Abstract
Individuals with latent tuberculosis infection (LTBI) live with a risk of reactivation, and several treatments for chronic inflammatory conditions are highly associated with such reactivation. A new Janus kinase inhibitor, tofacitinib (CP-690550), has shown promising results for treatment of inflammatory disorders, thus raising concerns of risk of active tuberculosis. Our goal was to characterize the impact of tofacitinib on LTBI using a mouse model of contained tuberculosis. Our data indicate that tofacitinib reduces host containment of Mycobacterium tuberculosis and promotes bacterial replication in the lungs, suggesting tuberculosis reactivation. Tofacitinib may carry a significant risk for LTBI reactivation in humans.
Tuberculosis is a devastating human infectious disease responsible for the death of nearly 2 million people worldwide every year [1]. Even more alarming, the World Health Organization has estimated that nearly 2 billion individuals harbor a latent infection with Mycobacterium tuberculosis, the etiologic agent of tuberculosis [1]. Thus, approximately one-third of the world's population is at risk to develop active disease and contribute to the continued spread of M. tuberculosis within communities.
Although the detailed mechanisms by which M. tuberculosis infection transitions from latent to active disease are poorly understood, epidemiologic studies have identified clear risk factors for activation of latent tuberculosis infection (LTBI), of which the most well known are human immunodeficiency virus infection and treatment with tumor necrosis factor alpha (TNF-α) inhibitors [2]. The TNF-α antagonists etanercept, infliximab, adalimumab, certolizumab, and golimumab are commonly used for the treatment of chronic inflammatory conditions, including rheumatoid arthritis, psoriasis, and Crohn's disease. Compared with individuals with LTBI who do not receive TNF-α blockers, patients with LTBI who use these anti-inflammatory treatments are at a significantly higher risk to develop active tuberculosis [3]. The screening of patients for LTBI prior to anti–TNF-α treatment can reduce the risk for active tuberculosis by 90% if patients testing positive are first treated with isoniazid preventive therapy prior to TNF-α antagonist administration [4].
Tofacitinib, an oral Janus kinase (JAK) inhibitor (CP-690550, formerly tasocitinib), is a new anti-inflammatory agent developed by Pfizer. Tofacitinib displays properties similar to TNF-α inhibitors, and a recent clinical trial of this drug in patients with rheumatoid arthritis showed significant benefit [5]. The new JAK inhibitor has also shown promising results against other inflammatory conditions [6, 7]. As an oral agent (as opposed to the intravenous or injectable TNF-α antagonists), tofacitinib may become a commonly used anti-inflammatory agent in the coming years. However, this new drug has not yet been evaluated as a risk factor for LTBI reactivation. In this study, we examined the influence of tofacitinib in a published mouse model of chronic paucibacillary tuberculosis that was developed to represent human LTBI [8, 9].
METHODS
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The Johns Hopkins Animal Care and Use Committee approved all procedures described in this article.
Animals
Six-week-old BALB/c mice were obtained from Charles River Laboratories and maintained in an animal biosafety level 3 facility at all times. The paucibacillary mouse model of LTBI was used as previously described [9]. Mice were immunized with Mycobacterium bovis bacille Calmette-Guérin (BCG) Copenhagen prior to infection with M. tuberculosis H37Rv, following the experimental design presented in Table 1.
Table 1.
Experimental Design and Mouse Sacrifice Schedule
| Number of BALB/c Mice Sacrificed at Each Time Point |
|||||
|---|---|---|---|---|---|
| Treatment Group | Week −12 (BCG Immunization) | Week −6 (H37Rv Infection) | Week 0 (Start Treatment) | Week 2 | Week 4 |
| BCG/H37Rv | 4 | 4 | 4 | 4 | 4 |
| Low Dose | |||||
| BCG/H37Rv | 4 | 4 | |||
| High Dose | |||||
| BCG/H37Rv | 4 | 4 | |||
| Untreated | |||||
| H37Rv only | … | 4 | 4 | 4 | 4 |
| TOTAL | 4 | 8 | 8 | 16 | 16 |
Week −12 represents the time of immunization with bacille Calmette-Guérin (BCG), and lung colony-forming units (CFUs) for BCG implantation were determined for these mice. For all other time points, lung CFUs for H37Rv were determined.
Aerosol Infection and Colony Counting Procedures
Bacterial strains were prepared in standard 7H9 Middlebrook liquid medium containing 0.2% Tween-80, and aerosol infections were performed using the Glas-col Inhalation Exposure System per the manufacturer's instructions. At each time of death (noted in Table 1), mice lungs were dissected and total lung colony-forming unit (CFU) counts were determined as previously described [8]. Following infection with M. tuberculosis, mouse lung homogenates were serially diluted and plated on selective 7H11 agar supplemented with 4 mg/mL of 2-thiophenecarboxylic acid hydrazide (TCH) (Sigma); M. tuberculosis but not M. bovis BCG can grow in the presence of TCH [9]. Thus, all CFUs reported in this article for M. tuberculosis–infected mice (whether also infected with BCG or not) represent the CFUs for H37Rv only.
Drug Preparation and Administration
Tofacitinib was purchased from Selleck Chemicals, and stock solutions were prepared weekly using 0.5% methylcellulose in water as vehicle and stored at 4°C. Tofacitinib was administered twice daily to the mice by oral gavage to achieve 1.5 or 15 mg/kg in volumes of 0.2 mL.
Data Analysis
Lung CFU counts were log10-transformed. Friedman's 2-way analysis of variance by ranks was used for statistical analyses, with significance set at P < .05.
RESULTS
Paucibacillary Model
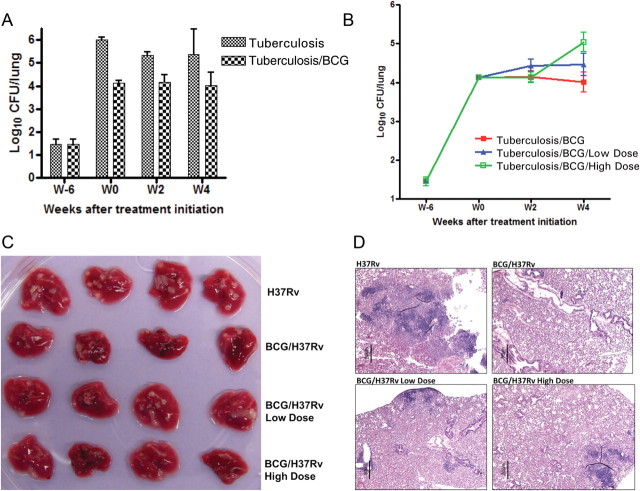
Mice were immunized with aerosol BCG infection yielding a day 1 (T = −12 weeks) implantation of mean 3.75 (standard deviation [SD] = 0.05) log10 CFUs. Six weeks following immunization (T = −6 weeks), BCG lung counts were mean 3.20 (SD = 0.17) log10 CFUs, at which point the immunized mice and a group of nonimmunized mice were infected with a low dose of H37Rv, with an implant of mean 1.45 (SD = 0.12) log10 CFUs. When tofacitinib therapy was initiated 6 weeks later (T = 0 weeks), the H37Rv lung counts had reached mean 4.13 (SD = 0.06) and mean 6.02 (SD = 0.04) log10 CFUs for immunized and nonimmunized mice, respectively. The immunized mice not treated with tofacitinib experienced a conserved 4 log10 CFU plateau, whereas the nonimmunized mice had 1–2 log10 more CFUs (Figure 1A), thus validating the paucibacillary model in this experiment.
Figure 1.
Tofacitinib treatment was associated with enhanced bacterial growth and pathology in mouse lungs. A, The paucibacillary model of latent tuberculosis. Mycobacterium tuberculosis H37Rv log10 colony-forming unit (CFU) counts in the lungs of bacille Calmette-Guérin (BCG)–vaccinated (Tuberculosis/BCG) and unvaccinated (Tuberculosis) mice. Error bars represent standard deviation. B, The impact of tofacitinib treatment on M. tuberculosis growth. M. tuberculosis H37Rv log10 CFU counts in the lungs of mice from vaccinated mice that were untreated (Tuberculosis/BCG), treated with 1.5 mg/kg/day (Tuberculosis/BCG/Low Dose), or treated with 15 mg/kg/day (Tuberculosis/BCG/High Dose). Error bars represent standard deviation. C, Gross pathology of mouse lungs from each treatment group. D, Representative histopathology sections from mouse lungs from each treatment group, stained using hematoxylin and eosin.
Tuberculosis Reactivation With Tofacitinib
After 14 days of tofacitinib treatment, both the low- and high-dose groups exhibited mean lung H37Rv CFU counts similar to the immunized, nontreated group (Figure 1B). However, after 28 days of treatment, the low-dose tofacitinib group had higher but nonstatistically significant lung CFU levels compared with the untreated mice, and the group receiving the high-dose tofacitinib treatment had a significantly higher lung H37Rv burden than the nontreated mice (mean, 5.04 [SD = 0.25] vs mean, 4.01 [SD = 0.26]; P = .045) (Figure 1B). These results demonstrate that 4 weeks of tofacitinib treatment promotes bacterial growth in a dose-dependent manner in the mouse lungs.
Gross pathology indicated that the lungs of the nonimmunized mice had more and larger lesions than the lungs of the BCG-immunized mice (Figure 1C). Lungs from the immunized, tofacitinib-treated mice exhibited intermediate gross lesions, which were indistinguishable between the low or high dose. Consistently, the most severe lesions, containing collapsed alveolar sacs and leukocyte aggregation, were found in the nonimmunized mouse lungs, whereas no obvious histopathological lesions were noted in the BCG-immunized, untreated mouse lungs (Figure 1D). Tofacitinib-treated mouse lungs had limited leukocyte aggregation on histopathology examination.
DISCUSSION
These data indicate that caution is needed when using the new JAK inhibitor tofacitinib in patients with chronic inflammation. Screening for LTBI should be required in all settings (ie, in both low- and high-burden tuberculosis regions) where tofacitinib is approved for use. Using the paucibacillary chronic mouse model of LTBI, we have shown that tofacitinib administration leads to increased bacterial replication, indicating a risk of tuberculosis reactivation. The 1.5 and 15 mg/kg/day doses used in this study have been previously reported in BALB/c mice to have an anti-inflammatory effect, and plasma levels of tofacitinib in mice receiving 15 mg/kg/day were reported to average 61.2 ± 5.75 ng/mL, which is similar to the levels reported in humans receiving a twice-daily 15 mg/kg dose of tofacitinib [10, 11]. Thus, our study conditions reflected those seen in human tofacitinib administration, and our results indicated that this drug promotes M. tuberculosis replication in a dose-dependent manner. These data are consistent with a recent report on tofacitinib phase 3 trial that found 2 cases of tuberculosis related to the use of the drug [12].
Tofacitinib signaling pathways involve both the adaptive and innate immune responses by inhibiting interleukin 4–dependent Th2 cell differentiation and interfering with Th17 cell differentiation [13]. Additionally, the fact that tofacitinib promotes bacterial growth indicates that this drug could be useful as host-directed adjunctive therapy in combination with tuberculosis antibiotics because replicating M. tuberculosis are readily killed by most first-line tuberculosis drugs and are also less likely to be sequestered within granulomas [14].
These findings have important clinical implications. Tofacitinib has been demonstrated to be an effective oral treatment for several inflammatory diseases for which other treatments are frequently inadequate or uncomfortable [15]. Our results show that like existing TNF-α inhibitors, tofacitinib reduces the ability of the host to contain LTBI and stimulates tuberculosis reactivation. Although this study hasn't evaluated the isoniazid prophylaxis prior to tofacitinib therapy, it strongly suggests that screening for LTBI will be valuable for patients prior to initiation of the therapy. If a patient is found to have LTBI, isoniazid prophylaxis may be considered. Further studies are needed to evaluate the benefit of isoniazid prophylaxis with tofacitinib.
Notes
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) (grant numbers AI30036, AI37856 and AI36973) and by the Howard Hughes Medical Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Global tuberculosis control 2010. Geneva, Switzerland: World Health Organization Press; 2010. [Google Scholar]
- 2.Lin PL, Flynn JL. Understanding latent tuberculosis: a moving target. J Immunol. 2010;185:15–22. doi: 10.4049/jimmunol.0903856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 4.Theis VS, Rhodes JM. Review article: minimizing tuberculosis during anti-tumour necrosis factor-alpha treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2008;27:19–30. doi: 10.1111/j.1365-2036.2007.03553.x. [DOI] [PubMed] [Google Scholar]
- 5.Kremer JM, Bloom BJ, Breedveld FC, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60:1895–905. doi: 10.1002/art.24567. [DOI] [PubMed] [Google Scholar]
- 6.van Gurp E, Weimar W, Gaston R, et al. Phase 1 dose-escalation study of CP-690 550 in stable renal allograft recipients: preliminary findings of safety, tolerability, effects on lymphocyte subsets and pharmacokinetics. Am J Transplant. 2008;8:1711–8. doi: 10.1111/j.1600-6143.2008.02307.x. [DOI] [PubMed] [Google Scholar]
- 7.Boy MG, Wang C, Wilkinson BE, et al. Double-blind, placebo-controlled, dose-escalation study to evaluate the pharmacologic effect of CP-690,550 in patients with psoriasis. J Invest Dermatol. 2009;129:2299–302. doi: 10.1038/jid.2009.25. [DOI] [PubMed] [Google Scholar]
- 8.Zhang T, Zhang M, Rosenthal IM, Grosset JH, Nuermberger EL. Short-course therapy with daily rifapentine in a murine model of latent tuberculosis infection. Am J Respir Crit Care Med. 2009;180:1151–7. doi: 10.1164/rccm.200905-0795OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nuermberger EL, Yoshimatsu T, Tyagi S, Bishai WR, Grosset JH. Paucibacillary tuberculosis in mice after prior aerosol immunization with Mycobacterium bovis BCG. Infect Immun. 2004;72:1065–71. doi: 10.1128/IAI.72.2.1065-1071.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishnaswami S, Gupta P, French J, Cook J, Gruben D, Chan G. The effect of CP-690,550, a Janus kinase inhibitor, on QTC interval and blood pressure: a concentration-response analysis of phase I data. Transplantation. 2008;86:414. [Google Scholar]
- 11.Kudlacz E, Conklyn M, Andresen C, Whitney-Pickett C, Changelian P. The JAK-3 inhibitor CP-690550 is a potent anti-inflammatory agent in a murine model of pulmonary eosinophilia. Eur J Pharmacol. 2008;582:154–61. doi: 10.1016/j.ejphar.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 12. Medpage Today. http://www.medpagetoday.com/MeetingCoverage/EULAR/26693. Accessed 12 October 2011. [Google Scholar]
- 13.Ghoreschi K, Jesson MI, Li X, et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550) J Immunol. 2011;186:4234–43. doi: 10.4049/jimmunol.1003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paige C, Bishai WR. Penitentiary or penthouse condo: the tuberculous granuloma from the microbe's point of view. Cell Microbiol. 2010;12:301–9. doi: 10.1111/j.1462-5822.2009.01424.x. [DOI] [PubMed] [Google Scholar]
- 15.Schuna AA, Megeff C. New drugs for the treatment of rheumatoid arthritis. Am J Health Syst Pharm. 2000;57:225–34. doi: 10.1093/ajhp/57.3.225. [DOI] [PubMed] [Google Scholar]