Abstract
Objective
To determine whether human immunodeficiency virus (HIV) infection is associated with increased risk of malaria incidence and recurrence in children.
Methods
Newborn infants of HIV-infected mothers were enrolled at 6 weeks and followed for 2 years. HIV status was assessed by enzyme-linked immunosorbant assay and confirmed by HIV DNA polymerase chain reaction. Malaria was defined as (1) physician-diagnosed clinical malaria; (2) probable malaria, in which laboratory testing is requested for parasitemia; and (3) blood smear–confirmed malaria. Cox proportional hazards models estimated hazard ratios (HRs) for development of first and second malaria episodes, and generalized estimating equation models estimated malaria rate differences per 100-child-years in relation to time-updated HIV status.
Results
Child HIV infection was associated with clinical (HR, 1.34; 95% confidence interval [CI], 1.12–1.61), probable (HR, 1.47; 95% CI, 1.19–1.81), and confirmed (HR, 1.67; 95% CI, 1.18–2.36) malaria episodes. Per 100 child-years, HIV-infected children experienced 88 (95% CI, 65–113), 36 (95% CI, 19–53), and 20 (95% CI, 9–31) more episodes of clinical, probable, and confirmed malaria episodes, respectively, than HIV-uninfected children. Among children with ≥1 malaria episodes, those with HIV infection developed second clinical (HR, 1.28; 95% CI, 1.04–1.57), probable (HR, 1.60; 95% CI, 1.26–2.14), and confirmed (HR, 2.27; 95% CI, 1.06–3.89) malaria sooner than HIV-uninfected children.
Conclusions
HIV infection is a risk factor for the development of malaria. Proactive malaria disease prevention and treatment is warranted for all children, particularly those with HIV infection in settings of coendemicity.
Nearly 22.5 million persons live with human immunodeficiency virus (HIV) in sub-Saharan Africa (SSA) [1], where 2.25 million cases of clinical malaria occur yearly [2]. More than 70% of AIDS deaths and 90% of malaria-related deaths occur in SSA. The disproportionate burden of these 2 infections in the region is well described [3], and simultaneous infection by both (so-called coinfection) may be as high as 30% [4]. Even modest negative interactions between them in coinfected populations could substantially amplify the morbidity and mortality associated with these infections in SSA [5].
In coinfected persons, HIV and Plasmodium organisms could interact with implications for malaria disease. HIV infection impairs T-cell immunity and antibody responses necessary for effective antimalarial response. These factors may explain the attenuation of age-acquired malaria immunity [6, 7], loss of gravidity-specific pattern of placental malaria [8, 9], and higher frequency and severity [10] of malaria infections observed in HIV-positive adults. Equally important, the prophylactic drug cotrimoxazole routinely prescribed to HIV-positive individuals may provide some protection from malaria by inhibiting the in vivo survival of some Plasmodium species [8].
Whether HIV infection affects the risk of malaria in children is unclear. Results from different epidemiologic studies produced conflicting results that suggested no association [9, 11, 12], protection [13, 14], or higher risk [15, 16] of malaria for HIV-positive versus HIV-negative children. Variation with respect to child age and the local malaria transmission pattern—2 factors with implication for the presence or lack of adaptive immunity to malaria infection [17]—may have contributed the heterogeneity observed across studies. In addition, some of these studies were conducted early in the HIV epidemic [9, 11, 12], and others were limited by the use of a cross-sectional [14] or hospital-based case-control design [14, 15].
Hence, we conducted a prospective cohort study among infants born to HIV-positive women to determine whether malaria incidence and frequency over 2 years of follow-up was associated with HIV infection. We also investigated whether HIV infection was associated with shorter time to malaria recurrence among children with ≥1 episode of malaria. We hypothesized that HIV-positive children would develop both first and second malaria episodes sooner than HIV-negative children.
METHODS
Study Location and Population
At the beginning of this study, the prevalence of HIV infection among patients at antenatal clinics in the mainland of Tanzania was 8.4%, with mother-to-child transmission accounting for 18% of new infections [18]. Per annum, 1 million clinical malaria episodes are reported by health facilities in Dar es Salaam [19], where malaria is endemic and stable with year-round transmission. Annual malaria prevalence is 12%, with 1.28 infectious bites per person per year [20]. Thirty-one percent of Tanzanian children aged <5 years regularly sleep under insecticide-treated bed nets; coverage is higher (≥65%) in the eastern region, which includes Dar es Salaam [21].
This study was nested in a randomized controlled trial evaluating the impact of child multimicronutrient supplementation among 2387 singleton live-born infants whose mothers were HIV-positive during pregnancy and intended to stay in Dar es Salaam for at least 2 years after delivery. Children were randomized at 6 weeks to receive a daily multivitamin regimen (vitamins B, C, and E) or placebo. Children whose mothers were unable to return for follow-up, infants with serious congenital anomalies that interfered with study procedure compliance (eg, inability to take a daily micronutrient regimen), and nonsingletons were excluded. The mother-child pair returned to the clinic monthly for scheduled visits during which health history and nutritional status were determined and laboratory assessments were made. Study participants were encouraged to return to the clinic for management of illnesses that occurred outside of scheduled visits. The study was conducted between February 2004 and May 2008.
Per standard of care, all mothers received folate, iron, and intermittent preventive malaria therapy with sulfadoxine-pyrimethamine at the 20th and 30th weeks of pregnancy. Initially, maternal antiretroviral (ARV) medication was limited to nevirapine that was administered prophylatically during labor to prevent intrapartum HIV transmission. From July 2005 on, mothers began to be routinely evaluated for ARV eligibility. Eligible mothers initiated ARV with drugs supplied by the President's Emergency Plan for AIDS Relief (PEPFAR) and other programs. All newborns received nevirapine within 72 hours of birth and were prescribed cotrimoxazole until 6 months old; thereafter, only breast-feeding and HIV-positive children continued to receive cotrimoxazole.
Malaria was diagnosed during monthly or unscheduled visits by clinicians as follows:
Clinical malaria: Confirmation of malaria diagnosis prior to treatment is recommended by the World Health Organization (WHO) [2]. However, WHO recognizes that confirmation may be inaccessible in some resource-limited settings. In that case, prompt treatment based on physician diagnosis is recommended, to minimize malaria-related deaths. This reflects the standard of clinical practice in the area where malaria is symptomologically diagnosed by a physician, with/without laboratory testing for parasitemia, following integrated management of childhood illnesses (IMCI) guidelines [22, 23]. This approach is sensitive for malaria diagnosis but limited by low specificity [23].
Probable malaria: Probable malaria was defined as a malaria episode for which results of a laboratory test for parasitemia had positive or unknown results. Physician ordering of laboratory tests is indicative of heightened clinical suspicion, as these were typically done for febrile episodes not responding to treatment, to rule out severe or cerebral malaria in this setting.
Confirmed malaria: Confirmed malaria was defined as a malaria episode for which clinical suspicion was confirmed by observation of any parasitemia in a blood smear. Thin smears stained with Giemsa were made from a finger or heel prick and left to air dry for about 30 minutes to assess malaria parasites in the blood. Trained laboratory technicians read each blood slide in 3 different fields, and the parasite density per cubic millimeter was estimated from the number of parasites per 200 leukocytes.
Clinical malaria or malaria-related death (MRD): MRDs are deaths for which malaria was the primary or secondary cause. One third of all child deaths in this study were MRDs. Therefore, we analyzed clinical malaria or MRD as a composite end point to determine its association with HIV status. A verbal autopsy was performed in all cases of child death. Coding of the cause of death from the verbal autopsies was performed independently by 2 pediatricians (K. P. M. and C. D.), and differences were adjudicated by a third pediatrician.
HIV status was determined at 6 weeks by HIV DNA polymerase chain reaction, using Amplicor HIV-1 DNA, version 1.5 (Roche Molecular Systems, Branchburg, NJ). At 18 months of age, HIV serostatus was determined using Murex HIV antigen/antibody (Abbott Murex, Dartford, United Kingdom), followed by the Enzygnost anti-HIV-1/2 Plus enzyme-linked immunosorbant assay (Dade Behring, Marburg, Germany), and the discordant results were resolved through Western blot assay [24]. For children who were HIV positive at 24 months, back-testing of blood samples from months 6, 12, and 18 were done to determine the window of seroconversion.
CD4 cell count and CD4 cell percentage were used as markers of maternal and child immunologic status, respectively, and were defined as high versus low (for mothers, absolute CD4 cell counts of <350 vs ≥350 cells/mL and for children CD4 cell percentages of <25% vs ≥25%), in light of CD4 distribution in our data and using earlier studies as precedents for determining biologically relevant CD4 thresholds [25]. For children, CD4 cell percentage was determined by the FACSCalibur system (Becton Dickinson, San Jose, CA) as the ratio of the CD4 cell count to the total lymphocyte count. Both child and maternal indicators of immunologic status were time updated within each follow-up interval.
Statistical Analyses
Children with no HIV or malaria data were excluded from all analyses. Incident malaria analyses were restricted to children without malaria at baseline. We conducted 3 sets of analyses. First, Cox proportional hazards models were used to estimate hazard ratios (HRs) for current HIV-positive versus HIV-negative status in relation to development of first malaria and to the composite end point, clinical malaria or MRD. Second, we estimated malaria rate differences per 100 child-years for current HIV-positive versus HIV-negative children by means of a generalized estimating equations (GEE) model with multiple observations per child, using an unstructured working correlation structure and identity link [26]. Within-subject clustering was specified for this GEE model to account for nonindependence of repeated malaria episodes for some children. Following previous analyses of Plasmodium falciparum malaria, we distinguished between malaria episodes that occurred >14-days apart [6, 27]. Third, we evaluated the potential for synergy between malaria and HIV infection in the development of subsequent malaria among children with a prior history of malaria. In this analysis, we estimated the extent to which children with both infections had a shorter time to the development of a subsequent malaria episode, using Cox proportional hazards models. For this analysis, the time of the first clinical, probable, and confirmed malaria diagnosis for each child was taken as baseline.
For the Cox proportional hazards models, we used an Anderson-Gill data structure to split each child's follow-up period into blocks of person-time reflecting the interval between 2 consecutive visits. Within each interval, beginning and end time were defined as age at the beginning and end of the interval respectively. All time-varying covariates including breast-feeding, HIV infection, cotrimoxazole compliance, and child and maternal indicators of immunologic status were updated to their values at the start of each interval.
We expected that maternal and child immunologic status (defined as CD4 cell count for mothers or CD4 cell percentage for children) could be modifiers, mediators, and confounders of the HIV infection and malaria association. Therefore, we examined the possibility of both confounding and effect modification in multivariate models. Immunologic status was deemed a confounder if its adjustment changed the magnitude of the HIV infection–malaria association by ≥10% in either direction. To examine effect modification, we separately introduced the product terms “HIV*maternal CD4 cell count” or “HIV*child CD4 cell percentage” in a multivariate model already containing separate main effects for HIV infection and maternal CD4 cell count or child CD4 cell percentage and examined improvement in overall model fit, using the likelihood ratio test. If the addition of the interaction term improved the model fit as judged by difference in likelihood ratio tests for models with and without the interaction term, analyses were repeated within the strata of high versus low maternal or child immunologic status.
All multivariate models adjusted for season of consultation (in 2 categories: long-duration rains [November–February] and short-duration rains [March–May] vs dry season [June–October]) and several child factors (sex, low birth weight, CD4 cell percentage of <25 vs ≥25, cotrimoxazole compliance, and breast-feeding status [yes vs no]), intervention regimen (multivitamin vs placebo), maternal factors (age, history of early neonatal mortality, CD4 cell count of <350 vs ≥350 cells/μL, years of education, and marital status), and household factors (socioeconomic status, defined as a family per-capita daily food expenditure of >500 Tanzanian shillings [equivalent to approximately US$0.37 at the time of writing). Cotrimoxazole compliance was determined on the basis of the mothers’ response to the question, “Did you give any cotrimoxazole to your child over the past month?” Final models included all potential confounders that changed the effect estimate by ≥10% in either direction, as well as above mentioned potential risk factors for malaria, even if these did not meet the strict criteria for confounding. All analyses were conducted in SAS, version 9.1 (SAS Institute, Cary, NC).
Ethical clearance for the conduct of the parent trial was provided by the institutional review boards of the Harvard School of Public Health and Muhimbili University of Health and Allied Sciences. The mothers of all children provided written informed consent for their own and their child's participation in the trial.
RESULTS
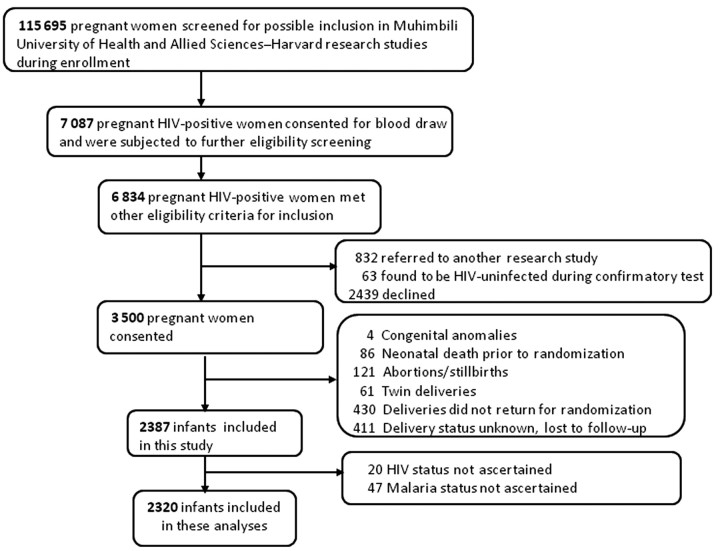
Included in this secondary analysis are 2320 children derived as specified in Figure 1. At baseline, 240 children (10%) had clinical malaria, 63 (3%) had probable malaria, and 21 (1%) had confirmed malaria, and 255 (11%) were HIV positive. Relative to HIV-negative children, HIV-positive children had more clinical malaria episodes and lower body weights and CD4 cell levels at baseline. There was no difference in the prevalence of probable and confirmed malaria by baseline HIV status (Table 1). Children were followed for a median of 92 weeks (interquartile range [IQR], 37–147 weeks), during which another 4% (91) became HIV positive. Forty percent (143) of children who were ever HIV positive were receiving ARV therapy.
Figure 1.
Screening, recruitment, and randomization into parent study, and derivation of analytic sample.
Table 1.
Characteristics of 2320 Enrolled Children at Baseline by Human Immunodeficiency Virus (HIV) Status
| Characteristic | HIV Positivea | HIV Negative | Pb |
|---|---|---|---|
| Enrolled subjects | 255 (11) | 2043 (89) | |
| Maternal and household factors | |||
| Maternal age at enrollment (years) | 28.5 ± 4.9 | 28.2 ± 5.0 | .52 |
| Maternal baseline CD4 T-cell count (cells/μL) | 467 ± 516 | 549 ± 351 | <.001 |
| Maternal education (years) | 7.2 ± 2.4 | 7.2 ± 2.8 | .80 |
| Parity, including current child | 2.6 ± 1.3 | 2.6 ± 1.3 | .62 |
| Maternal history of ≥1 early neonatal mortalityc | 15 (5.9) | 83 (4.1) | .17 |
| Married/living with partner | 212 (84.5) | 1764 (87.2) | .23 |
| Household daily food expenditure >500 T shillingd | 182 (71.4) | 1436 (70.3) | .72 |
| Child factors | |||
| Age at enrollment (days) | 41.0 ± 3.0 | 40.6 ± 2.9 | .025 |
| Birth weight (kg) | 2.9 ± 0.5 | 3.1 ± 0.5 | <.001 |
| Weight at baseline (kg) | 4.1 ± 0.7 | 4.5 ± 0.7 | <.001 |
| Baseline CD4 percentage (%) | 29.4 ± 10.3 | 38.0 ± 10.6 | <.001 |
| Sex (male) | 124 (48.6) | 1118 (54.8) | .066 |
| Low birth weight (<2500 g) | 42 (17.5) | 110 (5.6) | <.001 |
| Underweight (WAZ ≤ −2) | 52 (20.6) | 148 (7.3) | <.001 |
| CD4 cell percentage | |||
| <15% | 16 (7.7) | 37 (2.3) | <.001 |
| 15%–25% | 54 (26.0) | 132 (8.0) | |
| >25% | 138 (66.4) | 1475 (89.7) | |
| Malaria outcome | |||
| Clinical malaria | 42 (16.5) | 198 (9.7) | <.001 |
| Probable malaria with laboratory test | 9 (3.5) | 54 (2.6) | .41 |
| Confirmed malaria | 4 (1.6) | 17 (0.8) | .24 |
Data are no. (%) of children or mean ± SD.
Abbreviations: IQR, interquartile range; WAZ, weight-for-age z score.
a Baseline HIV positivity denotes child seroconversion ≤30 days of baseline.
b Estimated via t tests for differences in mean, by HIV status, for continuous variables; for tests of differences in proportion, χ2 tests with appropriate degrees of freedom are used.
c Number of children born alive but died ≤7 days of birth.
d At the time this study was conducted, 1350 Tanzanian shillings was equal to US$1 and 500 Tanzanian shillings was equal to approximately US$0.37.
During follow-up, 1380 of 2075 (66.6%), 899 of 2256 (41.8%), and 418 (19%) children developed ≥1 episode of clinical, probable, and confirmed malaria, respectively. Of the 768 laboratory tests for parasitemia in which results were available, 35 (5%) were negative. Overall mean parasitemia density (±SD) was 3.12 ± 3.56 trophozoites per 200 white blood cells. HIV-positive children were at higher risk of developing a first clinical, probable, and confirmed malaria episode, compared with HIV-negative children, with estimates of higher risk ranging from 34% (95% CI, 12%–61%) for clinical malaria to 67% (95% CI, 18%–136%) for confirmed malaria (Table 2). Including all malaria events, HIV-positive children experienced more episodes of malaria per 100 child-years than HIV-negative children. The estimated risk difference in malaria per 100 child-years for HIV-positive relative to HIV-negative children ranged from 20 (95% CI, 9–31) for confirmed malaria to 88 (95% CI, 65–148) for clinical malaria (Table 3). The difference in malaria rate per 100 child-years for HIV-positive versus HIV-negative children decreased with increasing specificity of malaria diagnosis; however, all estimates were robust to adjustment for season, per-capita daily food expenditure, child characteristics (sex, underweight, treatment group, breast-feeding status, and cotrimoxazole compliance), and maternal factors (age, educational status, baseline CD4 cell count, and early neonatal mortality history) (Tables 2 and 3).
Table 2.
Current Human Immunodeficiency Virus (HIV) Status in Relation to First Bout of Malaria in Children of HIV-Positive Women From Dar es Salaam, Tanzania
| No. of Children |
Univariate Model |
Multivariate Modela |
||||
|---|---|---|---|---|---|---|
| Outcome | With Event | Without Event | HRb | (95% CI) | HR | (95% CI) |
| Clinical malaria | 1380 | 851 | 1.26 | (1.07–1.48) | 1.34 | (1.12–1.61) |
| Probable malaria | 899 | 1438 | 1.39 | (1.15–1.66) | 1.47 | (1.19–1.81) |
| Confirmed malaria | 418 | 1949 | 1.42 | (1.05–1.93) | 1.67 | (1.18–2.36) |
| Combined malaria/mortality end point | ||||||
| Clinical malaria or malaria-related death | 1393 | 838 | 1.26 | (1.07–1.48) | 1.34 | (1.12–1.61) |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Multivariate models are adjusted for several time varying and non–time-varying child, maternal, climatic, and household factors. Baseline covariates adjusted include child sex, supplementation (micronutrient vs placebo), birth weight (<2500 vs ≥2500 g), per-capita household daily food expenditure (≤500 vs >500 Tanzanian shillings), maternal age (<25, 25–29, 30–34 vs ≥35 years), maternal education (<7, 7 vs >7 years), and maternal history of prior early neonatal mortality. The time-varying covariates adjusted for include current cotrimoxazole use, current breast-feeding status, season of visit, and current maternal CD4 cell count (<350 vs ≥350 cells/μL).
b Regression models show the relative hazard of developing a first malaria episode or the composite end point clinical malaria or malaria specific mortality for HIV-positive children versus HIV-negative children.
Table 3.
Malaria Episodes for Children With and Without Human Immunodeficiency Virus (HIV) Infection of HIV-Infected Women From Dar es Salaam, Tanzania
| No. of Malaria Episodes per 100 Child-Years |
Univariate Rate Difference per 100 Child-Years |
Adjusted Rate Difference per 100 Child-Yearsa |
|||||
|---|---|---|---|---|---|---|---|
| Outcome | All | HIV negative | HIV positive | RD | (95% CI) | RD | (95% CI) |
| Clinical malaria | 182.6 | 175.6 | 221.6 | 52.5 | (29.5–75.6) | 88.8 | (64.5–113.2) |
| Probable malaria | 62.3 | 56.3 | 96.1 | 32.9 | (17.4–48.5) | 35.8 | (18.8–52.8) |
| Confirmed malaria | 21.5 | 19.1 | 34.9 | 14.7 | (4.9–24.5) | 19.8 | (8.9–30.7) |
Abbreviations: CI, confidence interval; RD, rate difference.
a Estimates are derived from a normal generalized estimation equation model with identity link and unstructured working covariance. Multivariate models are adjusted for several time-varying and non–time-varying child, maternal, climatic, and household factors. Baseline covariates adjusted include child sex, supplementation (micronutrient vs placebo), birth weight (<2500 vs ≥2500 g), per-capita household daily food expenditure (≤500 vs >500 Tanzanian shillings), maternal age (<25, 25–29, 30–34 vs ≥35 years), maternal education (<7, 7 vs >7 years), and maternal history of prior early neonatal mortality. The time-varying covariates adjusted for include percentage with cotrimoxazole compliance to date, exclusive breast-feeding status, season of visit, maternal CD4 cell count (<350 vs ≥350 cells/μL), and time-updated child CD4 cell percentage (<25% vs ≥ 25%).
Among children with ≥1 malaria episodes during the study period, repeat infections were common, with 1121 of 1547 (72.5%), 442 of 942 (46.9%), and 140 of 439 (31.9%) experiencing ≥1 more clinical, probable, and confirmed malaria episode, respectively, during follow-up. The risk of developing a subsequent clinical, probable, and confirmed malaria episode was higher for HIV-positive relative to HIV-negative children, with relative hazard ratios ranging from 28% (95% CI, 4%–57%) for clinical malaria to 127% (95% CI, 32%–289%) for confirmed malaria episodes (Table 4). The association between HIV infection and the combined end point of clinical malaria or MRD were similar in magnitude and direction to that for HIV infection and clinical malaria (Tables 2 and 4).
Table 4.
Current Human Immunodeficiency Virus (HIV) Status in Relation to Development of a Second Malaria Episode Among Children With ≥1 Malaria Episode
| No. of Malaria Episodes |
Univariate Association |
Multivariate Modela |
||||
|---|---|---|---|---|---|---|
| Outcome | ≥1 | 1 | HRb | (95% CI) | HR | (95% CI) |
| Clinical malaria | 1119 | 426 | 1.24 | (1.03–1.48) | 1.28 | (1.04–1.57) |
| Probable malaria | 442 | 500 | 1.50 | (1.17–1.94) | 1.60 | (1.20–2.14) |
| Confirmed malaria | 140 | 299 | 1.58 | (1.02–2.47) | 2.27 | (1.32–3.89) |
| Subsequent clinical malaria or malaria-related death | 1121 | 424 | 1.25 | (1.05–1.50) | 1.30 | (1.06–1.54) |
Abbreviations: CI, confidence interval; HR, hazard ratio.
a Multivariate models are adjusted for several time-varying and non–time-varying child, maternal, climatic, and household factors. Baseline covariates adjusted include child sex, supplementation (micronutrient vs placebo), birth weight (<2500 vs ≥2500 g), per-capita household daily food expenditure (≤500 vs >500 Tanzanian shillings), maternal age (<25, 25–29, 30–34 vs ≥35 years), maternal education (<7, 7 vs >7 years) and maternal history of prior early neonatal mortality. The time-varying covariates adjusted for include current cotrimoxazole use, current breast-feeding status, season of visit, and current maternal CD4 cell count (<350 vs ≥350 cells/μL).
b Regression models show the relative hazard of developing a second malaria episode or the composite end point of clinical malaria or malaria specific mortality for HIV-positive children versus HIV-negative children.
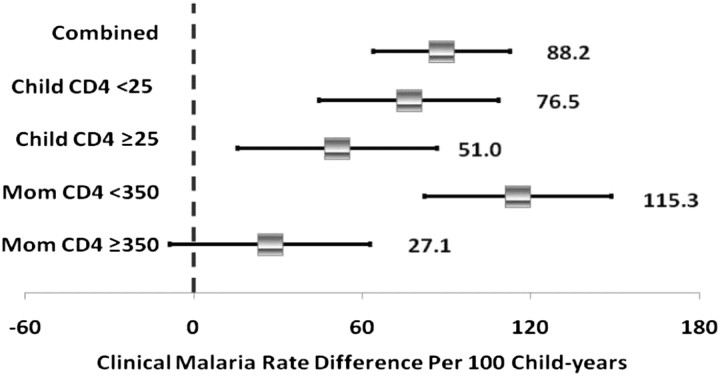
Among HIV-positive children, there was no difference in clinical (HR, 1.39; 95% CI, 0.67–2.86), probable (HR, 1.18; 95% CI, 0.62–2.23), and confirmed (HR, 0.86; 95% CI, 0.18–4.01) malaria incidence for children receiving ARVs, relative to those not receiving ARVs. The prevalence of any breast-feeding and cotrimoxazole use was 98.5% and 71%, respectively. Current breast-feeding (HR, 0.21; 95% CI, 0.13–0.34) and cotrimoxazole use (HR, 0.69; 95% CI, 0.57–0.83) protected against the development of malaria. Low versus high current maternal CD4 cell count (HR, 0.91; 95% CI, .82–1.02) and child CD4 cell percentage (HR, 0.90; 95% CI, .77–1.04) were not independent predictors of malaria incidence. However, the rate of clinical malaria per 100 child-years was modified by maternal (P value for interaction = .03) and child (P value for interaction = .02) immunologic status, such that the association between HIV infection and malaria rates was generally strongest in the strata of lower immunologic competence (Figure 2). For both probable and confirmed malaria episodes, trends of stronger associations between HIV infection and malaria rates per 100 child-years persisted among children with a CD4 cell percentage of <25%, compared with ≥25%, and among mothers with a CD4 cell count of <350, compared with ≥350 cells/μL, but these differences were not statistically significant (data not shown).
Figure 2.
Clinical malaria rates by strata of child CD4 cell percentage (in %) and maternal CD4 cell count (in cells/μL). Estimates are rate differences derived from a generalized estimation equation models with normal distribution, identity link, and unstructured working covariance. Baseline covariates adjusted for include child sex, supplementation (micronutrient vs placebo), birth weight (<2500 vs ≥2500 g), per-capita household daily food expenditure (≤500 vs >500 Tanzanian shillings), maternal age (<25, 25–29, 30–34 vs ≥35 years), maternal education (<7, 7 vs >7 years), and maternal history of prior early neonatal mortality. The time-varying covariates adjusted for include percentage with cotrimoxazole compliance to date, exclusive breast-feeding status, season of visit, maternal CD4 cell count (<350 vs ≥350 cells/μL), and time-updated child CD4 cell percentage (<25% vs ≥25%).
DISCUSSION
These recent data from Tanzania suggest that HIV-infected children were more likely than HIV-negative children to develop malaria over 2 years of follow-up. Repeat malaria episodes were common, and children coinfected with HIV had a 28%–127% higher risk of developing a second malaria episode as compared to HIV-negative children. This finding suggests the presence of synergistic negative interactions between HIV infection and malaria in coinfected children that may amplify malarial morbidity in such children. Equally important, we confirmed well-described protective roles of breast-feeding [28] and cotrimoxazole use [29] in child malarial morbidity. These results were generally robust to malaria diagnostic criteria, as well as to adjustment for potential confounding by seasonality and several child and maternal factors.
Our finding of a positive association between HIV-positive status and malaria incidence is consistent with reported findings among adults in Malawi and Uganda [6, 7] and corroborates findings from 2 previous reports of more severe malaria morbidity and mortality for HIV-positive versus HIV-negative Kenyan and Ugandan children [15, 16]. Our results are also consistent with findings from a Ugandan study of 77 HIV-infected and 232 HIV-uninfected children of HIV-positive mothers and 125 age-matched HIV-unexposed children followed from birth to 4 years old. In line with our findings, these investigators found that HIV-infected children developed malaria earlier and had more severe morbidity (hospitalization and requirement of blood transfusions) per malaria episode than HIV-uninfected children [13]. However, paradoxically and in contradiction with our results, the same study also found that HIV-positive status protected against the development of malaria in the first, second, third, and fourth years of life. Of note, follow up duration was much shorter for HIV-positive relative to HIV-negative children (median, 19.5 vs 31.4 months) because of AIDS-related death in HIV-positive children. Hence, this contradiction could be partially explained by mortality-driven noncomparability of malaria at-risk periods for HIV-positive relative to HIV-negative children.
Our finding of positive associations between HIV-positive status and malarial morbidity is also in conflict with findings from 3 other studies [9, 11, 12]. Unlike our study, these found no difference in malaria prevalence [11], malaria incidence [9], and malaria frequency by HIV status. There was also no difference in malaria parasitemia density [11] and response to treatment [12] for HIV-positive versus HIV-negative infants [9, 11, 12]. We note salient design differences between our study and these others, including short follow-up duration [9, 12], use of data from early instead of later stages of the HIV epidemic [9, 11, 12], statistical power limitation due to small sample sizes [9, 11, 12], and use of hospitalized pediatric HIV-positive populations and hospital-based HIV-negative controls [9, 11, 12] that may limit the direct comparison of our results with these studies. Future epidemiologic investigations of this relationship will be useful to confirm or refute our findings.
One mechanism by which HIV infection could increase malaria morbidity is via impairment of malaria-specific T-cell immunity [10]. This in turn raises the probability that concurrent HIV infection could increase the risk and severity of malaria in coinfected populations. In this study, even though maternal and child CD4 cell counts were not independently associated with malaria incidence, the estimated association between HIV infection and malaria incidence were attenuated with adjustment for these immunologic parameters, suggesting that the impairment of malaria specific T-cell immunity could be a mechanism of effect especially among children with low baseline CD4 cell levels. However, the fact that the higher risk of malaria for HIV-positive as compared to HIV-negative children was often robust to adjustment for both child and maternal CD4 cell counts also indicates that other mechanisms may be important.
We note 2 limitations of our study that should be considered in the interpretation of our results. First, we were unable to adjust for bed net use, which is a well-known factor protective against the risk of malaria and a possible surrogate marker of adaptive maternal health–seeking behavior that may be associated with child HIV status. If bed net use occurred more frequently among HIV-positive children, the lack of control for this variable may have underestimated the HIV infection–malaria association in this study. Alternatively, less frequent bed net use among HIV-positive children will result in overestimation of the HIV infection–malaria association in this study. Second, our findings may be affected by malaria detection bias to the extent that diagnosis was contingent on children exhibiting symptoms and their mothers seeking care at study clinics. However, asymptomatic malaria in P. falciparum–infected children <2 years old is rare because o the absence of age-acquired adaptive immunity. Therefore, failure to detect asymptomatic malarial morbidity is likely to have only limited effect on our findings.
In contrast, our study has the following design strengths: its large sample size, prospective study design, and ability to control for important baseline and time-varying confounders, including seasonality, breast-feeding, and cotrimoxazole use. In Tanzania, malaria-related morbidity and mortality account for >30% of the disease burden in the general population and represent the leading cause of both outpatient and in-hospital death among children <5 years old [30]. We examined the relationship between HIV infection and development of malarial morbidity in this region of high HIV infection prevalence and stable malaria transmission within a cohort highly susceptible to malarial morbidity. These factors enabled us to contribute much needed information regarding the interaction between HIV infection and development of malarial morbidity in HIV-exposed children. An additional strength of this study is the ability to compare the HIV infection–malaria association across different malaria definitions with increasing specificity. We found the IMCI malaria definition that was based on physician clinical diagnosis to be a useful index of malarial morbidity. Despite its expected higher rate of misclassification, the direction of associations with HIV infection and the ultimate inference regarding the association between HIV infection and malaria remained unchanged, lending support to the clinical usefulness of IMCI-based definitions for malaria in resource-limited settings where confirmation by laboratory tests may be difficult. However, to avoid potentially harmful and unnecessary malaria treatment, getting definitive malaria diagnosis via rapid diagnostic assays, which are increasingly available at reasonable cost, should be done whenever possible, even in the most resource-limited settings.
In conclusion, HIV-positive children have a higher risk of developing malaria as compared to HIV-negative children. For those with malaria, HIV-coinfected children developed subsequent episodes earlier than HIV-uninfected children, despite prophylactic use of cotrimoxazole and widespread infant breast-feeding in the study population. Hence, all children, particularly those exposed to HIV in utero, will benefit from malaria prevention efforts and proactive clinical management for malaria in regions of substantial HIV infection and malaria overlap. In this era of widening access to treatment for HIV-infected patients, health practitioners, and policy makers in setting where malaria and HIV infection are coendemic will benefit from definitive evaluation of whether HIV-positive individuals receiving malaria prophylaxis are further protected from malarial morbidity, using a randomized placebo-controlled trial.
Notes
Acknowledgments. We thank the study participants from Dar es Salaam, Tanzania, and the study staff, for making this study possible; and the following field staff, for their diligence and energy: Rehema Mtonga, Illuminata Ballonzi, Godwin Njiro, Frank Killa, Emily Denzer, Elizabeth Long, and Jenna Golan. A. E. E. is grateful to the Harvard School of Public Health Yerby Postdoctoral Fellowship Program, for salary and professional development support.
Financial support. This work was funded by the National Institute of Child Health and Development, National Institutes of Health (NIH; grant R01 HD043688) and the NIH (grant K24 HD058795 to C. D.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.UN Joint Programme on HIV/AIDS. Global Report: UNAIDS Report on the Global AIDS Epidemic: 2010. December 2010, ISBN 978-92-9173-871-7, available at: http://www.unhcr.org/refworld/docid/4cfca9c62.html . Accessed 3 April 2012. [Google Scholar]
- 2.WHO. World Malaria Report: 2010. 2011 Geneva, Switzerland: World Health Organization. [Google Scholar]
- 3.Hewitt K, Steketee R, Mwapasa V, Whitworth J, French N. Interactions between HIV and malaria in non-pregnant adults: evidence and implications. AIDS. 2006;20:1993–2004. doi: 10.1097/01.aids.0000247572.95880.92. [DOI] [PubMed] [Google Scholar]
- 4.Kamya MR, Gasasira AF, Yeka A, et al. Effect of HIV-1 infection on antimalarial treatment outcomes in Uganda: a population-based study. J Infect Dis. 2006;193:9–15. doi: 10.1086/498577. [DOI] [PubMed] [Google Scholar]
- 5.Abu-Raddad LJ, Patnaik P, Kublin JG. Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science. 2006;314:1603–6. doi: 10.1126/science.1132338. [DOI] [PubMed] [Google Scholar]
- 6.Patnaik P, Jere CS, Miller WC, et al. Effects of HIV-1 serostatus, HIV-1 RNA concentration, and CD4 cell count on the incidence of malaria infection in a cohort of adults in rural Malawi. J Infect Dis. 2005;192:984–91. doi: 10.1086/432730. [DOI] [PubMed] [Google Scholar]
- 7.Whitworth J, Morgan D, Quigley M, et al. Effect of HIV-1 and increasing immunosuppression on malaria parasitaemia and clinical episodes in adults in rural Uganda: a cohort study. Lancet. 2000;356:1051–6. doi: 10.1016/S0140-6736(00)02727-6. [DOI] [PubMed] [Google Scholar]
- 8.Mermin J, Lule J, Ekwaru JP, et al. Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet. 2004;364:1428–34. doi: 10.1016/S0140-6736(04)17225-5. [DOI] [PubMed] [Google Scholar]
- 9.Colebunders R, Bahwe Y, Nekwei W, et al. Incidence of malaria and efficacy of oral quinine in patients recently infected with human immunodeficiency virus in Kinshasa, Zaire. J Infect. 1990;21:167–73. doi: 10.1016/0163-4453(90)91701-e. [DOI] [PubMed] [Google Scholar]
- 10.Good MF, Doolan DL. Immune effector mechanisms in malaria. Curr Opin Immunol. 1999;11:412–9. doi: 10.1016/S0952-7915(99)80069-7. [DOI] [PubMed] [Google Scholar]
- 11.Muller O, Moser R. The clinical and parasitological presentation of Plasmodium falciparum malaria in Uganda is unaffected by HIV-1 infection. Trans R Soc Trop Med Hyg. 1990;84:336–8. doi: 10.1016/0035-9203(90)90306-y. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen-Dinh P, Greenberg AE, Mann JM, et al. Absence of association between Plasmodium falciparum malaria and human immunodeficiency virus infection in children in Kinshasa, Zaire. Bull World Health Organ. 1987;65:607–13. [PMC free article] [PubMed] [Google Scholar]
- 13.Kalyesubula I, Musoke-Mudido P, Marum L, et al. Effects of malaria infection in human immunodeficiency virus type 1-infected Ugandan children. Pediatr Infect Dis J. 1997;16:876–81. doi: 10.1097/00006454-199709000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Villamor E, Fataki MR, Mbise RL, Fawzi WW. Malaria parasitaemia in relation to HIV status and vitamin A supplementation among pre-school children. Trop Med Int Health. 2003;8:1051–61. doi: 10.1046/j.1360-2276.2003.01134.x. [DOI] [PubMed] [Google Scholar]
- 15.Malamba S, Hladik W, Reingold A, et al. The effect of HIV on morbidity and mortality in children with severe malarial anaemia. Malar J. 2007;6:143. doi: 10.1186/1475-2875-6-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otieno RO, Ouma C, Ong'echa JM, et al. Increased severe anemia in HIV-1-exposed and HIV-1-positive infants and children during acute malaria. AIDS. 2006;20:275–80. doi: 10.1097/01.aids.0000200533.56490.b7. [DOI] [PubMed] [Google Scholar]
- 17.Renia L, Potter SM. Co-infection of malaria with HIV: an immunological perspective. Parasite Immunol. 2006;28:589–95. doi: 10.1111/j.1365-3024.2006.00903.x. [DOI] [PubMed] [Google Scholar]
- 18.TCAIDS. Tanzania Commission for AIDS: UNGASS Country Progress Report Tanzania Mainland. 2008 Dar es Salaam, Tanzania: Tanzania Commission on AIDS. [Google Scholar]
- 19.Dongus S, Nyika D, Kannady K, et al. Urban agriculture and Anopheles habitats in Dar es Salaam, Tanzania. Geospat Health. 2009;3:189–210. doi: 10.4081/gh.2009.220. [DOI] [PubMed] [Google Scholar]
- 20.Geissbuhler Y, Kannady K, Chaki PP, et al. Microbial larvicide application by a large-scale, community-based program reduces malaria infection prevalence in urban Dar es Salaam, Tanzania. PLoS One. 2009;4:e5107. doi: 10.1371/journal.pone.0005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Bureau of Statistics (NBS) [Tanzania] and ORC Macro. Tanzania Demographic and Health Survey 2004–05. Dar es Salaam, Tanzania: National Bureau of Statistics and ORC Macro. 2008 [Google Scholar]
- 22.Erdman LK, Kain KC. Molecular diagnostic and surveillance tools for global malaria control. Travel Med Infect Dis. 2008;6:82–99. doi: 10.1016/j.tmaid.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Tangpukdee N, Duangdee C, Wilairatana P, Krudsood S. Malaria diagnosis: a brief review. Korean J Parasitol. 2009;47:93–102. doi: 10.3347/kjp.2009.47.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aboud S, Urassa W, Lyamuya E, Mhalu F, Biberfeld G. Evaluation of HIV antibody and antigen/antibody combination ELISAs for use in an alternative confirmatory HIV testing strategy in Dar es Salaam, Tanzania. J Virol Methods. 2006;135:192–6. doi: 10.1016/j.jviromet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 25.Callens SF, Kitetele F, Lusiama J, et al. Computed CD4 percentage as a low-cost method for determining pediatric antiretroviral treatment eligibility. BMC Infect Dis. 2008;8:31. doi: 10.1186/1471-2334-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeger SL, Liang KY. An overview of methods for the analysis of longitudinal data. Stat Med. 1992;11:1825–39. doi: 10.1002/sim.4780111406. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization (WHO) Geneva: WHO; 1996. Assessment of therapeutic efficacy of antimalarial drugs for uncomplicated falciparum malaria in areas with intense transmission [WHO/MAL/96.1077] [Google Scholar]
- 28.Vora N, Homsy J, Kakuru A, et al. Breastfeeding and the risk of malaria in children born to HIV-infected and uninfected mothers in rural Uganda. J Acquir Immune Defic Syndr. 2010;55:253–61. doi: 10.1097/QAI.0b013e3181eb4fd7. [DOI] [PubMed] [Google Scholar]
- 29.Sandison TG, Homsy J, Arinaitwe E, et al. Protective efficacy of co-trimoxazole prophylaxis against malaria in HIV exposed children in rural Uganda: a randomised clinical trial. BMJ. 2011;342:d1617. doi: 10.1136/bmj.d1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Republic of Tanzania. 2002. National Malaria Medium Term Strategic Plan, 2002–2007. 2003 2008. [Google Scholar]