Abstract
Background
Real time PCR has become the most common technique to monitor BCR-ABL transcript levels of patients treated with kinase inhibitors. The aim of this study was to evaluate BCR-ABL levels of chronic myeloid leukemia patients treated with imatinib in the chronic phase and correlate the response to therapy and event-free survival.
Methods
BCR-ABL levels were measured in peripheral blood cell samples using Real time PCR at diagnosis and then every 3 months after starting therapy with imatinib. Major molecular response was defined as a three-log reduction from the standardized baseline value. Major molecular response values were adjusted to international scale using a conversion factor of 1.19. The results are reported as a BCR-ABL/ABL ratio (%).
Results
Hematological, major cytogenetic and complete cytogenetic responses were achieved by 57 (95%), 45 (75%) and 38 (63%) patients, respectively. Twenty-four out of sixty patients achieved a major molecular response (40%) in a median time of 8.5 months. Overall survival and event free survival were higher for patients with (100%) versus patients without (77%) a complete cytogenetic response (p-value = 0.01) at 48 months. Patients with complete cytogenetic response and major molecular response had a higher event free survival compared to patients with complete cytogenetic response but without major molecular response (p-value = 0.007).
Conclusion
In conclusion, the prognostic impact of achieving complete cytogenetic response and a major molecular response and also the importance of molecular monitoring in the follow-up of chronic myeloid leukemia patients were demonstrated.s
Keywords: Polymerase chain reaction; Monitoring; Leukemia, myelogenous, chronic, BCR-ABL positive
Introduction
Chronic myeloid leukemia (CML) is a hematopoietic disorder characterized by the malignant expansion of bone marrow stem cells, with the presence of a reciprocal translocation between chromosomes 9 and 22 resulting in the fusion gene, BCR-ABL, whose product is a 210-kd protein with tyrosine kinase activity.(1)
The level of leukemic inhibition after treatment can be measured by quantitative real-time PCR (RT-PCR), which has become the main molecular technique used to monitor BCR-ABL transcript levels in CML during treatment with kinase inhibitors.(2-4) Increasing levels of BCR-ABL are strongly predictive of cytogenetic and hematologic relapse after allogeneic transplant.(5) Monitoring imatinib-treated CML patients by quantitative RT-PCR has proved to be effective to define patient response as was reported in the IRIS trial.(6) Attaining a major molecular response, defined as a three-log reduction in BCR-ABL levels from the standardized baseline, is associated with a favorable progression-free survival(6-8) and a longer duration of complete cytogenetic response (CCR).(9) Early reductions in BCR-ABL can predict a subsequent cytogenetic response.(3)
Standardization of all procedures involved in BCR-ABL quantification is important for the reproducibility and credibility of the results.
The aim of this study was to standardize RT-PCR in monitoring BCR-ABL levels in CML patients treated with tyrosine kinase inhibitors and correlate BCR-ABL levels with cytogenetic response, and event free and overall survival (OS).
Methods
Peripheral blood samples were collected from 60 patients with diagnosis of chronic phase CML from June 2005 until September 2008. Diagnosis of CML was determined by the presence of the Ph chromosome in cytogenetic evaluation and/or BCR-ABL transcripts by RT-PCR. Eligibility criteria included age of 18 years or more. Patients provided written informed consent and the study was approved by the local Research Ethics Committee. The median follow-up time was 22 months (Range: 0.9 -44.6 months).
Treatment procedures and definitions: patients received imatinib as first or second line therapy (53 and 7 patients, respectively) for chronic phase CML. Four patients participating in the TOPS trial were initially treated with imatinib 800 mg/day and another 56 patients were treated with imatinib 400 mg/day. Filgrastin 300 µg/day was given if the neutrophil count was below 1.00 x 109/L until recovery. Imatinib dose was escalated to 600 mg/day when a patient had sub-optimal response [less than major cytogenetic response (MCyR) at six months, less than CCR at 12 months, less than major molecular response at 18 months] or failure (no minimal cytogenetic response at six months, no MCyR at 12 months, loss of hematological response, progression to accelerated phase or blast crisis at any time) and was able to tolerate the increased dose. Second generation tyrosine kinase inhibitors (TKI) (nilotinib or dasatinib) were used for intolerance or resistance to imatinib.
The second generation TKI were available in our center only in clinical trials until 2008 when dasatinib was approved in Brazil. Blood cell counts were performed every two weeks until complete hematological response was achieved, then every one to three months. Cytogenetic evaluation was performed at diagnosis and then every 3-6 months until CCR was confirmed and then every 6-12 months. Peripheral blood samples were collected for the evaluation of BCR-ABL levels at diagnosis and then every three months after starting imatinib treatment. Criteria by the European Leukemia Net group were used to define response: complete hematologic response: normalization of blood cell counts with no immature cells, < 5% of basophils, no palpable spleen; cytogenetic response: partial cytogenetic response 1-35% of Ph+ metaphases; CCR: 0% of Ph+ metaphases; major molecular response: 3 or more log reduction of BCR-ABL transcript levels of a standardized baseline (< 0.1 in the international scale).(10) The standard procedures described by Brandford et al. 2006 and Hughes 2006(11,12) were followed.
RNA extraction and cDNA synthesis
Total leukocyte RNA was extracted from 16 mL of peripheral blood in tubes with EDTA. Samples were kept at room temperature and processed within 24 h after collection to avoid RNA degradation. Red cells were lysed and residual cells were homogenized in 1 mL Trizol RNA stabilization solution (Invitrogen®) and stored at -80ºC. RNA was extracted according to manufacturer's instructions. RNA integrity was assessed by electrophoresis in agarose gel and quantified by Nanodrop spectrophotometry (ND 1000-NanoDrop® 3.2.1). SuperScript II (Invitrogen®) reverse transcriptase was used for cDNA synthesis using 1 µg of total RNA and random hexamers.
Quantitative real-time PCR
cDNA was amplified by 50 cycles of RT-PCR using the ABI 7300 sequence detection system (Applied biosystems) and TAQMAN Universal Master Mix in accordance with the manufacturer's instructions in a final reaction volume of 15 µL. ABL was used as the control gene. The primers used were as followed:
- Forward (ABL-146F): 5'-GATACGAAGGGAGGG TGTACCA-3';
- Reverse (ABL-240R): 5'-CTCGGCCAGGGTGTTGAA-3'
The bcr-abl and abl probes were dual labeled with a reporter fluorochrome (FAM) and a quencher fluorochrome (TAMRA). Probe and primer concentrations were 5 µM.
A standard curve was generated using serial dilutions of a linearized plasmid containing a BCR-ABL insert (4-6 dilutions). Triplicates were performed for standard curves and patients. The threshold was adjusted to 0.05. The accepted coefficient to determine the standard curve (R2) was close to 1.0 (greater than 99%). Slopes were considered acceptable if the values were between 3.3 and 3.6. Quality controls at high and low levels were used in each assay (K562 lineage and a 3 log reduction sample). Negative controls: no template and HL60 lineage.
The results were reported as a BCR-ABL/ABL ratio (%). A baseline value for the laboratory was calculated using the median quantification of 30 pre-treatment samples. The standardized baseline value determined for our laboratory was 83.67%. Major molecular response (MMR) was considered a three log reduction from this baseline value. MMR values were adjusted to international scale using a conversion factor of 1.19.
Statistical analysis
The cutoff date for this analysis was April 2009. We performed analyses of OS and progression free survival (PFS), using the Kaplan-Meier method according to an "intention to treat" basis. Differences between subgroups receiving imatinib were calculated by the log-rank test and a p-value < 0.05 was considered significant. OS was measured from the beginning of imatinib treatment to the date of death or last follow-up. Event-free Survival included all patients and was calculated from beginning of imatinib treatment until any event (death, progression to accelerated phase or blast crisis CML, loss of CHR or CCyR). The prognostic scores were calculated according to the method of Sokal et al.(13) The analyses were performed using the SPSS software, version 14.0.
Results
Sixty CML patients in the chronic phase at diagnosis were analyzed. Patients' characteristics are described in Table 1. Imatinib was initiated in a median time of two months from diagnosis (0.5-18.5 months). Hydrea was used as cytoreduction therapy before imatinib until confirmation of diagnosis of CML or imatinib availability. Imatinib was initiated in early chronic phase (before 6 months from diagnosis) in 57 patients and in the late chronic phase in three patients (6, 8 and 18 months). Patients were treated with imatinib for a median time of 22 months (Range: 0.9-44.6 months). Imatinib was used as first line treatment in 53 patients (88.6%). Initial dose was 400 mg/day with the exception of four patients participating from the TOPS trial(13) who were treated with 800 mg/day up front.
Table 1.
Patients characteristics
| Variable | n = 60 | ||
| Age at diagnosis (median-range) | 47.6 | 17.9 - 79.4 | |
| Age at starting imatinib therapy | 48 | 18-79.5 | |
| (median-range) | |||
| Gender (male/female) | 28/32 | 47%/53% | |
| Hb (g/dL) (median-range) | 12.8 | 6.8- 17.1 | |
| White blood cells x 109/L | 18 | 2.9-234 | |
| (median-range) | |||
| Platelets x 109/L (median-range) | 322.5 | 30.2 - 1736 | |
| Sokal score | |||
| Low | 21 | 35% | |
| Intermediate | 16 | 27% | |
| High | 12 | 20% | |
| Missing | 11 | 18% | |
| Cytogenetics | |||
| Ph positive | 58 | 97% | |
| Ph negative | 2 | 3% | |
| Previous treatment | |||
| Hydrea | 53 | 88% | |
| Hydrea, IFN | 7 | 12% | |
| Last evaluation | |||
| CCR with MMR | 24 | 40% | |
| CCR without MMR | 17 | 28% | |
| No CCR | 19 | 32% | |
| Survivors | 58 | 97% |
Responses
Cumulative response rates: 57 (95%) patients achieved complete hematologic response (CHR) in a median time of 21 days (Range: 0 - 147 days). One patient was primarily resistant and two were intolerant with grade 3 hepatic toxicity; imatinib was then discontinued. Forty-five (75%) patients achieved a MCyR and 38 (63%) a CCR in a median time of 8 months (Range: 3.4 - 29 months). In three patients cytogenetic response could not be evaluated: one due to insufficient number of metaphases for analysis and two were Ph negative at diagnosis. In the group that achieved CCR, four patients lost response: one is currently on Hydrea, one on dasatinib and two are on imatinib 600 mg. None of them have achieved MMR, so far.
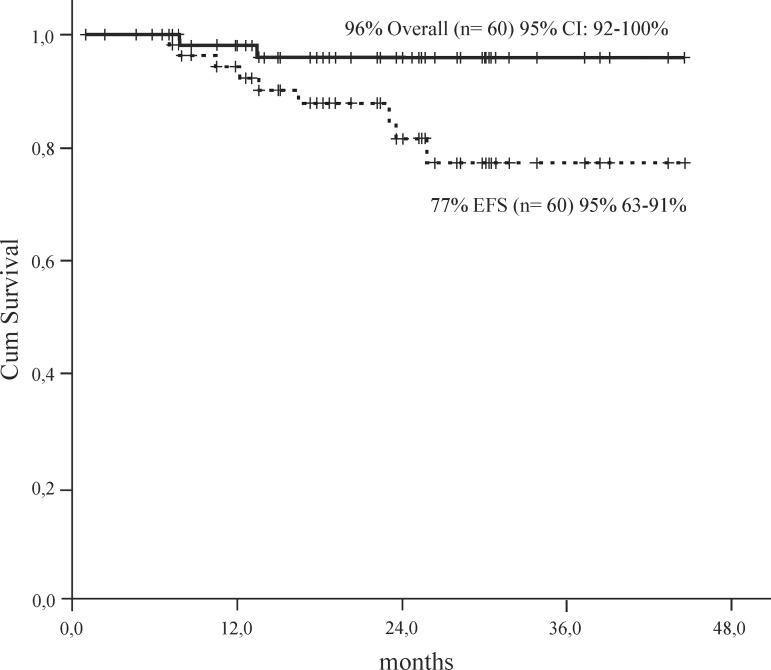
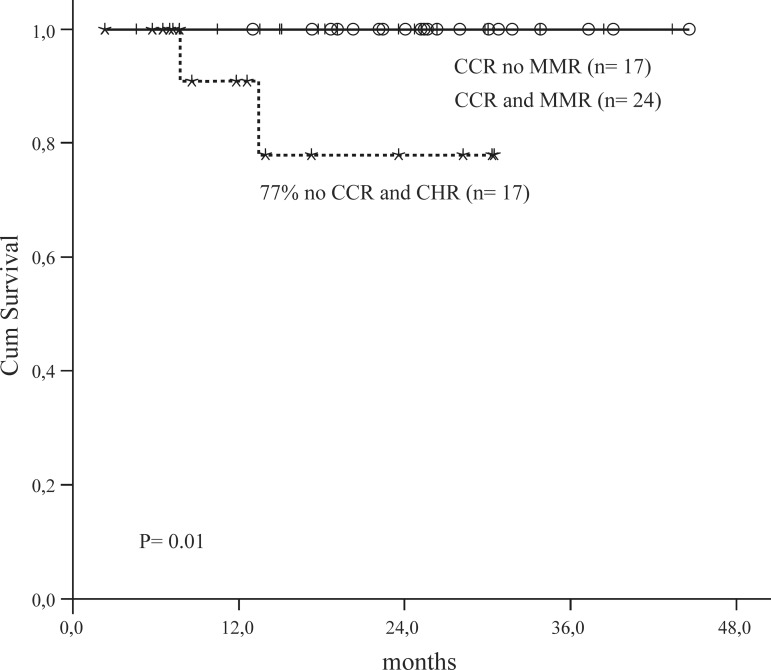
Twenty-four out of 60 patients achieved a MMR (40%) in a median time of 8.5 months (Range: 0.4-31 months). There was a good correlation between CCR and MMR (p-value = 0.04). Overall and event free survival for all patients was 96% and 77%, respectively at 48 months (Figure 1). OS and EFS was higher for patients with CCR (100%) versus patients with no CCR (77%; p-value = 0.01 - Figure 2).
Figure 1.
Overall Survival and Event Free Survival of CML patients in chronic phase
EFS = Event Free Survival
Figure 2.
Overall Survival by cytogenetic and molecular responses
CCR = Complete Cytogenetic Responsel
MMR = Major Molecular Response
CHR = Complete Hematological Response
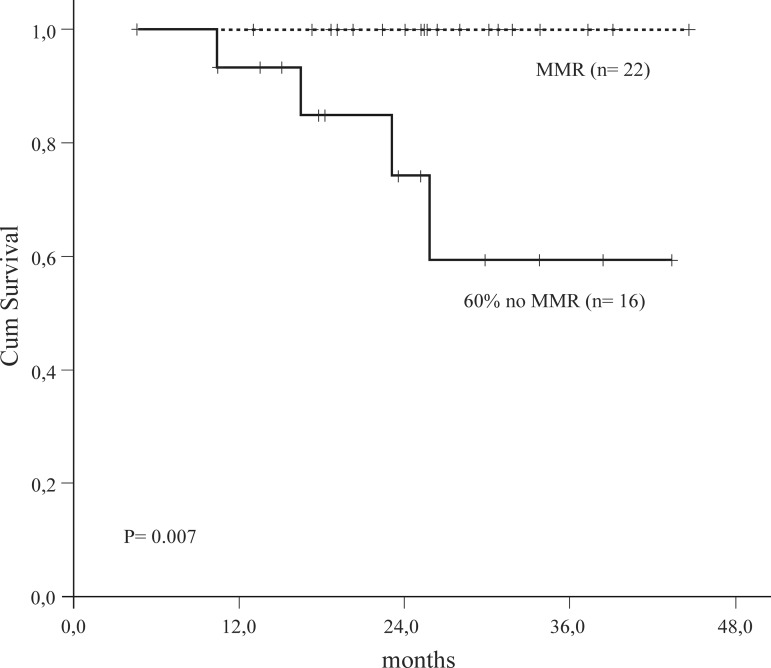
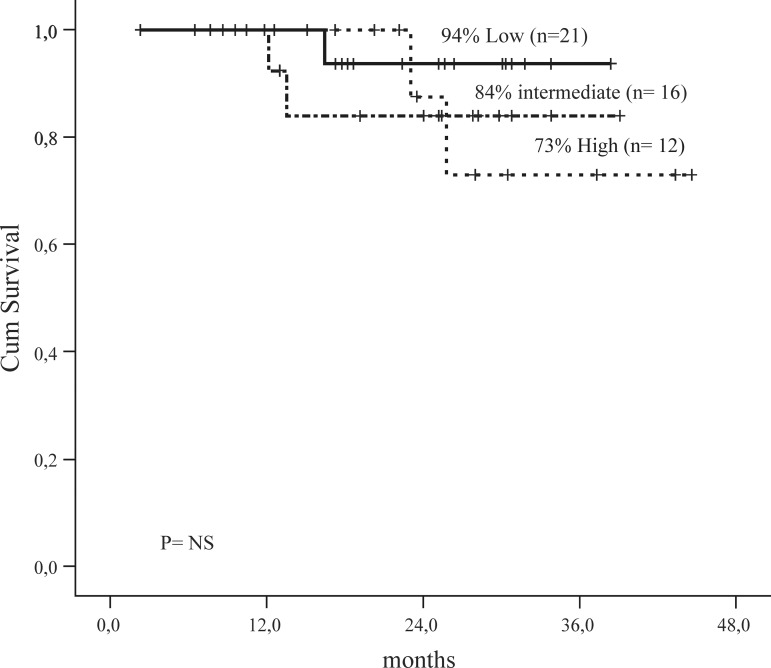
Patients with CCR and MMR had a higher EFS in comparison with patients with CCR and no MMR (p-value = 0.007) (Figure 3). There was no significant difference in survival according to Sokal groups (Figure 4) although intermediate and high scores had more events.
Figure 3.
Event Free Survival Complete Cytogenetic Response Group
MMR = Major Molecular Response
Figure 4.
Event Free Survival by Sokal
Three patients progressed: one to blast crisis, one to accelerated phase and one to lost hematological response in chronic phase. Median time to progression was 13.5 months.
Imatinib was discontinued in ten of sixty patients (16.6%): six (10%) due to intolerance (hepatic toxicity grade 3 in three patients; hematological toxicity grade 4 in three patients) and four (6.6%) due to resistance. The three patients with hepatic toxicity are currently on nilotinib. All achieved major cytogenetic response and two of them major molecular response. Patients who discontinued imatinib due to hematological toxicity were treated with dasatinib. One of them progressed to blast crisis and died of disease progression. Four patients discontinued imatinib due to resistance: one lost cytogenetic response and had T315I mutation, two had cytogenetic resistance and one progressed to accelerated phase. This last patient had the M244V mutation identified and died due to disease progression. The two patients with cytogenetic resistance are alive: one received hematopoietic stem cell transplantation and is in complete molecular response and the other one is in major cytogenetic response with dasatinib. So, during imatinib treatment only two patients died, one due to progression and the other due to causes not related to CML.
Discussion
In this study we monitored CML patients treated with imatinib in the chronic phase by RT-PCR. Samples were collected at diagnosis and then every three months after starting imatinib therapy.
Initially we performed the standardization of BCR-ABL quantification by RT-PCR. The standardization involved a series of procedures, such as appropriate sample collection, RNA extraction, cDNA synthesis, choice of control gene (ABL), equipment, standard curves of control gene and BCR-ABL and RT-PCR analysis. The recommendations for standardizing BCR-ABL measurement were reviewed by Hughes et al. in 2006.(11) Many of the procedures discussed in that study were used to standardize the procedures in our laboratory. Recently Branford et al. validated the use of an international scale to report PCR results which allows a comparison of molecular response rates between different laboratories.(14) Standardization is an important step to compare treatment in different populations. We calculated the laboratory baseline based on the median value of quantification of 30 CML samples at diagnosis. We found a good correlation between cytogenetic and molecular response as previously described.(4)
A significant proportion of patients achieved CCR and MMR (63% and 40%, respectively). In general, after 12 months of imatinib therapy, 69% of patients with CML in chronic phase achieved CCR, as described in IRIS study.(15) Among patients with CCR after 12 months of imatinib treatment, those with MMR have a significantly improved progression-free survival compared to those without MMR.(6) In our study, OS and EFS was higher for patients with CCR (100%) versus patients with no CCR (77%). We also observed that patients presenting CCR and MMR had a better event free survival compared to patients with CCR but no MMR (100% vs. 60%). The rate of progression was higher in patients who did not achieve MMR. In fact, patients who achieved MMR did not progress during the follow-up. Iacobucci et al. showed that attaining MMR at the time of first CCR is associated with longer cytogenetic remission compared to not achieving MMR.(9) Moreover, patients with a BCR-ABL ratio = 10% after three months on imatinib had a 92% of probability of CCR, but the risk of progression was almost 3-fold that of patients with a ratio < 1%, suggesting that patients not in CCR in the first year have a higher risk of progression. Similar results were found by Marin et al. who showed that patients with CCR but no MMR at 12 or 18 months were more likely to lose CCR than patients that achieved MMR (23.6% vs. 2.6% and (24.4% vs. 0%, respectively). However, they did not find any significant difference in OS or PFS in patients without MMR at 12 or 18 months.(16)
In summary, this study demonstrates that RT-PCR is an accurate and practical method to monitor BCR-ABL transcripts, that correlates well with clinical response. Attainment of MMR is an important goal in the treatment of CML and was related to a better EFS.
Acknowledgments
Melissa Pereira Machado was supported by scholarship from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
Funding Statement
Melissa Pereira Machado was supported by scholarship from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior).
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interest
References
- 1. Melo JV.The molecular biology of chronic myeloid leukaemia. Leukemia. 1996; 10(5): 751-6 [PubMed] [Google Scholar]
- 2. Wang L, Pearson K, Pillitteri L, Ferguson JE, Clark RE.Serial monitoring of BCR-ABL by peripheral blood real-time polymerase chain reaction predicts the marrow cytogenetic response to imatinib mesylate in chronic myeloid leukaemia. Br J Haematol. 2002; 118(3): 771-7 [DOI] [PubMed] [Google Scholar]
- 3. Muller MC, Gattermann N, Lahaye T, Deininger MW, Berndt A, Fruehauf S, et al. Dynamics of BCR-ABL mRNA expression in firstline therapy of chronic myelogenous leukemia patients with imatinib or interferon alpha/ara-C. Leukemia. 2003; 17(12): 2392-400 [DOI] [PubMed] [Google Scholar]
- 4. Branford S, Hughes TP, Rudzki Z.Monitoring chronic myeloid leukaemia therapy by real-time quantitative PCR in blood is a reliable alternative to bone marrow cytogenetics. Br J Haematol. 1999; 107(3): 587-99 [DOI] [PubMed] [Google Scholar]
- 5. Radich JP, Gooley T, Bryant E, Chauncey T, Clift R, Beppu L, et al. The significance of bcr-abl molecular detection in chronic myeloid leukemia patients "late," 18 months or more after transplantation. Blood. 2001; 98(6): 1701-7 [DOI] [PubMed] [Google Scholar]
- 6. Hughes TP, Kaeda J, Branford S, Rudzki Z, Hochhaus A, Hensley ML, et al. Frequency of major molecular responses to imatinib or interferon-alpha plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003; 349(15): 1423-32 [DOI] [PubMed] [Google Scholar]
- 7. Press RD, Love Z, Tronnes AA, Yang R, Tran T, Mongoue-Tchokote S, et al. BCR-ABL mRNA levels at and after the time of a complete cytogenetic response predict the duration of CCR in imatinib mesylate-treated patients with CML. Blood. 2006; 107(11): 4250-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cortes J, Talpaz M, O'Brien S, Jones D, Luthra R, Shan J, et al. Molecular responses in patients with chronic myelogenous leukemia in chronic phase treated with imatinib mesylate. Clin Cancer Res. 2005; 11(9): 3425-32 [DOI] [PubMed] [Google Scholar]
- 9. Iacobucci I, Saglio G, Rosti G, Testoni N, Pane F, Amabile M, et al. Achieving a major molecular response at the time of a complete cytogenetic response (CCgR) predicts a better duration of CCgR in imatinib-treated chronic myeloid leukemia patients. Clin Cancer Res. 2006; 12(10): 3037-42 [DOI] [PubMed] [Google Scholar]
- 10. Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006; 108(6): 1809-20 [DOI] [PubMed] [Google Scholar]
- 11. Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006; 108(1): 28-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Branford S, Cross NC, Hochhaus A, Radich J, Saglio G, Kaeda J, et al. Rationale for the recommendations for harmonizing current methodology for detecting BCR-ABL transcripts in patients with chronic myeloid leukaemia. Leukemia. 2006; 20(11): 1925-30 [DOI] [PubMed] [Google Scholar]
- 13. Cortes J, Baccarani M, Guilhot Fea.A phase III, randomized, openlabel study of 400 mg versus 800 mg of imatinib mesylate (IM) in patients with newly diagnosed, previously untreated chronic myeloid leukemia in chronic phase (CML-CP), using molecular endpoints: one year results of TOPS (Tyrosine kinase inhibitor optimization and Selectivity) Study [abstract]. 50th ASH Annual Meeting and Exposition Online program and Abstracts San Francisco, CA; 2008 [Google Scholar]
- 14. Branford S, Fletcher L, Cross NC, Muller MC, Hochhaus A, Kim DW, et al. Desirable performance characteristics for BCR-ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood. 2008; 112(8): 3330-8 [DOI] [PubMed] [Google Scholar]
- 15. O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and lowdose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003; 348(11): 994-1004 [DOI] [PubMed] [Google Scholar]
- 16. Marin D, Milojkovic D, Olavarria E, Khorashad JS, de Lavallade H, Reid AG, et al. European LeukemiaNet criteria for failure or suboptimal response reliably identify patients with CML in early chronic phase treated with imatinib whose eventual outcome is poor. Blood. 2008; 112(12): 4437-44 [DOI] [PMC free article] [PubMed] [Google Scholar]