Abstract
Background
The expression of CD56 is considered a bad prognostic factor for overall survival, lower rates or short complete remission and extramedullary invasion but the results are controversial. The importance of validating new prognostic parameters in acute leukemias was the reason to investigate the CD56 expression in blast cells of patients with acute myeloid leukemia.
Methods
A cohort of 48 patients treated at Hospital de Clinicas de Porto Alegre and diagnosed with acute myeloid leukemia as classified by the French-American-British group (FAB) criteria using cell morphology, cytochemistry and flow cytometry were evaluated.
Results
Eight cases (16.7%) were CD56 positive without correlation to age or gender. The highest incidence of CD56 positivity was in FAB subtypes M4 and M5. The death rate during induction was not significantly different between patients with and without CD56 expression (62.5% vs. 27.5%; p-value = 0.097). However, patients that expressed CD56 had significantly lower overall survival than those who did not (mean 4.0 months vs. 14.5 months; p-value = 0.03).
Conclusions
The data suggest that expression of CD56 in acute myeloid leukemia may be indicative of poor prognosis because it is associated with a shorter overall survival. The death rate during induction was not significantly different despite an apparent difference in proportions between groups.
Keywords: Antigens, CD56; Leukemia, Myeloid; Prognosis
Introduction
Acute myeloid leukemia (AML) is a clonal malignant disease of the hematopoietic tissue. Its estimated incidence is 30% of all adult leukemia; the relative survival time for adults diagnosed between 1990 and 1994 was one year in 34% of cases and five years in 15%.(1)
The commonly used method to classify the various subtypes of AML was developed in 1976 by the French-American-British (FAB) cooperative group, based on morphologic and cytochemical aspects.(2) Immunophenotypic methods were later incorporated as part of the diagnostic criteria. Conventional morphology supplemented by cytochemistry is often sufficient to differentiate leukemia types. Occasionally, myeloblasts have atypical, agranular lymphoblast morphology, which may impair diagnosis.(3) Cases such as minimally differentiated AML (FAB M0), acute erythroid leukemia (FAB M6) or acute megakaryoblastic leukemia (FAB M7) require complementary immunophenotyping.(1)
Immunophenotyping is widely used and complementary to morphological, cytochemical and karyotypic (cytogenetics) studies, allowing a more precise diagnosis and classification of AML.(4) The assessment of membrane or intracellular antigens (cluster differentiation - CD) using flow cytometry (FC) has enabled the identification of immunophenotypic peculiarities of cells. Distinctive characteristics have been studied for their possible prognostic value(5,6) in predicting response to treatment, rate and duration of complete remission (CR), disease-free survival, overall survival (OS) and potential of extramedullary invasion (EMI), as well as the correlation of molecular expression with cell resistance mechanisms or apoptosis-regulating proteins.(7) The use of these expressions was proposed to monitor minimal residual disease (MRD)(8) and more recently to develop and monitor antibody-based treatment strategies.(9)
CD56 is commonly expressed in natural killer cytotoxic lymphocytes (NK cells) and in other lymphocyte(10) and monocyte(11) subtypes. CD56 NK cells are an important cell component of the innate immune system as they are the major source of IFNg (interferongamma). Normal plasmocytes are CD56-negative and when present, this expression has been used to distinguish multiple myeloma (MM) from monoclonal gammopathies of undetermined significance or reactive plasmocytes.(12) CD56 can also be expressed in certain neoplastic cells such as AML cells,(10,13-17) acute myeloid/NK cell leukemia (myeloid/NK AL),(17) ALL(10) and lymphoma.(11,18-21)
The prognostic meaning of cell surface antigen expressions in AML is still a controversial matter.(7) CD56 expression is considered by some authors to have prognostic value in AML patients and is apparently associated with short overall survival,(14-16,22, 23) lower CR rates(14,22) and shorter duration of CR.(14,15, 23) Baer et al. demonstrated that CD56 expression in AML with t(8;21)(q22;q22), usually associated with a high CR rate and prolonged disease-free survival, was in fact significantly correlated with a short CR period and low OS.(15) Ferrara et al. reported that there was no association between CD56 expression and the rate of CR in patients with AML M3(23) differently from Murray et al. who found a lower CR rate in this subtype of CD56+ AML.(14) Both authors however observed shorter OS and duration of CR in AML M3 patients with CD56 expression. Raspadori et al.(22) found that CD56 expression was associated with a lower rate of CR. On the other hand, in a study including all LMA subtypes except M3, Chang et al.(13) demonstrated that the CR rate was not associated with CD56 expression, but with CD34 and HLA-DR expression.
Some authors report that the presence of the CD56 marker is associated with a higher incidence of central nervous system (CNS) disease in patients with AML,(15,16) myeloid/ NK AL,(24) ALL(10) and lymphoma.(11) Moreover, EMI patients have demonstrated a lower CR rate and short OS.(16) Because CD56 is an NCAM isoform that plays an important function in neuronal growth and migration through cell to cell adhesion, there is a hypothesis that CD56 expression in leukemic cells could allow infiltration through the adhesion of these cells to tissues that also express this marker.(10,25) Ravandi et al.(10) demonstrated a high incidence of CNS disease in adult ALL patients who expressed CD56.
While CD56 expression in MM had no prognostic value in one study,(26) it was associated with worse outcomes in another two studies.(27) NK cell or NK-like CD56+ lymphoma cells in extranodal sites such as the skin, which is not uncommon, have a bad prognostic value almost without exception.(28)
In the present study, we investigated whether CD56 expression in AML is a risk factor for shorter OS and a higher death rate during induction chemotherapy in patients treated at Hospital de Clínicas of Porto Alegre (HCPA). Also, the incidence of CD56 expression in different AML FAB subtypes was addressed.
Methods
Patients
This study involved a cohort of 48 AML patients admitted to HCPA who were diagnosed from July 2002 to July 2005. The group included 29 male and 19 female patients (proportion 1.4:1). The patients' ages varied from three to 78 years old with a median of 43 and a mean of 20.5 years old. The most frequent AML subtype observed was FAB M3 (25%) followed by FAB M1 (22.9%) and FAB M4 (14.6%).
Data were obtained by consulting the medical records of AML patients treated by the hematology team of Hospital de Clínicas in Porto Alegre. The data of interest were: gender, age, FAB classification, leukocyte count at diagnosis, CR rate, disease-free survival and OS.
All non-M3 AML patients received induction with Cytarabine 100 mg/m2/d as a continuous infusion, for seven days associated with Daunorubicine 45 mg/m2/day for three days ('7+3' scheme). The post-induction phase was carried out with one consolidation cycle (same drugs and doses as the induction phase) and two intensification cycles consisting of three days of Idarubicin (10 mg/m2/day) and high-dose Cytarabine (3 g/m2/day - three days). Patients with promyelocytic leukemia (M3) were treated with the AIDA protocol of the Italian group GIMEMA. Patients submitted to bone marrow transplant were not included in this study.
Morphological analysis
The cytomorphological analysis was performed using a bone marrow smear or a peripheral blood smear after anticoagulation with EDTA stained with May Grunwald-Giemsa. The bone marrow cytological exam subjectively assessed the cellularity, assessed hematopoiesis, and quantified megakaryocytes, blasts and the myeloid: erythroid ratio.
Cytochemical analysis
The PAS (periodic acid-Schiff) reaction and Sudan Black staining were used for the cytochemical analyses. PAS identifies cytoplasmic glycogen through the action of the periodic acid, which oxidizes protein carbohydrate radicals and converts them into aldehydes, which are stained red or a strong pink color by the Schiff's reagent; Sudan Black stains the lipid membrane of lysosomal granules in mature and immature myeloid cells of granulocytic and monocytic lineage due to the presence of intracellular phospholipids.(1)
Immunophenotyping
Immunophenotypic analysis of bone marrow blasts and of peripheral blood was performed according to a standard technique.(4) The sample was acquired using CellQuestT software in a FACSCalibur BD (Becton, Dickinson) flow cytometer, with five simultaneous reading parameters, three of which were fluorescence parameters (FL1, FL2, FL3) and then analyzed using Paint-a-Gate software in the mononuclear gate.
The monoclonal antibodies marked directly with phycoerythrin (PE) fluorochromes or fluorescein isothiocyanate (FITC) (Becton Dickson, Immunotech, DAKO, Marseille, France) were: IgG1PE, CD45 FITC, CD117 PE, CD10 FITC, CD19 PE, HLA DR FITC, CD13 PE, CD33 PE, CD56 PE, CD3 FITC, CD7 PE, cTDT FITC, cMPO FITC, CD14 PE, CD15 FITC, CD34 PE, CD61 FITC and Glycophorin PE. Monoclonal antibodies were combined in the immunophenotypic panel, when necessary, especially to assess the following coexpressions: CD33/CD56/CD45, HLA-DR/CD56/CD45, CD117/CD56/CD45 and CD14/CD56/CD45.
Schiff's reagent (Merck), erythrocyte lysis solution (FACS Lyse BD-Biosciences), perforating reagent Tween 20 (polyoxyethylene sorbitan monolaurate) and PBS solution (NaHPO.HO, NaHPO.NaCl) were used to prepare the solutions necessary to carry out the technique.
Results
The AML subtypes of the 48 patients were classified according to the FAB group criteria. Eight patients (16.7%) were defined as being CD56 positive as this antigen expression was found on the surface of over 20% of the blast population in the sample. Among the CD56+ samples, the percentage of CD56 expression on blasts varied from 40.8 to 90.9% (mean: 67.4%). The age, gender and AML subtype of control patients (without CD56 expression) are listed in Table 1 along with the CD56+ cases.
Table 1.
Age, gender and AML subtype of control patients (without CD56 expression) and of study cases (with CD56 expression)
| Control group (n = 40) | Cases with CD56 expression (n = 8) | |
| Age (years) | ||
| Average (standard deviation) | 43.14(21,77) | 50.5 (21.63) |
| Median (variation) | 49 (3 to 78) | 53 (17 to 75) |
| Gender | ||
| Male % (n) | 60.4 (22) | 87.5(7) |
| Female % (n) | 39.6(18) | 12.5(1) |
| Proportion M:F | 1.2:1 | 7:1 |
| Subtype LMA FAB % (n) | ||
| MO | 7.5 (3) | 0(0) |
| Ml | 27.5 (11) | 0(0) |
| M1/M2 | 5.0 (2) | 0(0) |
| M2 | 12.5 (5) | 12.5(1) |
| M3 | 30.0(12) | 12.5(1) |
| M4 | 17.5 (7) | 37.5 (3) |
| M5 | 7.5 (3) | 25.0 (2) |
| M6 | 2.5(1) | 0(0) |
| M7 | 5.0 (2) | 12.5(1) |
| ND* | 5.0 (2) | 0(0) |
*ND: subtype not determined
The median age of CD56+ patients was 53 years; none was aged 17 or under. The median age of the patients with no CD56 expression was 49 and eight (20%) patients were aged 21 or under. No significant age difference was identified between the groups (p-value = 0.301).
The male:female ratio of CD56+ patients was 7:1 and 1.22:1 (22 males and 18 females) for those not expressing CD56. The proportions did not show any significant difference (Fisher's Exact Test: p-value = 0.123). A total of 62.5% of CD56+ patients died soon after or during induction compared with 27.5% of patients without CD56 expression. In spite of the observed tendency, there was no significant difference between the two groups (Fisher's Exact Test: p-value = 0.097).
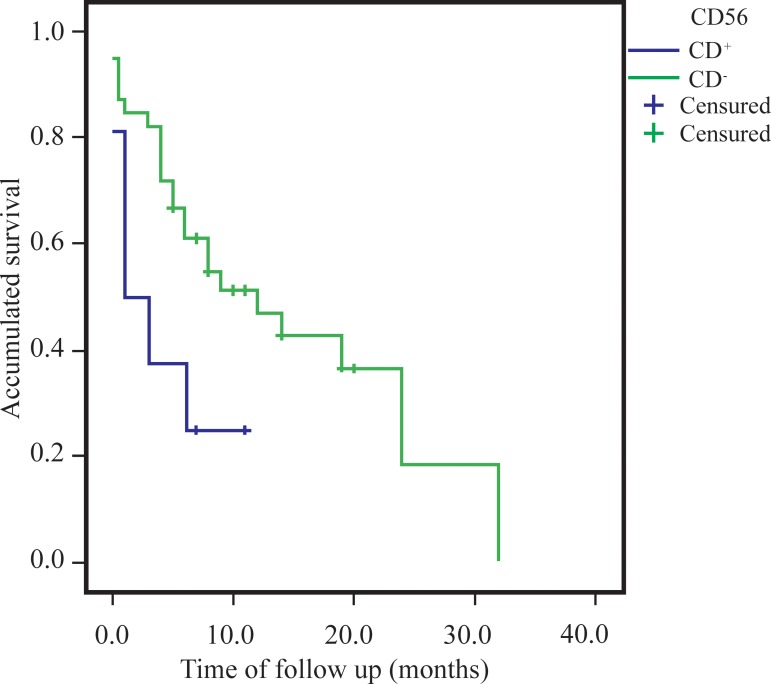
The difference between the OS of the groups was estimated using the Kaplan-Meier survival curve, comparing the survival of patients with or without CD56 expression using the Log-Rank test. As demonstrated in Figure 1, the time of OS was significantly shorter (p-value = 0.033) in CD56+ patients, with an average survival of 4.0 months (Range: 0.91 to 7.17 months; median: 1.0 month) compared to the mean survival of 14.5 months (Range: 10.0 to 18.9 months; median: 12.0 months) of patients with no CD56 expression.
Figure 1.
Overall survival estimated using the Kaplan-Meier method in CD56+ (n = 8) and CD56- (n = 39) acute myeloid leukemia patients
No studies were carried out to correlate CD56 expression and the rate and duration of complete remission, presence of extramedullary disease, resistance to multiple drugs, presence of genetic anomalies and investigation of aneuploidies through DNA analysis (phase S). Neither was the influence of other aberrant markers present in the immunophenotype investigated in respect to the induction death rate and OS.
Discussion
This study assessed a cohort of 48 patients admitted to HCPA, diagnosed as having AML and treated with standard chemotherapy protocols of the institution. The diagnosis was made based on clinical, cytomorphological, cytochemical and immunophenotypic data according to the FAB classification. Among the patients studied, 40 patients with no CD56 expression were considered a control group and eight CD56+ patients were considered the cases.
Our results demonstrated that the OS was significantly lower in the group expressing CD56 in leukemic blasts, which was compatible with published data.(13,16,22) Ferrara et al. demonstrated in a multivariate analysis that CD56 expression is an independent factor for low survival of AML subtype FAB M3 patients.(23) This same difference was observed in another study, which included all AML subtypes with the exception of AML M3 due to its different biology.(13)
In our study a higher proportion of CD56 expression was found in men (7:1) compared with the control group (1.2:1) but without significant difference, which confirmed published data.(15,23,28) Only one study reported a higher incidence in male patients with myeloid/NK AL.(24)
The incidence of CD56 expression in the patients assessed in our study was 16.7%, similar to rates found in the literature which range from 14 to 58% of AML patients.(13-16,23,29,30) The incidence of FAB subtypes of our patients evidenced a higher proportion in AML FAB M4 cases (37.5%) and FAB M5 cases (25.0%), which were compatible with those reported in literature.(13,16) Raspadori et al.(22) found that CD56 expression was detected more in M2 and M5 patients.
The death rate during chemotherapy induction was assessed in our study in order to verify whether the group expressing CD56 had a tendency to higher susceptibility to death; here this did not reach a statistically significant difference which may be attributed to the small study population. None of the authors referenced reported death rates in the induction of chemotherapy, nor did they assess any possible correlation with the CD56 expression.
Studies about the prognostic value of CD56 expression in AML are relatively recent and a number of clinical correlations are found in the literature. A few factors that contributed to the discrepancies are methodological differences in the detection of antigens, size of the population studied, patients from one or multiple institutions, as well as factors such as age, cytogenetics and a number of different treatment protocols.
Our results may contribute to more specific investigations of CD56 expression in AML at diagnosis, and they could help an assessment of prognostic value in a larger cohort submitted to a single treatment protocol. If the hypotheses of early mortality, lower CR rate, an equally early relapse and/or extramedullary invasion are confirmed in AML cases with CD56 expression, this data would be relevant to stratify patients, based on this risk factor. A possible use for the detection of this molecule in blasts through flow cytometry would be as a marker to assess MRD, as normal blasts do not express myeloid markers concurrently with CD56.
Our data demonstrated that CD56 expression in the blasts of AML patients is indicative of shorter OS, therefore confirming published findings. The power of this study may be increased with the inclusion of a larger number of individuals. If it is confirmed that the presence of CD56 is a risk factor, patients with this phenotype may be submitted to a different treatment regimen.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interest
References
- 1. Smith M, Barnett M, Bassan R, Gatta G, Tondini C, Kern W.Adult acute myeloid leukaemia. Crit Rev Oncol Hematol. 2004; 50(3): 197-222 [DOI] [PubMed] [Google Scholar]
- 2. Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med. 1985; 103(4): 620-5 [DOI] [PubMed] [Google Scholar]
- 3. Wood BL.Myeloid malignancies: myelodysplastic syndromes, myeloproliferative disorders, and acute myeloid leukemia. Clin Lab Med. 2007; 27(3): 551-75, vii. [DOI] [PubMed] [Google Scholar]
- 4. Stewart CC.Clinical applications of flow cytometry. Immunologic methods for measuring cell membrane and cytoplasmic antigens. Cancer. 1992; 69(6Suppl): 1543-52 [DOI] [PubMed] [Google Scholar]
- 5. Ball ED, Davis RB, Griffin JD, Mayer RJ, Davey FR, Arthur DC, et al. Prognostic value of lymphocyte surface markers in acute myeloid leukemia. Blood. 1991; 77(10): 2242-50 [PubMed] [Google Scholar]
- 6. Bradstock K, Matthews J, Benson E, Page F, Bishop J.Prognostic value of immunophenotyping in acute myeloid leukemia. Australian Leukaemia Study Group. Blood. 1994; 84(4): 1220-5 [PubMed] [Google Scholar]
- 7. Schabath R, Ratei R, Ludwig WD.The prognostic significance of antigen expression in leukaemia. Best Pract Res Clin Haematol. 2003; 16(4): 613-28 [DOI] [PubMed] [Google Scholar]
- 8. Zeng QT, Pratt JP, Pak J, Ravnic D, Huss H, Mentzer SJ.Featureguided clustering of multi-dimensional flow cytometry datasets. J Biomed Inform. 2007; 40(3): 325-31 [DOI] [PubMed] [Google Scholar]
- 9. Stone RM.Targeted agents in AML: much more to do. Best Pract Res Clin Haematol. 2007; 20(1):39-48. [DOI] [PubMed] [Google Scholar]
- 10. Ravandi F, Cortes J, Estrov Z, Thomas D, Giles FJ, Huh YO, et al. CD56 expression predicts occurrence of CNS disease in acute lymphoblastic leukemia. Leuk Res. 2002; 26(7): 643-9 [DOI] [PubMed] [Google Scholar]
- 11. Suzuki R, Kagami Y, Takeuchi K, Kami M, Okamoto M, Ichinohasama R, et al. Prognostic significance of CD56 expression for ALK-positive and ALK-negative anaplastic large-cell lymphoma of T/null cell phenotype. Blood. 2000; 96(9): 2993-3000 [PubMed] [Google Scholar]
- 12. Lima M, Teixeira MA, Fonseca S, Goncalves C, Guerra M, Queiros ML, et al. Immunophenotypic aberrations, DNA content, and cell cycle analysis of plasma cells in patients with myeloma and monoclonal gammopathies. Blood Cells Mol Dis. 2000; 26(6): 634-45 [DOI] [PubMed] [Google Scholar]
- 13. Chang H, Salma F, Yi QL, Patterson B, Brien B, Minden MD.Prognostic relevance of immunophenotyping in 379 patients with acute myeloid leukemia. Leuk Res. 2004; 28(1): 43-8 [DOI] [PubMed] [Google Scholar]
- 14. Murray CK, Estey E, Paietta E, Howard RS, Edenfield WJ, Pierce S, et al. CD56 expression in acute promyelocytic leukemia: a possible indicator of poor treatment outcome? J Clin Oncol. 1999; 17(1): 293-7 [DOI] [PubMed] [Google Scholar]
- 15. Baer MR, Stewart CC, Lawrence D, Arthur DC, Byrd JC, Davey FR, et al. Expression of the neural cell adhesion molecule CD56 is associated with short remission duration and survival in acute myeloid leukemia with t(8;21)(q22;q22). Blood. 1997; 90(4): 1643-8 [PubMed] [Google Scholar]
- 16. Chang H, Brandwein J, Yi QL, Chun K, Patterson B, Brien B.Extramedullary infiltrates of AML are associated with CD56 expression, 11q23 abnormalities and inferior clinical outcome. Leuk Res. 2004; 28(10): 1007-11 [DOI] [PubMed] [Google Scholar]
- 17. Suzuki R, Yamamoto K, Seto M, Kagami Y, Ogura M, Yatabe Y, et al. CD7+ and CD56+ myeloid/natural killer cell precursor acute leukemia: a distinct hematolymphoid disease entity. Blood. 1997; 90(6): 2417-28 [PubMed] [Google Scholar]
- 18. Hayashi K, Nakamura S, Koshikawa T, Kitoh K, Koike K, Komatsu H, et al. A case of neural cell adhesion molecule-positive peripheral T-cell lymphoma associated with human T-cell lymphotrophic virus type 1 showing an unusual involvement of the gastrointestinal tract during the course of the disease. Hum Pathol. 1994; 25 (11): 1251-3 [DOI] [PubMed] [Google Scholar]
- 19. Kern WF, Spier CM, Hanneman EH, Miller TP, Matzner M, Grogan TM.Neural cell adhesion molecule-positive peripheral T-cell lymphoma: a rare variant with a propensity for unusual sites of involvement. Blood. 1992; 79(9): 2432-7 [PubMed] [Google Scholar]
- 20. Radonich MA, Lazova R, Bolognia J.Cutaneous natural killer/Tcell lymphoma. J Am Acad Dermatol. 2002; 46(3): 451-6 [DOI] [PubMed] [Google Scholar]
- 21. Muroi K, Omine K, Kuribara R, Uchida M, Izumi T, Hatake K, et al. CD56 expression in B-cell lymphoma. Leuk Res. 1998; 22(2): 201-2 [DOI] [PubMed] [Google Scholar]
- 22. Raspadori D, Damiani D, Lenoci M, Rondelli D, Testoni N, Nardi G, et al. CD56 antigenic expression in acute myeloid leukemia identifies patients with poor clinical prognosis. Leukemia. 2001; 15(8): 1161-4 [DOI] [PubMed] [Google Scholar]
- 23. Ferrara F, Morabito F, Martino B, Specchia G, Liso V, Nobile F, et al. CD56 expression is an indicator of poor clinical outcome in patients with acute promyelocytic leukemia treated with simultaneous all-trans-retinoic acid and chemotherapy. J Clin Oncol. 2000; 18(6): 1295-300 [DOI] [PubMed] [Google Scholar]
- 24. Suzuki R, Nakamura S.Malignancies of natural killer (NK) cell precursor: myeloid/NK cell precursor acute leukemia and blastic NK cell lymphoma/leukemia. Leuk Res. 1999; 23(7): 615-24 [DOI] [PubMed] [Google Scholar]
- 25. Zocchi MR, Vidal M, Poggi A.Involvement of CD56/N-CAM molecule in the adhesion of human solid tumor cell lines to endothelial cells. Exp Cell Res. 1993; 204(1): 130-5 [DOI] [PubMed] [Google Scholar]
- 26. Poley S, Stieber P, Nussler V, Pahl H, Fateh-Moghadam A.Evaluation of serum neural cell adhesion molecule as a prognostic marker in multiple myeloma. Anticancer Res. 1997; 17(4B): 3021-4 [PubMed] [Google Scholar]
- 27. Garcia-Sanz R, Gonzalez M, Orfao A, Moro MJ, Hernandez JM, Borrego D, et al. Analysis of natural killer-associated antigens in peripheral blood and bone marrow of multiple myeloma patients and prognostic implications. Br J Haematol. 1996; 93(1): 81-8 [DOI] [PubMed] [Google Scholar]
- 28. Bekkenk MW, Jansen PM, Meijer CJ, Willemze R.CD56+ hematological neoplasms presenting in the skin: a retrospective analysis of 23 new cases and 130 cases from the literature. Ann Oncol. 2004; 15(7): 1097-108 [DOI] [PubMed] [Google Scholar]
- 29. Cruse JM, Lewis RE, Pierce S, Lam J, Tadros Y.Aberrant expression of CD7, CD56, and CD79a antigens in acute myeloid leukemias. Exp Mol Pathol. 2005; 79(1): 39-41 [DOI] [PubMed] [Google Scholar]
- 30. Suvannasankha A, Minderman H, OLoughlin KL, Sait SN, Stewart CC, Greco WR, et al. Expression of the neural cell adhesion molecule CD56 is not associated with P-glycoprotein overexpression in core-binding factor acute myeloid leukemia. Leuk Res. 2004; 28(5): 449-55 [DOI] [PubMed] [Google Scholar]