Warts, Hypogammaglobulinemia, Infections and Myelokathexis (WHIM) syndrome is a very rare form of severe congenital neutropenia with approximately only 40 cases reported until now. The name of this syndrome, WHIM, describes its main features that include, but are not limited to warts, hypogammaglobulinemia, recurrent bacterial Infections and myelokathexis (retention and apoptosis of mature neutrophils in the bone marrow).(1,2)
Although recently the molecular basis of this disease was better characterized, the diagnosis of this rare condition remains largely based on clinical features and bone marrow morphology; prompt recognition and initiation of treatment is crucial to avoid morbidity and mortality due to infections. Administration of granulocyte colony-stimulating factor (G-CSF) in this setting reportedly increases the number of neutrophils in circulation and leads to clinical improvement during episodes of bacterial infections.(3,4)
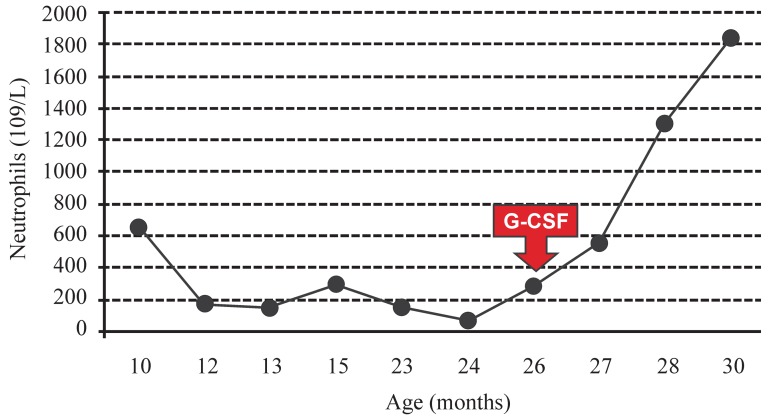
Recently a 2-year-old girl was referred to our hematology clinic with a past history of recurrent infections (3 episodes of pneumonia and 5 episodes of urinary tract infection) and persistent low neutrophil counts ranging from 64 to 650 cells/µL since she was 10 months old. Her previous blood counts showed that her hemoglobin concentration and platelet counts were within the normal range. There were no abnormal findings on physical examination. Investigational tests showed hypogammaglobulinemia (gamma globulin at 0.29g/dL) on protein electrophoresis and the examination of the bone marrow aspirate revealed a hypercellular bone marrow with marked granulocytic hyperplasia characterized by an increased number of mature neutrophils with hypersegmented nuclei and cytoplasmic vacuolization. Neutrophils showed nuclear lobes often separated by long strands of chromatin (Figure 1). These typical bone marrow morphologic findings of myelokathexis in association with the clinical picture were consistent with the diagnosis of WHIM syndrome. Treatment with G-CSF (5 µg/kg per day subcutaneously) was initiated after confirmation of diagnosis leading to an increase in neutrophil count to 550 cells/µL after 12 days of therapy, 1300 cells/µL after one month and to 1836 cells/µL after 4 months (Figure 2). No side effects or new episodes of bacterial infection have been observed so far after five months of therapy.
Figure 1.
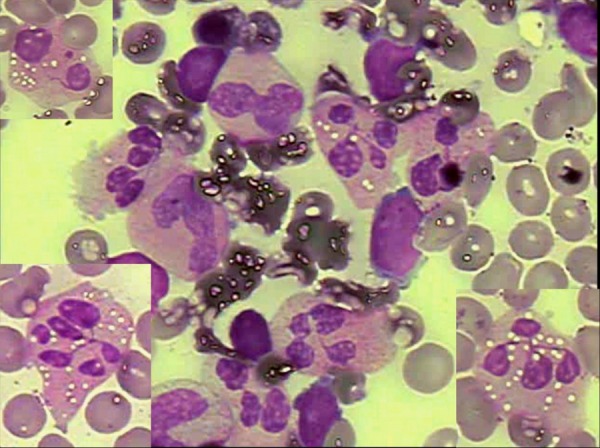
Morphological abnormalities of mature neutrophils on Leishman stained bone marrow aspirate film. Neutrophils show cytoplasmic vacuolization, nuclear hypersegmentation and nuclear lobes separated by long strands of chromatin
Figure 2.
Neutrophil counts before and after initiation of therapy with G-CSF at 5 µg/kg per day
The diagnosis of WHIM syndrome was mainly based on the patient's clinical background with recurrent infections and severe chronic neutropenia and on specific laboratorial findings including hypogammaglobulinemia and, more importantly, a bone marrow aspirate showing granulocytic hyperplasia and aberrant morphology of mature neutrophils. The granulocytic hyperplasia with specific neutrophil morphological abnormalities in the bone marrow help to distinguish myelokathexis from most other forms of severe chronic neutropenia.(5) Indeed, although verrucosis and hypogammaglobulinemia are not observed in all cases, neutropenia and a hypercellular bone marrow are typically observed. In this condition, myeloid and lymphoid cells are normally generated in bone marrow but are not released to the bloodstream resulting in a hypercellular bone marrow with hypersegmented and hypermature neutrophils showing cytoplasmic vacuolization and separated nuclear lobes connected by long strands of chromatin.(6)
In 2003, Hernandez et al.(7) reported that this syndrome is caused by a gain in function of a mutation in the CXC chemokine receptor 4 (CXCR4) causing partial truncation of this receptor. Expression of the truncated form of CXCR4 confers increased responsiveness of mature cells to the chemokine and prevents their marginalization to the bone marrow and subsequent release to the bloodstream. This would explain the paradoxical granulocytic hyperplasia with myelokathexis observed in our and other reports of WHIM syndrome, in which neutrophils are retained in the marrow beyond the normal time for release into circulation; they progress to apoptosis after the normal lifespan, but within the marrow rather than in the circulation and peripheral tissues.
In another important paper, Aprikyan et al.(4) studied apoptotic features in four patients with myelokathexis by electron microscopy and flow cytometry, including the expression of bcl-x on granulocyte precursor subpopulations. The authors demonstrated that the insufficient supply of neutrophils to the peripheral blood in myelokathexis is caused, at least in part, by accelerated spontaneous apoptosis of bone marrow progenitor cells. They also showed that these abnormalities, including low bcl-x expression, were partially corrected by the administration of G-CSF, providing evidence of a possible drug mediated protection against apoptotic cell death.
The satisfactory response to G-CSF therapy in our patient, represented by increased neutrophil counts and decreased infectious episodes, is in accordance with previous reports. In fact, G-CSF has been the standard initial therapy for all forms of severe congenital neutropenia because of the high rate of absolute neutrophil count responsiveness (= 90%) and reduced number and severity of life threatening infections prolonging survival.(8) Stem cell transplantation can be indicated for patients who are refractory to G-CSF or who develop genetic or cytogenetic abnormalities of bone marrow cells that are associated with increased risk of conversion to myelodysplastic syndrome or acute myeloid leukemia.
In summary, WHIM syndrome is a very rare disease in which careful morphological examination of bone marrow aspirate is determinant for diagnosis of myelokathexis, especially in clinical settings where genetic and molecular characterization is unavailable. Prompt diagnosis allows the initiation of G-CSF therapy, which seems to diminish the frequency and intensity of infections.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interest
References
- 1. Zuelzer WW.'Myelokathexis': a new form of chronic granulocytopenia: report of a case. N Engl J Med. 1964; 270: 699-704 [DOI] [PubMed] [Google Scholar]
- 2. Diaz G, Gulino AV.WHIM syndrome (Warts Hyopogammaglobulinemia infections myelokhatexis). Orphanet [Internet]. [cited 2004 June 27] Available from: http://www.orpha.net/data/patho/GB/ukWhim.pdf
- 3. Weston B, Axtell RA, Todd RF 3rd, Vincent M, Balazovich KJ, Suchard SJ, et al. Clinical and biologic effects of granulocyte colony stimulating factor in the treatment of myelokathexis. J Pediatr. 1991; 118(2): 229-34 [DOI] [PubMed] [Google Scholar]
- 4. Aprikyan AA, Liles WC, Park JR, Jonas M, Chi EY, Dale DC.Myelokathexis, a congenital disorder of severe neutropenia characterized by accelerated apoptosis and defective expression of bcl-x in neutrophil precursors. Blood. 2000; 95(1): 320-7 [PubMed] [Google Scholar]
- 5. Hess U, Ganser A, Schnürch HG, Seipelt G, Ottmann OG, Falk S, et al. Myelokathexis treated with recombinant human granulocytemacrophage colony stimulating factor (rhGM-CSF). Br J Haematol. 1992; 80(2): 254-6 [DOI] [PubMed] [Google Scholar]
- 6. Gorlin RJ, Gelb B, Diaz GA, Lofsness KG, Pittelkow MR, Fenyk JR Jr.WHIM syndrome, an autosomal dominant disorder: clinical, hematological, and molecular studies. Am J Med Genet. 2000; 91(5): 368-76 [PubMed] [Google Scholar]
- 7. Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, et al. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combine immunodeficiency disease. Nat Genet. 2003; 34(1): 70-4 [DOI] [PubMed] [Google Scholar]
- 8. Badolato R, Fontana S, Notarangelo LD, Savoldi G.Congenital neutropenia: advances in diagnosis and treatment. Curr Opin Allergy Clin Immunol. 2004; 4(6): 513-21 [DOI] [PubMed] [Google Scholar]