Abstract
Background
Sickle cell anemia is a hemoglobinopathy caused by a mutation that results in the production of an abnormal hemoglobin molecule, hemoglobin S (Hb S). This is responsible for profound physiological changes, such as the sickling of red blood cells. Several studies have shown that hydroxyurea protects against vaso-occlusive crises.
Objective
The aim of this study was to evaluate the oxidative stress associated with biochemical parameters in patients with sickle cell anemia treated with hydroxyurea.
Methods
The study was conducted with 20 male and 25 female patients at the Hospital Universitário Walter Cantídio. The patients were divided into two groups: a study group (n = 12), patients with sickle cell anemia who were receiving hydroxyurea and a control group (n = 33) of sickle cell anemia patients not submitted to hydroxyurea treatment. The biochemical parameters analyzed were ferritin, transferrin, and serum iron. Glutathione was measured in its reduced form to analyze the oxidative state.
Results
The results showed insignificant increases in the levels of serum iron, transferrin and ferritin in patients treated with hydroxyurea when compared with those who did not take the medication. However, the glutathione levels were significantly higher in patients taking hydroxyurea than in controls.
Conclusions
These results indicate that hydroxyurea possibly acts as an antioxidant by increasing glutathione levels.
Keywords: Glutathione; Iron overload; Anemia, sickle cell; Reactive oxygen species; Hydroxyurea/ therapeutic use
Introduction
Sickle cell anemia (SCA) is the most common monogenic, inherited disease in the world. It originated in Africa and occurs predominantly in people of African descent. The disease has spread heterogeneously in Brazil because of racial miscegenation, thereby facilitating the continuity of this type of anemia in Brazil. It is considered a serious public health problem by Brazilian scientific literature.(1)
The disease is caused because of a point mutation at position 6 in the beta-globin gene that gives rise to an abnormal hemoglobin molecule called hemoglobin S (Hb S).(2) This causes physiological changes that affect the hemoglobin molecule in its deoxygenated state through the sickling of red blood cells; this triggers the formation of Hb S polymers, oxidative degradation of the Hb S molecule and the generation of oxidizing free radicals.(3,4) These erythrocytes have a greater adherence to the vascular endothelium, thus contributing to episodes of vaso-occlusion which is associated with the disease.(5,6)
Hb S is more unstable than normal Hb because the former releases high amounts of reactive oxygen species (ROS) (O2, H2O2, HO)(7) and has reduced antioxidant capacity; this imbalance leads to oxidative stress. Previous studies have shown that the levels of glutathione, a major intracellular antioxidant, are lower in patients with SCA.(8,9) SCA patients require blood transfusions to improve oxygen transport and to improve blood volume. One of the complications of long-term transfusion therapy is iron overload.(10) Approximately 25% of the iron in the body of a normal adult is stored in the form of ferritin (each ferritin molecule has 4500 atoms of iron) and hemosiderin.(11) The main clinical manifestations of SCA are infections, acute chest syndrome (ACS), splenic sequestration, pain crises, renal disorders, cardiac disorders (heart failure), osteoarticular disorders (such as dactylitis or hand-foot syndrome), neurological disorders (stroke), ocular disorders, sores on the lower limbs and priapism.(12)
Several studies have shown that the use of hydroxyurea (HU) promotes higher levels of fetal hemoglobin (Hb F) in SCA patients and is useful in protecting against the sickling of red blood cells and vaso-occlusion. HU is a chemotherapeutic agent used to treat myeloproliferative disorders; it blocks DNA synthesis by interfering in the conversion of ribonucleotide to deoxyribonucleotide through the inhibition of ribonucleotide reductase and by keeping the cells in the S phase. HU began to be used in protocols to treat adult sickle cell disease patients in the 1980s.(13) This study aimed at evaluating the oxidative profile and biochemical parameters in SCA patients treated with HU and thus to assess the benefit of HU in improving the quality of life of patients.
Methods
Patient population
A cross-sectional, descriptive study was performed of 20 male and 25 female adult patients treated with HU for a period of 3 - 6 months (n = 12) or untreated (n = 33) as a follow-up activity at the outpatient clinic of the Hospital Universitário Walter Cantídio in Fortaleza between January and October 2009. This study was approved by the hospital's Research Ethics Committee.
Laboratory methods
Peripheral blood (5 mL) was collected by venipuncture using heparin as an anticoagulant (to measure glutathione and total iron) and in a separator gel blood collection tube without anticoagulant (to measure ferritin and transferrin). The plasma was separated after centrifugation (10 min. at 1500 rpm) and washed with NaCl (0.9% w/v). Reduced glutathione (GSH) was quantified by spectrophotometry at 412 nm in heparinized plasma; the appearance of a yellowish color indicates a product of 5,5-dithiobis-2-nitrobenzoic acid (DTNB) oxidation.(14) Ferritin in the serum was measured by the microparticle enzyme immunoassay (MEIA) method developed by Abbott® using turbidimetry for the final quantification and is expressed in ng/mL. Transferrin was quantified in the serum by the colorimetric assay method using a Roche-Hitachi 917® analyzer; the results are expressed as mg/dL. To measure the total iron in the plasma (200 µL), nitric acid (4% v/v final concentration) was added to digest the sample. Digestion was performed at 200ºC, until the volume was reduced to 1 mL. The iron was then released in its free form. The plasma iron content was determined using an atomic absorption spectrophotometer and an air-acetylene flame.(15)
Statistical analysis
A descriptive analysis of the data was conducted using the GraphPad Prism 5 statistics program. The t-test was used to analyze the association between variables. The level of significance was set at 5% (p-value < 0.05).
Results
In this study, we evaluated glutathione levels and the iron profile of 45 sickle cell anemia patients (age range: 19 66 years; mean age: 31.4 years). The majority of the patients (65%) were mulattos followed by black patients (24%).
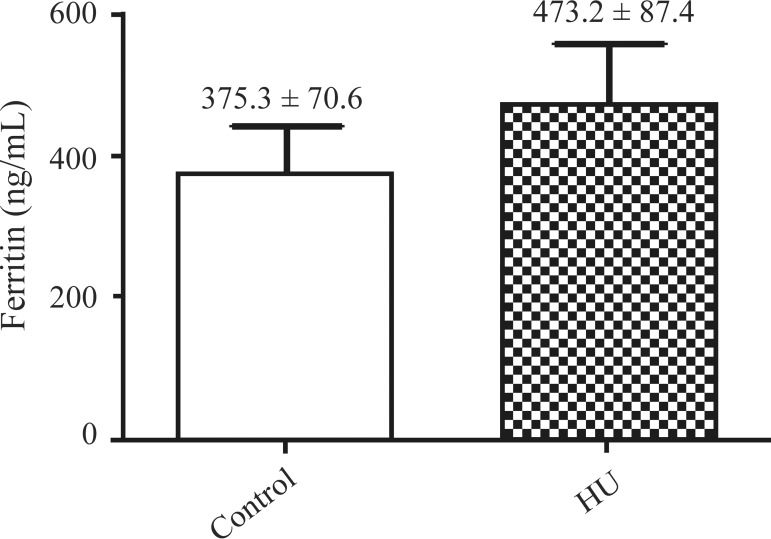
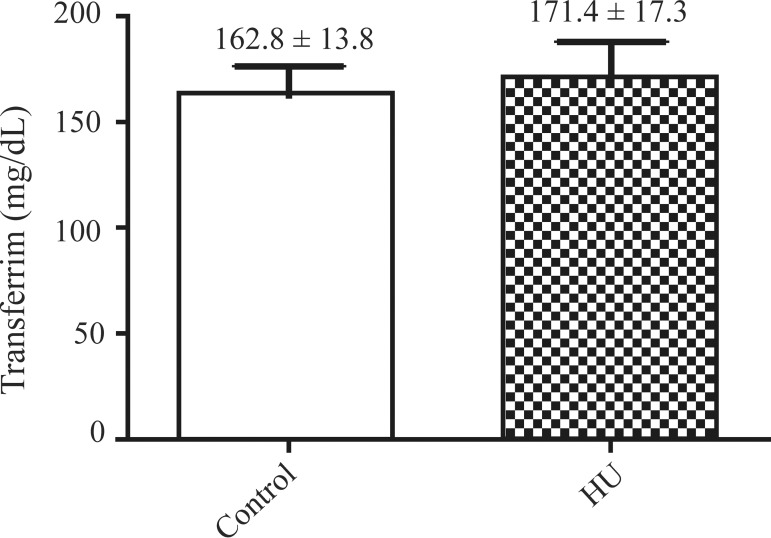
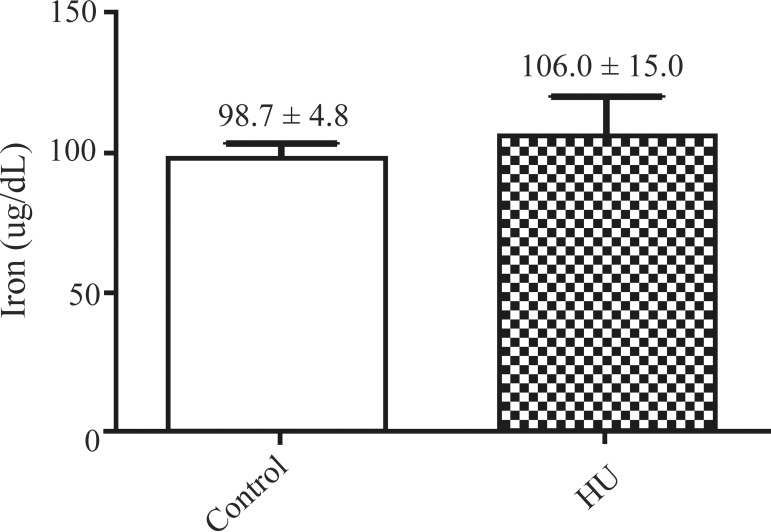
The mean serum levels of iron, transferrin and ferritin of the SCA patients treated with HU were 106.0 ± 15.0 µg/dL, 171.4 ± 17.3 mg/dL and 473.2 ± 87.4 ng/mL, respectively and for the untreated patients (control) they were 98.7 ± 4.8 µg/dL, 162.8 ± 13.8 mg/dL and 375.3 ± 70.6 ng/mL, respectively.
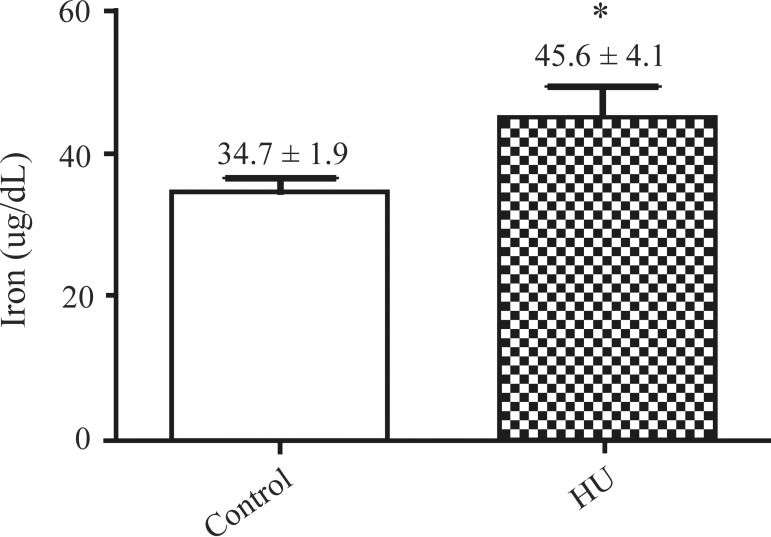
The average GSH levels in treated and untreated SCA patients were 45.6 ± 4.1 µM and 34.7 ± 1.9 µM, respectively. This difference was significant (p-value < 0.05).
Discussion
Oxidative stress is a condition that results in an imbalance between the concentrations of pro- and antioxidant species. There is a strong correlation between the levels of glutathione in its reduced form (i.e. GSH) and enzymatic defense mechanisms. The resistance of many cells against oxidative stress is associated with high intracellular levels of GSH. One of the roles of GSH is to control the levels of lipid hydroperoxides and thus prevent cell damage by these radicals. Hence, glutathione levels may provide important biochemical information on the oxidant-antioxidant balance in the body.(9,16)
The results of the study show a significant difference in the GSH levels of SCA patients treated with HU and those who were not given this medication and are thus in line with the results of another study,(17) in which the antioxidant effect of HU on the disease was demonstrated.
The results also showed an insignificant increase in the mean levels of iron, transferrin and ferritin in the serum of patients treated with HU when compared with those who did not take the medication. These results are similar to those reported by Manfredini(12) who demonstrated high levels of ferritin and serum iron and reduced levels of transferrin in SCA patients who did not take HU. The serum ferritin level is an indicator of iron stores and is considered the most important prognostic factor in patients with iron overload.(18) The accumulation of iron in the body is associated with the development and progression of various comorbidities with secondary iron overload being commonly found in SCA.(19) In terms of toxicity, chronic deposition of iron is often associated with the frequent blood transfusions required to treat certain types of anemia including SCA.(20,21)
The study showed that the use of HU may possibly act as a form of protection against oxidative stress by increasing the levels of GSH, the main intracellular antioxidant compound.
Figure 1.
Ferritin levels of patients taking hydroxyurea compared to controls (ng/mL)
Figure 2.
Transferrin levels of patients taking hydroxyurea compared to controls (mg/dL)
Figure 3.
Total iron levels of patients taking hydroxyurea compared to controls (µg/dL)
Figure 4.
GSH levels of patients taking hydroxyurea compared to controls (µM)
*Data are reported as means (± SEM). *P < 0.05 vs. control group (ANOVA and Student-Newman-Keuls Test)
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interest
References
- 1. Akerboom TP, Sies H.Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981;(77): 373-82 [DOI] [PubMed] [Google Scholar]
- 2. Appoloni CR, Estevam M.Uso da fluorescência de raios X portátil (XRF) in vivo como técnica alternativa para acompanhamento dos níveis de ferro em pacientes com sobrecarga de ferro. Rev Bras Hematol Hemoter. 2009; 31(3): 153-9 [Google Scholar]
- 3. Bandeira FM, Peres JC, Carvalho EJ, Bezerra I, Araújo AS, Mello MR, et al. Hidroxiuréia em pacientes com síndromes falciformes acompanhados no Hospital Hemope, Recife-PE. Rev Bras Hematol Hemoter. 2004; 26(3): 189-94 [Google Scholar]
- 4. Cançado RD, Chiattone CS.Anemia de doença crônica. RevBras Hematol Hemoter. 2002; 24(2): 127-36 [Google Scholar]
- 5. Cançado RD.Sobrecarga e quelação de ferro na anemia falciforme. Rev Bras Hematol Hemoter. 2007; 29(3): 316-26 [Google Scholar]
- 6. Doner G, Ege A.Evaluation of digestion procedures of determination of iron and zinc in biscuits by flame atomic absorption spectrometry. Anal Chim Acta. 2004; 46(520): 217-22 [Google Scholar]
- 7. Fasola F, Adedapo K, Anetor J, Kuti M.Total Antioxidants status and some hematological values in sickle cell disease patients in steady state. J Natl Med Assoc. 2007; 99(8): 891-4 [PMC free article] [PubMed] [Google Scholar]
- 8. Ferreira AL, Matsubara LS.Radicais livres: conceitos, doenças relacionadas, sistema de defesa e estresse oxidativo. Rev Ass Med Brasil. 1997; 43(1): 61-8 [DOI] [PubMed] [Google Scholar]
- 9. Grotto HZ.Metabolismo do ferro: uma revisão sobre os principais mecanismos envolvidos em sua homeostase. Rev Bras Hematol Hemoter. 2008; 30(5): 390-7 [Google Scholar]
- 10. Hiorushi K, Ballas SK, Asakura T.The effect of deoxygenating rate on the formation of irreversibly sickled cells. Blood. 1988; 71(1): 46-51 [PubMed] [Google Scholar]
- 11. Kohgo Y, Ikuta K, Ohtake T, Torimoto Y, Kato J.Body iron metabolism and pathophysiology of iron overload. Int J Haematol. 2008; 88(1): 7-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manfredini V, Lazzaretti LL, Griebeler IH, Santin AP, Brandão VD, Wagner S, et al. Blood antioxidant parametres in sickle cell anemia patients in steady state. J Natl Med Assoc. 2008; 100(8): 897-902 [DOI] [PubMed] [Google Scholar]
- 13. Martins VD, Manfredini V, Peralba MC, Benfato MS.Alpha-lipoic acid modifies oxidative stress parameters in sickle cell patients. Clin Nutr. 2009;28(2):192-7. [DOI] [PubMed] [Google Scholar]
- 14. Naoum PC.Interferentes eritrocitários e ambientais na anemia falciforme. Rev Bras Hematol Hemoter. 2000; 22(1): 5-22 [Google Scholar]
- 15. Naoum PC, Souza PC.Avaliação dos produtos da degradação oxidativa da Hb S nos genótipos SS, SF (S/ß0 talassemia) e AS, em comparação com hemoglobinas normais. J Bras Patol Med Lab. 2004; 40(4): 249-59 [Google Scholar]
- 16. Quinn CT, Shull EP, Ahmad N, Lee NJ, Rogers ZR, Buchanan GR.Prognostic significance of early vaso-occlusive complications in children with sickle cell anemia. Blood. 2007; 109(1): 40-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rover Júnior L, Hoehr NF, Vellasco AP, Kubota LT.Sistema antioxidante envolvendo o ciclo metabólico da glutationa. Quim Nova. 2001; 24(1): 112-9 [Google Scholar]
- 18. Pinheiro LS, Gonçalves RP, Tomé CA, Alcântara AE, Marques AR, Silva MM.Prevalência de hemoglobina S em recém-nascidos de Fortaleza: importância da investigação neonatal. Rev Bras Ginecol Obstet. 2006; 28(2): 122-5 [Google Scholar]
- 19. Red M, Badaloo A, Forrester T, Jahoor F.In vivo rates of erythrocyte glutathione synthesis in adults with sickle cell disease. Am J Physiol Endocrinol Metab. 2006; 291(1): E73-9 [DOI] [PubMed] [Google Scholar]
- 20. Shander A, Cappellini MD, Goodnough LT.Iron overload and toxicity: the hidden risk of multiple blood transfusions. Vox Sanguinis. 2009; 97(3): 185-97 [DOI] [PubMed] [Google Scholar]
- 21. Stuart MJ, Nagel RL.Sickle cell disease. Lancet. 2004; 364 (9442): 1343-60 Comment in: Lancet. 2005; 365(9457):382-3 [DOI] [PubMed] [Google Scholar]