors. Cut the skin and underlying membrane to expose the abdominal contents.
Identify the small intestine, cecum and colon. Grab the cecum with forceps and pull out of abdominal cavity. With another pair of forceps push small intestine over to the right exposing the mesenteric lymph nodes (mLNs). Remove the mLNs being careful not to cut into the intestines and place in a 15 ml falcon containing 2 mls of media. If desired these can be processed and restimulated in vitro (4x106/ml in 1 ml in 24 well plates with 1 μg/ml αCD3/CD28 or with 50μg/ml 4h Trichuris Ag) for 72 hrs for cytokine analysis by ELISA and intracellular flow cytometry.
Cut out the cecum and colon.
Using forceps, push out fecal pellets from distal colon and place in a 2 ml Axygen tube. Freeze at -20°C. Fecal pellets can be analyzed by western blot for expression of resistin-like molecule-β (RELM-β).
Remove the tip of the cecum (˜0.5 cm). To remove feces hold cecum tip with forceps and flush with PBS using a 3 ml syringe and 22-gauge needle in a petri dish. Place in fresh 4% paraformaldehyde in a 5 ml Falcon snap cap tube. Store at 4°C. These can be paraffin-embedded, sectioned and mounted on slides for histological analysis.
Remove a piece of proximal colon (˜ 1 cm) for RNA. Put sample into 2 ml Axygen tube with 500 μl RNAlater. Store at 4°C. These samples can be analyzed for mRNA expression by qPCR.
Place the rest of the cecum in a labeled petri dish and freeze at -20°C.
4. Enumeration of Trichuris muris worms in the cecum
Remove cecum from freezer and place in a petri dish containing ˜5 ml water.
Using scissors, cut open cecum to expose the lumen.
Hold the cecum with forceps and vigorously shake the cecum to remove the majority of the feces.
Transfer cecum to another petri dish containing water and using smooth curved forceps gently scrape off the IECs the underlying tissue.
Transfer cecum to a new petri dish containing water and vigorously scrape remaining tissue with forceps, ripping the tissue into small pieces.
Use a dissecting microscope to count all of the worms in all 3 of the petri dishes. As you find a worm remove it from the dish to ensure the same worm isn’t counted twice. If worms are damaged (i.e. broken in half) count only thick or thin ends to get an accurate count.
5. Representative Results
The Trichuris muris infection model allows researchers to quantify parasite burden and systemic immune responses, as well as to histologically examine the inflammatory response within the distal cecum. When the lumen of the harvested cecum is exposed, Trichuris worms can be enumerated using a dissecting microscope (Figure 1A-B). Parameters of immunity to Trichuris also include antibody isotype switching to IgG1 (associated with Th2-type immunity) in resistant animals, and IgG2a (associated with Th1-type immunity) in susceptible animals (Figure 1C). Expression of the goblet cell-specific protein resistin-like molecule-β (RELM-β) is associated with clearance of Trichuris and is detectable by Western blot analysis (Figure 2). The distal portion of the cecum can be stained with Periodic Acid-Schiff (PAS) to assess goblet cell responses and the extent of intestinal inflammation in response to infection (Figure 3).
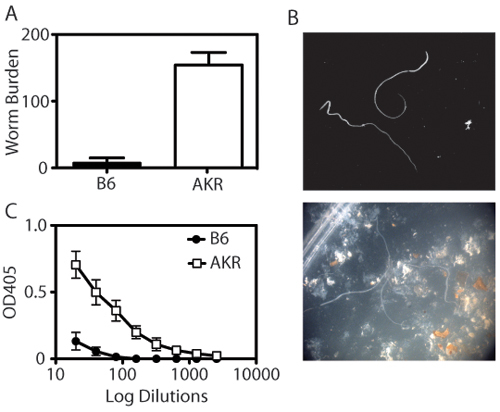 Figure 1. Quantification of immunity to Trichuris
muris. (A) Resistant (B6) mice expel Trichuris muris by day 21 post-infection. By contrast, susceptible (AKR/J) mice become chronically infected
with Trichuris, resulting in parasite establishment. Each bar represents the mean ± SEM of 4-8 animals per group. (B) Worms are counted using a
dissecting microscope. Top panel shows worms in isolation, bottom panel shows worms in the presence of IECs and feces. (C) Trichuris-specific IgG2a
titres are determined by ELISA of diluted serum (1:2 dilution, starting at 1:20 dilution and ending at 1:2560) on plates coated with excretory Trichuris
antigen (O/N Ag, 5 μg/ml). Susceptible AKR/J mice have notably higher levels of Trichuris-specific serum IgG2a than resistant B6 mice.
Figure 1. Quantification of immunity to Trichuris
muris. (A) Resistant (B6) mice expel Trichuris muris by day 21 post-infection. By contrast, susceptible (AKR/J) mice become chronically infected
with Trichuris, resulting in parasite establishment. Each bar represents the mean ± SEM of 4-8 animals per group. (B) Worms are counted using a
dissecting microscope. Top panel shows worms in isolation, bottom panel shows worms in the presence of IECs and feces. (C) Trichuris-specific IgG2a
titres are determined by ELISA of diluted serum (1:2 dilution, starting at 1:20 dilution and ending at 1:2560) on plates coated with excretory Trichuris
antigen (O/N Ag, 5 μg/ml). Susceptible AKR/J mice have notably higher levels of Trichuris-specific serum IgG2a than resistant B6 mice.
 Figure 2. Clearance of Trichuris muris is associated with expression of the goblet cell-specific protein RELM-β7.
RELM-β production can be detected from fecal pellets from Trichuris-infected resistant (B6), but not susceptible (AKR/J) mice by western blot
analysis. Each lane is representative of a single animal, (N)=Naive, (Tm)=21-day Trichuris infected mice.
Figure 2. Clearance of Trichuris muris is associated with expression of the goblet cell-specific protein RELM-β7.
RELM-β production can be detected from fecal pellets from Trichuris-infected resistant (B6), but not susceptible (AKR/J) mice by western blot
analysis. Each lane is representative of a single animal, (N)=Naive, (Tm)=21-day Trichuris infected mice.
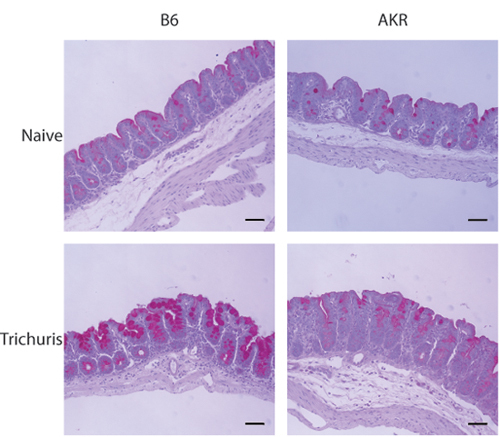 Figure 3. Histological examination of the distal cecum
following Trichuris infection. 5-7 μm sections of the distal cecum stained with PAS. Goblet cell hyperplasia and mucus production are
evident in resistant (B6) but not susceptible (AKR/J) mice following Trichuris infection.
Figure 3. Histological examination of the distal cecum
following Trichuris infection. 5-7 μm sections of the distal cecum stained with PAS. Goblet cell hyperplasia and mucus production are
evident in resistant (B6) but not susceptible (AKR/J) mice following Trichuris infection.
Discussion
This protocol details a standard high dose acute Trichuris muris infection which can be modified as required by the investigator. For example, mice can be sacrificed and tissues harvested on different days. To determine that the mice have successfully established full worm burden they can be sacrificed on day 14, at which point all mice should carry a burden of approximately 200 worms. Mice can also be infected for 32 days where any worm detected will have reached maturity and would remain with host for the duration of its life. In addition, the protocol can be adapted to model chronic Trichuris muris infection. To do so, the administered egg dose can be adjusted to a low dose infection (approximately 30 eggs). At this dose, normally resistant mice fail to induce a protective Th2 response, but rather generate a Th1 response and maintain a burden of approximately 20 worms17.
One advantage of the Trichuris muris infection model over other infection models is that in general the infected mice do not exhibit any morbidity or mortality. There are however factors that can affect experimental outcome that researchers should be aware. The first factor is the background strain of the mice. As outlined in the results, C57BL/6 mice are resistant and AKR/J mice are susceptible, and, likewise, other inbred strains may have a different infection profiles. Therefore it is important that the researcher be aware of the background strain of their mice and use appropriate control mice. In addition, there can be mouse-to-mouse variation in parasite clearance so there is no need for concern if some normally resistant control mice have up to 30 worms at 21 days post infection. As well, susceptible mice will not always maintain a full 200-count worm burden, but can range from approximately 75 to 200 worms per cecum. This variation can also be accounted for by natural variation in gut flora of mice housed in different facilities.
A factor that adversely affects Trichuris muris infection is the presence of pinworm. Pinworms infect the same anatomical site as Trichuris and induce an immune response that can confound the interpretation of the Trichuris studies. Furthermore, pinworm treatment (albendazole, fenbendazole) also kills Trichuris. In this experimental procedure, we use mice that are maintained under specific-pathogen free conditions, and it is paramount that experimental mice be kept pinworm free prior to and during Trichuris muris infection.
Some minor points researchers should be aware of:
Eggs should be maintained in water, not PBS.
Eggs are very heavy and therefore should be resuspended before delivery to each mouse. Also, load only enough volume of eggs to infect one mouse at a time, as they will settle in the syringe.
Some rare strains show sex differences in infectivity but in most cases, either males or females can be used for Trichuris muris infections.
Naïve mice can be housed in the same cage as infected mice as there is no concern of horizontal transfer of infection with this model.
Overall, Trichuris muris infection is a simple procedure to perform that models Th2-dependent immunity and can be adapted to suit individual researchers needs.
Disclosures
Experiments on animals were performed in accordance with the guidelines and regulations set forth by The University of British Columbia.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (MSH-95368, MOP-89773 and MOP-106623 to C.Z.) and the Canada Foundation for Innovation. S.C.M. is the recipient of a CIHR/Canadian Association of Gastroenterology postdoctoral fellowship. C.Z. is a CIHR New Investigator.
References
- Cliffe LJ, Grencis RK. The Trichuris muris system: a paradigm of resistance and susceptibility to intestinal nematode infection. Adv. Parasitol. 2004;57:255–307. doi: 10.1016/S0065-308X(04)57004-5. [DOI] [PubMed] [Google Scholar]
- Else KJ, Finkelman FD, Maliszewski CR, Grencis RK. Cytokine-mediated regulation of chronic intestinal helminth infection. J. Exp. Med. 1994;179:347–351. doi: 10.1084/jem.179.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft AJ, Grencis RK. Th1 and Th2 cells and immunity to intestinal helminths. Chem. Immunol. 1998;71:192–208. doi: 10.1159/000058711. [DOI] [PubMed] [Google Scholar]
- Bancroft AJ, McKenzie AN, Grencis RK. A critical role for IL-13 in resistance to intestinal nematode infection. J. Immunol. 1998;160:3453–3461. [PubMed] [Google Scholar]
- Helmby H, Takeda K, Akira S, Grencis RK. Interleukin (IL)-18 promotes the development of chronic gastrointestinal helminth infection by downregulating IL-13. J. Exp. Med. 2001;194:355–364. doi: 10.1084/jem.194.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owyang AM. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J. Exp. Med. 2006;203:843–849. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artis D. RELMβ/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc. Natl. Acad. Sci. U. S. A. 2004;101:13596–13600. doi: 10.1073/pnas.0404034101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta R. Identification of novel genes in intestinal tissue that are regulated after infection with an intestinal nematode parasite. Infect. Immun. 2005;73:4025–4033. doi: 10.1128/IAI.73.7.4025-4033.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliffe LJ. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science. 2005;308:1463–1465. doi: 10.1126/science.1108661. [DOI] [PubMed] [Google Scholar]
- Artis D. New weapons in the war on worms: identification of putative mechanisms of immune-mediated expulsion of gastrointestinal nematodes. Int. J. Parasitol. 2006;36:723–733. doi: 10.1016/j.ijpara.2006.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman FD. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu. Rev. Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- Grencis RK. Enteric helminth infection: immunopathology and resistance during intestinal nematode infection. Chem. Immunol. 1997;66:41–61. doi: 10.1159/000058665. [DOI] [PubMed] [Google Scholar]
- Khan WI. Modulation of intestinal muscle contraction by interleukin-9 (IL-9) or IL-9 neutralization: correlation with worm expulsion in murine nematode infections. Infect. Immun. 2003;71:2430–2438. doi: 10.1128/IAI.71.5.2430-2438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan WI, Blennerhasset P, Ma C, Matthaei KI, Collins SM. Stat6 dependent goblet cell hyperplasia during intestinal nematode infection. Parasite Immunol. 2001;23:39–42. doi: 10.1046/j.1365-3024.2001.00353.x. [DOI] [PubMed] [Google Scholar]
- Akiho H, Blennerhassett P, Deng Y, Collins SM. Role of IL-4, IL-13, and STAT6 in inflammation-induced hypercontractility of murine smooth muscle cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282:226–2232. doi: 10.1152/ajpgi.2002.282.2.G226. [DOI] [PubMed] [Google Scholar]
- Kopper JJ, Mansfield LS. Development of improved methods for delivery of Trichuris muris to the laboratory mouse. Parasitol. Res. 2010;107:1103–1113. doi: 10.1007/s00436-010-1978-8. [DOI] [PubMed] [Google Scholar]
- Bancroft AJ, Else KJ, Grencis RK. Low-level infection with Trichuris muris significantly affects the polarization of the CD4 response. Eur. J. Immunol. 1994;24:3113–3118. doi: 10.1002/eji.1830241230. [DOI] [PubMed] [Google Scholar]


