Abstract
The plasma and tissue disposition of two novel dextran prodrugs of methylprednisolone (MP) containing one (DMP-1) or five (DMP-5) amino acids as linkers were studied in rats. Single 5-mg/kg doses (MP equivalent) of each prodrug or MP were administered intravenously, and blood and tissue samples were collected. Prodrug and drug concentrations were quantitated using HPLC, and non-compartmental pharmacokinetic parameters were estimated. Whereas conjugation of MP with dextran in both prodrugs substantially decreased the clearance of the drug by ~200 fold, the accumulations of the drug in the liver, spleen, and kidneys were significantly increased by conjugation. However, the extent of accumulation of DMP-1 in these tissues was substantially greater than that for DMP-5. Substantial amounts of MP were regenerated from both prodrugs in the liver and spleen, with the rate of release from DMP-5 being twice as fast as that from DMP-1. However, the AUCs of MP regenerated from DMP-1 in the liver and spleen were substantially higher than those after DMP-5. In contrast, in the kidneys, the AUC of MP regenerated from DMP-5 was higher than that after DMP-1 administration. These data suggest that DMP-1 may be more suitable than DMP-5 for targeting immunosuppression to the liver and spleen.
Keywords: dextran prodrugs, macromolecular prodrugs, peptide linkers, methylprednisolone, pharmacokinetics, tissue distribution, immunosuppression, liver targeting
INTRODUCTION
Intravenous mega doses (e.g., three 1-g pulses) of methylprednisolone (MP) succinate are currently the treatment of choice, and the most widely used protocol, for the treatment of acute cellular rejection1–4 that occurs in 60-80% of liver transplant patients.2,5 However, this treatment has been fatal in several cases6–11 and/or associated with severe life-threatening toxicities related to the cardiovascular system (cardiac arrest and arrhythmia),8,10–12 central nervous system (seizure and blindness),6,13–16 severe infections (viral and bacterial),2,3,5,17 or metabolic complications (hypokalamia).18 Therefore, a targeted delivery of MP to the liver may afford administration of smaller doses, resulting in prolonged local effects in the liver, without significant toxicity to other organs and sudden death observed with the current protocols.6–11
Recently, we developed a macromolecular prodrug of MP by attaching the drug to dextran 70 kDa through a succinate linker, producing two ester bonds at both ends of the linker. The pharmacokinetics19 and systemic20 and local21 immunosuppressive activities of the conjugate confirmed the feasibility of the use of dextrans for delivery of MP to the liver and spleen. These studies demonstrated that the prodrug would selectively accumulate and gradually release MP in the liver and spleen, resulting in a more intense and sustained immunosuppressive activity in these organs, compared to administration of an equal dose of the parent drug. Additionally, studies in a rat transplantation model showed that this dextran prodrug of MP is more effective than the parent drug in preventing rejection of the liver allograft.22 However, despite very high accumulation of the prodrug in the liver and spleen, the regeneration of MP (via enzymatic hydrolysis of the ester bonds) in these tissues was very slow and incomplete.20,21 For example, although the concentration of the conjugate in the spleen was still very high at 96 hr after its administration (84.2 μg/g; 50% of the peak concentration), no free MP was detected at this time, resulting in the return of lymphocyte proliferation activity to the baseline levels. In fact, the ratio of the regenerated MP: 70 kDa-succinic acid-MP concentrations in the spleen progressively and rapidly declined with time after the administration of the conjugate.20 Similarly, despite the fact that the conjugate concentration in the liver at 72 hr after its administration was ~40% of its peak concentration, it did not result in any MP regeneration or immunosuppressive activities in this organ at this time point.21 This was attributed to the type of linker (succinic acid) and steric hindrance exerted by the 70 kDa dextran.23 The slow and incomplete regeneration of MP from the dextran 70 kDa-succinic acid-MP is undesirable because the full potentials of the prodrug in terms of both intensity and duration of the effect are not realized. Therefore, newer prodrugs of MP with faster and more controllable rate of release are desirable.
In an effort to improve and control the release profile of MP from its macromolecular prodrugs, second-generation dextran-MP prodrugs were recently24 synthesized containing a lower Mw dextran (~25 kDa) and peptide linkers of varying lengths (1–5 amino acids). In vitro studies24 indicated that these prodrugs are subject to hydrolysis by lysosomal enzymes, with the rate of hydrolysis being proportional to the length of the peptide linker. Therefore, it may be possible to control the in vivo rate of release of MP from its dextran prodrugs by using different peptide linkers. In the current study, we selected two second-generation dextran prodrugs of MP with the shortest (one amino acid) and longest (five amino acids) linkers for in vivo pharmacokinetic studies in rats. Single doses of dextran-MP conjugates with one (DMP-1) or five (DMP-5) amino acid linkers (Scheme 1) or the parent drug MP were administered to rats and their plasma pharmacokinetics and tissue disposition were determined. Our hypothesis was that the in vivo rate of regeneration of MP from DMP-5 is faster than that after the DMP-1 administration.
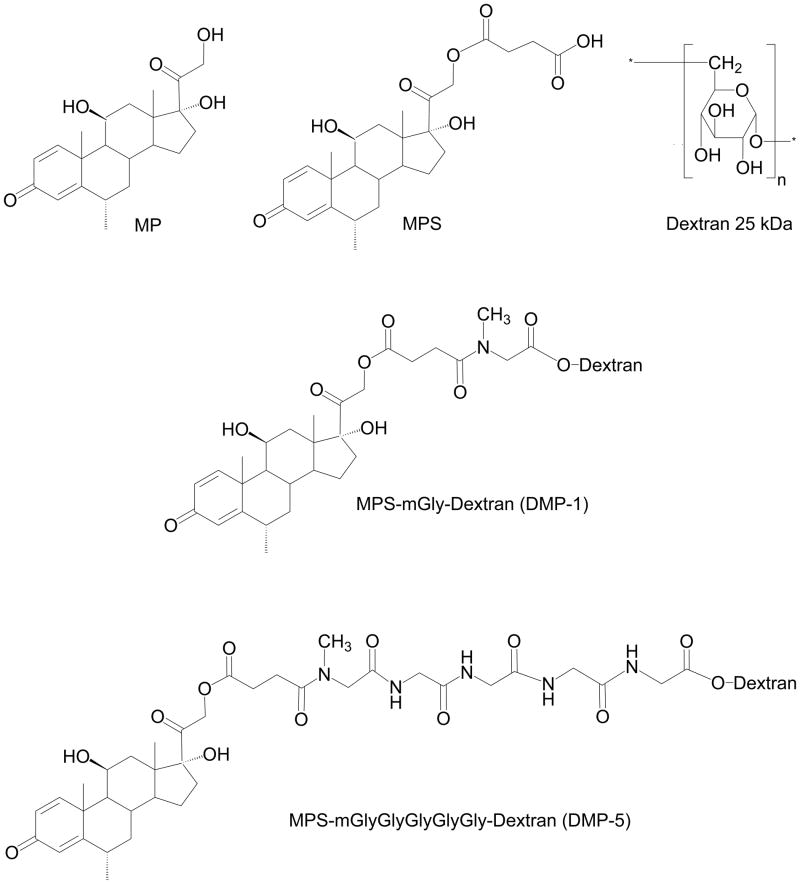
Scheme 1.
Chemical structures of methylprednisolone (MP), MP succinate (MPS), dextran, and the two dextran prodrugs of MP with methyl (m) Gly (DMP-1) or mGly-Gly-Gly-Gly-Gly (DMP-5) as linkers.
MATERIALS AND METHODS
Chemicals
Dextran with an average Mw of 23500 was obtained from Dextran Products Ltd. (Scarborough, Ontario, Canada). The degree of polydispersity of dextran was 2.3. 6α-Methylprednisolone (MP) was purchased from Steraloids (Newport, RI, USA). Internal standard (triamcinolone acetonide) was purchased from Sigma (St. Louis, MO). For chromatography, HPLC grade acetonitrile (EMD) was obtained from VWR Scientific (Minneapolis, MN, USA). All other reagents were analytical grade and obtained through commercial sources.
DMP conjugates (Scheme 1) with methyl Gly (mGly) (DMP-1) or mGly-Gly-Gly-Gly-Gly (DMP-5) were synthesized and characterized as reported by us before.24 The degrees of MP substitution (w/w) of the conjugates were 9.4% and 6.9% for DMP-1 and DMP-5, respectively, with purities of >90%.
Animals, Dosing, and Sampling
All procedures involving animals used in this study were consistent with the guidelines set by the National Institute of Health (NIH Publication No. 85–23, revised 1985) and approved by our Institutional Animal Care and Use Committee. Adult male Sprague-Dawley rats were obtained from Charles River Lab (Wilmington, MA) and housed in a 12-h light-dark cycle and temperature-controlled facility prior to the study. A total of 64 animals were used for this study that consisted of 3 groups. Two groups (21 rats/group) were treated with DMP-1 or DMP-5 and the third group (18 rats) was treated with MP. The remaining four rats were used as organ donors for blank sample preparation. The mean ± standard deviation (SD) of the body weights of rats were 250 ± 12, 236 ± 26, and 246 ± 12 g for DMP-1, DMP-5, and MP groups, respectively.
A single 5-mg/kg (MP equivalent) dose of either MP or dextran prodrugs of MP were administered into the penile veins of rats under isoflurane anesthesia. The drugs were injected as bolus doses over ~15 sec. At various times after dosing, different groups of animals were euthanized using carbon dioxide, and blood (cardiac puncture) and tissues were collected. The sampling times were 1 min and 0.5, 1.5, 3, 5, 12, and 24 h after DMP-1 or DMP-5 injection, and 1, 10, 20, 40, 60, and 120 min after MP injection (n = 3/time point). Also, total urine output was collected from the 24-hr groups after the DMP-1 or DMP-5 injections. Immediately after excision, the collected tissues were rinsed in ice-cold saline and blotted dry and kept frozen until analysis. The blood was immediately centrifuged in heparin-coated microcentrifuge tubes to obtain plasma. One hundred microliters of plasma sample was transferred to a silanized microcentrifuge tube for DMP analysis. For MP analysis, 500 μL of plasma sample was transferred to a silanized microcentrifuge tube containing 100 μL of 10% acetic acid solution to prevent DMP hydrolysis in vitro.25 All the samples were stored at −800C until analysis.
Sample Analysis
Before analysis, tissues were homogenized with 3 volumes of 2% (v/v) acetic acid, and the homogenate was used for the HPLC analysis of DMP and/or free MP. Previous studies have shown that the in vitro hydrolysis of DMP conjugates with peptide linkers in the liver homogenates containing 2% acetic acid is negligible during sample preparation.25
The concentrations of free MP in plasma and tissue homogenates were determined using a previously reported reversed-phase HPLC method26 with a modified extraction procedure. Briefly, to 240 μL of plasma and/or tissue homogenates were added 600 μL of cold acetonitrile containing 5 μg/mL of triamcinolone acetonide as internal standard. After vortex mixing and centrifugation, the supernatant was evaporated, and the analytes were first dissolved in 120 μl of methanol, followed by the addition of 120 μL of 10 mM sodium acetate buffer (pH 4.5). After a brief centrifugation, the resultant supernatants were injected into the HPLC.
The concentrations of DMP in plasma were measured using a slightly modified size-exclusion HPLC (SEC) method described before.27 To precipitate proteins, 80 μL of cold methanol and 20 μL of 20% (v/v) perchloric acid were added to 100 μL of plasma. After a brief vortex mixing and centrifugation, 150 μL of the supernatant was transferred to a new microcentrifuge tube and 175 μL of a 0.2 M phosphate buffer (pH 7.0) were added. Subsequently, a 100-μL aliquot was subjected to the SEC method.
The concentrations of DMP in tissue homogenates were quantitated according to a previously reported assay,19 with two minor modifications. Instead of ethanol, dextran conjugates were precipitated with methanol, and the acetonitrile proportion in the mobile phase was 35%, instead of 25%.
Pharmacokinetic Analysis
Non-compartmental pharmacokinetic parameters were calculated using WinNonlin 5.2.1 (Pharsight Company; Mount View, CA). The pharmacokinetic parameters obtained included the area under the plasma concentration-time curve from time zero to the last measurable concentration without (AUClast) and with (AUC0-∞) extrapolation to infinity, total body clearance (CL), initial volume of distribution (V0), steady-state volume of distribution (Vss), mean residence time (MRT), maximum observed drug concentration (Cmax), and time to reach Cmax (Tmax). The terminal elimination rate constant (λz) was calculated from the log-linear portion of plasma or tissue concentration-time curves. The maximum concentrations of DMP or MP in plasma after the injection of the conjugate or parent drug (C0) were assumed to be the same as the concentrations at the first sampling time (1 min). The concentrations of drugs in tissues were corrected28 for the contribution from the residual blood using the volume fraction of blood (VB) in different organs; VB values of 0.0135, 0.061, 0.0459, 0.0572, 0.175, and 0.321 were used for brain, heart, kidney, liver, lung, and spleen, respectively.29
Statistical Analysis
Because of the destructive nature of sampling, the variance could not be estimated by normal methods for most pharmacokinetic parameters except for Cmax, C0, V0, and the cumulative amount excreted unchanged in urine within 24 hr ( ), which are presented as mean ± SD. Additionally, the variance of AUClast was estimated using the Bailer method, and the AUClast values are presented as mean ± SE.30
Statistical comparisons were conducted either between DMP-1 and DMP-5 or among parent MP, MP regenerated from DMP-1, and MP regenerated from DMP-5. For parameters with known variances (e.g., Cmax), statistical comparisons among three groups were tested by one-way ANOVA, followed by Tukey’s post-hoc analysis, whereas unpaired, two-tailed t-test was used for comparisons involving two groups (DMP-1 vs. DMP-5). The statistical differences among different groups in their AUC values were analyzed using a two-sided Z-test after Bonferroni adjustment for the appropriate number of comparisons, as described in detail before.19
RESULTS
Plasma Pharmacokinetics
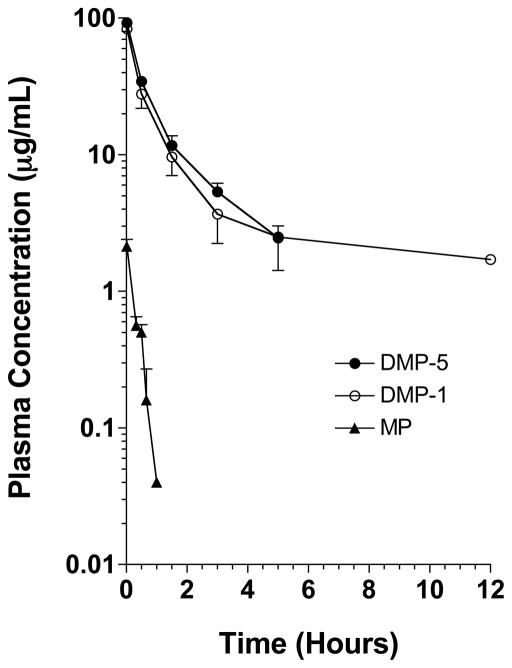
The plasma concentration-time courses of DMP-1, DMP-5, and MP after intravenous doses of 5 mg/kg (MP equivalent) of the prodrugs or the parent drug are presented in Figure 1. The plasma concentrations of MP after the administration of the parent drug were relatively low and not detectable beyond 60 min after the drug administration. In contrast, both DMP-1 and DMP-5 demonstrated very high concentrations, which were quantifiable for at least 5 hr. Whereas the plasma concentrations of DMP-1 were also measurable at 12 hr in all the rats, no detectable traces of DMP-5 were found at this time point (Fig. 1).
Figure 1.
The plasma concentration-time courses of DMP-1, DMP-5, and MP after intravenous administration of single 5 mg/kg (MP equivalent) doses of the prodrugs or the parent drug to rats. The symbols and bars represent the mean and SD values, respectively (n = 3 for each point).
After the administration of DMP-1, no regenerated MP was detectable in all the plasma samples, except for the 1-min sample, which contained an MP concentration of 0.180 ± 0.020 μg/mL. The plasma concentrations of the regenerated MP after DMP-5 injection, which ranged from 2.94 ± 0.83 μg/mL at 1 min to 0.239 ± 0.122 μg/mL at 3 hr (data not shown), were higher than those after the administration of the parent drug (Fig. 1), suggesting in vitro regeneration of MP from DMP-5 during sample collection, storage, and/or analysis.
The pharmacokinetic parameters of MP, DMP-1, and DMP-5 are presented in Table 1. Conjugation of MP to dextran using the mGly (DMP-1) or mGly-Gly-Gly-Gly-Gly (DMP-5) linker resulted in a substantial (170–210 fold) reduction in the plasma clearance of the drug. However, the CL values of the two conjugates were relatively close (Table 1). Additionally, dextran conjugation caused substantial reductions in the overall extent of distribution of the drug, as reflected in both V0 and VSS. However, the magnitudes of the decrease in the volume of distribution of MP as a result of conjugation were much smaller than those observed for the CL (Table 1). Furthermore, although the initial volumes of distribution (V0) of the two conjugates were similar, the VSS of DMP-1 was ~4 fold larger than that for DMP-5. The higher VSS of DMP-1 was also reflected in the longer terminal half life and MRT for this conjugate (Table 1). Twenty four hours after the drug injection, 15.4% and 24.7% of the dose of DMP-1 and DMP-5, respectively, were recovered unchanged in the urine (Table 1).
Table 1.
The pharmacokinetic parameters of the parent MP, DMP-1, and DMP-5 after single 5 mg/kg (MP equivalent) intravenous doses of the parent drug or the prodrugs
| Injected Drug | MP | DMP-1 | DMP-5 |
|---|---|---|---|
| Measured Analyte | MP | DMP-1 | DMP-5 |
| C0 μg/mL | 2.13 ± 0.27 | 83.4 ± 1.7 | 92.5 ± 8.6 |
| V0, mL/kg | 2370 ± 286 | 60.0 ± 1.2 | 54.4 ± 5.2 |
| Vss, mL/kg | 2470 | 365 | 89.5 |
| AUClast, μg h/mL | 0.468 ± 0.028 | 77.8 ± 4.5 | 74.7 ± 2.5 |
| AUC0-∞, μg h/mL | 0.480 | 100 | 80.3 |
| CL, mL/min/kg | 174 | 0.832 | 1.04 |
| MRT, h | 0.237 | 7.32 | 1.44 |
| T1/2, h | 0.183 | 9.02 | 1.57 |
| , %Dose | -a | 15.4 ± 6.1 | 24.7 ± 8.5 |
Not determined.
Tissue Disposition
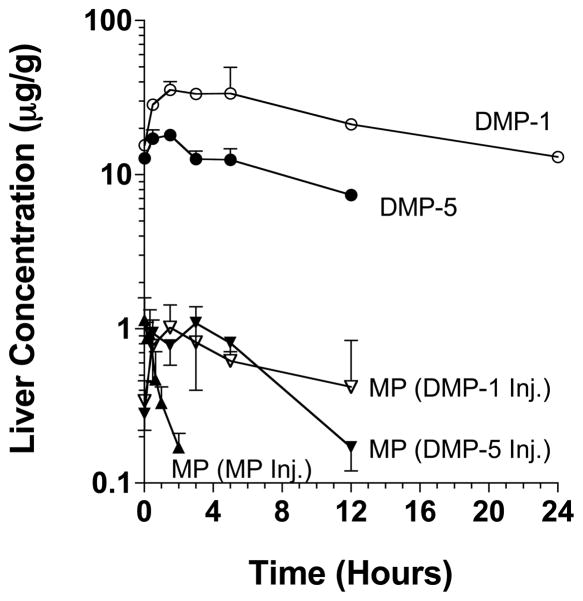
The hepatic concentration-time profiles of the administered MP, DMP-1, and DMP-5 and MP regenerated in vivo from DMP-1 and DMP-5 are presented in Figure 2. Additionally, the respective pharmacokinetic parameters are presented in Table 2. Following the administration of the parent drug MP to rats, the maximum liver concentrations of MP (1.14 ± 0.45 μg/g) occurred within the first sample (1 min), and, thereafter, the concentrations declined very rapidly (Fig. 2) with a terminal half life of 0.708 h (Table 2). In contrast, the hepatic concentrations of both prodrugs DMP-1 and DMP-5, after the administration of equivalent MP doses, were much higher than those of the parent drug and declined relatively slowly with terminal half lives of 15.0 and 9.07 h, respectively (Fig. 2 and Table 2). Indeed, the hepatic AUC0-∞ values of DMP-1 and DMP-5 were 760- and 220-fold, respectively, larger than that of the parent drug (Table 2). Also, although the Cmax values of the regenerated MP after the administration of both prodrugs were similar to that after the administration of the parent drug (p > 0.05), they occurred at a later time, and the decline in the hepatic concentrations of the regenerated MP after DMP-1 and DMP-5 were much more sustained than that after the parent drug (Table 2 and Fig. 2). Therefore, the hepatic AUC0-∞ of regenerated MP after DMP-1 and DMP-5 were 14 and 10 fold, respectively, higher than that after the administration of the parent drug (Table 2). We also estimated the liver:plasma AUC ratios after the parent drug or prodrug administration as a measure of liver targeting. Whereas the ratio for DMP-5 (2.98) was close to that for the parent drug (2.29), the ratio for DMP-1 (8.40) was ~4-fold higher than that for the parent drug (Table 2).
Figure 2.
The hepatic concentration-time courses of the administered DMP-1, DMP-5, and MP and the MP regenerated from DMP-1 and DMP-5 after intravenous administration of single 5 mg/kg (MP equivalent) doses of the prodrugs or the parent drug to rats. The symbols and bars represent the mean and SD values, respectively (n = 3 for each point).
Table 2.
The hepatic disposition parameters of the parent MP, DMP-1, and DMP-5 and the MP regenerated from DMP-1 or DMP-5 after single 5 mg/kg (MP equivalent) intravenous doses of the parent drug or the prodrugs
| Injected Drug | MP | DMP-1 | DMP-5 | ||
|---|---|---|---|---|---|
| Measured Analyte | MP | MP | DMP-1 | MP | DMP-5 |
| Cmax, μg/g | 1.14 ± 0.45 | 1.02 ± 0.41 | 35.6 ± 4.49a | 1.09 ± 0.69 | 18.1 ± 1.44a |
| Tmax, h | 0 | 1.5 | 1.5 | 3 | 1.5 |
| AUClast, μg h/g | 0.927 ± 0.088b | 7.63 ± 1.10b | 560 ± 42a | 9.57 ± 0.87b | 143 ± 6 a |
| AUC0-∞, μg h/g | 1.10 | 15.0 | 842 | 10.8 | 239 |
| Liver:Plasma AUC Ratio | 2.29 | -c | 8.40 | -c | 2.98 |
| MRT, h | 1.02 | 17.2 | 21.6 | 9.79 | 13.0 |
| T1/2, h | 0.708 | 12.3 | 15.0 | 7.47 | 9.07 |
Significant difference between DMP-1 and DMP-5.
Significant differences between parent MP and MP regenerated from DMP-1 or between parent MP and MP regenerated from DMP-5.
Not estimated because of the lack of accurate estimate of plasma AUC.
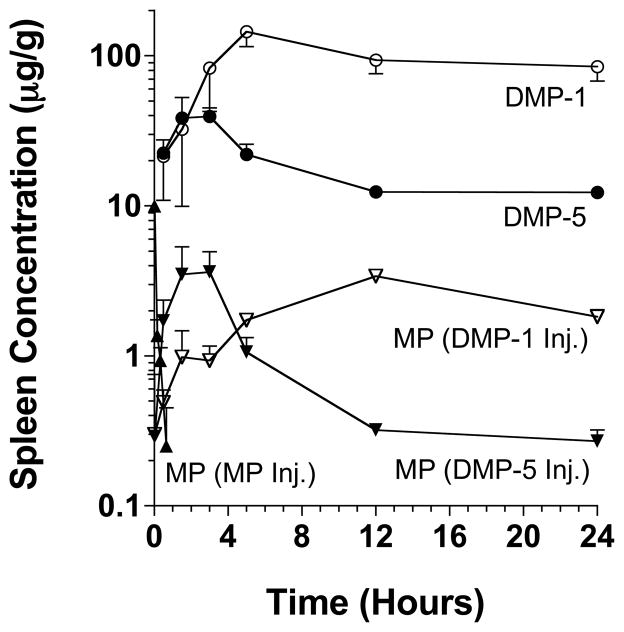
The splenic concentration-time profiles and disposition parameters of all the administered drugs (MP, DM-1, and DM-5) and MP regenerated from the two prodrugs are presented in Figure 3 and Table 3, respectively. Compared with the parent drug MP, the concentrations of the two prodrugs in the spleen were very high, with the Cmax values occurring much later. Similar to the liver profiles (Fig. 2), DMP-1 achieved much higher concentrations in the spleen, when compared with DMP-5 (Fig. 3). Both DMP-1 and DMP-5 released MP in the spleen (Fig. 3). Although the Cmax values of the regenerated MP were similar after DMP-1 and DMP-5 administration, the Tmax was much longer and the AUC was substantially higher after the DMP-1 injection (Table 3). Furthermore, the spleen: plasma AUC ratios, a measure of targetability to the spleen, increased from 2.90 for the parent drug to 54.8 and 10.9 for DMP-1 and DMP-5, respectively (Table 3).
Figure 3.
The splenic concentration-time courses of the administered DMP-1, DMP-5, and MP and the MP regenerated from DMP-1 and DMP-5 after intravenous administration of single 5 mg/kg (MP equivalent) doses of the prodrugs or the parent drug to rats. The symbols and bars represent the mean and SD values, respectively (n = 3 for each point).
Table 3.
The splenic disposition parameters of the parent MP, DMP-1, and DMP-5 and the MP regenerated from DMP-1 or DMP-5 after single 5 mg/kg (MP equivalent) intravenous doses of the parent drug or the prodrugs
| Injected Drug | MP | DMP-1 | DMP-5 | ||
|---|---|---|---|---|---|
| Measured Analyte | MP | MP | DMP-1 | MP | DMP-5 |
| Cmax, μg/g | 9.94 ± 0.81a | 3.43 ± 0.07a | 145 ± 30b | 3.62 ± 1.32a | 39.4 ± 5.5b |
| Tmax, h | 0 | 12 | 5 | 3 | 3 |
| AUClast, μg h/g | 1.3 ± 0.06a | 54.3 ± 1.0a,c | 2250 ± 145b | 21.5 ± 2.1a,c | 423 ± 15.4b |
| AUC0-∞, μg h/g | 1.39 | 89.2 | 5500 | 25.7 | 873 |
| Spleen:Plasma AUC Ratio | 2.90 | -d | 54.8 | -d | 10.9 |
| MRT, h | 0.162 | 24.7 | 41.7 | 11.3 | 35.7 |
| T1/2, h | 0.202 | 13.3 | 26.5 | 10.6 | 25.3 |
Significant differences between parent MP and MP regenerated from DMP-1 or between parent MP and MP regenerated from DMP-5.
Significant difference between DMP-1 and DMP-5.
Significant difference between MP generated from DMP-1 and MP generated from DMP-5.
Not estimated because of the lack of accurate estimate of plasma AUC.
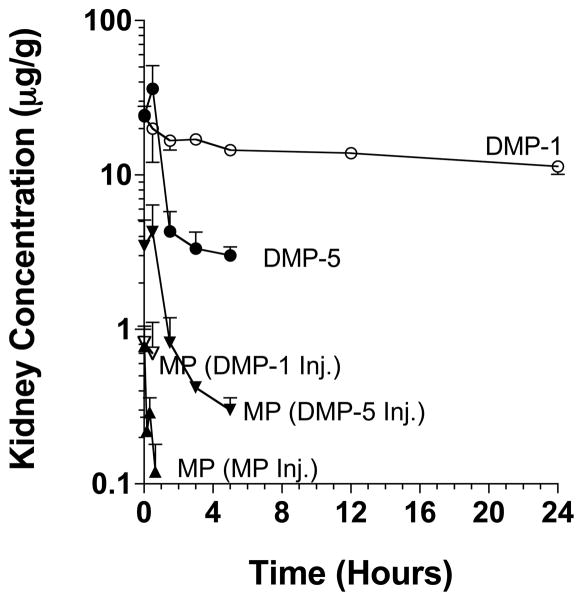
In terms of tissues other than the liver and spleen, the concentrations of DMP-1 and DMP-5 in the heart and lungs were below the level of quantitation for most samples. Additionally, no detectable peaks of DMP-1 or DMP-5 were found in any of the brain samples. However, relatively significant concentrations of DMP-1 and DMP-5 were found in the kidneys (Figure 4 and Table 4). Similar to the pattern in the liver and spleen, the DMP-1 AUC in the kidney was several-fold larger than that of DMP-5. However, in contrast to the liver and spleen, the higher and sustained concentrations of DMP-1 in the kidneys were associated with relatively low levels of regenerated MP, which were only detectable within the first 30 min of DMP-1 injection (Figure 4).
Figure 4.
The kidney concentration-time courses of the administered DMP-1, DMP-5, and MP and the MP regenerated from DMP-1 and DMP-5 after intravenous administration of single 5 mg/kg (MP equivalent) doses of the prodrugs or the parent drug to rats. The symbols and bars represent the mean and SD values, respectively (n = 3 for each point).
Table 4.
The kidney disposition parameters of the parent MP, DMP-1, and DMP-5 and the MP regenerated from DMP-1 or DMP-5 after single 5 mg/kg (MP equivalent) intravenous doses of the parent drug or the prodrugs
| Injected Drug | MP | DMP-1 | DMP-5 | ||
|---|---|---|---|---|---|
| Measured Analyte | MP | MP | DMP-1 | MP | DMP-5 |
| Cmax, μg/g | 0.779 ± 0.274a | 0.823 ± 0.179b | 24.0 ± 0.3 | 4.26 ± 2.13a,b | 36.2 ± 14.9 |
| Tmax, h | 0 | 0 | 0 | 0.5 | 0.5 |
| AUClast, μg h/g | 0.195 ± 0.018a | -c | 337± 6d | 6.10 ± 0.98a | 47.1 ± 6.5d |
| AUC0-∞, μg h/g | 0.285 | -c | 1020 | 7.17 | 77.5 |
| Kidney:Plasma AUC Ratio | 0.591 | -c | 10.3 | -e | 0.953 |
| MRT, h | 0.614 | -c | 59.6 | 2.23 | 6.60 |
| T1/2, h | 0.5 | -c | 41.5 | 2.47 | 6.98 |
Significant difference between parent MP and MP regenerated from DMP-5.
Significant difference between MP regenerated from DMP-1 and MP regenerated from DMP-5.
ND: Not determined because only two kidney samples showed measurable concentrations of MP.
Significant difference between DMP-1 and DMP-5
Not estimated because of the lack of accurate estimate of plasma AUC.
DISCUSSION
Our recent in vitro studies24 indicated that dextran prodrugs of MP with linkers containing 1-5 (methyl) Gly residues were degraded by rat liver lysosomal fractions at a rate directly proportional to the length of the linker. The present work was then undertaken to study the in vivo pharmacokinetics, tissue distribution, and extent of MP regeneration of the two prodrugs of MP with the shortest (DMP-1) and longest (DMP-5) linkers. Although both prodrugs significantly altered the pharmacokinetics and tissue disposition of MP qualitatively in a similar manner, substantial differences were observed between the two prodrugs, most notably in their extent of tissue distribution and regeneration of MP (Figs. 2–4 and Tables 2–4).
In all the studied tissues, DMP-1 achieved significantly higher concentrations (Figs. 2–4) and AUCs (Tables 2–4), compared with those observed after DMP-5 administration, suggesting a larger extent of distribution for this prodrug. This difference was also reflected in a four-fold larger VSS for DMP-1, estimated from the plasma concentration data (Table 1). The significant differences between the two prodrugs in their extent of tissue distribution suggest that the linker affects the physicochemical properties of the prodrug that are critical in its distribution.
Alternatively, the observed differences between DMP-1 and DMP-5 in their VSS may have been influenced by their different degrees of MP substitution (9.4% versus 6.9%, respectively). Whereas dextrans are extremely water soluble, MP is very lipophilic. Therefore, a 27% lower MP content of DMP-5, relative to DMP-1, may have contributed, in part, to a lower lipophilicity of DMP-5 and a smaller VSS for this conjugate. Additionally, because of the higher MP content, the dose of dextran backbone was 27% lower after DMP-1 injection. However, it is unlikely that a 27% difference in the dose of the carrier would be responsible for the 4-10 fold observed higher accumulation of DMP-1 in the tissues (Tables 2–4).
Although less accumulated in the tissue than DMP-1, DMP-5 regenerated MP faster than did DMP-1 in all the tissues. This is demonstrated by comparisons of the AUCs of the regenerated MP with those of their prodrugs in each tissue. In the liver, the AUC of the regenerated MP comprised 1.7% of the AUC of the prodrug after DMP-1 administration and 4.5% of the AUC of the prodrug after DMP-5 administration (Table 2). Similarly, in the spleen the ratios were 1.62% and 2.94% after the DMP-1 and DMP-5 injections, respectively (Table 3). In the kidneys, the ratio was the highest for DMP-5 (9.25%) among all the studied tissues, whereas virtually no release of MP was observed after DMP-1 administration (Table 4). These data suggest that the in vivo release of MP from the prodrugs is faster for DMP-5 than DMP-1, an observation that is in complete agreement with the in vitro release data reported by us previously.24
The faster in vivo release of MP from its dextran prodrug with the longer peptidyl linker (DMP-5) is in agreement with the literature data on a dextran prodrug of a camptothecin analog (T-2513), containing peptidyl linkers.31 Harada et al.31 studied the in vivo release of T-2513 in the liver and tumor of rats bearing Walker-256 carcinomas at 6 hr after a single 1-mg/kg dose of T-2513 conjugated to carboxymethyldextran (Mw of 130 kDa) via linkers comprising of one to five Gly amino acids. Similar to our studies, an increase in the length of the linker progressively increased the in vivo rate of release of T-2513. However, they did not detect any release of T-2513 from the prodrug with Gly linker. This is in contrast to a relatively significant release of MP from DMP-1 in the liver in our studies (Fig. 2). This difference could be attributed to the differences in the two prodrugs in the chemistry of the bond between the drug and the carrier and/or the much larger size of the dextran carrier used in their study (130 kDa), compared with ours (~25 kDa).
It may be argued that the lower concentrations of DMP-5 in the tissues, compared with DMP-1 (Figs 2-4), are due to a faster release of MP from this prodrug and not because of differences in the tissue accumulation between the two prodrugs. Although a faster MP release might have contributed to the lower tissue concentrations of DMP-1, in particular in the kidneys, it cannot explain the substantial differences between the tissue AUCs of DMP-1 and DMP-5 in the liver and spleen. This is because in both the liver (Table 2) and spleen (Table 3), the AUC of the generated MP after DMP-1 was higher than that after the administration of DMP-5. Therefore, the differences in the tissue accumulation of the two prodrugs, at least in the case of liver and spleen, are likely due to a selective accumulation of DMP-1 in these tissues.
The tissue:plasma AUC ratio is a more appropriate index of tissue targeting, compared with the absolute AUC values in the tissues. Based on these AUC ratios, both dextran prodrugs of MP are superior to the parent drug in terms of targeting MP to the liver (Table 2), spleen (Table 3), and kidneys (Table 4). Further, a comparison of the ratios for the two prodrugs indicate that DMP-1 has 3, 5, and 11 fold higher selectivity for the liver, spleen, and kidneys, respectively, compared with DMP-5. It should be noted the true index of tissue targetability for prodrugs is the tissue:plasma AUC ratios of the regenerated drug. This parameter was not estimated in our study because of the lack of regeneration of MP in the plasma after DMP-1. However, it is clear that based on this parameter, the selectivity of DMP-1 for targeting to the liver and spleen would have been even more dramatic (because of the very low plasma concentrations of MP regenerated from DMP-1, compared with DMP-5). However, the same may not be true for the kidneys because in this tissue, the concentrations of MP regenerated from DMP-5 were higher than those after DMP-1 (Fig. 4). Overall, our data indicate that although releasing MP at a slower rate, DMP-1 is superior to DMP-5 in terms of targeting MP to the liver and spleen.
In addition to the MP prodrug developed by us, at least three other examples are available in the literature with regard to the use of peptide linkers for the attachment of drugs to dextran carriers.31–37 In all of these examples, carboxymethyldextran, a negatively charged derivative of dextran, with high Mw was used as a carrier with a peptide linker with three,31–33 four,34,35 or six36,37 amino acids to deliver anticancer drugs camptothecin derivatives31–35 or methotrexate36,37 to the tumor. These prodrugs exhibit increased circulation time, relative to that of the parent drug, allowing higher accumulation of the anticancer drugs in the tumors, where they gradually release the parent drug, resulting in higher efficacy and/or lower toxicity in animal and/or human studies. Our prodrugs intended for the delivery of MP to the liver and spleen differ from these anticancer prodrugs mainly in that we used a neutral dextran, instead of the negatively charged carboxymethyldextran used for the delivery of the anticancer drugs. This was because previous studies38 have shown that negatively-charged dextrans have a lower accumulation in the liver, compared with neutral dextrans. Therefore, neutral dextrans are more suitable than negatively-charged dextrans for delivery to the liver, which is intended for our MP prodrug.
The two dextran prodrugs of MP tested in the present in vivo study (DMP-1 and DMP-5) are part of a second-generation dextran prodrugs of MP that were recently synthesized and characterized in vitro.24 A major difference between these prodrugs and a prodrug of MP developed earlier (dextran 70 kDa-succinic acid-MP) is the use of amino acid/peptide linker (instead of a succinate linker) in the new prodrugs. The peptide linkers were selected to circumvent the inflexible and slow rate of MP regeneration observed with dextran 70 kDa-succinic acid-MP.19,20 Indeed, whereas the ratio of the AUC of the regenerated MP over that of the prodrug in the liver was only 0.60% for dextran 70 kDa-succinic acid-MP,19 the corresponding ratios were 1.8% and 4.5% for DMP-1 and DMP-5, respectively (Table 2). Similarly, the ratios for DMP-1 and DMP-5 in the spleen (1.6%, and 2.9%, respectively; Table 3) were higher than that for dextran 70 kDa-succinic acid-MP (0.93%).19 These data indicate that not only is the rate of MP regeneration faster with the new prodrugs, but also can be controlled by varying the length of the linker.
The second major difference between the newly-developed prodrugs described here and dextran 70 kDa-succinic acid-MP is the lower Mw of the new prodrugs (25 kDa instead of 70 kDa). Our previous work with dextran carriers demonstrated that high Mw dextrans (Mw ≥ 20 kDa) preferentially accumulated in the liver and spleen, whereas low Mw dextrans (e.g., Mw of 4 kDa) were rapidly excreted into the urine without achieving significant concentrations in the liver or spleen.28 Among dextrans with a Mw in the range of 4 kDa to 150 kDa, dextran with a Mw of 70 kDa achieved the highest accumulation in the liver. Therefore, in our initial studies designing a prodrug of MP, we used the 70 kDa dextran as a carrier. However, the elimination of dextran 70 kDa is strictly via non-renal pathways,28 making it susceptible to nonlinearity in its pharmacokinetics.23,39 Therefore, in designing the newer dextran prodrugs of MP, we selected a dextran with a Mw of 25 kDa, which is expected to be subject to some degree of linear renal excretion,39 in addition to a significant accumulation in the liver.28 Therefore, another major difference in the pharmacokinetics of the newer prodrugs (DMP-1 and DMP-5) and dextran 70 kDa-succinic acid-MP is that in contrast to no renal excretion for dextran 70 kDa-succinic acid-MP, between 15% to 25% of the administered dose of DMP-1 or DMP-5 was recovered in the 24-h urine of rats as intact prodrug (Table 1). These data suggest that the potential for the long-term, undesired accumulation of the carrier dextran in the tissues is less for these novel dextran prodrugs of MP.
Our recent in vitro studies24 showed that DMP-1 and DMP-5 are degraded in the rat blood in vitro (37°C) with half lives of 24.5 and 4.67 h, respectively. Additional studies25 also showed that the addition of acetic acid to the plasma samples during the sample preparation and storage prevents in vitro degradation of the prodrugs in plasma. Although blood samples in our study were centrifuged immediately to separate plasma before the addition of acetic acid and storage, the MP concentrations in the plasma samples of DMP-5-treated animals were substantially higher than the corresponding concentrations after the administration of the same dose of the parent drug (See Results). Pharmacokinetically, the AUC of a drug after the administration of its prodrug cannot be higher than that after the administration of the same dose of the parent drug, suggesting that despite our precautions DMP-5 was converted to MP in the blood in vitro during centrifugation. This is a plausible explanation because in vitro degradation of even 1-2% of the very high concentrations of the prodrug in the plasma (Fig. 1) can easily result in the values observed after DMP-5. Consequently, we did not report here the plasma AUC values of MP after DMP-5 administration. This problem did not occur with DMP-1 because of much higher stability of this prodrug, compared with DMP-5, in blood.24 Nevertheless, our data clearly show that DMP-1 does not regenerate MP to a significant degree in the blood (in vivo or in vitro), whereas DMP-5 is prone to the release of MP in the blood both in vivo and in vitro.
An ideal macromolecular prodrug of a drug with its site of action in the tissue is expected to remain intact in the circulation, enter the tissues and cells of interest, and release the drug intracellularly. If the drug is released prematurely in the circulation, the prodrug will lose its targeting effect, resulting in no change in drug exposure. Our tissue data for the liver (Fig. 2 and Table 2) and spleen (Fig. 3 and Table 3) for both DMP-1 and DMP-5 conjugates clearly show that the tissue exposure to released MP was much higher, compared with the parent drug administration. This suggests that the MP present in these tissues after the conjugate administration is most likely regenerated from the conjugate intracellularly. This is consistent with previous studies40,41 demonstrating that dextrans enter the cells by endocytosis, ending up in lysosomes, which contain enzymes capable of degrading peptide bonds such as those in DMP-1 and DMP-5.24
In conclusion, our data show that the novel dextran prodrugs of MP with amino acid/peptide linkers are suitable for delivery of the drug to the liver and spleen for the purpose of immunosuppression. In agreement with the reported in vitro data,24 our current in vivo study shows that the regeneration of MP from the prodrug with the five amino acid linker (DMP-5) is much faster than that from the prodrug with the one amino acid linker (DMP-1) in all the tissues. However, the AUC of MP regenerated from DMP-1 is substantially higher than that regenerated from DMP-5 in the liver and spleen. In contrast to the liver and spleen, minimal regeneration of MP occurred in the kidneys after DMP-1 administration. Therefore, DMP-1 may be more suitable than DMP-5 for targeting immunosuppression to the liver and spleen. Further pharmacodynamic studies are required to determine the effects of these prodrugs on the local immunosuppression in the liver after liver transplantation.
Acknowledgments
This study was supported by a grant from the National Institute of General Medical Sciences of NIH (R01 GM069869). The authors acknowledge the use of WinNonlin program as part of a Pharsight Academic Licensing Program.
References
- 1.Evans R, Manninen D, Dong F, Ascher N, Frist W, Hansen J, Kirklin J, Perkins J, Pirsch J, Sanfilippo F. Immunosuppressive therapy as a determinant of transplantation outcomes. Transplantation. 1993;55:1297–1305. doi: 10.1097/00007890-199306000-00017. [DOI] [PubMed] [Google Scholar]
- 2.Wiesner R, Ludwig J, Krom R, Steers J, Porayko M, Gores G, Hay J. Treatment of early cellular rejection following liver transplantation with intravenous methylprednisolone. The effect of dose on response. Transplantation. 1994;58:1053–1056. doi: 10.1097/00007890-199411150-00015. [DOI] [PubMed] [Google Scholar]
- 3.Volpin R, Angeli P, Galioto A, Fasolato S, Neri D, Barbazza F, Merenda R, Del Piccolo F, Strazzabosco M, Casagrande F, Feltracco P, Sticca A, Merkel C, Gerunda G, Gatta A. Comparison between two high-dose methylprednisolone schedules in the treatment of acute hepatic cellular rejection in liver transplant recipients: a controlled clinical trial. Liver Transpl. 2002;8:527–534. doi: 10.1053/jlts.2002.33456. [DOI] [PubMed] [Google Scholar]
- 4.Millis JM. Treatment of liver allograft rejection. Liver Transpl Surg. 1999;5:S98–S106. doi: 10.1053/JTLS005s00098. [DOI] [PubMed] [Google Scholar]
- 5.Adams D, Neuberger JM. Treatment of acute rejection. Semin Liver Dis. 1992;12:80–87. doi: 10.1055/s-2007-1007379. [DOI] [PubMed] [Google Scholar]
- 6.Stubbs S, Morrell R. Intravenous methylprednisolone sodium succinate: adverse reactions reported in association with immunosuppressive therapy. Transplant Proc. 1973;5:1145–1146. [PubMed] [Google Scholar]
- 7.McDougal BA, Whittier FC, Cross DE. Sudden death after bolus steroid therapy for acute rejection. Transplant Proc. 1976;8:493–496. [PubMed] [Google Scholar]
- 8.Bocanegra TS, Castaneda MO, Espinoza LR, Vasey FB, Germain BF. Sudden death after methylprednisolone pulse therapy. Ann Intern Med. 1981;95:122. doi: 10.7326/0003-4819-95-1-122_1. [DOI] [PubMed] [Google Scholar]
- 9.Gardiner PV, Griffiths ID. Sudden death after treatment with pulsed methylprednisolone. Br Med J. 1990;300:125. doi: 10.1136/bmj.300.6717.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moses RE, McCormick A, Nickey W. Fatal arrhythmia after pulse methylprednisolone therapy. Ann Intern Med. 1981;95:781–782. doi: 10.7326/0003-4819-95-6-781_3. [DOI] [PubMed] [Google Scholar]
- 11.Guillen EL, Ruiz AM, Bugallo JB. Hypotension, bradycardia, and asystole after high-dose intravenous methylprednisolone in a monitored patient. Am J Kidney Dis. 1998;32:E4. doi: 10.1053/ajkd.1998.v32.pm10074612. [DOI] [PubMed] [Google Scholar]
- 12.McLuckie AE, Savage RW. Atrial fibrillation following pulse methylprednisolone therapy in an adult. Chest. 1993;104:622–623. doi: 10.1378/chest.104.2.622. [DOI] [PubMed] [Google Scholar]
- 13.Ayoub WT, Torretti D, Harrington TM. Central nervous system manifestations after pulse therapy for systemic lupus erythematosus. Arthritis Rheum. 1983;26:809–810. doi: 10.1002/art.1780260621. [DOI] [PubMed] [Google Scholar]
- 14.Suchman AL, Condemi JJ, Leddy JP. Seizure after pulse therapy with methyl prednisolone. Arthritis Rheum. 1983;26:117. doi: 10.1002/art.1780260123. [DOI] [PubMed] [Google Scholar]
- 15.Cerilli J, Miller JA. The effect of massive pulse steroid therapy on the water content of the rat brain. Transplantation. 1972;14:403–405. [PubMed] [Google Scholar]
- 16.El-Dahr S, Chevalier RL, Gomez RA, Campbell FG. Seizures and blindness following intravenous pulse methylprednisolone in a renal transplant patient. Int J Pediatr Nephrol. 1987;8:87–90. [PubMed] [Google Scholar]
- 17.Kozeny GA, Quinn JP, Bansal VK, Vertuno LL, Hano E. Pneumocystis carinii pneumonia: a lethal complication of "pulse" methylprednisolone therapy. Int J Artif Organs. 1987;10:304–306. [PubMed] [Google Scholar]
- 18.Bonnotte B, Chauffert B, Martin F, Lorcerie B. Side-effects of high-dose intravenous (pulse) methylprednisolone therapy cured by potassium infusion. Br J Rheumatol. 1998;37:109. doi: 10.1093/rheumatology/37.1.109a. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Mehvar R. Dextran-methylprednisolone succinate as a prodrug of methylprednisolone: plasma and tissue disposition. J Pharm Sci. 2001;90:2078–2087. doi: 10.1002/jps.1158. [DOI] [PubMed] [Google Scholar]
- 20.Mehvar R, Hoganson DA. Dextran-methylprednisolone succinate as a prodrug of methylprednisolone: immunosuppressive effects after in vivo administration to rats. Pharm Res. 2000;17:1402–1407. doi: 10.1023/a:1007555107691. [DOI] [PubMed] [Google Scholar]
- 21.Chimalakonda AP, Mehvar R. Dextran-methylprednisolone succinate as a prodrug of methylprednisolone: local immunosuppressive effects in liver after systemic administration to rats. Pharm Res. 2003;20:198–204. doi: 10.1023/a:1022358702643. [DOI] [PubMed] [Google Scholar]
- 22.Chimalakonda AP, Montgomery DL, Weidanz JA, Shaik IH, Nguyen JH, Lemasters JJ, Kobayashi E, Mehvar R. Attenuation of acute rejection in a rat liver transplantation model by a liver-targeted dextran prodrug of methylprednisolone. Transplantation. 2006;81:678–685. doi: 10.1097/01.tp.0000177654.48112.b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Mehvar R. Dextran-methylprednisolone succinate as a prodrug of methylprednisolone: dose-dependent pharmacokinetics in rats. Int J Pharm. 2001;229:173–182. doi: 10.1016/s0378-5173(01)00854-7. [DOI] [PubMed] [Google Scholar]
- 24.Penugonda S, Kumar A, Agarwal HK, Parang K, Mehvar R. Synthesis and in vitro characterization of novel dextran-methylprednisolone conjugates with peptide linkers: Effects of linker length on hydrolytic and enzymatic release of methylprednisolone and its peptidyl intermediates. J Pharm Sci. 2008;97:2649–2664. doi: 10.1002/jps.21161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang SQ, Thorsheim HR, Penugonda S, Pillai VC, Smith QR, Mehvar R. Liquid chromatography-tandem mass spectrometry for the determination of methylprednisolone in rat plasma and liver after intravenous administration of its liver-targeted dextran prodrug. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:927–932. doi: 10.1016/j.jchromb.2009.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehvar R, Dann RO, Hoganson DA. Simultaneous analysis of methylprednisolone, methylprednisolone succinate, and endogenous corticosterone in rat plasma. J Pharmaceut Biomed Anal. 2000;22:1015–1022. doi: 10.1016/s0731-7085(00)00253-3. [DOI] [PubMed] [Google Scholar]
- 27.Mehvar R. High-performance size-exclusion chromatographic analysis of dextran- methylprednisolone hemisuccinate in rat plasma. J Chromatogr B. 2000;744:293–298. doi: 10.1016/s0378-4347(00)00256-5. [DOI] [PubMed] [Google Scholar]
- 28.Mehvar R, Robinson MA, Reynolds JM. Molecular weight dependent tissue accumulation of dextrans: in vivo studies in rats. J Pharm Sci. 1994;83:1495–1499. doi: 10.1002/jps.2600831024. [DOI] [PubMed] [Google Scholar]
- 29.Bernareggi A, Rowland M. Physiologic modeling of cyclosporine kinetics in rat and man. J Pharmacokinet Biopharm. 1991;19:21–50. doi: 10.1007/BF01062191. [DOI] [PubMed] [Google Scholar]
- 30.Bailer AJ. Testing for the equality of area under the curves when using destructive measurement techniques. J Pharmacokinet Biopharm. 1988;16:303–309. doi: 10.1007/BF01062139. [DOI] [PubMed] [Google Scholar]
- 31.Harada M, Sakakibara H, Yano T, Suzuki T, Okuno S. Determinants for the drug release from T-0128, camptothecin analogue-carboxymethyl dextran conjugate. J Control Release. 2000;69:399–412. doi: 10.1016/s0168-3659(00)00321-7. [DOI] [PubMed] [Google Scholar]
- 32.Okuno S, Harada M, Yano T, Yano S, Kiuchi S, Tsuda N, Sakamura Y, Imai J, Kawaguchi T, Tsujihara K. Complete regression of xenografted human carcinomas by camptothecin analogue-carboxymethyl dextran conjugate (T-0128) Cancer Res. 2000;60:2988–2995. [PubMed] [Google Scholar]
- 33.Veltkamp SA, Witteveen EO, Capriati A, Crea A, Animati F, Voogel-Fuchs M, van den Heuvel IJ, Beijnen JH, Voest EE, Schellens JH. Clinical and pharmacologic study of the novel prodrug delimotecan (MEN 4901/T-0128) in patients with solid tumors. Clin Cancer Res. 2008;14:7535–7544. doi: 10.1158/1078-0432.CCR-08-0438. [DOI] [PubMed] [Google Scholar]
- 34.Kumazawa E, Ochi Y. DE-310, a novel macromolecular carrier system for the camptothecin analog DX-8951f: Potent antitumor activities in various murine tumor models. Cancer Sci. 2004;95:168–175. doi: 10.1111/j.1349-7006.2004.tb03199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soepenberg O, de Jonge MJ, Sparreboom A, de Bruin P, Eskens FA, de Heus G, Wanders J, Cheverton P, Ducharme MP, Verweij J. Phase I and pharmacokinetic study of DE-310 in patients with advanced solid tumors. Clin Cancer Res. 2005;11:703–711. [PubMed] [Google Scholar]
- 36.Chau Y, Tan FE, Langer R. Synthesis and characterization of dextran-peptide-methotrexate conjugates for tumor targeting via mediation by matrix metalloproteinase II and matrix metalloproteinase IX. Bioconjug Chem. 2004;15:931–941. doi: 10.1021/bc0499174. [DOI] [PubMed] [Google Scholar]
- 37.Chau Y, Dang NM, Tan FE, Langer R. Investigation of targeting mechanism of new dextran-peptide-methotrexate conjugates using biodistribution study in matrix-metalloproteinase-overexpressing tumor xenograft model. J Pharm Sci. 2006;95:542–551. doi: 10.1002/jps.20548. [DOI] [PubMed] [Google Scholar]
- 38.Takakura Y, Fujita T, Hashida M, Sezaki H. Disposition characteristics of macromolecules in tumor-bearing mice. Pharm Res. 1990;7:339–346. doi: 10.1023/a:1015807119753. [DOI] [PubMed] [Google Scholar]
- 39.Mehvar R, Robinson MA, Reynolds JM. Dose dependency of the kinetics of dextrans in rats: effects of molecular weight. J Pharm Sci. 1995;84:815–818. doi: 10.1002/jps.2600840706. [DOI] [PubMed] [Google Scholar]
- 40.Lake JR, Licko V, Van Dyke RW, Scharschmidt BF. Biliary secretion of fluid-phase markers by the isolated perfused rat liver. Role of transcellular vesicular transport. J Clin Invest. 1985;76:676–684. doi: 10.1172/JCI112021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stock RJ, Cilento EV, McCuskey RS. A quantitative study of fluorescein isothiocyanate-dextran transport in the microcirculation of the isolated perfused rat liver. Hepatology. 1989;9:75–82. doi: 10.1002/hep.1840090112. [DOI] [PubMed] [Google Scholar]