Abstract
Background
New therapeutic targets for antibiotic-resistant bacterial pathogens are desperately needed. The bacterial surface polysaccharide poly-β-(1-6)-N-acetyl-glucosamine (PNAG) mediates biofilm formation by some bacterial species, and antibodies to PNAG can confer protective immunity. By analyzing sequenced genomes, we found that potentially multidrug-resistant bacterial species such as Klebsiella pneumoniae, Enterobacter cloacae, Stenotrophomonas maltophilia, and the Burkholderia cepacia complex (BCC) may be able to produce PNAG. Among patients with cystic fibrosis patients, highly antibiotic-resistant bacteria in the BCC have emerged as problematic pathogens, providing an impetus to study the potential of PNAG to be targeted for immunotherapy against pan-resistant bacterial pathogens.
Methods
The presence of PNAG on BCC was assessed using a combination of bacterial genetics, microscopy, and immunochemical approaches. Antibodies to PNAG were tested using opsonophagocytic assays and for protective efficacy against lethal peritonitis in mice.
Results
PNAG is expressed in vitro and in vivo by the BCC, and cystic fibrosis patients infected by the BCC species B. dolosa mounted a PNAG-specific opsonophagocytic antibody response. Antisera to PNAG mediated opsonophagocytic killing of BCC and were protective against lethal BCC peritonitis even during coinfection with methicillin-resistant Staphylococcus aureus.
Conclusions
Our findings raise potential new therapeutic options against PNAG-producing bacteria, including even pan-resistant pathogens.
The crisis of bacterial resistance to antibiotics, which is manifest as difficult-to-treat infections in both hospital and community settings, has presented clinicians worldwide with an increasing frequency of encountering patients with infections for which there are either no effective antibiotics or only a single class of antibiotics available for treatment. Examples of these nearly pan-resistant organisms are Enterobacteriaceae such as Klebsiella pneumoniae and Escherichia coli carrying the K. pneumoniae carbapenemases or the CTX-M extended-spectrum β-lactamase plasmids, Enterobacteriaceae producing the New Delhi metallo-β-lactamase-1 [1], and resistant gram-positive bacteria such as methicillin-resistant Staphylococcus aureus (MRSA). Coincident with these increases in antibiotic resistance is the lack of development in the pharmaceutical pipeline of new antibiotics for treating highly resistant infections, particularly for gram-negative bacteria.
Patients with cystic fibrosis (CF) are particularly at risk for serious infections with antibiotic-resistant bacteria [2, 3]. For example, recent data suggest that infection with MRSA hastens the decline in lung function compared to methicillin-sensitive S. aureus [4]. In recent years, a nearly pan-resistant Burkholderia dolosa strain caused an outbreak in a CF center in Boston [5]. Burkholderia dolosa is a member of the Burkholderia cepacia complex (BCC), the members of which are known to cause a small but significant proportion of infections in CF patients [6, 7] that are linked with accelerated loss of lung function, decreased survival, and the cepacia syndrome [8]. New therapeutic approaches are desperately needed for these pan-resistant bacteria.
Capitalizing on the recent microbial genome sequencing initiatives and protein homology algorithms, we noted that B. dolosa, other members of the BCC, and a number of bacteria known to harbor multidrug-resistant (MDR) and extensively drug-resistant mobile genetic elements (K. pneumoniae and Enterobacter cloacae) possess a putative pga (polyglucosamine) locus, which encodes the biosynthetic genes for a surface polysaccharide called poly-β-(1-6)-N-acetylglucosamine (PNAG) [9, 10]. PNAG was first described as a biofilm component in S. aureus and Staphylococcus epidermidis [10, 11] but is now known also to be produced by several bacterial pathogens, including antibiotic-resistant strains of E. coli [12] and Acinetobacter baumannii [13]. Because antibodies to PNAG have been shown to be opsonic and protective against S. aureus and E. coli infections [10, 14], we hypothesized that these antibodies might be a new therapeutic option for the pan-resistant B. dolosa and potentially all the other resistant bacteria possessing the pga locus.
We show here that B. dolosa and the other BCC species found in CF patients produce PNAG both in vitro and in vivo and that antibodies to PNAG can kill these bacteria in opsonophagocytic assays. We also demonstrate that antibodies to PNAG can protect mice from lethal peritonitis caused by BCC strains or a combination of BCC and MRSA, chosen as another example of a PNAG-producing pathogenic and resistant bacterium. Thus, we describe a potentially effective strategy, not related to antibiotics, that could protect against even pan-resistant bacterial pathogens and show that antibodies directed to this shared, single-surface polysaccharide can simultaneously protect against infection due to multiple bacterial species.
METHODS
For a detailed description of the genetic, biofilm, and opsonophagocytic assay methods, please see the Supplementary Data.
Antibiotic Susceptibility Testing
Clinical isolates of B. dolosa were tested for antibiotic susceptibility by the Clinical Microbiology Laboratory at Children's Hospital Boston using standard methods per the Clinical and Laboratory Standards Institute (http://www.clsi.org/source/orders/free/m100-s20.pdf). The minimum inhibitory concentrations are in micrograms per milliliter.
Genetic: Presence of a Locus Encoding PNAG in BCC Strains
We constructed a pgaC mutant in Burkholderia cenocepacia strain K56-2 as well as a complemented mutant utilizing modifications of previously described methods [15] (Supplementary Data).
Confocal Microscopy
Paraffin-embedded lung sections were heated at 65°C for 1 hour and then deparaffinized with xylene followed by rehydration with ethanol–water mixtures. After permeabilization with 0.2% Triton X-100 in phosphate-buffered saline (PBS) for 10 minutes at room temperature, sections were blocked overnight at 4°C in PBS containing 1% bovine serum albumin (BSA) and 2% horse serum (Invitrogen) (2% goat serum [Invitrogen] was also added for human lung sections). Primary antibodies were used at 1:100 dilutions in blocking buffer, with incubation for 1 hour at room temperature. Burkholderia dolosa–specific antiserum was obtained from FVB/NJ mice infected 3 weeks previously with B. dolosa via intranasal inoculation. PNAG-specific antiserum was raised in a rabbit immunized with the 9-mer glucosamine–tetanus toxoid (TT) conjugate vaccine (9GlcNH2-TT) [16]. Nuclear staining was with TO-PRO-3 (Invitrogen) at 1:300 dilution. Secondary antibodies (all from Invitrogen) were Alexa Fluor 568 donkey antimouse immunoglobulin G (IgG; for B. dolosa staining) and Alexa Fluor 488 goat antirabbit IgG (for PNAG staining). Secondary antibodies were used at 1:200 dilution in blocking buffer, with incubation for 1 hour at room temperature. After washing in PBS, sections were mounted with Aqua-Poly/Mount (Polysciences) and then analyzed by confocal microscopy with a Zeiss LSM5 Pascal instrument equipped with a krypton/argon laser. Images were analyzed with PASCAL software (version 5; Zeiss). Human tissue was from autopsy samples of a CF patient who died of cepacia syndrome caused by B. dolosa and was obtained under a protocol approved by the Children's Hospital Boston Institutional Review Board. Images depicted in the figures are representative of multiple fields viewed.
Bacteria were grown in tryptic soy broth (TSB) for 72 hours at room temperature without shaking. Samples were harvested from the air-medium interface and washed. Aliquots of 10 µL were air-dried onto glass slides, heat-fixed, and then reacted with 50 µg/mL of a human IgG1 monoclonal antibody (mAb), F598, specifically reactive with PNAG [17] or a recombinant human IgG1 mAb to Pseudomonas aeruginosa alginate, mAb F429 [18], as a negative control. The mAbs were diluted in PBS containing 0.5% BSA (PBS/BSA) and left on the slides overnight at 4°C. Samples were washed with PBS and incubated with 4 μM of the DNA-binding dye Syto83 (Invitrogen) to visualize bacterial cells and a 1:250 dilution of antihuman IgG conjugated to Alexa Fluor 488 (Invitrogen) in PBS/BSA for 1 hour at room temperature. Slides were washed and mounted for viewing. To confirm the specificity of the binding of mAb F598 to PNAG, additional samples were treated with either 50 μg chitinase/mL, an enzyme specific for poly-β-(1-4)-N-acetylglucosamine and thus ineffective against PNAG, or 50 mg dispersin B/mL [19], an enzyme that specifically degrades PNAG, in tris-buffered saline, pH 6.4, for 24 hours at 37°C, then processed for viewing by confocal microscopy. Images depicted in the figures are representative of multiple fields viewed.
PNAG Extraction and Immunoblots
Immunoblotting to detect PNAG was performed essentially as described previously [13], with minor modifications as detailed in the Supplementary Data.
Biofilm Assays
Biofilm production was assessed by measuring the incorporation of crystal violet after growth on glass tubes [13].
PNAG Enzyme-Linked Immunosorbent Assay
Overnight cultures in TSB plus isopropyl-β-d-thiogalactopyranoside were diluted to an optical density at 650 nm of 0.2, and 100 µL of a 1:100 dilution was transferred into 96-well polystyrene microtiter plates that were sealed and incubated at 37°C for 2 days. Wells were then washed and PNAG detected using anti-dPNAG-DT rabbit serum [14] (1:500) followed by alkaline phosphatase-conjugated goat antirabbit IgG secondary (1:1000). Here, we used rabbit (rather than goat) antiserum to the staphylococcal PNAG owing to high background staining of goat sera in this assay.
Immunoelectron Microscopy
Detailed methods for immunoelectron microscopy are provided in the Supplementary Data.
Opsonophagocytic Activity of PNAG-Specific Antibodies Against the Major Species of BCC
The opsonophagocytic assays followed published protocols [9], with minor modifications (Supplementary Data). To achieve antigenic specificity in the opsonophagocytic assay, polyclonal PNAG-specific goat antiserum to 9GlcNH2-TT was adsorbed with ∼1010 colony-forming units (CFUs)/mL of the B. cenocepacia K56-2 pgaC mutant to remove antibodies reactive with antigens on the surface of BCC strains, except those to PNAG.
Protection Studies
Mice were housed under virus-free conditions, and all animal experiments complied with institutional and federal guidelines regarding the use of animals in research.
Lethal Peritonitis Model
To evaluate the in vivo protective efficacy of antibody to PNAG, we used an intraperitoneal infection model in mice, as described previously [14]. In brief, groups of 8 mice (FVB/N, female, 6–8 weeks of age) were immunized intraperitoneally 24 hours before and 4 hours after infection with 0.2 mL of normal goat serum or PNAG-specific goat antiserum to 9GlcNH2-TT [16]. Bacteria were grown overnight in TSB and then resuspended in sterile PBS to approximately 5 × 109 to 5 × 1010 CFU/0.2 mL, depending on the strain. Mice were challenged intraperitoneally and monitored at least twice daily. When mice became moribund, as defined by hunched appearance, markedly increased respiration rate, piloerection, and lack of an ability to move in response to being touched, they were euthanized and counted as dead for these experimental outcomes.
Statistical Analysis
Nonparametric data were evaluated by Mann–Whitney U test for 2-group comparisons and Kruskal–Wallis test with Dunn multiple comparison test for pairwise comparisons. Parametric data were analyzed by t test (for 2-group comparisons) or analysis of variance with Tukey multiple comparison test for pairwise comparisons. Survival analysis utilized the Kaplan–Meier method. All analyses used the GraphPad Prism software program.
RESULTS
The outbreak strain of B. dolosa has remarkably high antibiotic resistance: of 56 B. dolosa isolates tested for antimicrobial susceptibility at a Boston hospital from 2007 to 2009, none were susceptible to meropenem, ceftazidime, piperacillin/tazobactam, trimethoprim/sulfamethoxazole, or ciprofloxacin; only 29% were susceptible to minocycline. An antibiogram of a representative clinical isolate is shown in Figure 1A. This resistance profile has made these infections very difficult to control and is the underpinning of efforts to uncover new targets for immunotherapy. Because a number of pathogenic bacteria have been shown to produce PNAG and antibodies to PNAG have been shown to be protective against S. aureus and E. coli infections in murine models [10, 14], we investigated whether PNAG was expressed by B. dolosa infecting the lungs of a CF patient. Using immunofluorescence, we found that most of the B. dolosa cells expressed PNAG, although some cells were PNAG-negative (Figure 1B).
Figure 1.
Production of poly-β-(1-6)-N-acetylglucosamine (PNAG) by the Burkholderia cepacia complex. A, Antibiotic susceptibility testing of a pan-resistant B. dolosa determined by disk diffusion and Etest. B, Confocal microscopic images of lung tissue sections from a CF patient who died of B. dolosa pneumonia and sepsis. A. Nuclear staining with TO-PRO-3 (blue). B. B. dolosa-specific mouse serum followed by a red secondary antibody. C. Rabbit antiserum to PNAG followed by a green secondary antibody. D. Phase contrast. E. Overlay of all channels. F. Negative control (normal rabbit serum) with TO-PRO-3 nuclear stain. Bar is 10 microns for A-E. C, B. dolosa pga locus. D, Results of a BLAST search using the sequence of the pgaC gene. E, Detection of PNAG production by confocal microscopy and specific enzymatic digestion using chitinase (top row) or dispersin B (bottom row) using red DNA stain (left panels), anti-PNAG human IgG1 mAb F598 with green secondary antibody (middle panels). Right panels are overlays of red and green channels. Bar is 10 microns.
Upon analysis of the genome of B. dolosa strain AU0158, we discovered a locus homologous to the E. coli pga locus (Figure 1C and 1D). By polymerase chain reaction (PCR), the appropriately sized fragment of the pgaC gene was detected in 100% of 68 BCC clinical and laboratory isolates (B. dolosa, n = 57; B. cenocepacia, n = 4; and B. multivorans, n = 7). In comparison, previous studies of E. coli strains evaluated in this manner found that 13% (4 of 30 clinical isolates) were negative for the pga locus by PCR [14]. Although the B. dolosa isolates all belong to the outbreak genotype SLC6 [20], the B. cenocepacia and B. multivorans isolates tested were from different genotypes as assessed by BOX-PCR [21], with the exception of B. cenocepacia strains J2315 (the positive control strain upon which the primers were based) and K56-2, which are both of the ET12 lineage. Remarkably, among 112 B. dolosa SLC6 isolates obtained from 14 CF patients during a period of 16 years that were recently sequenced, no single-nucleotide polymorphisms were observed in the pga locus [22]. BLAST analysis also found homologs of the pgaC gene in other BCC species and several other pathogenic MDR bacteria including K. pneumoniae, E. cloacae, and Stenotrophomonas maltophilia (Figure 1D), suggesting that most of the MDR and even pan-resistant bacteria could be producers of PNAG, thus making all of them a potential target for immunotherapy mediated by antibodies to PNAG.
We next assessed PNAG expression by BCC strains using immunochemical methods. By confocal microscopy, it was seen that the PNAG-specific mAb F598 bound to cells of the major BCC species (Figure 1E). The specificity of this binding was confirmed by showing decreased binding following enzymatic digestion of PNAG with the PNAG-specific degradative enzyme dispersin B (Figure 1E). Immunoelectron microscopy using goat polyclonal antibodies raised to the 9GlcNH2-TT conjugate vaccine [16, 23] detected PNAG on the surface of the wild-type B. cenocepacia strain K56-2, whereas no gold particles were detected using normal goat serum as a primary antibody (Supplementary Figure 1A and 1B).
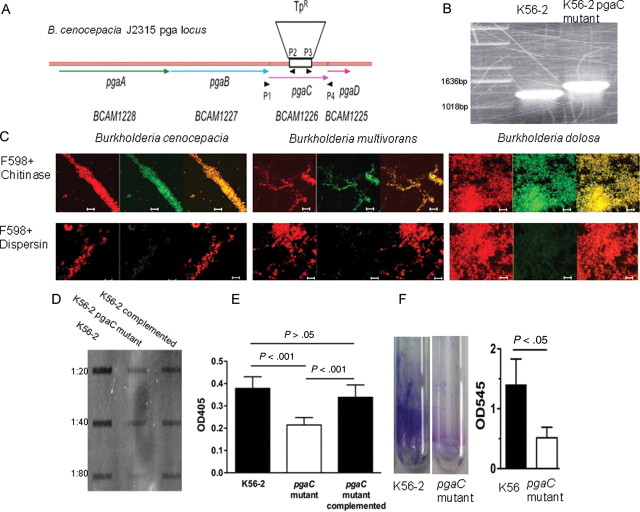
We next confirmed the association of PNAG expression with biofilm production and its dependence on the pga locus using the B. cenocepacia strain K56-2 along with a pgaC mutant generated in strain K56-2 (Figure 2A and 2B). We constructed the pgaC mutant in B. cenocepacia K56-2 rather than in B. dolosa due to the very high antibiotic resistance of B. dolosa strains, which precluded standard genetic manipulations. By confocal microscopy, it was seen that the PNAG-specific mAb did not bind to cells of the pgaC mutant, confirming the lack of background staining that could have been associated with nonspecific binding, a common property of antibodies to polysaccharides (Figure 2C). Immunoblot analysis of ethylenediaminetetraacetic acid extracts of bacterial cells probed with goat anti-PNAG antibodies (raised to a 9-mer glucosamine-TT conjugate vaccine [9GlcNH2-TT] [16]) showed that the B. cenocepacia pgaC mutant had less reactivity than the wild-type and pgaC-complemented strain (Figure 2D). The residual production of antibody-reactive material observed by immunoblot from extracted cells of the pgaC mutant is likely due to synthesis of a cross-reactive antigen. The difference in PNAG production between the wild-type strain and its mutant was confirmed by enzyme-linked immunosorbent assay on bacterial cells grown in 96-well plates for 2 days (Figure 2E). A biofilm assay using crystal violet (Figure 2F) also showed that the pgaC mutant produced much less biofilm. Immunoelectron microscopic analysis of the mutant strain complemented in trans with an intact pgaC gene revealed a higher level of gold-stained cells than that observed for the pgaC mutant strain (Supplementary Figure 1C and 1D).
Figure 2.
Poly-β-(1-6)-N-acetylglucosamine (PNAG) expression by the Burkholderia cepacia complex is dependent on the pga locus and associated with biofilm production. A, Burkholderia cenocepacia pga locus in strain J2315. DNA sequence was obtained from the Sanger Center (http://www.sanger.ac.uk/Projects/B_cenocepacia/) and analyzed using the Gene Construction Kit (Textco Biotech). Open reading frames are marked, as are primer sites and the trimethoprim resistance (TpR) insert in pgaC. B, Polymerase chain reaction confirmation of the pgaC insertional mutant in B. cenocepacia K56-2. C, Confocal microscopic images of B. cenocepacia K56-2 and K56-2 pgaC mutant using red DNA stain (left panel), anti-PNAG human immunoglobulin G1 (IgG1) monoclonal antibody F598 [17], or a control anti–Pseudomonas aeruginosa alginate IgG1 antibody with green secondary antibody (middle panel). Right panel is overlay of red and green channels. Bar is 10 μm. D, Expression of PNAG as assessed by immunoblot of B. cenocepacia K56-2, K56-2 pgaC mutant, and complemented K56-2 pgaC mutant. E, Detection of PNAG production by enzyme-linked immunosorbent assay after 2 days of growth at 30°C, comparing B. cenocepacia K56-2, its pgaC mutant, and the complemented mutant. The values shown are the means ± SD of 9 wells of a representative experiment. P values by analysis of variance with Tukey multiple comparisons test. F, Biofilm assay using crystal violet staining of biofilm formed after 2 days of growth at room temperature comparing B. cenocepacia K56-2 and its pgaC mutant, visualized in left panel and quantified in right panel. Right panel bars represent means (n = 3), error bars SD. P value by unpaired t test.
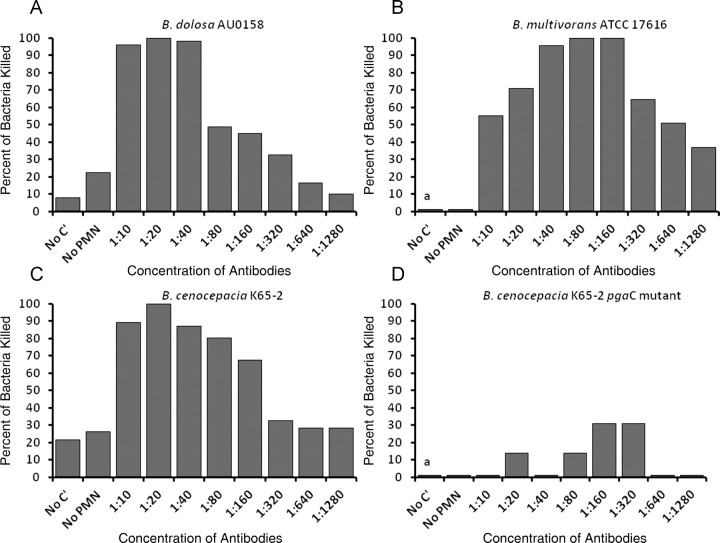
Expression of PNAG on the BCC strains suggested that this antigen might serve as a target of opsonophagocytic killing, a major in vitro property of protective antibodies. Serum samples from 13 CF patients chronically infected by B. dolosa showed a PNAG-specific opsonophagocytic killing activity against B. dolosa and the other major BCC species at a 1:10 serum dilution (Figure 3A–C). This killing activity was dependent on the presence of complement and was absent at 1:160 dilution (P < .001 by Kruskal–Wallis test with Dunn multiple comparison test). No killing was present against the B. cenocepacia pgaC mutant (Figure 3D). The lack of killing against the B. cenocepacia K56-2 pgaC strain confirmed the specificity of the opsonophagocytic killing and that we achieved depletion of any nonspecific opsonic antibodies potentially present in the sera studied. There was minimal killing with pooled normal human sera (<20% against all BCC species tested, not shown). We speculate that the presence of PNAG-specific opsonophagocytic antibodies in these patients, although not able to eliminate lung infection, may be protective against dissemination from the lung and the usually lethal cepacia syndrome. Indeed, none of these patients had the cepacia syndrome. This situation is somewhat analogous to that described in older CF patients having opsonophagocytic antibody directed against mucoid Pseudomonas aeruginosa who are less likely to be colonized with mucoid P. aeruginosa and have slower decline in lung function [24]. Although high levels of antibodies directed against bacteria in CF patients have sometimes been associated with immune complexes [25], this is generally not a phenomenon seen with opsonophagocytic antibodies [26]. Finally, demonstrating that humans can mount an opsonophagocytic antibody response to PNAG is an important prerequisite for the potential success of PNAG-based vaccines, indicative of the lack of immune nonresponsiveness to this antigen.
Figure 3.
Presence of opsonophagocytic killing activity specific to poly-β-(1-6)-N-acetylglucosamine (PNAG) in sera from cystic fibrosis (CF) patients chronically infected with Burkholderia dolosa. Opsonophagocytic killing (OPK) of Burkholderia cepacia complex by human sera from 13 CF patients chronically infected by B. dolosa. A: OPK of B. dolosa. B: OPK of B. multivorans. C: OPK of B. cenocepacia. D: OPK of B. cenocepacia pgaC mutant. Sera were adsorbed to remove antibodies not specific for PNAG. *P < .001 in comparison to any of the other groups by Kruskal-Wallis test with Dunn multiple comparison test. Abbreviations: C′, complement; PMN, polymorphonuclear leukocyte.
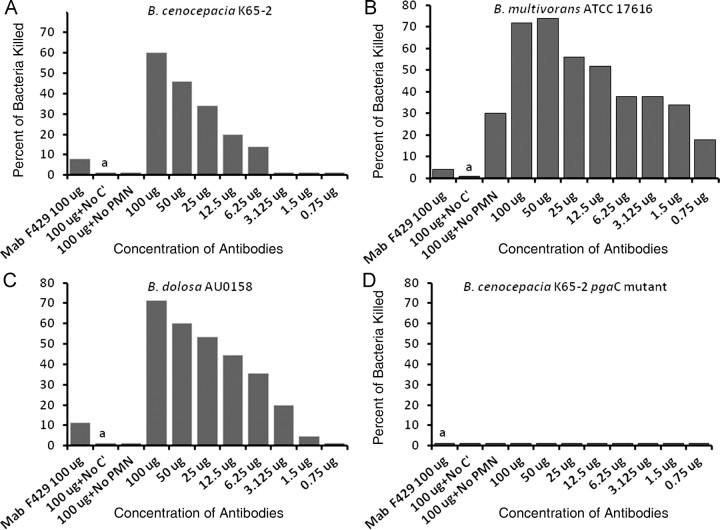
We also found excellent (>80%) opsonic killing by PNAG-specific goat antiserum against 3 BCC strains (Figure 4): B. dolosa AU0158, B. multivorans ATCC 17616, and B. cenocepacia K56-2. The pgaC mutant of B. cenocepacia K56-2 was not killed (Figures 3D, 4D, and 5D), indicating the specificity of the antibodies for the PNAG antigen. There was no bacterial killing without polymorphonuclear leukocytes or complement. These results were confirmed using the PNAG-specific human IgG1 mAb F598 [17] (Figure 5).
Figure 4.
Animal antibodies to poly-β-(1-6)-N-acetylglucosamine (PNAG) mediate opsonic killing of Burkholderia cepacia complex strains. Opsonophagocytic killing of B. dolosa AU0158 K56-2 (A), B. multivorans ATCC17616 (B), B. cenocepacia K56-2 (C), and B. cenocepacia K56-2 pgaC mutant (D) by PNAG-specific goat antiserum. Bars represent mean percentage of killing relative to control containing normal goat serum. All SDs (not shown) were <15%. Assays done in duplicate. Abbreviations: C′, complement; PMN, polymorphonuclear leukocyte; a, absence of killing arbitrarily assigned as 1% killing.
Figure 5.
Human monoclonal antibody (mAb) to poly-β-(1-6)-N-acetylglucosamine (PNAG) mediates opsonic killing of Burkholderia cepacia complex (BCC) isolates. Opsonophagocytic killing of BCC isolates (B. cenocepacia K56-2, 1a; B. multivorans ATCC17616, 1b; B. dolosa AU0158, 1c; B. cenocepacia K56-2 pgaC mutant, 1d) by human immunoglobulin G1 (IgG1) mAb F598 to PNAG. Control is human IgG1 mAb to Pseudomonas alginate (F429). Bars represent mean percentage of killing relative to the starting inoculum. All SDs (not shown) were <12%. Abbreviations: C′, complement; PMN, polymorphonuclear leukocyte; a, absence of killing arbitrarily assigned as 1% killing.
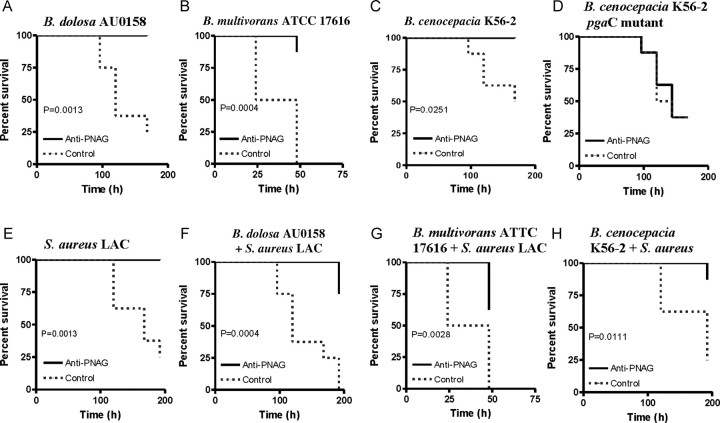
We next assessed the protective efficacy of PNAG-specific antibodies against BCC in a murine model of lethal peritonitis, which has been shown by the Holland group [27] to lead to bacteremia and dissemination to spleen and thus simulates the cepacia syndrome. We passively immunized mice intraperitoneally with the PNAG-specific goat antiserum (2 doses of 0.2 mL) and challenged with either a single strain of BCC or BCC mixed with a MRSA strain, the latter representing the frequent presence of polymicrobial infection in CF. As shown in Figure 6, the PNAG-specific serum enhanced survival from infection with B. cenocepacia K56-2 and B. dolosa AU0158 but not from the pgaC mutant of K56-2 (Figure 6D), indicating a PNAG-specific protective effect. Infection with B. multivorans ATCC17616 resulted in no lethality at a dose of 5 × 109 CFU/mouse (not shown), but protection was observed following a 10-fold higher challenge dose. Very interestingly, high-level protection was also seen during coinfection of BCC and S. aureus using the MRSA strain LAC (Figure 6E–H).
Figure 6.
Antibodies to poly-β-(1-6)-N-acetylglucosamine (PNAG) protect against lethal peritonitis from PNAG-producing Burkholderia cepacia complex (BCC) even during coinfection with methicillin-resistant Staphylococcus aureus. Mice were passively immunized with PNAG-specific goat antiserum given intraperitoneally 24 hours before and 4 hours after intraperitoneal challenge with the indicated strains. Doses were 5 × 109 colony-forming units (CFUs) per mouse for BCC other than B. multivorans, which was 5 × 1010 CFU/mouse. S. aureus strain LAC was used at 109 CFU/mouse (E–H). Controls received normal goat serum. P values by log rank test, n = 8 mice per group.
DISCUSSION
In summary, we found that strains of the BCC, including the highly antibiotic-resistant B. dolosa, express the broadly conserved surface polysaccharide PNAG both in vitro and in vivo. We also found that PNAG is a target for opsonophagocytic killing mediated completely by a human monoclonal antibody that has successfully undergone a phase 1 safety and dose-ranging study in humans and also by antisera produced in animals to a leading glycoform that could be used as an active vaccine. Although PNAG was first described as a biofilm component in S. epidermidis [11], subsequent work showed that PNAG is also produced by several gram-negative, often antibiotic-resistant human pathogens such as E. coli [12] and A. baumannii [13]. Antibodies to the deacetylated glycoform of PNAG are known to be opsonic and protective against S. aureus and E. coli infections [10, 14]. The current studies now extend this activity and protection to the BCC as well, including the nearly pan-resistant B. dolosa.
We focus here on passive rather than active vaccination both as proof of principle and due to the more practical therapeutic aspects of passive therapy. Notably, the PNAG-specific antibodies mediated protective immunity against all BCC strains but not against one genetically engineered to lack PNAG. Furthermore, antibodies to this single-surface polysaccharide can, remarkably, also protect against coinfection with a MRSA strain, suggesting the therapeutic potential of using PNAG-directed antibodies to target multiple bacterial species for which there are few antibiotic options. Determination of the concentration of PNAG-specific antibodies required for protection and assessment, whether protection can be achieved by a physiologic dose of antibodies, will be critical for the potential future use of antibodies to PNAG as passive immunotherapy in humans. These questions will be further explored using sera from the ongoing phase 2 clinical trial with a human monoclonal antibody to PNAG.
One worrisome aspect related to the overall problem of antibiotic resistance in human bacterial pathogens is that even as new drugs are developed, resistance can rapidly emerge. Thus, immunotherapeutic approaches offer an alternative that might be less prone to emergence of resistant organisms. In the case of PNAG, resistance to immunotherapies could emerge if strains can maintain virulence when not producing PNAG. Staphylococcus aureus [16, 28] and Bordetella pertussis [29] studies in mice have suggested that PNAG is an important virulence factor. However, in our mouse peritonitis model, we did not detect a difference in virulence between the PNAG-proficient B. cenocepacia strain and its PNAG-deficient mutant. Whether this indicates a lack of importance of PNAG production by BCC for virulence or reflects the limitations of results from mouse studies with poorly virulent bacterial pathogens is not clear. Although the broad expression of PNAG among bacterial pathogens presents a potential welcome single target and also suggests an important role in bacterial biology due to its common expression, if there is not much of a compromise in virulence in humans from loss of PNAG expression, then there may be limitations to targeting PNAG by immunotherapeutic approaches.
The approach described might also be effective against other pan-resistant bacteria because a putative pga locus was found, as described in this work, in K. pneumoniae, E. cloacae, and S. maltophilia. An immune approach potentially targeting a variety of multiresistant bacterial pathogens possessing a functional biosynthetic loci for PNAG synthesis has obvious appeal, but extensive additional studies are needed for preclinical evaluation of this potential. Notably, we did find that antibody to PNAG could protect against coinfection with PNAG-expressing B. dolosa and PNAG-expressing S. aureus, indicative of sufficient similarity in the PNAG antigen between a gram-negative and gram-positive strain. This suggests that antibodies to PNAG (raised to a 9-mer glucosamine-TT conjugate vaccine, 9GlcNH2-TT) could be opsonic and protective against both bacterial pathogens. Overall, our finding of a shared antigenic target that could be used to treat or prevent infections by MDR bacterial pathogens that express PNAG is very promising and supports the development of PNAG-based vaccines and passive therapies to address the vexing problem of antibiotic resistance and the inexorable loss of effective antibacterial therapies.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://www.oxfordjournals.org/our_journals/jid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Disclaimer. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Financial support. This work was supported by grants from the Cystic Fibrosis Foundation (PRIEBE06G0 to G. P. P.); the National Institutes of Health, National Institute of Allergy and Infectious Diseases (AI46706 and AI057159 to G. B. P.), a component of award U54 AI057159 (G. B. P.); and the University of Virginia Infectious Diseases program (training grant AI07406 to M. R. D. and S. K. C.).
Potential conflicts of interest. G. B. P. and T. M.-L. have developed intellectual property that has been licensed for development of PNAG and PNAG-based vaccines as well as for monoclonal antibodies to the PNAG antigen and have received consulting income and licensing income from these arrangements. G. B. P. also holds equity interest in Alopexx Pharmaceuticals LLC, which has licensed the rights to develop monoclonal antibody therapies for PNAG-expressing microbes. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waters V, Ratjen F. Multidrug-resistant organisms in cystic fibrosis: management and infection-control issues. Expert Rev Anti Infect Ther. 2006;4:807–19. doi: 10.1586/14787210.4.5.807. [DOI] [PubMed] [Google Scholar]
- 3.Aaron SD, Vandemheen KL, Ramotar K, et al. Infection with transmissible strains of Pseudomonas aeruginosa and clinical outcomes in adults with cystic fibrosis. JAMA. 2010;304:2145–53. doi: 10.1001/jama.2010.1665. [DOI] [PubMed] [Google Scholar]
- 4.Dasenbrook EC, Checkley W, Merlo CA, Konstan MW, Lechtzin N, Boyle MP. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA. 2010;303:2386–92. doi: 10.1001/jama.2010.791. [DOI] [PubMed] [Google Scholar]
- 5.Kalish LA, Waltz DA, Dovey M, et al. Impact of Burkholderia dolosa on lung function and survival in cystic fibrosis. Am J Respir Crit Care Med. 2006;173:421–5. doi: 10.1164/rccm.200503-344OC. [DOI] [PubMed] [Google Scholar]
- 6.Mahenthiralingam E, Urban TA, Goldberg JB. The multifarious, multireplicon Burkholderia cepacia complex. Nat Rev Microbiol. 2005;3:144–56. doi: 10.1038/nrmicro1085. [DOI] [PubMed] [Google Scholar]
- 7.Drevinek P, Mahenthiralingam E. Burkholderia cenocepacia in cystic fibrosis: epidemiology and molecular mechanisms of virulence. Clin Microbiol Infect. 2010;16:821–30. doi: 10.1111/j.1469-0691.2010.03237.x. [DOI] [PubMed] [Google Scholar]
- 8.Isles A, Maclusky I, Corey M, et al. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J Pediatr. 1984;104:206–10. doi: 10.1016/s0022-3476(84)80993-2. [DOI] [PubMed] [Google Scholar]
- 9.Maira-Litran T, Kropec A, Goldmann DA, Pier GB. Comparative opsonic and protective activities of Staphylococcus aureus conjugate vaccines containing native or deacetylated staphylococcal poly-N-acetyl-beta-(1-6)-glucosamine. Infect Immun. 2005;73:6752–62. doi: 10.1128/IAI.73.10.6752-6762.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKenney D, Pouliot KL, Wang Y, et al. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science. 1999;284:1523–7. doi: 10.1126/science.284.5419.1523. [DOI] [PubMed] [Google Scholar]
- 11.Mack D, Fischer W, Krokotsch A, et al. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol. 1996;178:175–83. doi: 10.1128/jb.178.1.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Preston JF, 3rd, Romeo T. The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J Bacteriol. 2004;186:2724–34. doi: 10.1128/JB.186.9.2724-2734.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi AH, Slamti L, Avci FY, Pier GB, Maira-Litran T. The pgaABCD locus of Acinetobacter baumannii encodes the production of poly-beta-1-6-N-acetylglucosamine, which is critical for biofilm formation. J Bacteriol. 2009;191:5953–63. doi: 10.1128/JB.00647-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerca N, Maira-Litran T, Jefferson KK, Grout M, Goldmann DA, Pier GB. Protection against Escherichia coli infection by antibody to the Staphylococcus aureus poly-N-acetylglucosamine surface polysaccharide. Proc Natl Acad Sci U S A. 2007;104:7528–33. doi: 10.1073/pnas.0700630104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urban TA, Griffith A, Torok AM, Smolkin ME, Burns JL, Goldberg JB. Contribution of Burkholderia cenocepacia flagella to infectivity and inflammation. Infect Immun. 2004;72:5126–34. doi: 10.1128/IAI.72.9.5126-5134.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gening ML, Maira-Litran T, Kropec A, et al. Synthetic β-(1→6)-linked N-acetylated and nonacetylated oligoglucosamines used to produce conjugate vaccines for bacterial pathogens. Infect Immun. 2010;78:764–72. doi: 10.1128/IAI.01093-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly-Quintos C, Cavacini LA, Posner MR, Goldmann D, Pier GB. Characterization of the opsonic and protective activity against Staphy530 lococcus aureus of fully human monoclonal antibodies specific for the bacterial surface polysaccharide poly-N-acetylglucosamine. Infect Immun. 2006;74:2742–50. doi: 10.1128/IAI.74.5.2742-2750.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pier GB, Boyer D, Preston M, et al. Human monoclonal antibodies to Pseudomonas aeruginosa alginate that protect against infection by both mucoid and nonmucoid strains. J Immunol. 2004;173:5671–8. doi: 10.4049/jimmunol.173.9.5671. [DOI] [PubMed] [Google Scholar]
- 19.Manuel SG, Ragunath C, Sait HB, Izano EA, Kaplan JB, Ramasubbu N. Role of active-site residues of dispersin B, a biofilm-releasing beta-hexosaminidase from a periodontal pathogen, in substrate hydrolysis. FEBS J. 2007;274:5987–99. doi: 10.1111/j.1742-4658.2007.06121.x. [DOI] [PubMed] [Google Scholar]
- 20.Biddick R, Spilker T, Martin A, LiPuma JJ. Evidence of transmission of Burkholderia cepacia, Burkholderia multivorans and Burkholderia dolosa among persons with cystic fibrosis. FEMS Microbiol Lett. 2003;228:57–62. doi: 10.1016/S0378-1097(03)00724-9. [DOI] [PubMed] [Google Scholar]
- 21.Coenye T, Spilker T, Martin A, LiPuma JJ. Comparative assessment of genotyping methods for epidemiologic study of Burkholderia cepacia genomovar III. J Clin Microbiol. 2002;40:3300–7. doi: 10.1128/JCM.40.9.3300-3307.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lieberman TD, Michel J-B, Aingaran M, et al. Parallel bacterial evolution within multiple patients ties novel genes to pathogenesis. Nat Genetics. 2011;43:1275–80. doi: 10.1038/ng.997. doi:10.1038/ng.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gening ML, Tsvetkov YE, Pier GB, Nifantiev NE. Synthesis of β-(1→6)-linked glucosamine oligosaccharides corresponding to fragments of the bacterial surface polysaccharide poly-N-acetylglucosamine. Carbohydr Res. 2007;342:567–75. doi: 10.1016/j.carres.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Pier GB, Saunders JM, Ames P, et al. Opsonophagocytic killing antibody to Pseudomonas aeruginosa mucoid exopolysaccharide in older noncolonized patients with cystic fibrosis. New Engl J Med. 1987;317:793–8. doi: 10.1056/NEJM198709243171303. [DOI] [PubMed] [Google Scholar]
- 25.Van Bever HP, Gigase PL, De Clerck LS, Bridts CH, Franckx H, Stevens WJ. Immune complexes and Pseudomonas aeruginosa antibodies in cystic fibrosis. Arch Dis Child. 1988;63:1222–8. doi: 10.1136/adc.63.10.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pier GB, Takeda S, Grout M, Markham RB. Immune complexes from immunized mice and infected cystic fibrosis patients mediate murine and human T cell killing of hybridomas producing protective, opsonic antibody to Pseudomonas aeruginosa. J Clin Invest. 1993;91:1079–87. doi: 10.1172/JCI116265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segal BH, Ding L, Holland SM. Phagocyte NADPH oxidase, but not inducible nitric oxide synthase, is essential for early control of Burkholderia cepacia and Chromobacterium violaceum infection in mice. Infect Immun. 2003;71:205–10. doi: 10.1128/IAI.71.1.205-210.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kropec A, Maira-Litran T, Jefferson KK, et al. Poly-N-acetylglucosamine production in Staphylococcus aureus is essential for virulence in murine models of systemic infection. Infect Immun. 2005;73:6868–76. doi: 10.1128/IAI.73.10.6868-6876.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conover MS, Sloan GP, Love CF, Sukumar N, Deora R. The Bps polysaccharide of Bordetella pertussis promotes colonization and biofilm formation in the nose by functioning as an adhesin. Molec Microbiol. 2010;77:1439–55. doi: 10.1111/j.1365-2958.2010.07297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.