Abstract
Retinoic acid receptor-β2 (RAR-β2) is a putative tumor suppressor gene in various cancers. To determine the underlying molecular mechanisms, we transfected RAR-β2 cDNA into esophageal cancer TE-1 and TE-8 cells and found that RAR-β2 suppressed tumor cell growth in vitro and tumor formation in nude mice in TE-8 cells, whereas the stable transfection of RAR-β2 did not restore retinoid sensitivity or inhibit tumor formation in nude mouse in TE-1 cells. Molecularly, we revealed that RAR-β2 antitumor activity was associated with expression and suppression of cyclooxygenase-2 (COX-2) in these tumor cell lines. Moreover, antisense RAR-β2 cDNA induced COX-2 expression in TE-3 cells. Furthermore, when COX-2 expression is first blocked by using antisense COX-2 expression vector, the effect of RAR-β2 is diminished in these tumor cells. In addition, we analyzed expression of RAR-β2 and COX-2 mRNA in tissue specimens and found that RAR-β2 expression is associated with low levels of COX-2 expression in esophageal cancer tissues. Induction of RAR-β2 expression in oral leukoplakia tissues after the patients treated with 13-cis RA correlated with a reduction in COX-2 expression and clinical response. Our findings indicate that some of RAR-β2 antitumor activities are mediated by suppression of COX-2 expression in some of these esophageal cancer cells. After correlating antitumor effect of RAR-β2 with COX-2 expression in the published studies, we also found the association. Thus, further studies will determine whether manipulation of COX-2 expression in different cancers can antagonize RAR-β2 activity.
A great number of studies have shown that the expression of retinoic acid receptor-β2 (RAR-β2) is frequently and progressively lost in various premalignant and malignant human cells and tissues and that this loss is associated with the process of malignant transformation of different epithelial cells (for reviews, see refs. 1, 2) and with the poor prognosis of patients with neuroblastoma (3). Furthermore, cigarette smoke has been shown to cause morphology changes and the loss of RAR-β2 expression in the lung tissues of animals (4), and cigarette smoke and the tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone have been shown to induce the methylation of the RAR-β2 gene promoter in murine lung cancer (5). Our previous studies similarly showed that benzo[a] pyrene diol epoxide, a carcinogen present in tobacco smoke and environment pollution, and bile acid, a tumor promoter in the gastrointestinal tract, suppressed RAR-β2 expression in premalignant and malignant esophageal cells and that benzo[ a]pyrene diol epoxide induced the methylation of the RAR-β2 gene promoter (6, 7). In addition, the apparent immortality of cultures isolated from oral dysplastic lesions was associated with the loss of RAR-β2, p16, and mutated p53 expression and with increased levels of telomerase reverse transcriptase mRNA, but only the loss of RAR-β2 and of p16 was responsible for the immortalization of the cultures (8, 9). This finding showed the importance of RAR-β2 expression in suppressing tumorigenesis. Indeed, the restoration of RAR-β2 expression inhibited the growth of various cancers, induced tumor cells to undergo apoptosis, and suppressed cancer development in vitro and in vivo (for reviews, see refs. 1, 2). Notably, epigallocatechin gallate, a chemopreventive agent extracted from the tea plant, has been shown to bind to DNA methyltransferases and reactivate RAR-β2 expression in esophageal cancer cells (10).
Although the precise molecular mechanisms responsible for these RAR-β2–mediated effects on antitumor activities are as yet not fully understood, our previous studies showed that RAR-β2 suppressed the expression of the epidermal growth factor receptor, activating protein-1, and cyclooxy-genase-2 (COX-2) and the phosphorylation of extracellular signal-regulated protein kinases 1 and 2 (7, 11). The purpose of our current study was to further elucidate the molecular mechanisms underlying the antitumor effects of RAR-β2. We found that some RAR-β2 antitumor activities are mediated by suppression of COX-2 expression in some of esophageal cancer cell lines. If tumor cells do not express COX-2, then the induction of RAR-β2 expression has no effect in them.
Materials and Methods
Stable transfection of RAR-β2
The expression vector pRC/cytomegalovirus (CMV; Invitrogen) containing RAR-β2 cDNA was stably transfected with Lipofectin (Invitrogen) into esophageal cancer TE-1 cells (to make TE-1S4 and TE-1S16 cells) and TE-8 cells (to make TE-8S20 and TE-8S22 cells) or antisense RAR-β2 cDNA into TE-3 cells (to make TE-3A3 and TE-3A5 cells). pRC/CMV vector only transfectants in TE-1, TE-3, and TE-8 cells, named TE-1V1, TE-3V1, and TE-8V1, respectively, were also established. The stable cells were selected by using 400 µg/mL G418 (Invitrogen). The expression of exogenous RAR-β2 cDNA or knockdown of RAR-β2 expression was measured by Northern and Western blotting (see ref. 11). These sublines were grown in a monolayer in DMEM with 10% fetal bovine serum and 200 µg/mL G418 at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Protein extraction and Western blotting
Nuclear or cellular protein was extracted as described previously (6, 7, 11). Samples containing 50 µg of protein from each subline were then separated by 10% to 14% on SDS-PAGE gels and transferred electrophoretically to a Hybond-C nitrocellulose membrane (Amersham Pharmacia) at 500 mA for 2 h at 4°C. The membrane was subsequently stained with 0.5% Ponceau S containing 1% acetic acid to confirm that proteins were loaded equally and to verify transfer efficiency. The membranes were subjected to Western blotting by overnight incubation in a blocking solution containing 5% bovine skim milk and 0.1% Tween 20 in PBS at 4°C. The next day, the membranes were incubated first with primary antibodies and then with horse anti-mouse or goat anti-rabbit secondary antibodies (Amersham Pharmacia) for enhanced chemiluminescence detection of antibody signals. The antibodies used were anti-RAR-β (Santa Cruz Biotechnology, Inc.), anti-COX-2 (BD Transduction Laboratories), and anti-β-actin (Sigma-Aldrich).
Cell viability assay
Stable RAR-β2–transfected and vector only–transfected esophageal cancer cell sublines were maintained in 200 µg/mL G418 and then treated with or without 1 µmol/L all-trans RA (ATRA; Sigma-Al-drich), NS398 (12.5 µmol/L; Biomol Research Laboratories, Inc.), or their combination for 5 d, with the medium refreshed at day 3. For assaying the cell viability, the cells were fixed with 10% trichloroacetic acid and stained with 0.4% sulforhodamine B (Sigma-Aldrich) in 1% acetic acid, and the absorbances were determined by using an automated spectrophotometric plate reader at a single wavelength of 490 nm. The experiments were done in triplicate and repeated thrice. The percentage of control cells was determined by the following equation: ODt/ODc × 100, where ODt and ODc are absorbances of treated and control cultures, respectively.
Colony formation assay
Two thousand cells from the sublines were mixed in low-temperature melting agarose (0.35%); they were then placed on top of solidified agarose in 60-mm-diameter dishes and incubated in a humidified atmosphere of 95% air and 5% CO2 at 37°C for 21 d. DMEM at both the bottom and the top of the agarose contained either 0.01% DMSO (solvent, as a control) or 1 µmol/L ATRA. DMEM containing 10% fetal bovine serum (with or without ATRA) in a 0.5-mL volume was added on top of the dishes after 3 d and then refreshed every 3 d. At the end of the experiments, the dishes were stained with 0.2 mL of 0.2% p-io-donitrotetrazolium violet at 37°C overnight, and the colonies were counted under an inverted microscope at a ×20 magnification.
Animal experiments
In accordance with our Institution-approved Animal Care and Usage protocol, nu/nu nude mice (6–8 wk of age) were each injected s.c. in the right flank through a 22-gauge needle with 1 × 107 tumor cells mixed with 50% Matrigel (BD Biosciences) for a total volume of 200 µL per mouse. The animals were then monitored for tumor formation until most tumor burdens from the RAR-β2–transfected TE-8 sublines disappeared. The tumor mass volumes, measured weekly with a Vernier caliper, were calculated as follows: length × width2 / 2. At the end of the experiments, these volumes were plotted as the mean ± SD.
Immunohistochemical staining of Ki67 after transient gene transfection
Esophageal cancer cell lines TE-1, TE-8, HCE-4, and SKGT-4 were grown in monolayer overnight and transiently transfected with either pCMS/enhanced green fluorescent protein (EGFP; BD Clontech) plus pRC/CMV vector or pCMS/EGFP plus pRC/CMV/RAR-β2 by using Lipofectamine 2000. After 36 h, the cells were treated with 400 µg/mL G418 for additional 24 h. Similarly, these cells were first transfected with pCMS/EGFP vector plus pcDNA3.1 vector or pCMS/EGFP plus pcDNA3.1/COX2-AS. When GFP was expressed in these cells 2 d later, RAR-β2 expression vector was then transfected into these tumor cells for 48 h. After that, the cells were then fixed with 4% paraformaldehyde for 10 min at room temperature to preserve the GFP and permeabilized in 0.5% Triton X-100 for 10 min at room temperature. The cells were then subjected to Ki67 immunostaining as described previously (12). Ki67 antibody was purchased from Vector Laboratories and diluted at 1:50 in PBS. Afterwards, more than 200 cells in 10 fields of 20× objective were counted for positive staining of GFP (green) as well as for positive or negative Ki67 (red) staining in these cells. The percentage of control of cell proliferation was calculated from the following equation: % control = NT/NV × 100, where NT and NV are the numbers of Ki67-positive cells in GFP-positive cells of COX-2 antisense– transfected or RAR-β2–transfected and vector control cultures, respectively.
Tissue samples and real-time quantitative reverse transcription-PCR
We obtained 31 fresh tissue specimens of esophageal squamous cell carcinoma from Taishan Medical College (Taizhou, China; ref. 16). The tissue was subjected to RNA isolation and quantitative reverse transcription- PCR (Q-RT-PCR) analysis for RAR-β2 and COX-2 expression. The Q-RT-PCR was done in the core facility of The University of Texas-Houston Medical School by using 7700 Prism real-time PCR machine (Perkin-Elmer/Applied Biosystems; see details in ref. 16) with a fee. The data are presented as mean of the percentage of β-actin for each RNA sample. The primers for RAR-β2 were 5′-TTCCCTCACTCTGCCAGCT-3′ and 5′-CGAGTTCCTCAGAGCTGGTG-3′, which generated an 86-bp fragment, and primers for COX-2 were 5′-GCAGGGTTGCTGGTGGTAG-3′ and 5′-ATTTCATCTGCCTGCTCTGG-3′, which generated an 84-bp fragment.
Tissue samples and in situ hybridization
Our institutional review board approved our protocol for the use of patient samples in this study. Briefly, we examined paraffin-embedded sections of tissue biopsy specimens left over from our previous study of oral premalignant lesions before and after a 3-mo treatment with isotretinoin (13-cis RA; ref. 13) to determine the presence of RAR-β2 and COX-2 mRNA expression. These sections, which included five paired pretreatment and posttreatment sections, seven individual pretreatment sections, and six individual posttreatment sections, were subjected to in situ hybridization according to our established protocol (14, 15).
Review and scoring of sections
The stained sections were reviewed and scored for RAR-β2 and COX-2 expression with a microscope (Olympus America, Inc.). The sections were scored as negative (0), weakly positive (1), positive (2), or strongly positive (3) based on the staining intensity and the percentage of cells with staining.
Results
Tumor-suppressive effects of RAR-β2 associated with expression and suppression of COX-2
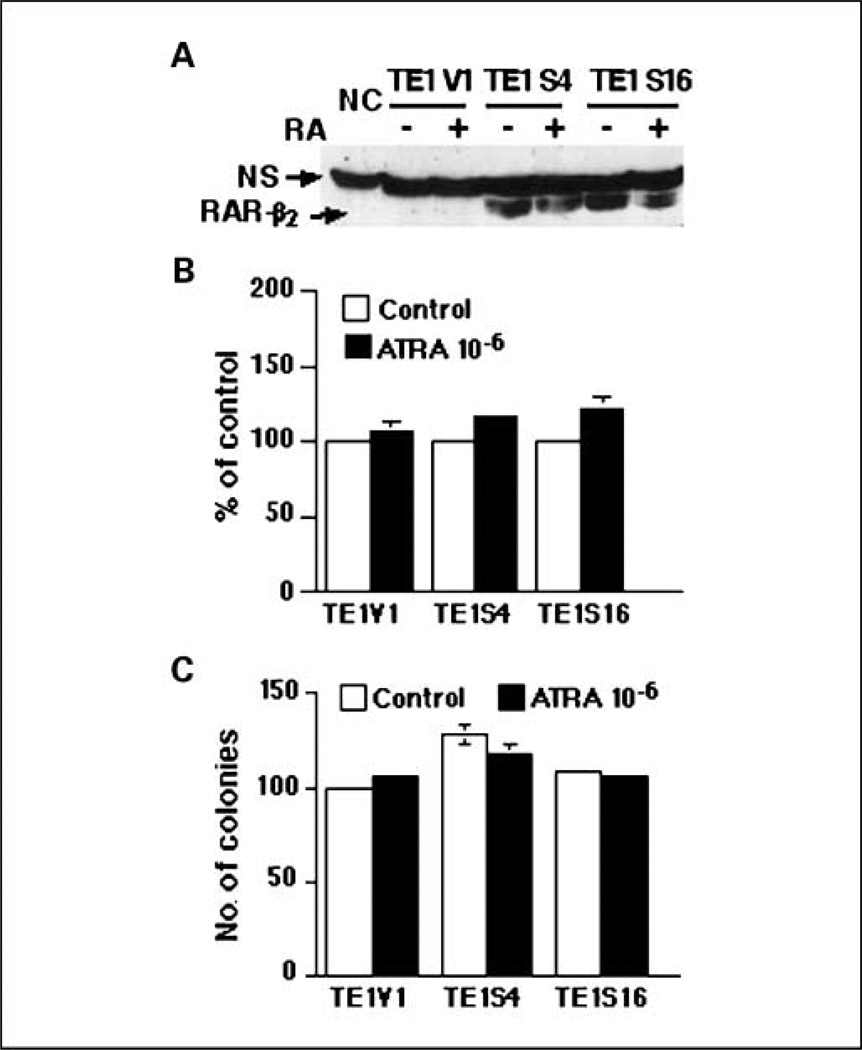
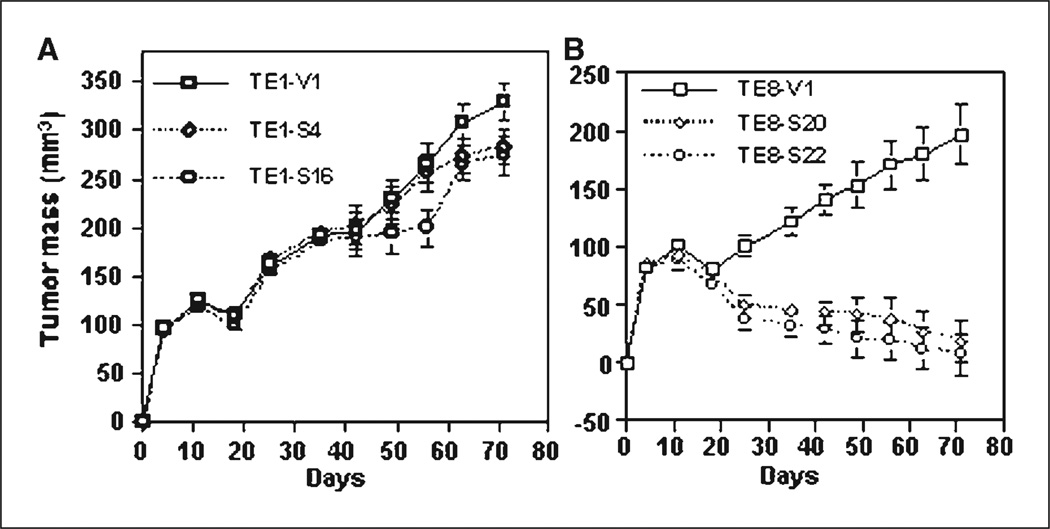
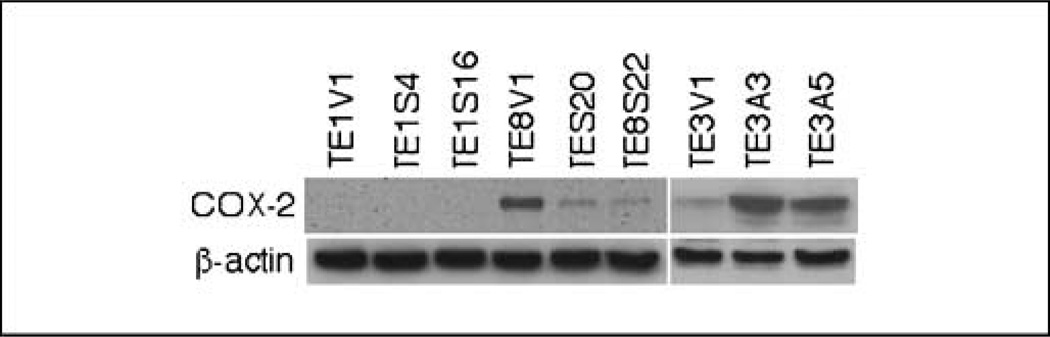
After we introduced RAR-β2 cDNA into COX-2–negative TE-1 esophageal cancer cells, the stable transfection of RAR-β2 restored RAR-β2 reexpression in the sublines (Fig. 1A) but surprisingly did not restore the sensitivity of these tumor cells to RA treatment (Fig. 1B and C), in contrast to what occurs in TE-8 cells (see ref. 11). By doing nude mouse xenograft experiments to test RAR-β2 antitumor activity in TE-1 and TE-8 sublines, we found that RAR-β2 did not suppress tumor development in RAR-β2–transfected TE-1 sublines but did in RAR-β2–transfected TE-8 sublines (Fig. 2). We then analyzed COX-2 expression in the sublines from these two cell lines and found that COX-2 is expressed in TE-8V1 cells but significantly reduced in TE-8S20 and TE-8S22 cells. In contrast, TE-1 sublines do not express COX-2 at all (Fig. 3), indicating that RAR-β2 does not suppress tumor cell growth and colony formation in vitro and tumor formation in vivo in TE-1 cells if COX-2 is not expressed. Moreover, after we introduced antisense RAR-β2 cDNA into TE-3 cells that express high level of RAR-β2 but low level of COX-2 (see refs. 11, 14), COX-2 expression was up-regulated in the antisense RAR-β2 stable sublines (Fig. 3), which was also associated with induction of tumor cell growth and resistance to retinoid treatment (see refs. 5, 11).
Fig. 1.
Characterization of RAR-β2–transfected esophageal cancer cells. TE-1V1 is the vector only–transfected subline and TE-1S4 and TE-1S16 are RAR-β2–transfected sublines. A, Western blotting analysis of RAR-β2 expression in RAR-β2–transfected TE-1 cells. PC, positive control; NS, nonspecific band. B, cell viability assay. The cells were treated with or without ATRA for 5 d, and cell viability was measured by a sulforhodamine B assay and then summarized as percentage of control (see Materials and Methods for details). C, colony formation assay. The cells were grown in soft agar and treated with or without ATRA for 21 d, after which colonies were counted and summarized as the mean ± SD. These experiments were repeated at least once.
Fig. 2.
Nude mouse xenograft assay. A, growth curve of tumor masses in nude mice injected with RAR-β2–transfected TE-1S4 and TE-1S16 esophageal cancer cells or with TE-1V1 vector control cells. B, growth curve of tumor masses in nude mice injected with RAR-β2–transfected TE-8S20 and TE-8S22 esophageal cancer cells or with TE-8V1 vector only–transfected cells. The results showed that the role of RAR-β2 in suppressing tumor formation is dependent on COX-2 expression.
Fig. 3.
Western blot analysis of gene expression in RAR-β2–transfected esophageal cancer cells. TE-1V1, TE-8V1, and TE-3V1 are stable vector only–transfected sublines, whereas TE-1S4, TE-1S16, TE-8S20, and TE-8S22 are RAR-β2–transfected sublines and TE-3A3 and TE-3A5 are antisense RAR-β2–transfected sublines of TE-1, TE-3, and TE-8, respectively. The cells were maintained in 200 µg/mL G418, and total cellular protein was extracted and subjected to Western blot analysis of COX-2 expression.
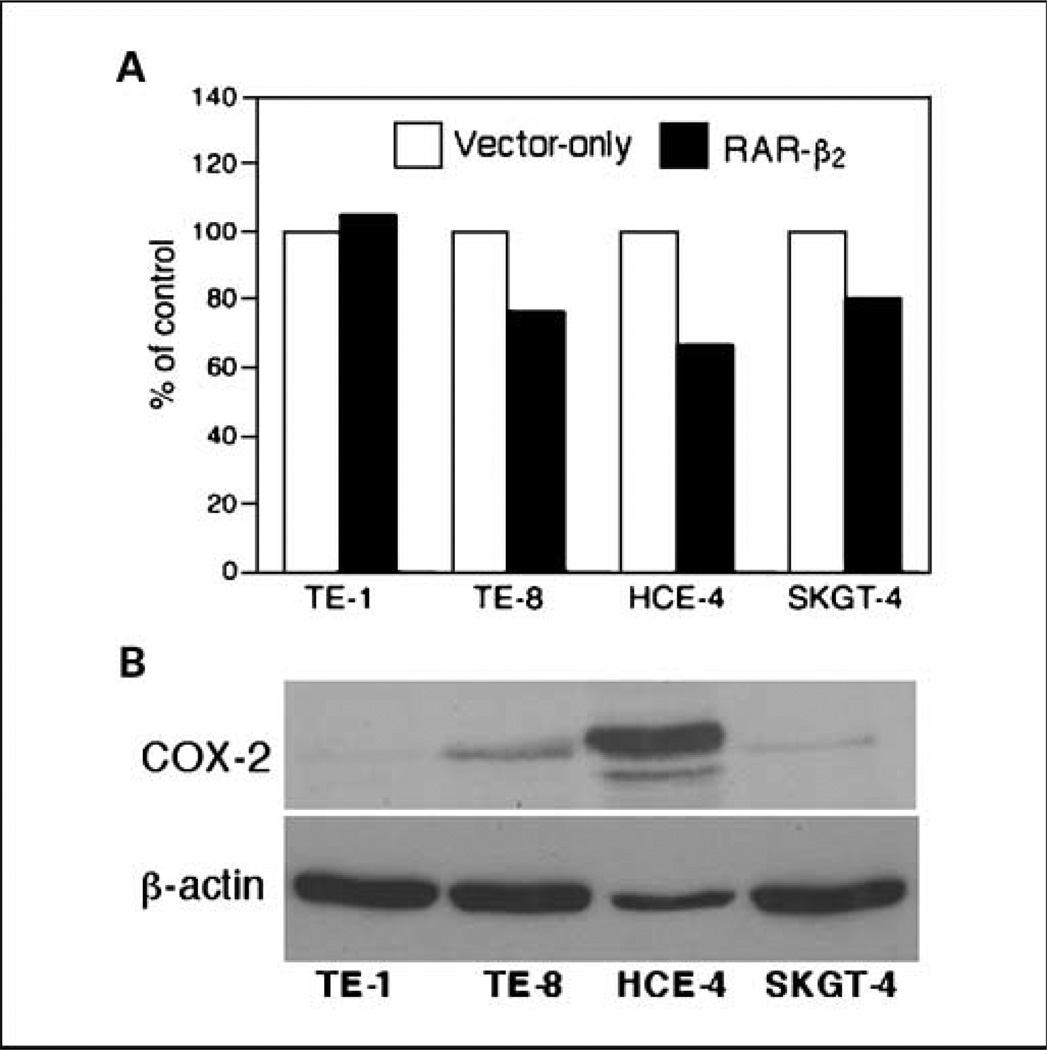
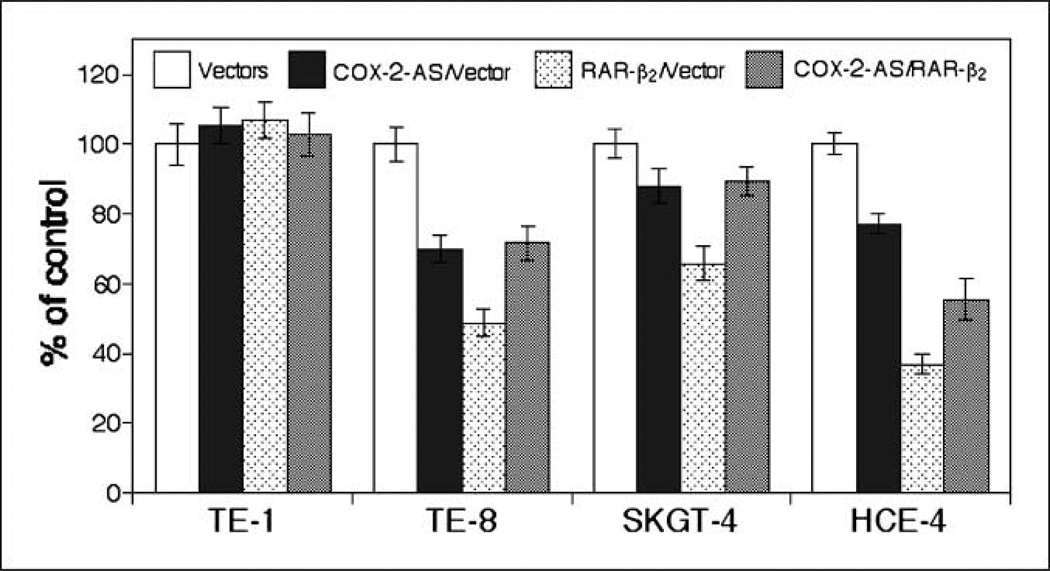
We then surveyed additional esophageal cancer cell lines by using transient transfection of RAR-β2 expression vector, which has eliminated the selection bias of stable transfection. Our data found that RAR-β2–reduced tumor cell proliferation (Ki67-positive staining) was indeed associated with the levels of COX-2 expression (e.g., HCE-4 cells expressed higher levels of COX-2 and RAR-β2 shows more reduction in Ki67-positive cells), whereas SKGT-4 cells expressed lower level of COX-2 and RAR-β2 shows less effects in the cells (Fig. 4).
Fig. 4.
Reduced Ki67 positivity by transient transfection of RAR-β2 and association of COX-2 expression. A, cell proliferation assay. Esophageal cancer cell lines TE-1, TE-8, HCE-4, and SKGT-4 were transiently transfected with either pCMS/EGFP plus pRC/CMV vector or pCMS/EGFP plus pRC/CMV/ RAR-β2 for 2 d. The cells were then treated with 400 µg/mL G418 for additional 24 h and stained for Ki67 immunohistochemically. After that, more than 200 cells in 10 fields of 20× objective were counted for positive staining of GFP (green) as well as for positive or negative Ki67 (red) staining in these cells. The percentage of control of cell proliferation was calculated from the following equation: % control = NT/NV × 100, where NT and NV are the numbers of Ki67-positive cells in GFP-positive cells of RAR-β2–transfected and vector control cultures, respectively. B, Western blot analysis of COX-2 expression. The cells were grown in monolayer for 5 d and total cellular protein was extracted and subjected to Western blot analysis.
Furthermore, we did additional experiments to block COX-2 expression in these esophageal cancer cell lines using transient transfection of a COX-2 antisense expression vector, and 2 days later, we transfected a RAR-β2 expression vector into these cells. We found that the antitumor effect of RAR-β2 is diminished in all three COX-2–positive esophageal cancer cell lines, although the separated transfection of RAR-β2 or anti-sense COX-2 had effects in suppression of these tumor cell proliferation (Fig. 5). However, in COX-2–negative TE-1 cells, these transfections do not have any effects in suppression of tumor cell growth (Fig. 5), indicating that some of RAR-β2 antitumor activities are mediated by suppression of COX-2 in these cell lines.
Fig. 5.
Antisense COX-2–antagonized RAR-β2 effects in esophageal cancer cell lines. Esophageal cancer cell lines TE-1, TE-8, HCE-4, and SKGT-4 were first transfected with pCMS/EGFP vector plus pcDNA3.1 vector or pCMS/EGFP plus pcDNA3.1/COX2-AS. Two days later, GFP was expressed in these cells. We then transfected RAR-β2 expression vector into these tumor cells for 48 h. After that, the cells were stained for Ki67 immunohistochemically. More than 200 cells in 10 fields of 20× objective were counted for positive staining of GFP (green) as well as for positive or negative Ki67 (red) staining in these cells. The percentage of control of cell proliferation was calculated from the following equation: %control = NT/NV × 100, where NT and NV are the numbers of Ki67-positive cells in GFP-positive cells of COX-2 antisense–transfected or RAR-β2–transfected and control cultures, respectively.
Expression of RAR-β2 mRNA associated with low levels of COX-2 mRNA in ex vivo
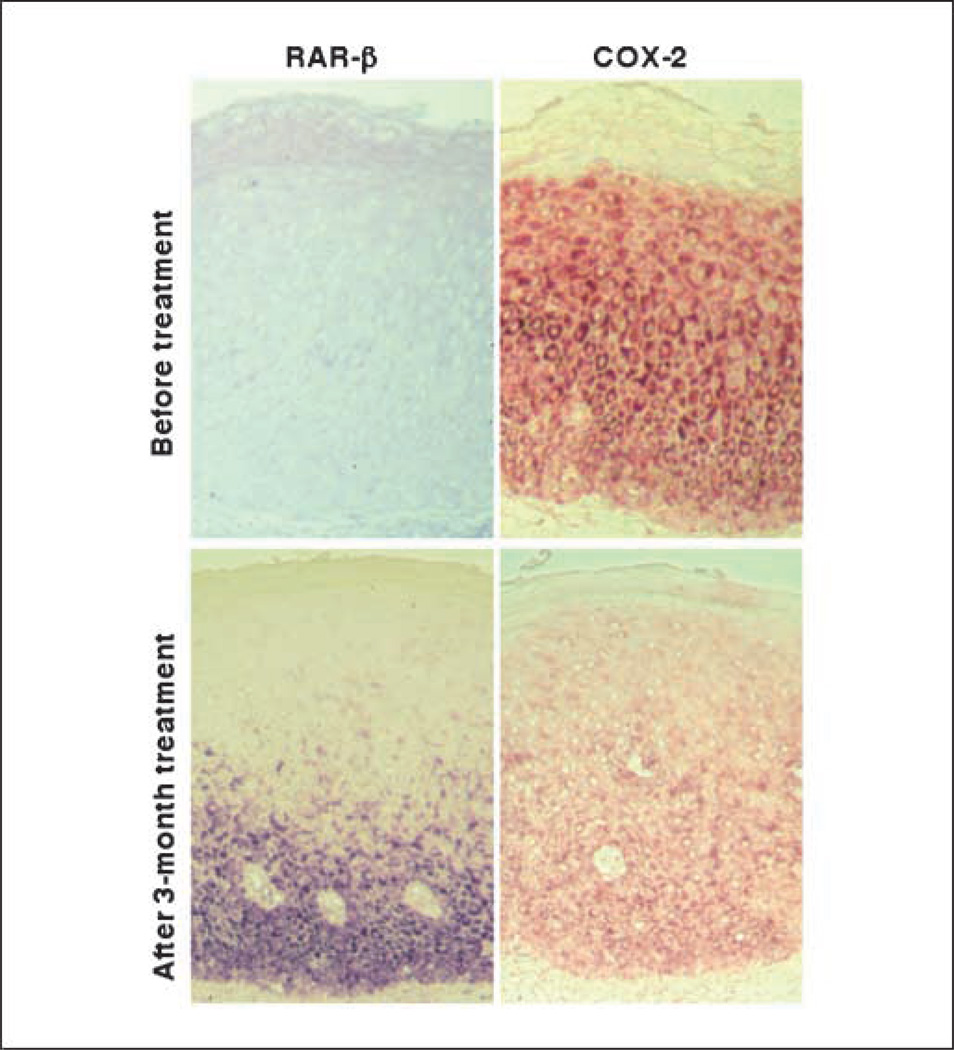
Using Q-RT-PCR assay, we analyzed expression of RAR-β2 and COX-2 mRNA in esophageal cancer tissue specimens and found that the expression of RAR-β2 mRNA is associated with the low levels of COX-2 mRNA in the tissues (Table 1). Moreover, when we examined tissue biopsy material from our previous clinical retinoid chemoprevention study to confirm this finding, we found that RAR-β2 mRNA was expressed in 4 of 12 (33.3%) pretreatment sections and 9 of 11 (81.8%) posttreatment sections. These data matched those of the original study, in which we found that RAR-β2 expression was lost in oral premalignant lesions but that 13-cis RA could up-regulate it (13); there was a correlation between these results and the clinical responses of the patients. Furthermore, COX-2 mRNAwas expressed in eight (66.7%) of the pretreatment sections and five (45.5%) of the posttreatment sections. Treatment with 13-cis RA up-regulated RAR-β2 expression in four of the five paired sections, which correlated with a reduction in COX-2 expression and with the clinical responses of the patients. In only one case did 13-cis RA not induce RAR-β2 expression or reduce COX-2 expression, and the patient from whom the biopsy material was obtained progressed to oral cancer. Photomicrographs from one representative case are shown in Fig. 6.
Table 1.
Expression of RAR-β2 mRNA associated with the low levels of COX-2 in esophageal squamous cell carcinoma tissue specimens
From two-sided Fisher's exact test.
High or low expression was compared with the mRNA levels from each other.
Fig. 6.
In situ detection of RAR-β2 and COX-2 mRNA expression. Paraffinembedded sections were from patients with oral premalignant lesions treated with 13-cis RA for 3 mo. Biopsy specimens were collected both before and after treatment and processed for in situ hybridization analysis of RAR-β2 and COX-2 mRNA expression by using digoxigenin-labeled probes.
Effects of combination of COX-2 inhibitor with RA in esophageal cancer cells
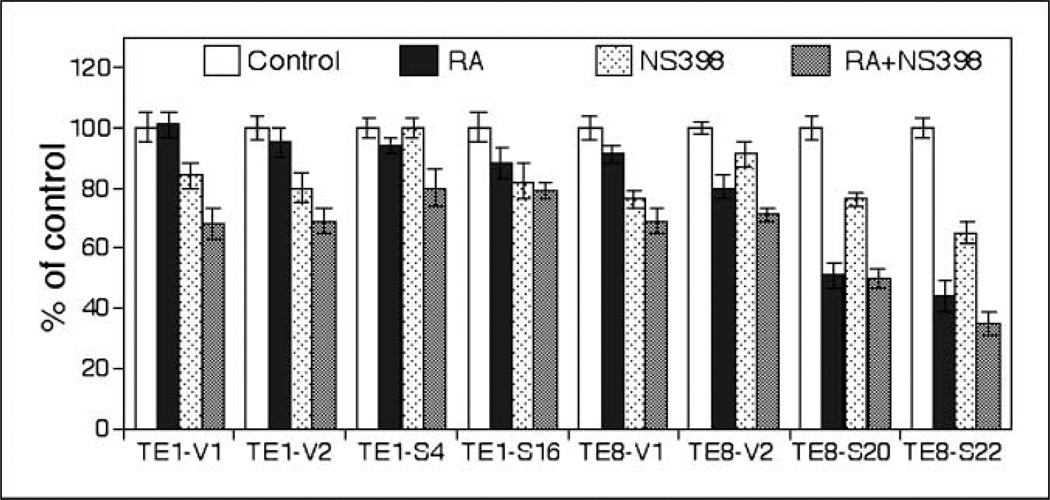
The results from the current study have shown that RAR-β2 can suppress COX-2 expression, whereas knockdown of RAR-β2 protein up-regulated COX-2 expression in COX-2–positive esophageal cancer cell lines. Therefore, if we combine COX-2 inhibitor, such as NS398 that blocks COX-2 enzymatic activity, with RA that can induce RAR-β2 expression, their antitumor activities should increase. Indeed, our experiments found that a low dose of NS398 (12.5 µmol/L) itself does not have much antitumor activity, but it can additively increase the effect of ATRA in inhibiting growth of some of esophageal cancer cells (Fig. 7).
Fig. 7.
Cell viability assay. Stable RAR-β2–transfected TE-1 and TE-8 sublines were grown in monolayer and treated with ATRA (1 µmol/L), COX-2 inhibitor NS398 (12.5 µmol/L), or their combination for 5 d. Cell viability assay was then done (see Materials and Methods). The experiments were repeated thrice with similar results.
Discussion
From the results of the current study, we can conclude that some of RAR-β2 antitumor activities are mediated by suppression of COX-2 expression in some of esophageal cancer cells and in patients with oral premalignant lesions. If COX-2 is not expressed in TE-1 cells, RAR-β2 does not suppress the growth of these esophageal cancer cells in vitro and in nude mouse xenografts. However, knockdown of RAR-β2 expression up-regulated COX-2 expression in RAR-β2–positive esophageal cancer cells. Furthermore, combination of COX-2 inhibitor, NS398, with RA can additively increase their antitumor effects in some of esophageal cancer cell lines.
It is well documented in the literature that the induction of RAR-β2 expression inhibited growth of different cancer cell lines in vitro and in nude mice. Our current study found that the antitumor effects of RAR-β2 were associated with expression and suppression of COX-2 in some of esophageal cancer cell lines. It may also be true in other studies, although such an association was not reported in the original publications. For examples, an earlier study showed that lung cancer cell lines H157 and Calu-1 expressing transfected RAR-β2 exhibited the decreased tumorigenicity in nude mice (17) and these tumor cells expressed COX-2 protein (18). RAR-β2 also suppressed growth of breast cancer cells (19, 20) in vitro or metastases in a mouse xenograft model (21) and these breast cancer cell lines (such as T-47D, MDA-MB231, and MDA-MB435) all expressed COX-2 protein when they are cultivated in serum-containing growth medium (22, 23). Nevertheless, it is an exception (i.e., MCF-7 cells usually expressed undetectable levels of COX-2 protein but it can express COX-2 in serum-containing medium; refs. 22, 24). RAR-β2 had an effect on inhibiting the growth of breast cancer MCF-7 cells (20). In addition, RA induced RAR-β2 expression in various premalignant lesions in different clinical chemoprevention trials (13, 25–28). For instance, in our study that provided the biopsy sections examined in this one, we showed that the expression of RAR-β2 mRNA was selectively lost in premalignant oral lesions but could be restored by treatment with 13-cis RA and that the restoration of RAR-β2 expression was associated with a clinical response (13) and COX-2 suppression (shown in current study). However, human lung cancer cell lines exhibited resistance to RA treatment, which were either due to noninducibility of RAR-β2 expression or lack of COX-2 expression in these cells (18, 29). In particular, non–small cell lung cancer H1944 and H460 cells expressed both RAR-β2 and COX-2, which showed growth-inhibitory effects by RA treatment, whereas H522 cells expressed very low levels of COX-2 and showed little response to RA treatment (compared the data from refs. 18, 29).
Indeed, our previous studies showed that induction of RAR-β2 can suppress COX-2 expression in esophageal cancer cells (7, 11) and the current study showed that RA can inhibit COX-2 expression in patients with oral premalignant lesions. An earlier study also reported the suppression of COX-2 expression by RA (30). Furthermore, RAR-β2 suppression of COX-2 expression is known to occur through the epidermal growth factor receptor/extracellular signal-regulated protein kinases 1 and 2/activating protein-1 molecular pathway (7), although further investigation is needed to thoroughly understand the underlying molecular events responsible for this suppression.
COX is the key enzyme in arachidonate metabolism and catalyzes the biosynthesis of prostaglandin H2, the precursor for prostanoids (for a review, see ref. 31). Prostanoids, such as prostaglandin E2, have been thought to play a role in controlling neoplastic and nonneoplastic cell proliferation, angiogenesis, and immune functions (32). In addition to COX-1, which is constitutively expressed in many tissues and thought to be involved in the homeostasis of various physiologic functions, the level of the isozyme COX-2 is elevated in numerous human cancers, including head and neck and esophageal cancers, and is inducible by various agents, such as growth factors and tumor promoters (30, 31). COX-2 has been thought to promote carcinogenesis and the malignant phenotype of tumor cells because of its ability to increase the production of prostaglandins, convert procarcinogens to carcinogens, inhibit apoptosis, promote angiogenesis, modulate inflammation and immune functions, and increase tumor cell invasiveness (31). Indeed, many studies have shown the antitumor effects of nonsteroidal anti-inflammatory drugs (NSAID) on different cancer cell lines, including cancers of the colon, lung, prostate, head and neck, breast, and pancreas (31). Our previous studies have shown that the inhibition of COX-2 enzymatic activity with NSAIDs, such as NS398 or aspirin, induced apoptosis of esophageal and colorectal cancer cells by inducing mitochondrial release of cytochrome c and then activating caspase-9 and caspase-3 in these cells (32, 33). The inhibition of COX-2 by different NSAIDs has shown promise in preventing different cancers in patients despite their side effects on the cardiovascular system (34). In addition, it was reported that black raspberries reduced mRNA and protein expression levels of COX-2, inducible nitric oxide synthase, and c-Jun as well as the level of prostaglandin E2 in esophageal preneoplastic lesions in animals treated with the N-nitrosomethylbenzylamine (35). Most recently, Brown et al. (36) reported combination chemoprevention study of COX-2 inhibitor with retinoid in breast cancer and their data showed that the combination was substantially more effective than either drug individually. Molecularly, the combination treatment of celecoxib with LGD1069 significantly reduced mammary prostaglandin E2 level (36). These studies will have significant effect on our future cancer prevention. The results from our current study also support the findings (36). However, there seem to be retinoid-sensitive or COX-2 inhibitor–sensitive or COX-2 inhibitor–resistant tumor cells. More studies, therefore, need to determine how these tumor cell lines respond to this combined treatment.
Collectively, our current and previous findings linking RAR-β2 to the suppression of COX-2 expression highlight a novel molecular pathway. If tumor cells do not express COX-2, induction of RAR-β2 expression has little effects in some of these tumor cells and this finding may help us to explain some failure of RA in clinical cancer prevention trials (37, 38). However, a full understanding of this pathway may translate into better control of different cancers.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Soprano DR, Qin P, Soprano KJ. Retinoic acid receptors and cancers. Annu Rev Nutr. 2004;24:201–221. doi: 10.1146/annurev.nutr.24.012003.132407. [DOI] [PubMed] [Google Scholar]
- 2.Xu X-C. Tumor-suppressive activity of retinoic acid receptor-β in cancer. Cancer Lett. 2007;253:14–24. doi: 10.1016/j.canlet.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung B, Hocker JE, Smith SA, Norris MD, Haber M, Marshall GM. Favorable prognostic significance of high-level retinoic acid receptor β expression in neuroblastoma mediated by effects on cell cycle regulation. Oncogene. 1998;17:751–759. doi: 10.1038/sj.onc.1201982. [DOI] [PubMed] [Google Scholar]
- 4.Wang XD, Liu C, Bronson RT, Smith DE, Krinsky NI, Russell M. Retinoid signaling and activator protein-1 expression in ferrets given β-carotene supplements and exposed to tobacco smoke. J Natl Cancer Inst. 1999;91:60–66. doi: 10.1093/jnci/91.1.60. [DOI] [PubMed] [Google Scholar]
- 5.Vuillemenot BR, Pulling LC, Palmisano WA, Hutt JA, Belinsky SA. Carcinogen exposure differentially modulates RAR-β promoter hypermethylation, an early and frequent event in mouse lung carcinogenesis. Carcinogenesis. 2004;25:623–629. doi: 10.1093/carcin/bgh038. [DOI] [PubMed] [Google Scholar]
- 6.Song S, Xu XC. Effect of benzo[a]pyrene diol epoxide on expression of retinoic acid receptor-β in immortalized esophageal epithelial cells and esophageal cancer cells. Biochem Biophys Res Commun. 2001;281:872–877. doi: 10.1006/bbrc.2001.4433. [DOI] [PubMed] [Google Scholar]
- 7.Song S, Lippman SM, Zou Y, Ye X, Xu X-C. Induction of cyclooxygenase-2 by benzo[a]pyrene diol epoxide through inhibition of retinoic acid receptor-β2 expression. Oncogene. 2005;24:8268–8276. doi: 10.1038/sj.onc.1208992. [DOI] [PubMed] [Google Scholar]
- 8.McGregor F, Wagner E, Felix D, Soutar D, Parkinson K, Harrison PR. Inappropriate retinoic acid receptor-β expression in oral dysplasias: correlation with acquisition of the immortal phenotype. Cancer Res. 1997;57:3886–3889. [PubMed] [Google Scholar]
- 9.Muntoni A, Fleming J, Gordon KE, et al. Senescing oral dysplasias are not immortalized by ectopic expression of hTERT alone without other molecular changes, such as loss of INK4A and/or retinoic acid receptor-β: but p53 mutations are not necessarily required. Oncogene. 2003;22:7804–7808. doi: 10.1038/sj.onc.1207085. [DOI] [PubMed] [Google Scholar]
- 10.Fang MZ, Wang Y, Ai N, et al. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63:7563–7570. [PubMed] [Google Scholar]
- 11.Li M, Song S, Lippman SM, et al. Induction of retinoic acid receptor-β suppresses cyclooxygenase-2 expression in esophageal cancer cells. Oncogene. 2002;21:411–418. doi: 10.1038/sj.onc.1205106. [DOI] [PubMed] [Google Scholar]
- 12.Liang ZD, Lippman SM, Wu TT, Lotan R, Xu X-C. RRIG1 mediates effects of retinoic acid receptor-β2 on tumor cell growth and gene expression through binding to and inhibiting RhoA. Cancer Res. 2006;66:7111–7118. doi: 10.1158/0008-5472.CAN-06-0812. [DOI] [PubMed] [Google Scholar]
- 13.Lotan R, Xu XC, Lippman SM, et al. Suppression of retinoic acid receptor-β in premalignant oral lesions and its up-regulation by isotretinoin. N Engl J Med. 1995;332:1405–1410. doi: 10.1056/NEJM199505253322103. [DOI] [PubMed] [Google Scholar]
- 14.Xu XC, Liu X, Tahara E, Lippman SM, Lotan R. Expression and up-regulation of retinoic acid receptor-β is associated with retinoid sensitivity and colony formation in esophageal cancer cell lines. Cancer Res. 1999;59:2477–2483. [PubMed] [Google Scholar]
- 15.Khuri FR, Wu H, Lee JJ, et al. Cyclooxygenase-2 overexpression is a marker of poor prognosis in stage I non-small cell lung cancer. Clin Cancer Res. 2001;7:861–867. [PubMed] [Google Scholar]
- 16.Xu X-C, Lee JJ, Wu TT, Hoque A, Ajani JA, Lippman SM. Increased retinoic acid receptor-β4 correlates in vivo with reduced retinoic acid receptor-β2 in esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2005;14:826–829. doi: 10.1158/1055-9965.EPI-04-0500. [DOI] [PubMed] [Google Scholar]
- 17.Houle B, Rochette-Egly C, Bradley WE. Tumor-suppressive effect of the retinoic acid receptor β in human epidermoid lung cancer cells. Proc Natl Acad Sci U S A. 1993;90:985–989. doi: 10.1073/pnas.90.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun SY, Schroeder CP, Yue P, Lotan D, Hong WK, Lotan R. Enhanced growth inhibition and apoptosis induction in NSCLC cell lines by combination of celecoxib and 4HPR at clinically relevant concentrations. Cancer Biol Ther. 2005;4:407–413. doi: 10.4161/cbt.4.4.1618. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Lee MO, Wang HG, et al. Retinoic acid receptor β mediates the growth-inhibitory effect of retinoic acid by promoting apoptosis in human breast cancer cells. Mol Cell Biol. 1996;16:1138–1149. doi: 10.1128/mcb.16.3.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seewaldt VL, Johnson BS, Parker MB, Collins SJ, Swisshelm K. Expression of retinoic acid receptor β mediates retinoic acid-induced growth arrest and apoptosis in breast cancer cells. Cell Growth Differ. 1995;6:1077–1088. [PubMed] [Google Scholar]
- 21.Treutin PM, Chen LI, Buetow BS, et al. Retinoic acid receptor β2 inhibition of metastasis in mouse mammary gland xenografts. Breast Cancer Res Treat. 2002;72:79–88. doi: 10.1023/a:1014906529407. [DOI] [PubMed] [Google Scholar]
- 22.Timoshenko AV, Chakraborty C, Wagner GF, Lala PK. COX-2-mediated stimulation of the lymphangiogenic factor VEGF-C in human breast cancer. Br J Cancer. 2006;94:1154–1163. doi: 10.1038/sj.bjc.6603067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basu GD, Liang WS, Stephan DA, et al. A novel role for cyclooxygenase-2 in regulating vascular channel formation by human breast cancer cells. Breast Cancer Res. 2006;8:R69. doi: 10.1186/bcr1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teh SH, Hill AK, Foley DA, McDermott EW, O'Higgins NJ, Young LS. COX inhibitors modulate bFGF-induced cell survival in MCF-7 breast cancer cells. J Cell Biochem. 2004;91:796–807. doi: 10.1002/jcb.10767. [DOI] [PubMed] [Google Scholar]
- 25.Kurie JM, Lotan R, Lee JJ, et al. Treatment of former smokers with 9-cis-retinoic acid reverses loss of retinoic acid receptor-β expression in the bronchial epithelium: results from a randomized placebo-controlled trial. J Natl Cancer Inst. 2003;95:206–214. doi: 10.1093/jnci/95.3.206. [DOI] [PubMed] [Google Scholar]
- 26.Ayoub J, Jean-Francois R, Cormier Y, et al. Placebo-controlled trial of 13-cis-retinoic acid activity on retinoic acid receptor-β expression in a population at high risk: implications for chemoprevention of lung cancer. J Clin Oncol. 1999;17:3546–3552. doi: 10.1200/JCO.1999.17.11.3546. [DOI] [PubMed] [Google Scholar]
- 27.Lam S, Xu X-C, Parker-Klein H, et al. Surrogate end-point biomarker analysis in a retinol chemoprevention trial in current and former smokers with bronchial dysplasia. Int J Oncol. 2003;23:1607–1613. [PubMed] [Google Scholar]
- 28.Alberts D, Ranger-Moore J, Einspahr J, et al. Safety and efficacy of dose-intensive oral vitamin A in subjects with sun-damaged skin. Clin Cancer Res. 2004;10:1875–1880. doi: 10.1158/1078-0432.ccr-03-0188. [DOI] [PubMed] [Google Scholar]
- 29.Geradts J, Chen J-Y, Russell EK, Yankaskas JR, Nieves L, Minna JD. Human lung cancer cell lines exhibit resistance to retinoic acid treatment. Cell Growth Differ. 1993;4:799–809. [PubMed] [Google Scholar]
- 30.Mestre JR, Subbaramaiah K, Sacks PG, et al. Retinoids suppress epidermal growth factor-induced transcription of cyclooxygenase-2 in human oral squamous carcinoma cells. Cancer Res. 1997;57:2890–2895. [PubMed] [Google Scholar]
- 31.Xu XC. COX-2 inhibitors in cancer treatment and prevention, a recent development. Anticancer Drugs. 2002;13:127–137. doi: 10.1097/00001813-200202000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Li M, Wu X, Xu X-C. Induction of apoptosis by cyclooxygenase-2 inhibitor NS398 through a cytochrome C-dependent pathway in esophageal cancer cells. Int J Cancer. 2001;93:218–223. doi: 10.1002/ijc.1322. [DOI] [PubMed] [Google Scholar]
- 33.Li M, Lotan R, Levin B, Tahara E, Lippman SM, Xu XC. Aspirin induction of apoptosis in esophageal cancer: a potential for chemoprevention. Cancer Epidemiol Biomarkers Prev. 2000;9:545–549. [PubMed] [Google Scholar]
- 34.Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 35.Chen T, Hwang H, Rose ME, Nines RG, Stoner GD. Chemopreventive properties of black raspberries in N-nitrosomethylbenzylamine-induced rat esophageal tumorigenesis: down-regulation of COX-2, iNOS and c-Jun. Cancer Res. 2006;66:2853–2859. doi: 10.1158/0008-5472.CAN-05-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown PH, Subbaramaiah K, Salmon AP, et al. Combination chemoprevention of HER2/neu-induced breast cancer using a cyclooxygenase-2 inhibitor and a retinoid X receptor-selective retinoid. Cancer Prev Res (Phila Pa) 2008;1:208–214. doi: 10.1158/1940-6207.CAPR-08-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Zandwijk N, Dalesio O, Pastorino U, de Vries N, van Tinteren H. EUROSCAN, a randomized trial of vitamin A and N-acetylcysteine in patients with head and neck cancer or lung cancer. For the European Organization for Research and Treatment of Cancer Head and Neck and Lung Cancer Cooperative Groups. J Natl Cancer Inst. 2000;92:977–986. doi: 10.1093/jnci/92.12.977. [DOI] [PubMed] [Google Scholar]
- 38.Lippman SM, Lee JJ, Karp DD, et al. Randomized phase III intergroup trial of isotretinoin to prevent second primary tumors in stage I non-small-cell lung cancer. J Natl Cancer Inst. 2001;93:605–618. doi: 10.1093/jnci/93.8.605. [DOI] [PubMed] [Google Scholar]