Abstract
Background
Bacterial vaginosis (BV) represents shifts in microbiota from Lactobacillus spp. to diverse anaerobes. Although antibiotics relieve symptoms and temporarily eradicate BV-associated bacteria (BVAB), BV usually recurs. We investigated the role of extravaginal BVAB reservoirs in recurrence.
Methods
Risks for BV acquisition over the course of 1 year were defined. DNA in vaginal, anal, and oral swab samples from enrollment was subjected to quantitative polymerase chain reaction assays targeting 16S ribosomal RNA genes of Gardnerella vaginalis, Lactobacillus crispatus, BVAB1, BVAB2, BVAB3, Megasphaera spp., Lactobacillus jensenii, and Leptotrichia/Sneathia spp. A case-control approach analyzed BVAB detection at enrollment for case patients (BV acquisition) versus controls (none).
Results
Of 239 women enrolled without BV, 199 were seen in follow-up, and 40 experienced BV; 15 had all samples for analysis. Detection of G. vaginalis in oral cavity or anal samples and Leptotrichia/Sneathia spp. in anal samples was more common at enrollment among case patients, who also had higher concentrations of these bacteria and Megasphaera relative to 30 controls at each site. In contrast, L. crispatus was detected more frequently in anal samples among controls.
Conclusions
Women who acquire BV are more likely have previous colonization of extravaginal reservoirs with some BVAB, and less likely to have L. crispatus, suggesting that BVAB may be acquired vaginally from extravaginal reservoirs.
Bacterial vaginosis (BV) is the most prevalent vaginal infection in reproductive-age women, and has been consistently associated with adverse outcomes related to upper genital tract complications and with increased risk of human immunodeficiency virus acquisition [1–3]. Of 3739 women enrolled during 2001–2004 in a nationally representative sample of the US civilian noninstitutionalized population, almost 1 in 3 (29.2%; 95% confidence interval, 27.2–31.3) had BV by Gram stain of vaginal fluid samples [4, 5].
Microbiologically, BV is distinguished by depletion of hydrogen peroxide–producing lactobacilli that characterize normal vaginal microbiota, with profound overgrowth of anaerobic bacteria [6]. However, the etiology of BV remains unclear; few studies have followed women prospectively for incident BV. These have reported that African American race, report of the same male or any female sex partner during follow-up, and higher Nugent score at initial evaluation were risks [7, 8]. One study found that BV acquisition was associated with African American race, cigarette smoking, vaginal intercourse, receptive anal sex before vaginal intercourse, sex with an uncircumcised male partner, lack of precedent hydrogen peroxide–producing vaginal lactobacilli, and detection of herpes simplex virus type serum antibodies at the visit before BV diagnosis [9].
Women who have sex with women have had a high prevalence of BV (25%–52%) in several cross-sectional studies [4, 10–14]. We recently reported risks for incident BV in a cohort of women who reported sex with women in the previous year [15]. Although most participants (80%) without BV at enrollment had vaginal Lactobacillus crispatus detected by polymerase chain reaction (PCR), 20% acquired BV in the year of follow-up. Risks for incident BV included detection of several BV-associated bacteria (BVAB) or absence of L. crispatus in vaginal fluid at the enrollment (BV-negative) visit, and evaluation for incident BV in the 2 weeks after menses. Despite collection of extensive sexual behaviors using computer-assisted self-interview (CASI), only report of a new female sex partner with a history of BV was associated with increased risk of BV acquisition in univariate analysis. A dose-response relationship was observed between risk of BV acquisition and increasing reported number of episodes of receptive vulvovaginal sex. The association between detection of BVAB at enrollment and BV acquisition remained significant in multivariate analysis.
We sought to evaluate a potential role for extravaginal colonization with BVAB for incident BV, namely, detection in the oral cavity or anus. The rationale was 2-fold. First, the presence of BVAB in these areas could represent recent acquisition from an intimate partner through kissing or anal or vaginal intercourse, which might precede BV development. Second, BVAB presence at these sites could reflect enhanced likelihood of colonization at multiple mucosal sites due to host immune characteristics. To pursue this, we applied species-specific quantitative 16S ribosomal RNA (rRNA) gene polymerase PCR assays for various BVAB [16] to vaginal, oral, and anal swab samples obtained at the enrollment visit for a cohort of women who did not have BV and considered these as baseline characteristics in analysis of risks for subsequent acquisition of BV. The results represent a nested case-control analysis that is part of our large prospective cohort study evaluating the natural history of vaginal microbiota in women who have sex with women.
METHODS
Study Population and Sample Collection
The study population was derived from a larger cohort of women who have been described elsewhere [14, 15, 17]. Briefly, 335 women aged 16–35 years who reported sex with ≥1 other woman in the previous year were enrolled from October 2003 through December 2006. All had vaginal swab samples collected at enrollment; collection of rectal and oral swab samples was instituted approximately halfway into enrollment and was completed for 128 women (38.2% of the overall cohort). Participants completed extensive CASI on demographics and medical, reproductive, and sexual history, and underwent standardized examination including collection of vaginal fluid for Gram stain, saline microscopy, pH measurement, potassium hydroxide evaluation, and culture for Trichomonas vaginalis.
To obtain specimens for bacterium-specific PCR assays, a polyurethane foam swab (Catch-All; Epicentre Biotechnologies) was brushed against the lateral vaginal wall and resheathed and frozen immediately in a −80°C freezer until DNA extraction. Oral swab samples were obtained by asking participants to brush the foam swab lightly along their gums as if brushing their teeth for approximately 30 seconds; our intent was to optimize collection of bacteria from the subgingival space, a microenvironment that has yielded many newly described fastidious anaerobes. Anal swab samples were obtained by inserting a Dacron swab 1–2 cm into the anus and rotating it 2 full circumferences. All swab samples were frozen immediately at −20°C and subsequently held at −80°C.
BV was diagnosed if 3 of 4 clinical (Amsel) criteria (vaginal pH of >4.5, clue cells on saline microscopy being >20% of epithelial cells, amine odor on addition of potassium hydroxide, and homogeneous vaginal discharge) were present [18] and Gram stain of vaginal fluid confirmed abnormal microbiota (Nugent score, >3) [5]. All participants were asked to return for 3 subsequent quarterly visits (total duration of follow-up, 1 year) or for evaluation any time genitourinary symptoms occurred. Women with BV were treated with vaginal metronidazole gel (37.5 mg nightly for 5 days). Participants were tested at enrollment for Chlamydia trachomatis and Neisseria gonorrhoeae using the Aptima Combo 2 assay (Gen-Probe) on urine samples, and at follow-up visits if they reported interim risk behavior (new sex partner and/or >1 partner) or genitourinary symptoms.
DNA Extraction
Vaginal swab samples for DNA extraction were placed in 15-mL conical vials with 2 mL of saline and vortex mixed for 1 minute to dislodge cells. Swabs were removed and sample material centrifuged at a relative centrifugal force of 18<thin space>000 for 10 minutes. Cell pellets were stored at −80°C until DNA extraction. DNA extraction was performed using the BiOstic Bacteremia DNA isolation kit (MoBio). DNA was eluted in 150 µL of buffer. Blank swabs without human contact were included as controls to assess contamination from reagents or collection swabs. DNA from vaginal samples was diluted 1:1 with 1 mmol/L Tris and 0.1 mmol/L ethylenediaminetetraacetic acid buffer. Human 18S rRNA gene quantitative PCR (qPCR) or broad-range 16S rRNA qPCR were used to confirm that the swab was used to collect material from a mucosal site [19]. Each DNA sample was tested for PCR inhibitors using a qPCR targeting a segment of exogenously added jellyfish DNA, and inhibition was defined as a delay in the threshold cycle of >2 cycles compared with the no-template controls [19].
Quantitative PCR
DNA from each sample was subjected to a panel of 8 qPCR assays targeting the 16S rRNA gene of key vaginal bacteria, as described elsewhere [20, 21]. Bacteria targeted included 3 novel bacteria from the Clostridiales order designated BV-associated bacterium 1 (BVAB-1), BVAB- 2, and BVAB-3 as well as Gardnerella vaginalis, Leptotrichia/Sneathia spp., Megasphaera-like sp. (type 1 and 2), L. crispatus, and Lactobacillus jensenii. Two microliters of DNA was added to each qPCR reaction, and samples were analyzed in duplicate. Values were reported as 16S rRNA gene copies per swab sample.
Statistical Analysis
We performed a case-control analysis to assess the role of extravaginal BVAB in predicting subsequent BV acquisition. Case patients were defined as women who developed incident BV over the course of follow-up. A rectal swab sample was obtained in the study period noted above from 15 of the 40 women who developed incident BV during follow-up (35 were evaluated before institution of rectal or oral swab sample collection); thus, these 15 women comprised the case patients for the analysis. For each case patient, 2 control participants, defined as any woman who remained free of BV during follow-up, were randomly selected and matched to case patients for the duration of follow-up. For the analysis, incident BV was defined by the first visit at which a woman was found to have BV (at a quarterly routine visit or at one initiated for symptoms), as defined by Amsel criteria and confirmed by Gram stain. After detection of an incident BV episode in a participant, that participant was censored from the analysis. Poisson regression with a χ2 scale parameter was used to estimate risk ratios for the detection of bacteria in oral, anal, and vaginal niches for case patients relative to controls. Quantities of bacteria detected by qPCR assay (16S rRNA gene copies per swab sample) were compared using Wilcoxon rank sum tests. To determine whether bacteria were more likely to be detected at multiple anatomic sites among case patients compared with controls, proportions of participants with each bacteria detected at all 3 sites (oral, vaginal, and anal) or at ≥2 sites were compared by case-control status. All statistical tests were 2-sided, and a level of P < .05 was considered statistically significant. Analyses were performed using SAS version 9.2 and Stata version 9.2.
Ethics
Written informed consent was obtained. Conduct of the study adhered to standard guidelines for research involving human subjects, and approval was granted annually by the University of Washington Human Subjects Review Committee.
RESULTS
The characteristics of the 335 women enrolled in the parent cohort have been described elsewhere [14, 15, 17]. Briefly, the median age was 27 years, 24% of women categorized themselves as nonwhite, one-third reported a prior diagnosis of BV, and 24% reported recent (within the past 90 days) sex with a male partner. At enrollment, 2 women (0.6%) had C. trachomatis infection, and 9 (2.7%) had symptomatic vulvovaginal candidiasis. None had N. gonorrhoeae infection, trichomoniasis, or clinically evident genital herpes. Ninety-six women (28.7%) had BV at enrollment. Of the 239 women who did not have BV at the enrollment visit, 199 returned for ≥1 follow-up visit. The median duration of follow-up for these 199 women was 381 days (interquartile range, 361–412 days), with a median number of 4 follow-up visits. Forty episodes of BV occurred among these 199 women over a total of 172.6 woman-years at risk (overall incidence, 0.23 episode per woman-year), with a median number of days to BV diagnosis after a visit at which BV was not detected of 92 days (interquartile range, 89–104 days) [15].
Characteristics of the 45 women who comprised the subgroup for this analysis (15 women who developed incident BV [case patients] and 30 women who did not [controls]) are shown in Table 1. These women did not differ from the remaining women in the parent cohort, nor did case patients differ from controls in any of the measures shown.
Table 1.
Characteristics of 45 Participants at Enrollment Visit, According to Case or Control Status
| Characteristic | All Participants (n = 45) | Case Patients (n = 15) | Controls (n = 30) |
|---|---|---|---|
| Age, median (range), y | 26 (18–34) | 25 (18–34) | 27 (18–34) |
| Race (self-defined)a | |||
| White | 32 (71) | 9 (60) | 23 (77) |
| Nonwhite | 11 (24) | 5 (33) | 6 (20) |
| Declined to provide race data | 2 (4) | 1 (7) | 1 (3) |
| Vaginal douching, past month | 2 (4) | 1 (7) | 1 (3) |
| Current cigarette smoking | 10 (22) | 3 (20) | 7 (70) |
| Antibiotic use in past month | 6 (13) | 3 (20) | 3 (10) |
| Hormonal contraceptive use in past 60 d | 5 (11) | 1 (7) | 4 (13) |
| Vaginal symptoms presentb | 6 (13) | 3 (20) | 3 (10) |
| History of BV | 14 (31) | 7 (47) | 7 (23) |
| Sexual behaviors in past 3 mo | |||
| Any sex | 44 (98) | 15 (100) | 29 (97) |
| Sex with women | 42 (93) | 15 (100) | 27 (90) |
| Shared sex toys | 10 (22) | 5 (33) | 5 (17) |
| Sex with men | 12 (27) | 5 (33) | 7 (23) |
| Condom use at last vaginal intercoursec | 1 (2) | 1 (7) | 0 (0) |
| Receptive anal sex | 15 (33) | 4 (27) | 11 (37) |
| Receptive (vaginal) oral sex | 41 (91) | 14 (93) | 27 (90) |
| Receptive digital-vaginal sex | 41 (91) | 15 (100) | 26 (87) |
| Vaginal lubricant use | 25 (56) | 10 (67) | 15 (50) |
Data represent No. (%) of participants, unless otherwise indicated. Case patients were defined as women who developed incident bacterial vaginosis (BV) over the course of follow-up. For each case patient, 2 control participants, defined as women who remained free of BV over follow-up, were selected and matched to case patients for the duration of follow-up.
a Subjects were invited to choose >1 category to describe their race. “White” includes those who chose only this category.
b Defined as change in the amount, color, or odor of vaginal discharge.
c Among women who reported vaginal intercourse with a male partner in the prior 3 months.
Table 2 depicts the results of the individual bacterium-specific qPCR assays by anatomic site for women who acquired BV during follow-up (case patients) and those who did not (controls), along with the relative risks for detection in case patients relative to controls and a comparison of the quantities (16S rRNA gene copies per swab sample) for each bacterium. Results for each anatomic niche are discussed below.
Table 2.
Comparison of Bacterial Vaginosis–Associated Bacteria (BVAB) Detected in Case Patients Versus Controls in the Oral, Anal, and Vaginal Niches
| Women Testing Positive, % |
|||||
|---|---|---|---|---|---|
| BVAB-Specific qPCR assay | Case Patients (n = 15) | Controls (n = 30) | Pa | Risk Ratiob (95% CI) | Pc |
| Oral niche | |||||
| Gardnerella vaginalis | 100 | 43 | <.001 | 2.3 (1.4–3.7) | <.001 |
| Lactobacillus crispatus | 7 | 27 | .15 | 0.3 (.04–1.7) | .15 |
| Lactobacillus jensenii | 0 | 3 | … | … | … |
| Leptotrichia/Sneathia | 20 | 10 | .37 | 2.0 (.4–9.2) | .32 |
| Anal niche | |||||
| G. vaginalis | 100 | 53 | .002 | 1.9 (1.3–2.8) | .02 |
| L. crispatus | 47 | 80 | .03 | 0.6 (.4–.9) | .006 |
| BVAB-2 | 27 | 10 | .17 | 2.7 (.7–10.9) | .2 |
| BVAB-3 | 7 | 3 | .62 | 2.0 (.1–32.0) | .07 |
| Megasphaera spp. | 27 | 3 | .05 | 8.0 (1.0–66.2) | .02 |
| L. jensenii | 27 | 40 | .39 | 0.7 (.3–1.7) | .69 |
| Leptotrichia/Sneathia | 47 | 17 | .04 | 2.8 (1.0–7.7) | .07 |
| BVAB-2 or Megasphaera spp. | 40 | 10 | .03 | 4.0 (1.1–14.2) | … |
| Vaginal niche | |||||
| G. vaginalis | 67 | 37 | .07 | 1.8 (1.0–3.4) | <.001 |
| L. crispatus | 60 | 87 | .05 | 0.7 (.5–1.0) | .27 |
| BVAB-2 | 33 | 3 | .03 | 10.0 (1.3–77.3) | .005 |
| BVAB-3 | 7 | 0 | … | … | … |
| Megasphaera spp. | 33 | 7 | .04 | 5.0 (1.1–23.4) | .006 |
| L. jensenii | 47 | 80 | .03 | 0.6 (.4–.9) | .1 |
| Leptotrichia/Sneathia | 27 | 10 | .17 | 2.7 (.7–10.9) | .01 |
| BVAB-2 or Megasphaera spp. | 40 | 7 | .02 | 6.0 (1.4–26.5) | … |
Case patients were defined as women who developed incident bacterial vaginosis (BV) during the course of follow-up. For each case patient, 2 control participants, defined as women who remained free of BV during follow-up, were selected and matched to case patients for the duration of follow-up. Species-specific polymerase chain reaction assays were applied to samples from all 3 niches; results are shown in the table only if the assay detected that bacterium.
Abbreviations: CI, confidence interval; qPCR, quantitative PCR.
a Categorical comparison of detection of bacteria in case patients relative to controls (Poisson regression).
b Risk of case patients having the individual BVAB detected at enrollment relative to controls.
c Comparison of the quantity of bacteria detected in case patients relative to controls (Wilcoxon rank sum test).
Detection of Bacteria in the Oral Cavity
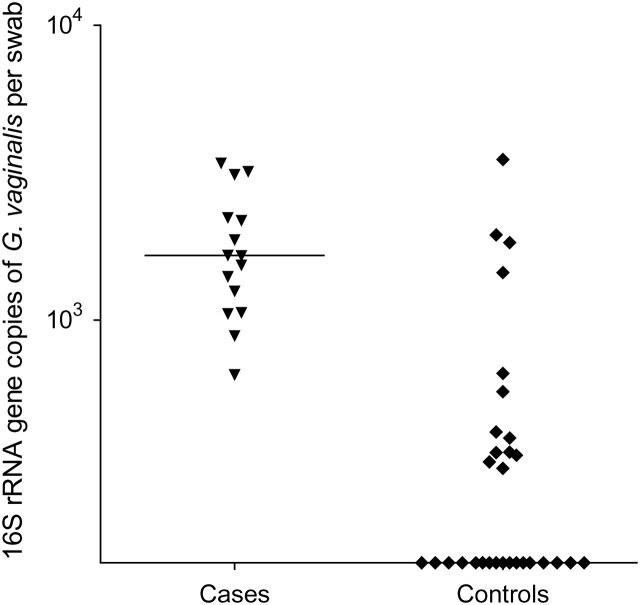
A total of 28 (62%) of 45 women had G. vaginalis detected in oral swab samples at the enrollment visit. Only 9 women (20%) had L. crispatus and 1 (2%) had L. jensenii detected in oral swab samples at baseline. Six (13%) women had Leptotrichia/Sneathia detected in oral swab samples at baseline. BVAB1, BVAB2, BVAB3, or Megasphaera-like spp. were not detected in the oral cavity in any woman. G. vaginalis was 2.3 times more likely to be detected among women who acquired BV during follow-up than among those who did not (P < .001). L. crispatus was less likely to be detected in the oral cavity among women who subsequently acquired BV; however, this did not reach statistical significance. As shown in Figure 1, the quantities of G. vaginalis in the oral cavity as measured by gene copies per swab sample were significantly greater among women who subsequently acquired BV.
Figure 1.
Numbers of 16S ribosomal RNA (rRNA) gene copies of Gardnerella vaginalis per swab sample in the oral cavities of case patients and controls. Horizontal lines represent median numbers of 16S rRNA gene copies for each bacterium.
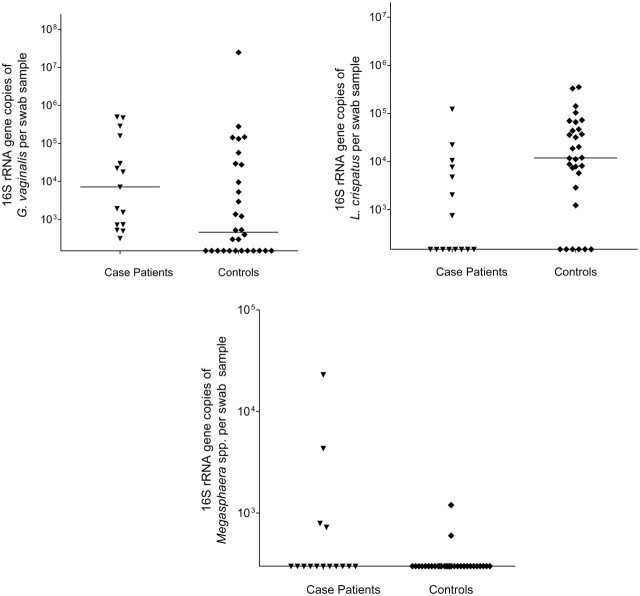
Detection of Bacteria in the Anus
Both G. vaginalis and L. crispatus were commonly detected in anal swab samples (69% of women for each bacterium). Detection was significantly different among those who acquired BV: risk of G. vaginalis was nearly 2-fold higher (P = .002), and risk of L. crispatus was nearly halved (P = .03) (Table 2). More than one-quarter of women had Leptotrichia/Sneathia species detected; women who acquired BV were more likely to have these bacteria detected. Although BVAB2 (16% of women), BVAB3 (4%), and L. jensenii (36%) were detected in the anus at baseline, none were associated with subsequent BV acquisition. BVAB1 was not detected in any anal swab samples. Figure 2 depicts the quantity of bacterium-specific 16S rRNA gene copies per anal swab sample by case patient and control status. Case patients had significantly higher quantities of G. vaginalis and Megasphaera-like spp. DNA, and lower quantities of L. crispatus, relative to controls. Although Megasphaera species were detected in only 11% of women overall, bacterial quantity was significantly higher in women who developed subsequent BV.
Figure 2.
Numbers of 16S ribosomal RNA (rRNA) gene copies of bacterial vaginosis–associated bacteria per swab sample in the anal niches of case patients and controls. Horizontal lines represent median numbers of 16S rRNA gene copies for each bacterium. Abbreviations: G. vaginalis, Gardnerella vaginalis; L. crispatus, Lactobacillus crispatus.
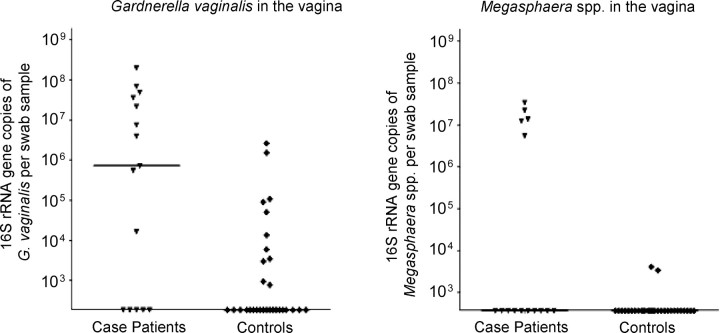
Detection of Bacteria in the Vagina
Most women (78%) were colonized in the vagina with L. crispatus at the enrollment visit; however, detection of L. crispatus in the vagina was 30% less likely among those who acquired BV compared with those who did not develop BV. Nearly one-half (47%) of the women at enrollment had G. vaginalis detected in the vagina, and women with subsequent BV were almost twice as likely to have G. vaginalis detected. Although only 13% of women had BVAB2 in the vagina at enrollment, the risk of vaginal BVAB2 detection was 10 times greater among those who acquired BV compared with women who did not. Other BVAB that were present at enrollment and that were significantly associated with subsequent BV acquisition were Megasphaera spp. (increased risk) and L. jensenii (decreased risk). BVAB3 was detected in vaginal swab samples from only 1 woman at enrollment, and BVAB1 was not detected in any woman. As shown in Figure 3, case patients had significantly higher quantities of BVAB DNA (16S rRNA gene copies per swab sample) for G. vaginalis and Megasphaera spp. than did controls.
Figure 3.
Numbers of 16S ribosomal RNA (rRNA) gene copies of bacterial vaginosis–associated bacteria per swab sample in the vaginas of case patients and controls. Horizontal lines represent median numbers of 16S rRNA gene copies for each bacterium.
Among case patients, detection by PCR assay at enrollment in a given anatomic niche of any of the BVAB listed in Table 2 was highly correlated with the likelihood of detection of the same bacteria by PCR assay performed at another anatomic site (Table 3). Case patients were more likely than controls to have G. vaginalis detected at ≥2 sites (P < .001) or all 3 sites (P < .001) compared with controls, and less likely to have L. crispatus detected at ≥2 sites (P = .02).
Table 3.
Detection of Gardnerella vaginalis and Lactobacillus crispatus at Multiple Sites by Case and Control Status
| Bacteria | Case Patients, No. (%) (n = 15) | Controls, No. (%) (n = 30) | Pa |
|---|---|---|---|
| G. vaginalis | |||
| Detected at all 3 sites | 10 (67) | 4 (13) | .001 |
| Detected at ≥2 sites | 15 (100) | 10 (33) | <.001 |
| L. crispatus | |||
| Detected at all 3 sites | 1 (7) | 7 (23) | .17 |
| Detected at ≥2 sites | 7 (47) | 25 (83) | .02 |
a From 1-sided Fisher exact test.
DISCUSSION
Among women without BV who were subsequently followed up for a mean of 1 year, we previously reported that detection of several BVAB in the vagina at the enrollment (BV-negative) visit was independently associated with subsequent acquisition of BV and that detection of L. crispatus at enrollment was associated with reduced risk of subsequent BV acquisition [15]. In extending the analysis to participants’ oral and anal BV-specific microbiology at the enrollment (BV-negative) visit, we found that the women who acquired BV during follow-up were more likely to have previous colonization of these reservoirs with key BVAB. Detection of G. vaginalis in the oral cavity or anus and of Leptotrichia/Sneathia spp. in the anus was significantly more common at enrollment among women who subsequently acquired BV. In contrast, L. crispatus was detected more frequently in the anus among controls. When these bacteria were present, they were typically detected at higher concentrations of bacterial DNA relative to controls at each site as measured by qPCR assays. These data suggest that some BVAB may be acquired vaginally from preexisting reservoirs at extravaginal sites and that changes in the microbiota of these reservoirs may precede the development of BV by a considerable period.
Prior analyses in this cohort provide one hypothesis to explain these findings. In prospective analysis of risks for BV acquisition, we carefully examined the role of sexual behaviors using CASI during follow-up [15]. Only report of a new female sex partner who provided a history of BV was associated with increased risk of BV acquisition in univariate analysis. We also observed a dose-response relationship between risk of BV acquisition and higher reported number of episodes of receptive vulvovaginal sex, and a less significant but directionally similar association with receptive oral-anal sex. These findings are consistent with a potential role for these anatomic niches as a reservoir for BVAB, not only in affected women but also in their sex partners. In a large cross-sectional study, Antonio and colleagues also reported that rectal colonization with L. crispatus or L. jensenii was significantly associated with lower likelihood of BV [22]. We have reported elsewhere that the majority of monogamous female sex partners shared unique Lactobacillus strains as assessed by DNA homology to type strains and fingerprinting by repetitive sequence-based polymerase chain reaction, and that detection of Lactobacillus gasseri at the rectum or vagina was associated with recent receptive digital-vaginal sex and increased BV risk (odds ratio, 4.2; 95% confidence interval, 1.4–13.4) [23]. Colonization of the oral cavity of neonates with maternal vaginal bacteria occurs and is directly related to exposure through vaginal birth [24]; G. vaginalis and various Lactobacillus species, as well as many anaerobes that are found in BV, have been reported to be part of the oral microbiome [25, 26].
Our findings may also reflect differences in innate immune response to colonizing bacteria. The host environment determined by innate immunity may permit substantial colonization by BVAB not only at the vagina but at extravaginal sites as well. Some in vitro studies have indicated that certain Toll-like receptor (TLR) polymorphisms (specifically in TLR2 and TLR4), which modify cellular immune response and production of cytokines, may be involved in BV pathogenesis [27–29]. TLR2 is expressed by epithelial cells in the vagina and cervix and by hematopoietic cells. TLR4 is expressed by leukocytes (monocytes, macrophages, and neutrophils) present in the lower female genital tract [30, 31]. Although no association between BV and the TLR4 299 and TLR4 399 gene polymorphisms was seen in 497 pregnant women with and 388 without BV [27], other investigators have observed that the TLR4 896A>G polymorphism may modify the vaginal immune response to G. vaginalis and anaerobic gram-negative rods [32]. Increases in interleukin 1β levels observed in the vaginas of TLR4 896A homozygotes colonized with these bacteria were not observed in carriers of the TLR4 896G allele also colonized, despite the latter group's having significantly higher quantities of these bacteria. These investigators hypothesized that a blunted proinflammatory cytokine response in the latter group was permissive for proliferation of these BVAB. In pregnant women, 2 TLR4 single-nucleotide polymorphisms were significantly associated with cervical interleukin 1 concentrations in European American but not African American women; this relationship was strengthened in women with BV [33]. The TLR studies offer a glimpse into how innate immunity may modify risk of acquiring BV, likelihood of cure, and adverse consequences.
Our study has limitations. First, PCR assays for BVAB were obtained only at enrollment; more proximate detection of these bacteria in women without BV relative to the BV episode might demonstrate even higher risk for BV acquisition. Prospective studies that apply PCR assays to frequent serial measurements—ideally through self-collection of extravaginal swab samples from the women being followed up and from of sex partners—would be ideal for clarifying the sequence of events that lead to BV. Nonetheless, detection of several BVAB at the enrollment visit was associated with subsequent BV acquisition. Second, our analysis is limited by relatively small numbers of women who had some BVAB detected by PCR assay. For this reason, we did not analyze individual BVAB in multivariate analysis. Third, our subjects were selected on the basis of reporting sex with other women. Although 24% also reported recent sex with men, they are unlikely to be representative of exclusively heterosexual women, to whom our findings may not apply. However, the fact that our subjects infrequently reported vaginal intercourse with male partners in the month after treatment might be advantageous in helping to determine whether antecedent vaginal colonization of these bacteria have an independent role in predicting future BV acquisition. Moreover, although >80% of participants without BV at enrollment had L. crispatus at enrollment as detected by PCR assay, 20% acquired BV in the year of follow-up. The incidence of BV that we observed in our participants—0.23 episode per woman-year—was only slightly lower than that reported in a larger year-long prospective study of young heterosexual women [9]. Finally, it is of interest that despite their very high predictive value for presence of BV or future development of BV when they are found in the vagina, BVAB1, BVAB2, and BVAB2 were not detected in the oral cavity. One possibility is that these bacteria are more fastidious than those we detected, and that identification through the methods we used might require deeper sampling in the subgingival crevice than our participants were asked to perform. Alternatively, these Clostridiales order bacteria may not thrive in the mouth.
Our findings raise important areas for future research. First, comparative assessments of risks for acquisition of BV, including molecular characterization of baseline microbiology of BVAB, need to be performed in large and diverse groups of women, ideally with frequent sampling (eg, daily or weekly) for application of bacterium-specific PCR assays. In some women assessed frequently (eg, daily), rapid shifts in vaginal microbiota, as measured by qPCR assays and by Nugent score, are evident [21, 34, 35]. Studies focused on the kinetics of acquisition of vaginal bacterial communities are likely to be useful for understanding BV pathogenesis. These measures should be carefully integrated with concomitant collection of behavioral data to further explore potential links with sexual practices [36]. Moreover, careful assessment of strain types, particularly for G. vaginalis, might shed light on whether differences exist in the vaginal, oral, and anal isolates.
In summary, our findings suggest that extravaginal colonization with key BVABs among women with no BV is an independent risk factor for incident BV. BV is a heterogeneous syndrome characterized by diverse microbiota, some of which might be essential “founder” species necessary to establish an environment favorable to progression to BV. For example, a tenacious biofilm on vaginal epithelium may lead to antibiotic failure or create a persistent reservoir of bacteria, further increasing risk of treatment failure [37]. Concentrations of bacteria at earliest stages of biofilm formation may be insufficient to cause BV, yet they may provide a critical layer that facilitates attachment of other community members. These early colonizers may be detected by species-specific PCR assays, as described above.
Notes
Acknowledgments. We are grateful to the study staff, including Kathleen Ringwood, Susan Heideke, Nancy Dorn, Lauren Asaba, and Dana Varon; to Kathy Agnew for performance of Gram stains on vaginal fluid samples; and to the women who enrolled.
Financial support. This work was supported by the National Institutes of health (grants R01-AI52228-01 to J. M. M. and R01 AI061628 to D. F.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am J Obstet Gynecol. 2003;189:139–47. doi: 10.1067/mob.2003.339. [DOI] [PubMed] [Google Scholar]
- 2.Myer L, Denny L, Telerant R, Souza M, Wright TC, Jr, Kuhn L. Bacterial vaginosis and susceptibility to HIV infection in South African women: a nested case-control study. J Infect Dis. 2005;192:1372–80. doi: 10.1086/462427. [DOI] [PubMed] [Google Scholar]
- 3.Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS (London, England) 2008;22:1493–501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koumans EH, Sternberg M, Bruce C, et al. The prevalence of bacterial vaginosis in the United States, 2001-2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007;34:864–9. doi: 10.1097/OLQ.0b013e318074e565. [DOI] [PubMed] [Google Scholar]
- 5.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol. 1991;29:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hillier SL, Marrazzo JM, Holmes KK. Bacterial vaginosis. In: Holmes KK, Sparling PF, Mardh P-A, et al., editors. Sexually transmitted diseases. 4th ed. New York, NY: McGraw-Hill; 2008. pp. 737–68. [Google Scholar]
- 7.Bradshaw CS, Morton AN, Hocking J, et al. High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J Infect Dis. 2006;193:1478–86. doi: 10.1086/503780. [DOI] [PubMed] [Google Scholar]
- 8.Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194:1283–9. doi: 10.1016/j.ajog.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 9.Cherpes TL, Hillier SL, Meyn LA, Busch JL, Krohn MA. A delicate balance: risk factors for acquisition of bacterial vaginosis include sexual activity, absence of hydrogen peroxide-producing lactobacilli, black race, and positive herpes simplex virus type 2 serology. Sex Transm Dis. 2008;35:78–83. doi: 10.1097/OLQ.0b013e318156a5d0. [DOI] [PubMed] [Google Scholar]
- 10.Berger BJ, Kolton S, Zenilman JM, Cummings MC, Feldman J, McCormack WM. Bacterial vaginosis in lesbians: a sexually transmitted disease. Clin Infect Dis. 1995;21:1402–5. doi: 10.1093/clinids/21.6.1402. [DOI] [PubMed] [Google Scholar]
- 11.Marrazzo JM, Koutsky LA, Eschenbach DA, Agnew K, Stine K, Hillier SL. Characterization of vaginal flora and bacterial vaginosis in women who have sex with women. J Infect Dis. 2002;185:1307–13. doi: 10.1086/339884. [DOI] [PubMed] [Google Scholar]
- 12.Bailey JV, Farquhar C, Owen C. Bacterial vaginosis in lesbians and bisexual women. Sex Transm Dis. 2004;31:691–4. doi: 10.1097/01.olq.0000143093.70899.68. [DOI] [PubMed] [Google Scholar]
- 13.Evans AL, Scally AJ, Wellard SJ, Wilson JD. Prevalence of bacterial vaginosis in lesbians and heterosexual women in a community setting. Sex Transm Infect. 2007;83:470–5. doi: 10.1136/sti.2006.022277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marrazzo JM, Thomas KK, Agnew K, Ringwood K. Prevalence and risks for bacterial vaginosis in women who have sex with women. Sex Transm Dis. 2010;37:335–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Marrazzo JM, Thomas KK, Fiedler TL, Ringwood K, Fredricks DN. Risks for acquisition of bacterial vaginosis among women who report sex with women: a cohort study. PLoS One. 2010;5:e11139. doi: 10.1371/journal.pone.0011139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fredricks DN, Fiedler TL, Thomas KK, Oakley BB, Marrazzo JM. Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. J Clin Microbiol. 2007;45:3270–6. doi: 10.1128/JCM.01272-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marrazzo JM, Thomas KK, Fiedler TL, Ringwood K, Fredricks DN. Relationship of specific vaginal bacteria and bacterial vaginosis treatment failure in women who have sex with women. Ann Intern Med. 2008;149:20–8. doi: 10.7326/0003-4819-149-1-200807010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 19.Khot PD, Ko DL, Hackman RC, Fredricks DN. Development and optimization of quantitative PCR for the diagnosis of invasive aspergillosis with bronchoalveolar lavage fluid. BMC infectious diseases. 2008;8:73. doi: 10.1186/1471-2334-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fredricks DN, Fiedler TL, Thomas KK, Mitchell CM, Marrazzo JM. Changes in vaginal bacterial concentrations with intravaginal metronidazole therapy for bacterial vaginosis as assessed by quantitative PCR. J Clin Microbiol. 2009;47:721–6. doi: 10.1128/JCM.01384-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srinivasan S, Liu C, Mitchell CM, et al. Temporal variability of human vaginal bacteria and relationship with bacterial vaginosis. PLoS One. 2010;5:e10197. doi: 10.1371/journal.pone.0010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antonio MA, Rabe LK, Hillier SL. Colonization of the rectum by Lactobacillus species and decreased risk of bacterial vaginosis. J Infect Dis. 2005;192:394–8. doi: 10.1086/430926. [DOI] [PubMed] [Google Scholar]
- 23.Marrazzo JM, Antonio M, Agnew K, Hillier SL. Distribution of genital Lactobacillus strains shared by female sex partners. J Infect Dis. 2009;199:680–3. doi: 10.1086/596632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107:11971–5. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaura E, Keijser BJ, Huse SM, Crielaard W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiology. 2009;9:259. doi: 10.1186/1471-2180-9-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroes I, Lepp PW, Relman DA. Bacterial diversity within the human subgingival crevice. Proc Natl Acad Sci U S A. 1999;96:14547–52. doi: 10.1073/pnas.96.25.14547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goepfert AR, Varner M, Ward K, et al. Differences in inflammatory cytokine and Toll-like receptor genes and bacterial vaginosis in pregnancy. Am J Obstet Gynecol. 2005;193:1478–85. doi: 10.1016/j.ajog.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 28.Mares D, Simoes JA, Novak RM, Spear GT. TLR2-mediated cell stimulation in bacterial vaginosis. J Reprod Immunol. 2008;77:91–9. doi: 10.1016/j.jri.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zariffard MR, Novak RM, Lurain N, Sha BE, Graham P, Spear GT. Induction of tumor necrosis factor-alpha secretion and Toll-like receptor 2 and 4 mRNA expression by genital mucosal fluids from women with bacterial vaginosis. J Infect Dis. 2005;191:1913–21. doi: 10.1086/429922. [DOI] [PubMed] [Google Scholar]
- 30.Fichorova RN, Cronin AO, Lien E, Anderson DJ, Ingalls RR. Response to Neisseria gonorrhoeae by cervicovaginal epithelial cells occurs in the absence of Toll-like receptor 4-mediated signaling. J Immunol. 2002;168:2424–32. doi: 10.4049/jimmunol.168.5.2424. [DOI] [PubMed] [Google Scholar]
- 31.Givan AL, White HD, Stern JE, et al. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol. 1997;38:350–9. doi: 10.1111/j.1600-0897.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 32.Genc MR, Vardhana S, Delaney ML, et al. Relationship between a Toll-like receptor-4 gene polymorphism, bacterial vaginosis-related flora and vaginal cytokine responses in pregnant women. Eur J Obstet Gynecol Reprod Biol. 2004;116:152–6. doi: 10.1016/j.ejogrb.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Ryckman KK, Williams SM, Krohn MA, Simhan HN. Genetic association of Toll-like receptor 4 with cervical cytokine concentrations during pregnancy. Genes Immun. 2009;10:636–40. doi: 10.1038/gene.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srinavasan S, Liu C, Mitchell CM, et al., editors. Human vaginal bacterial community dynamics assessed by quantitative PCR and relationship with bacterial vaginosis. Philadelphia, PA: American Society of Microbiology; 2008. [Google Scholar]
- 35.Brotman RM, Ravel J, Cone RA, Zenilman JM. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex Transm Infect. 2010;86:297–302. doi: 10.1136/sti.2009.040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marrazzo JM, Martin DH, Watts DH, et al. Bacterial vaginosis: identifying research gaps proceedings of a workshop sponsored by DHHS/NIH/NIAID. Sex Transm Dis. 2010;37:732–44. doi: 10.1097/OLQ.0b013e3181fbbc95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swidsinski A, Mendling W, Loening-Baucke V, et al. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol. 2008;198:e1–6. doi: 10.1016/j.ajog.2007.06.039. [DOI] [PubMed] [Google Scholar]