Abstract
We investigated a norovirus outbreak (genotype GII.2) affecting 9 members of a soccer team. Illness was associated with touching a reusable grocery bag or consuming its packaged food contents (risk difference, 0.636; P < .01). By polymerase chain reaction, GII norovirus was recovered from the bag, which had been stored in a bathroom used before the outbreak by a person with norovirus-like illness. Airborne contamination of fomites can lead to subsequent point-source outbreaks. When feasible, we recommend dedicated bathrooms for sick persons and informing cleaning staff (professional or otherwise) about the need for adequate environmental sanitation of surfaces and fomites to prevent spread.
(See the editorial commentary by Hall, on pages 1622–4.)
Noroviruses are a leading cause of gastroenteritis worldwide and the most common cause of foodborne outbreaks in the United States [1, 2]. The low infectious dose and the high viral load in vomit and feces [3] lead to efficient transmission through typical fecal-oral routes as well as airborne spread and environmental contamination of fomites [4]. Persistent, multigenerational outbreaks have been linked to fomites and reported on cruise ships [5], hotels [3], and institutional settings [3, 6] despite aggressive housekeeping [7], and point-source outbreaks from fomes exposure are rarely identified [8]. The role of fomites in transmission can be difficult to assess owing to lack of established protocol for testing fomites and environmental surfaces. We investigated a point-source norovirus outbreak caused by exposure to fomites.
In October 2010, the Oregon Public Health Division was notified by colleagues from public health agencies in Washington State that a parent-chaperone had reported a cluster of acute gastroenteritis among persons who had recently participated in a soccer tournament held in King County, Washington. The weekend tournament comprised about 2000 children in approximately 120 teams from Washington and Oregon. The Oregon group comprised 17 Oregon girls who were 13–14 years old and 4 adult chaperones who had traveled to the tournament on Friday afternoon in private automobiles. They shared rooms at a hotel in Washington on Friday and Saturday nights, eating at local restaurants and in their hotel rooms, and they returned to Oregon after the tournament ended on Sunday afternoon. We investigated to determine the scope of the outbreak and its etiology and to take appropriate control measures.
METHODS
Tournament organizers and contacts for other teams were canvassed by telephone and email to determine the extent of illness. Complaint logs were reviewed for reports of any contemporaneous illness among patrons of the restaurants and hotel visited by the Oregon group. This was a public health investigation to control a disease outbreak and therefore did not require approval by an institutional review board.
We conducted a retrospective cohort study of the Oregon group. Persons were interviewed by telephone or in person using a standardized questionnaire with questions about potential exposures (foods, hotel roommates, travel partners, etc), clinical history, and contemporary household illness.
A case was defined as a delegate of the Oregon group who developed vomiting or diarrhea (≥3 loose stools within a 24-hour period) within 72 hours of their return from the tournament. Household members of cases who developed similar symptoms within the following week but who did not attend the tournament were considered secondary cases.
Risk differences were calculated for all exposures using EXTSIG and CID2BP software (MD Anderson Cancer Center, The University of Texas) with Cox-Snell 95% confidence intervals [CIs] and Fisher exact test P values [9]. Relative risks (not presented) are less informative due to small sample size and zero-count cells.
Stool specimens were solicited from persons who reported illness. A reusable grocery bag was tested for norovirus by vigorously swabbing small patches (∼25 cm2) of the bag surface with sterile polyester swabs wetted with sterile nuclease-free water. The swabs were extracted using the MagAttract viral M48 RNA kit (Qiagen 955235) on an automated BioRobot M48 Extractor. All specimens were tested for the presence of norovirus RNA genogroups GI and GII by real-time reverse-transcriptase polymerase chain reaction [10, 11] and were further characterized using genetic sequencing of region C of the ORF2 gene [12].
RESULTS
There were no reports of similarly clustered illness among any other teams at the tournament, nor were there any coincident reports of illness among patrons of any of the restaurants or hotel patronized by the Oregon group.
All 21 members of the group were interviewed; however, 1 healthy person refused to answer exposure questions and 1 ill person was excluded due to direct exposure to case 1 and her vomit. We identified 7 cases who ranged from 13 to 48 years old (median, 13). All 7 (100%) reported vomiting; 4 (57%) also reported diarrhea. The reported duration of symptoms ranged from 1 to 7 days (median, 3). One case sought medical care, but there were no hospitalizations. There were no reports of mild illness not meeting the case definition. We identified at least 5 presumptive secondary infections among household members.
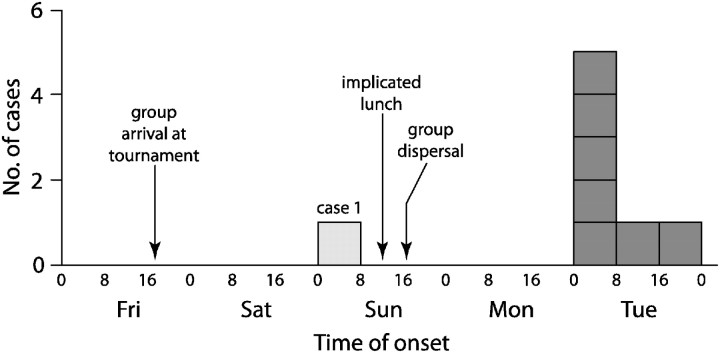
Case 1 initially became nauseated and developed abdominal pain late Saturday evening, at which time she left her room and moved in with one of the chaperones. Shortly after midnight, she began vomiting and having diarrhea that continued throughout the night. In the morning she was taken back to Oregon by this chaperone, who later became ill. Neither individual rejoined the group or participated in any of the Sunday group activities or meals; both were excluded from analysis for Sunday exposures. All other cases reported symptom onset on Tuesday (Figure 1).
Figure 1.
Epidemic curve of gastroenteritis among attendees of a soccer tournament in Washington in October 2010. Presumptive secondary infections are not shown.
The following variables were assessed for association with illness: age; hotel roommates and hotel room; transportation groups for activities, soccer games, and car groups returning from the tournament; and all reported food exposures on Friday, Saturday, and Sunday. Logistic modeling was not possible due to small sample size. No significant association with illness was identified for any Friday or Saturday exposure. On initial analysis, consumption of sealed packaged cookies from the Sunday lunch was significantly associated with illness (risk difference [RD], 0.750; 95% confidence interval [CI], .24–.91, P = .01); 3 of 7 cases (43%) and none of the 12 healthy attendees reported cookie consumption. The cookies and other lunch supplies had been purchased in Oregon and stored at the hotel until use.
On reinterview, we learned that the cookies, along with packaged chips and fresh grapes, had been stored in a reusable open-top grocery bag made from laminated woven polypropylene. This bag had been stored in the hotel bathroom of the chaperone who had cared for case 1. Case 1 reported never touching or handling the grocery bag, but it was in the bathroom she used throughout the night. At lunchtime on Sunday—hours after case 1 had departed—the bag was taken to another hotel room where the contents (cookies, chips, and grapes) were passed around as part of the lunch. The cookies and chips were in unopened commercial packages. We did not ascertain how many Oregon group members handled the grocery bag. Illness was associated with a composite exposure variable of any item in the bag (ie, cookies, chips, or grapes; 7 of 7 cases with exposure and 4 of 12 controls with exposure; RD, 0.636; 95% CI, .32–.87; P < .01). No single item in the bag was reportedly consumed by more than 4 of 7 cases. Assuming exposure at the Sunday lunch, incubation periods ranged from 36 to 57 hours (median, 38.5 hours).
All 3 stool specimens collected from ill persons were positive for norovirus (genotype GII.2). No specimen was available from case 1. Viral sequences from the 3 stool specimens were identical and a 98% match to a GII.2 reference sequence (GII.2.Vaals NLD05). Two of 10 swabs taken from the grocery bag 2 weeks after the implicated meal were positive (genogroup GII). The grocery bag samples were insufficient to sequence; no leftover food was available.
There were no reports of subsequent illness among guests or staff reported to hotel management.
DISCUSSION
Initial concerns that this outbreak may have involved other persons from the tournament or local restaurant patrons were quickly allayed. The distribution of incubation times for the Oregon group indicated that the larger group was exposed at the Sunday lunch. By that time, however, case 1 had been absent for over 12 hours, and because she had no contact with any of the other cases after her onset of vomiting or diarrhea and no direct contact with any of the lunch food, it was initially unclear as to how these illnesses could be connected. Only when we learned about the bag in the bathroom did a coherent story emerge.
The data indicate that virus aerosolized within the hotel bathroom settled upon the grocery bag and its contents, and it was touching the bag and consumption of its contents that led to the outbreak. Touching the bag could not be analyzed separately from consumption of food items from within the bag. Consumption of food from the grocery bag was strongly associated with illness, as was handling the grocery bag. The nature of the contaminated foods—a bag of chips, grapes, and a package of cookies—facilitated transmission. Fingers contaminated with norovirus have been shown to sequentially transfer virus to up to 7 clean surfaces [7], and environmental contamination with transmission via fomites has been documented [7, 8]. Incidentally, this also illustrates one of the less obvious hazards of reusable grocery bags.
Aerosolization of vomit and feces has been demonstrated to be of major importance in norovirus outbreaks [13]. Even viruses aerosolized from flushing a toilet can contaminate surfaces throughout a bathroom [14]. Once a fomes is contaminated, transfer to hands and other animate objects can readily occur [15]. The more confined the space (eg, most bathrooms), the more intense would be the “fallout” [13].
This investigation confirms the potential for aerosol contamination of fomites in norovirus outbreaks, which has long been suspected to contribute to persistent problems on cruise ships, in nursing homes, and other settings [5, 6, 13]. Although we certainly recommend not storing food in bathrooms, it is more important to emphasize that areas where aerosol exposures may have occurred should be thoroughly disinfected; this includes not only exposed surfaces but also objects in the environment that could serve as fomites. If multiple bathrooms are available, it would be prudent to dedicate one for use by sick persons. We also recommend that persons with responsibilities for cleaning (eg, housekeeping staff or family members) be informed about incidents of vomiting or diarrhea and best practices for disinfection.
Notes
Acknowledgments. We gratefully acknowledge the invaluable assistance of James Terry and LaDonna Grenz (Oregon State Public Health Laboratory), Kathryn MacDonald (Washington State Department of Health), Jenny Koepsell Lloyd (Public Health–Seattle & King County) and Ellen Stevenson (Departments of Pediatrics and Public Health & Preventive Medicine, Oregon Health and Science University).
Financial support. Authors were funded by their respective organizations. This work was also supported by the Pacific Northwest Regional Center of Excellence funded by the National Institutes of Health (grant U54AI 081680 to K. K. R.) and the Emerging Infections Program Cooperative Agreement (grant 3U01CI000306 to W. E. K.) with the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Patel MM, Hall AJ, Vinjé J, Parashar UD. Noroviruses: a comprehensive review. J Clin Virol. 2009;44:1–8. doi: 10.1016/j.jcv.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Gould LH, Nisler AL, Herman KM, et al. Surveillance for foodborne disease outbreaks–United States, 2008. MMWR Morb Mortal Wkly Rep. 2011;60:1197–202. [PubMed] [Google Scholar]
- 3.Cheesebrough JS, Green J, Gallimore CI, Wright PA, Brown DWG. Widespread environmental contamination with Norwalk-like viruses (NLV) detected in a prolonged hotel outbreak of gastroenteritis. Epidemiol Infect. 2000;125:93–8. doi: 10.1017/s095026889900432x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glass RL, Parashar UD, Estes MK. Norovirus gastroenteritis. N Engl J Med. 2009;361:1776–85. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho M-S, Monroe SS, Stine S, et al. Viral gastroenteritis aboard a cruise ship. Lancet. 1989;334:935–96. doi: 10.1016/s0140-6736(89)90964-1. [DOI] [PubMed] [Google Scholar]
- 6.Wu HM, Fornek M, Schwab KJ, et al. A norovirus outbreak at a long-term-care facility: the role of environmental surface contamination. Infect Control Hosp Epidemiol. 2005;26:802–10. doi: 10.1086/502497. [DOI] [PubMed] [Google Scholar]
- 7.Barker J, Vipond IB, Bloomfield SF. Effects of cleaning and disinfection in reducing the spread of norovirus contamination via environmental surfaces. J Hosp Infect. 2004;58:42–9. doi: 10.1016/j.jhin.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 8.Diggs R, Diallo A, Kan H, Glymph C, Furness B. Norovirus outbreak in an elementary school—District of Columbia, February 2007. MMWR Morbid Mortal Wkly Rep. 2008;56:1340–3. [PubMed] [Google Scholar]
- 9.Lee JJ, Serachitopol DM, Brown BW. Likelihood-weighted confidence intervals for the difference of two binomial proportions. Biometrics. 1997;39:387–407. [Google Scholar]
- 10.Kageyama T, Kojima S, Shinohara M, et al. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J Clin Microbiol. 2003;41:1548–57. doi: 10.1128/JCM.41.4.1548-1557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trujillo A, McCaustalnd K, Zheng D, et al. Use of TaqMan real-time reverse transcription-PCR for rapid detection, quantification, and typing of norovirus. J Clin Microbiol. 2006;44:1405–12. doi: 10.1128/JCM.44.4.1405-1412.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kojima S, Kageyama T, Fukushi S, et al. Genogroup-specific PCR primers for detection of Norwalk-like viruses. J Virol Methods. 2002;100:107–14. doi: 10.1016/s0166-0934(01)00404-9. [DOI] [PubMed] [Google Scholar]
- 13.Jones EL, Kramer A, Gaither M, Gerba CP. Role of fomite contamination during an outbreak of norovirus on houseboats. Int J Environ Health Res. 2007;17:123–31. doi: 10.1080/09603120701219394. [DOI] [PubMed] [Google Scholar]
- 14.Goldmann DA. Transmission of viral respiratory infections in the home. Pediatr Infect Dis J. 2000;19(Suppl 10):S97–102. doi: 10.1097/00006454-200010001-00002. [DOI] [PubMed] [Google Scholar]
- 15.Boone SA, Gerba CP. Significance of fomites in the spread of respiratory and enteric viral disease. Appl Environ Microbiol. 2007;73:1687–96. doi: 10.1128/AEM.02051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]