Abstract
Background
Individuals infected with human immunodeficiency virus (HIV) have higher risk than HIV-negative individuals for diseases associated with aging. T-cell senescence, characterized by expansion of cells lacking the costimulatory molecule CD28, has been hypothesized to mediate these risks.
Methods
We measured the percentage of CD28−CD4+ and CD8+ T cells from HIV-infected treatment-naive adults from 5 Adult Clinical Trials Group (ACTG) antiretroviral therapy (ART) studies and the ALLRT (ACTG Longitudinal Linked Randomized Trials) cohort, and from 48 HIV-negative adults. Pretreatment and 96-week posttreatment %CD28− cells were assessed using linear regression for associations with age, sex, race/ethnicity, CD4 count, HIV RNA, ART regimen, and hepatitis C virus (HCV) infection.
Results
In total, 1291 chronically HIV-infected adults were studied. Pretreatment, lower CD4 count was associated with higher %CD28−CD4+ and %CD28−CD8+ cells. For CD8+ cells, younger age and HCV infection were associated with a lower %CD28−. ART reduced %CD28− levels at week 96 among virally suppressed individuals. Older age was strongly predictive of higher %CD28−CD8+. Compared to HIV-uninfected individuals, HIV-infected individuals maintained significantly higher %CD28−.
Conclusions
Effective ART reduced the proportion of CD28− T cells. However, levels remained abnormally high and closer to levels in older HIV-uninfected individuals. This finding may inform future research of increased rates of age-associated disease in HIV-infected adults.
Human immunodeficiency virus (HIV) infection and aging are both implicated in the impairment of immunity [1], with T cells the component of immunity most affected. Chronic activation of T cells as a consequence of infections, inflammatory diseases, or increasing age may lead to a progressive loss of T-cell function and proliferative capacity. This process, commonly referred to as T-cell replicative senescence, is often characterized by loss of the costimulatory molecule CD28 on the surface of T cells, a molecule essential for effective T-cell activation and upregulation of cytokine expression [2].
The link between CD28 expression, aging, and HIV infection was first suggested more than a decade ago [3]. Loss of CD28 expression on T cells of HIV-infected individuals and changes in this expression after antiretroviral treatment (ART) initiation have not, however, been extensively studied. Previous studies indicate that reduced expression of CD28 is associated with HIV progression in both untreated [4] and treated [5, 6] adults. There is emerging evidence that treatment decreases the frequency of CD28− T cells. For example, in a cross-sectional study comparing 14 treated to 14 age- and CD4 count–matched treatment-naive adults, the former had higher levels of expression [7]. A longitudinal study of HIV-infected individuals with pre- and 6-month posttreatment samples found an increase in CD28 expression on CD8+ T cells after treatment [5]. Another study of 20 HIV-infected individuals observed a progressive increase in CD28 expression for both CD4+ and CD8+ T cells through 3 years of treatment [6]. These studies were small and hence not able to fully assess the factors associated with changes in the frequency of these cells.
Another important question is whether expression normalizes during long-term effective ART. Valdez et al [6] observed a trend toward higher CD4+ and CD8+ senescence for aviremic, treated HIV-infected adults than for HIV-uninfected adults, and Desai et al [8] found that effectively treated adults had significantly higher CD8+ senescence than uninfected adults. A recent study of women aged ≥40 years found that virally suppressed HIV-infected women had higher CD8+ senescence than did age- and race-matched uninfected controls [9].
Within several Adult Clinical Trials Group (ACTG) studies and the ACTG Longitudinal Linked Randomized Trials (ALLRT) cohort, advanced flow cytometry was performed in almost 1300 individuals pre- or post-ART. With this large sample size, we had the opportunity to expand our knowledge of CD28 expression in HIV-infected adults. Our objectives in this study were to (1) describe pre-ART expression among treatment-naive HIV-infected individuals; (2) describe the changes in CD28 expression after virally suppressive treatment; and (3) compare posttreatment expression to that observed among HIV-uninfected individuals. As a secondary objective we also examined correlations between CD28 expression and T-cell activation, and explored whether pre-ART expression was associated with activation pre- and posttreatment.
METHODS
Study Population
This analysis incorporated information from adults enrolled in ACTG treatment studies as well as the ALLRT cohort. ALLRT is an observational study of HIV-infected individuals previously randomized to therapeutic interventions in approved parent ACTG clinical trials who are followed for the purpose of evaluating outcomes associated with long-term treatment with potent antiretroviral drugs [10]. Individuals from 5 randomized ART studies (384, 388 immunology substudy 737, A5014, A5095, and A5142) [11–15] who were HIV-infected and treatment naive at the time of study entry and who had baseline or follow-up advanced flow data quantifying the percentage of CD4+ and CD8+ T cells negative for CD28 were included in these analyses. We also incorporated information for 48 individuals from A5113 (the HIV-negative control study for A5015) from 2 distinct age groups (18–30 and 45–66 years) as a comparison group of uninfected adults [16] and information from ACTG study 371 for comparison with acutely/recently infected adults [17].
Flow Cytometry
All subjects had pre- or post-ART immunophenotyping data quantifying %CD28−. The majority also had information on CD8+ T-cell activation (%CD38+/HLA-DR+) and a smaller proportion had information on CD4+ T-cell activation. All flow cytometry was conducted using ACTG consensus methods: 5 mL of fresh whole blood was stained and analyzed on the day it was obtained or collected into potassium ethylenediaminetetraacetic acid tubes and shipped at room temperature overnight to an ACTG-approved flow laboratory where it was immediately stained and analyzed. Parent studies and ALLRT used multiple laboratories for flow cytometry.
All subjects provided written informed consent, and each study site received approval from its designated institutional review board prior to protocol initiation.
Outcome Variables
Two outcomes were defined—the percentages of CD4+ and CD8+ T cells negative for CD28 (%CD28−CD4+, %CD28−CD8+). These percentages were analyzed as continuous variables.
Covariates
We examined the association of %CD28− with demographic variables (age, sex, race/ethnicity [black non-Hispanic, Hispanic, white/other]), initial ART regimen (protease inhibitor [PI]–based, no nonnucleoside reverse transcriptase inhibitor [NNRTI]; NNRTI, no PI; PI + NNRTI; nucleoside reverse transcriptase inhibitor only), disease severity (baseline CD4 count, log10 baseline HIV RNA), hepatitis C (HCV) status, and HIV status.
Statistical Analysis
All post-ART analyses were restricted to virally suppressed individuals at the time of CD28 measurement (HIV RNA <1000 copies/mL at week 16; HIV RNA <50 copies/mL at weeks 32–144).
Differences in CD28 expression by categorical variables over time were first assessed graphically, plotting mean levels of %CD28− by week, stratifying by age and then separately by baseline CD4 count. Differences between groups were assessed using t tests.
Associations between demographic and disease characteristics with pre-ART %CD28− were first assessed in unadjusted linear regression models, separately for CD4+ and CD8+ T cells to test the contribution of each covariate. All variables were then included together in a multivariable model to determine their independent effect on the pretreatment %CD28−. To examine the association of pre-ART factors with week 96 post-ART %CD28−, the same model-building procedures were used, with the addition of initial ART regimen as a covariate.
To examine CD28 expression by HIV status, 48 HIV-negative individuals from A5113, with flow cytometry data measured at 1 time point, were used as a comparison group. The %CD28−CD4+ and CD8+ T cells for HIV-negative individuals were compared to the 96-week post-ART %CD28− for HIV-infected individuals in linear regression models adjusting for sex, age, and race/ethnicity.
Supplementary data on T-cell expression from subjects identified during acute or recent HIV infection were analyzed to explore whether individuals identified and treated shortly after infection have higher expression than those chronically infected. We did not adjust for pretreatment CD4 count or HIV RNA in this comparison because these parameters are not in a steady state during primary HIV infection.
Correlations between CD28 expression and activation for each cell subtype were assessed with the Pearson correlation test. Associations between pre-ART %CD28− and (1) level of activation at weeks 0 and 96 and (2) change in activation between weeks 0 and 96 were assessed in linear regression models that included age, sex, pre-ART CD4 count, and log10 pre-ART viral load.
All analyses were conducted using SAS for Unix (version 9.2); statistical significance was defined as P < .05.
RESULTS
A total of 1291 chronically infected individuals with virally suppressive treatment had ≥1 measures of CD28 expression on CD4+ and CD8+ T cells. Because of changes in protocol evaluations over time, not all individuals had measurements at each time point. For example, at week 0, 690 participants had %CD28−CD4+ and 701 had %CD28−CD8+, whereas at week 96 numbers for both cell subtypes were 395. A comparison group of 48 HIV-uninfected individuals had 1 measure of CD28 expression for both T-cell subtypes. Supplementary analyses included 119 individuals with primary HIV infection, each with week 0 and week 48 measures.
Pretreatment CD28 Expression
Prior to ART initiation, the mean age of the study sample was 37 years, and the majority of subjects were male and white, non-Hispanic. The mean CD4 count was 264 cells/μL (SD, 206). In Table 1 we summarize multivariable associations between pre-ART factors and pre-ART %CD28−. Lower pre-ART CD4 count was strongly associated with higher %CD28−CD4+ and CD8+ T cells. Younger age was associated with significantly lower %CD28− for CD8+, but not CD4+, T cells. HCV-coinfected individuals had a lower pretreatment %CD28−CD8s than those monoinfected. Race/ethnicity and viral load were not associated with pre-ART expression.
Table 1.
Factors Associated With Pre–Antiretroviral Therapy T-Cell CD28 Expression in Chronically HIV-Infected Adults
| %CD28−CD4+ T Cells (n = 690) |
%CD28−CD8+ T Cells (n = 701) |
|||||||
|---|---|---|---|---|---|---|---|---|
| No. | Mean %CD28− | Multivariable Estimate (95% CI) | P Value | No. | Mean %CD28− | Multivariable Estimate (95% CI) | P Value | |
| Age, years | ||||||||
| ≤30 | 153 | 22.2 | 0.8 (−4.2, 5.8) | .76 | 156 | 63.3 | −4.5 (−8.1, −0.9) | .02 |
| 31–44 | 406 | 23.1 | 1.4 (−2.7, 5.6) | .50 | 410 | 64.9 | −3.1 (−6.0, −0.1) | .05 |
| ≥45 | 131 | 22.3 | Ref | … | 135 | 67.5 | Ref | … |
| Sex | ||||||||
| Male | 579 | 23.0 | 2.8 (−1.5, 7.2) | .20 | 590 | 65.5 | 3.0 (−0.2, 6.1) | .07 |
| Female | 111 | 21.2 | Ref | … | 111 | 62.9 | Ref | … |
| Wk 0 CD4+ cells/μL | ||||||||
| ≤50 | 132 | 44.8 | 31 (25, 38) | <.01 | 139 | 69.6 | 9.6 (5.1, 14) | <.01 |
| 51–200 | 160 | 23.9 | 11 (5.1, 17) | <.01 | 160 | 70.0 | 12 (7.4, 16) | <.01 |
| 201–350 | 184 | 16.8 | 3.2 (−2.1, 8.5) | .23 | 186 | 64.4 | 6.4 (2.5, 10) | <.01 |
| 351–500 | 122 | 13.1 | −0.5 (−6.2, 5.1) | .86 | 123 | 60.1 | 2.6 (−1.5, 6.7) | .21 |
| >500 | 92 | 13.7 | Ref | … | 93 | 57.8 | Ref | … |
| Wk 0 log10 copies/mL HIV RNA | 690 | … | −0.5 (−3.0, 2.0) | .69 | 701 | … | 1.6 (−0.2, 3.4) | .09 |
| Hepatitis C statusa | ||||||||
| Positive | 63 | 18.0 | −2.3 (−7.9, 3.3) | .42 | 64 | 59.3 | −6.2 (−10, −2.1) | <.01 |
| Negative | 495 | 22.4 | Ref | 504 | 64.8 | Ref | ||
Multivariable models include covariates shown as well as race/ethnicity.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus.
a Models include indicator term for missing hepatitis C status (no. missing for CD4+: n = 132; CD8+: n = 133).
Effect of Virally Suppressive Treatment on CD28 Expression
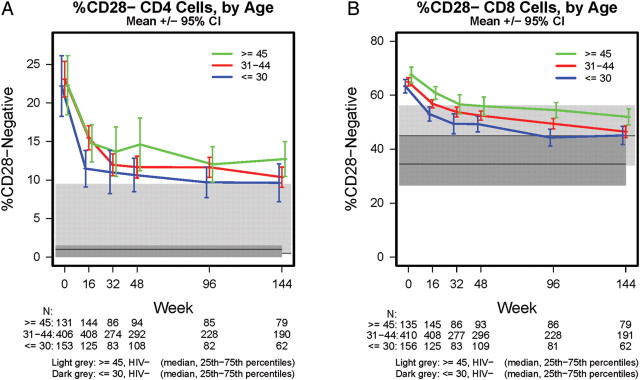
Figure 1 illustrates the change in %CD28−CD4+ and CD28−CD8+ T cells after treatment initiation through week 144. For both cell subtypes, %CD28− decreased significantly shortly after treatment initiation (t test P value for change from week 0 to week 16 <.01, both cell subtypes). The mean %CD28−CD4+ T cells were similarly high by age group prior to treatment initiation (23%) but decreased rapidly during the first 16 weeks, with a more gradual decrease thereafter (Figure 1A). The frequency of CD28−CD4+ T cells remained significantly higher than those for similarly aged HIV-negative individuals at weeks 48, 96, and 144 (P < .01 for both ≤30 and ≥45 years at each time point). The mean %CD28−CD8+ T cells differed by age prior to treatment initiation and remained higher with increasing age even after 144 weeks (Figure 1B). The frequency of CD28−CD8+ T cells dropped steadily after treatment, although, as with CD28−CD4+ T cells, they remained significantly higher than for HIV-negative individuals (P < .01, both age groups) at weeks 48, 96, and 144.
Figure 1.
A, Percentages of CD4+ T cells negative for CD28 by age and HIV infection status. B, Percentages of CD8+ T cells negative for CD28 by age and HIV infection status. Mean (95% CI) %CD28- are presented for HIV-infected adults through 144 weeks posttreatment; median (IQR) %CD28- are presented for HIV-uninfected adults ≤30 years (n = 24) and ≥45 years (n = 24), who had flow cytometry data measured at one time point. Abbreviations: CI, confidence interval; IQR, interquartile range.
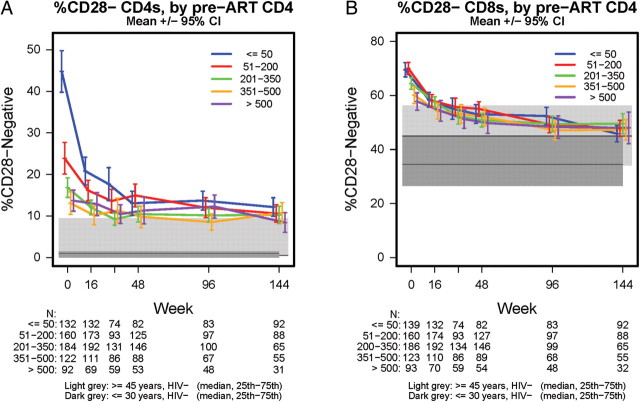
The impact of pretherapy CD4+ T-cell counts is shown in Figure 2. Individuals with pretreatment CD4 count ≤50 cells/μL, and to a lesser extent those with CD4 count ≤200 cells/μL, had a higher pretreatment %CD28−CD4+ T cells than those with higher CD4; posttreatment levels decreased to a mean of 11% CD28− (SD, 10) with no remaining difference by pretreatment CD4 at week 144 (Figure 2A). Individuals with pretreatment CD4 count ≤200 cells/μL had higher pretreatment %CD28−CD8+ T cells than those with higher CD4 counts; as with CD4+ T cells, levels decreased after treatment with no remaining difference by pretreatment CD4 count at week 144 (mean, 48% CD28−; SD, 15; Figure 2B).
Figure 2.
A, Percentages of CD4+ T cells negative for CD28 by pre-ART CD4 count and HIV infection status. B, Percentages of CD8+ T cells negative for CD28 by pre-ART CD4 count and HIV infection status. Mean (95% CI) %CD28- are presented for HIV-infected adults through 144 weeks posttreatment; median (IQR) %CD28- are presented for HIV-uninfected adults ≤30 years (n = 24) and ≥45 years (n = 24), who had flow cytometry data measured at one time point. Abbreviations: CI, confidence interval; IQR, interquartile range.
In Table 2 we summarize multivariable associations between pre-ART factors and week 96 post-ART %CD28−. Younger individuals had significantly lower %CD28−CD8+ but not CD4+ T cells at week 96. Males had higher %CD28− T cells than did females; this difference was statistically significant for CD4+ T cells. HCV-coinfected individuals also had lower %CD28−CD4+ T cells. There were no associations with initial ART regimen or race/ethnicity for either cell subtype.
Table 2.
Factors Associated With Post–Antiretroviral Therapy T-Cell CD28 Expression in Virally Suppressed Chronically HIV-Infected Adults
| %CD28−CD4+ T Cells at Wk 96 (n = 395) |
%CD28−CD8+ T Cells at Wk 96 (n = 395) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Variable | No. | Mean %CD28− | Multivariable Estimate (95% CI) | P Value | No. | Mean %CD28− | Multivariable Estimate (95% CI) | P Value |
| Age, years | ||||||||
| ≤30 | 82 | 9.7 | −2.1 (−5.2, 1.0) | .18 | 81 | 44.3 | −11 (−15, −6.5) | <.01 |
| 31–44 | 228 | 11.7 | −0.7 (−3.1, 1.8) | .59 | 228 | 49.4 | −5.3 (−8.9, −1.7) | <.01 |
| ≥45 | 85 | 12.0 | Ref | … | 86 | 54.5 | Ref | … |
| Sex | ||||||||
| Male | 330 | 12.0 | 4.5 (1.8, 7.1) | <.01 | 331 | 50.2 | 3.7 (−0.3, 7.6) | .07 |
| Female | 65 | 7.7 | Ref | 64 | 45.7 | Ref | … | |
| Wk 0 CD4+ cells/μL | ||||||||
| ≤50 | 83 | 13.7 | 1.4 (−2.6, 5.4) | .49 | 83 | 52.4 | 2.8 (−3.1, 8.7) | .35 |
| 51–200 | 97 | 12.0 | −0.1 (−3.7, 3.6) | .98 | 97 | 49.3 | 0.01 (−5.3, 5.4) | .99 |
| 201–350 | 100 | 10.2 | −1.5 (−5.0, 2.0) | .39 | 99 | 49.4 | 1.1 (−4.0, 6.2) | .68 |
| 351–500 | 67 | 8.5 | −3.4 (−7.0, 0.3) | .07 | 68 | 47.1 | −0.3 (−5.6, 5.0) | .91 |
| >500 | 48 | 12.3 | Ref | … | 48 | 48.3 | Ref | … |
| Wk 0 log10 copies/mL HIV RNA | 395 | … | −0.5 (−2.0, 1.1) | .56 | 395 | … | 1.4 (−0.9, 3.7) | .23 |
| Hepatitis C statusa | ||||||||
| Positive | 40 | 8.9 | −3.4 (−6.7, −0.1) | .05 | 41 | 46.0 | −4.5 (−9.4, 0.3) | .06 |
| Negative | 313 | 11.7 | Ref | 312 | 49.7 | Ref | ||
Multivariable models include covariates shown as well as race/ethnicity and initial antiretroviral therapy regimen.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus.
a Models include indicator term for missing hepatitis C status (n = 42).
For clinical utility, a pre-ART %CD28− was not included as a predictor of week 96 levels in primary analyses. However, in separate models, higher pre-ART levels of %CD28− were associated with higher levels of week 96 %CD28−, adjusting for the factors in Table 2 (per 10 percentage point increase in pre-ART %CD28−: week 96 %CD28−CD4+ = 2.1, 95% confidence interval [CI], 1.4–2.9; %CD28−CD8+ = 5.6, 95% CI, 4.2–7.0). In similar, separate multivariable models, week 96 CD4 count was significantly correlated with week 96 %CD28−CD4+ T cells (CD4+: ≤200 vs >500 cells/μL = 4.4, 95% CI, 0.4–8.4) but not CD8+ T cells.
Posttreatment CD28 Expression and Immunologic Response to Therapy
We also compared week 96 %CD28− for individuals with good CD4 responses posttreatment (≥100 cells/μL increase in cell count) versus those with poor responses (<100 increase). In analyses restricted to individuals with pretreatment CD4 count ≤300 cells/μL, poor responders (n = 26) had 7.4 percentage points higher CD28−CD8+ T cells (95% CI, 1.7–13) than responders (n = 24). Poor responders also had 3.7 percentage points higher CD28−CD4+ T cells (95% CI, −0.6 to 7.9) than did responders, although this difference was not statistically significant.
Posttreatment CD28 Expression by HIV Status
Figures 1 and 2 also illustrate the persistent elevation of CD28− T cells in HIV-infected individuals relative to uninfected individuals, even after 144 weeks of suppressive therapy. At week 96, in models adjusting for age, sex, and race/ethnicity, HIV-infected individuals had 5.4 percentage points higher CD28−CD4+ T cells (95% CI, 2.2–8.5) and 7.7 percentage points higher CD28−CD8+ T cells (95% CI, 3.1–12). The lower levels of expression in HIV-infected individuals were also observed for the subgroup of responders described above (≥100 cells/μL increase in CD4 count; data not shown), in analyses restricted to those with week 96 CD4+ T-cell counts >500 cells/μL, and in the subset of these subjects with pre-ART CD4+ T-cell counts ≥350 cells/μL (Table 3).
Table 3.
Comparisons Between HIV-Uninfected and Virally Suppressed HIV-Infected Adults 96 Weeks After Antiretroviral Treatment
| Unadjusted (95% CI) | Multivariable (95% CI) | |
|---|---|---|
| Parameter | P Value | P Value |
| Including all subjects (n = 443) | ||
| %CD28−CD4+ | −7.1 (−10, −4.2) | −5.4 (−8.5, −2.2) |
| <.01 | <.01 | |
| %CD28−CD8+ | −9.6 (−14, −5.2) | −7.7 (−12, −3.1) |
| <.01 | <.01 | |
| Restricted to those with posttreatment CD4+ >500 cells/μL (n = 234) | ||
| %CD28−CD4+ | −5.0 (−7.6, −2.3) | −3.9 (−7.0, −0.9) |
| <.01 | .01 | |
| %CD28−CD8+ | −8.8 (−13, −4.3) | −7.0 (−12, −2.1) |
| <.01 | .01 | |
| Restricted to those with posttreatment CD4+ >500 cells/μL and pretreatment CD4+ ≥350 cells/μL (n = 146) | ||
| %CD28−CD4+ | −5.6 (−8.6, −2.6) | −5.1 (−8.6, −1.6) |
| <.01 | .01 | |
| %CD28−CD8+ | −9.1 (−14, −4.2) | −7.5 (−13, −1.9) |
| <.01 | .01 | |
Models adjusted for age, race/ethnicity, and sex.
In all models reference group = chronically HIV infected.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus.
Pretreatment CD28 Expression and Duration of Infection
CD28 expression data were available for 119 individuals who started ART during primary HIV infection. The mean pretreatment %CD28−CD4+ was 8.9 (SD, 8.5) and mean %CD28−CD8+ was 54.6 (SD, 12.5), significantly lower (P < .001) than pretreatment levels for chronically infected individuals (see Figure 2). This difference was attenuated but still significant when compared to chronically infected individuals with pretreatment CD4+T-cell count >200. After 48 weeks of ART and HIV RNA <50 copies/mL, mean posttreatment %CD28−CD4+ and %CD28−CD8+ were 10.9 and 55.7, respectively, which did not significantly differ from week 48 levels for those starting ART during chronic infection (P > .10).
Association Between CD28 Expression and T-Cell Activation
Week 0 %CD28− and activation (%CD38+/HLA-DR+) were significantly correlated for the CD4+T-cell subset only (r = 0.54, P < .001; r = 0.46, P < .001 after adjusting for baseline CD4 count). This association remained strong in a linear regression model adjusting for age, sex, pre-ART CD4 count, and pre-ART viral load (P < .001).
Pretreatment %CD28− was also associated with a greater decrease in activation to week 96, only for CD4+ T cells (mean change from week 0 to week 96 activation per unit increase in week 0 %CD28− = −0.5 [95% CI, −0.6 to −0.3]). Pretreatment %CD28−CD4+ was not associated with week 96 levels of CD4 activation; however, for CD8 cells there was a significant multivariable association (mean change in week 96 activation per unit increase in week 0% CD28− = 0.2 [95% CI, 0.1–0.4]).
DISCUSSION
In the largest study to our knowledge to our knowledge of CD28 expression on T cells of HIV-infected individuals, we addressed questions related to changes in expression posttreatment and also expanded upon findings of previous studies while controlling for several potential confounders.
As previously observed in 2 small studies [5, 6], we found that successful ART substantially increased levels of CD28 expression for both CD4+ and CD8+ T-cell subtypes. In contrast to previous work from a subset of this cohort on T-cell activation [18], posttreatment levels of CD28− T cells were not associated with pretreatment CD4 count or viral load. Expression was also not associated with initial ART regimen. A decrease in CD28− T cells was even observed in those with early HIV disease (defined as having pretherapy CD4+ T-cell count >500 cells/μL).
Despite improvement in CD28 expression with virally suppressive ART, CD28 expression for both CD4+ and CD8+ T-cell subsets was abnormally low relative to those of HIV-uninfected individuals after adjusting for age, sex, and race/ethnicity. These reduced levels were observed even among individuals who achieved significant CD4 recovery and those initiating treatment during primary infection. CD28 signaling is essential for multiple T-cell functions including telomerase activation. T-cell senescence likely requires recurrent activation and division until cells reach their Hayflick limit or run out of telomerase. This is a natural consequence of increasing age due to the number of divisions that T cells, in particular CD8+ memory cells, undergo. It has also been shown that CD28−CD8+ T cells have the shortest telomeres of any subset within the peripheral blood mononuclear cell population [19]. In HIV this process is shortened, likely because of chronic activation that drives the continued division occurring with both CD4+ and CD8+ memory cells. Although therapy significantly slows this process by removing the stimulus of very high viral load, it does not completely stop the process because chronic activation persists due to continued presence of virus and microbial translocation. Therefore, the T-cell environment never completely returns to normal. The functional consequences of elevated senescence remain to be determined. A recent study of HIV-infected individuals found that although T cells of untreated individuals were highly susceptible to apoptosis and proliferated poorly, treated individuals had strongly proliferative T cells with lower susceptibility to apoptosis. The investigators concluded that while the proportion of T cells that are CD28− are elevated during treated infection, they may have preserved effector function [20]. Regardless, our data suggest that even patients considered immunological successes with ART may remain at risk for diseases associated with the presence of senescent T cells, and argue for the further development of immune-based therapeutics for this patient population.
Although CD8+ CD28 expression and activation were not correlated prior to treatment, after 96 weeks of treatment and viral suppression a positive association was observed even after adjusting for other potential confounders including baseline CD4 count. Ongoing antigenic stimulation could be the joint underlying mechanism, and future research would benefit from examining factors such as microbial translocation and cytomegalovirus (CMV) coinfection, which were not available for our analyses.
As observed previously, we found that older age was a strong predictor of CD8+, but not CD4+, CD28 expression in both untreated and treated HIV disease. Kalayjian et al [16] identified reduced CD28 expression on CD8+ T cells as a potential immune correlate of the interaction between age and HIV disease progression because older age was found to be associated with lower expression among both untreated infected and uninfected adults. We expanded on these findings by showing that older age remained significantly associated with higher levels of CD28−CD8+ T cells among treated, virally suppressed HIV-infected individuals even after adjusting for multiple potential confounders.
We also identified a lower level of posttreatment expression (particularly for the CD4+ T-cell subset) among men than women, a finding to our knowledge not previously observed. Multiple other immune differences have been seen between HIV-uninfected women and men [21], and sex differences in pretreatment CD8+ activation in HIV-infected individuals have been shown as well [22].
Our counterintuitive finding that HCV coinfection was associated with higher levels of expression, seen also by others [23, 24], warrants further investigation, perhaps as part of studies using the new, direct-acting HCV drugs. A limitation of our analysis is that HCV antibodies were used to define HCV status; HCV RNA was not uniformly available. (However, analyses restricted to those with HCV RNA available [n = 112 at week 0; n = 73 at week 96], although not statistically significant, yielded similar trends [data not shown].)
There are several limitations to our study. Flow cytometry was conducted using different laboratories, so some assay variability is likely. However, the effect of such variability is minimized by the study's large sample size. All patients did not have advanced flow data at all time points; therefore, we did not examine changes from baseline in our models but present the results for week 96 posttreatment when modeling the association between baseline factors and posttreatment %CD28− and also when comparing posttreatment %CD28− to that for HIV-uninfected individuals. Repeating these analyses using week 144 posttreatment instead of week 96 yielded substantively similar results (with the exception that, when modeling associations with posttreatment %CD28−, sex was no longer associated with expression on CD4+ T cells [data not shown]). Also, we focused on a homogeneous population of virally suppressed individuals, which may limit the generalizability of our findings.
We did not have information on the proportion of T cells that were CD57+ as well as CD28−. Because CD28−CD57+ cells are considered more definitive indicators of terminal senescence, we include both intermediate and terminally differentiated T cells in our analysis. We did not have information on duration of HIV infection and therefore cannot rule out the possibility that the observed association between older age and %CD28−CD8+ T cells is due to some extent to longer duration of disease among older infected individuals. However, this association remained strong after adjusting for pre-ART CD4 count and viral load.
This analysis focused on CD28 expression and changes in expression after ART. We examined but found no association between posttreatment expression and CD4 count. Few studies have examined T-cell senescence and chronic disease outcomes, although CD28− T cells have been associated with diabetes [25], coronary artery disease [26], and renal disease [27], conditions prevalent in HIV-infected persons. A recent study reported that CD8+, but not CD4+, T-cell senescence predicted subclinical carotid artery abnormalities in HIV-infected women [9]. An ongoing case-control study nested in ALLRT is examining the senescence markers CD28 and CD57 on CD4+ and CD8+ T cells for association with non-AIDS-defining clinical endpoints among virally suppressed individuals.
Our comparison group of uninfected individuals differed from our HIV-infected cohort in that the former had a higher proportion of female participants (83% vs 47%) and white, non-Hispanic participants (56% vs 17%); adjustment by these factors did not change the associations observed. A previous study found that seronegative homosexual men had more immune activation and more CD28− cells in both the CD4+ and CD8+ compartments than did seronegative heterosexual men [28]. This suggests that additional factors might explain the lower expression we observed posttreatment in our infected cohort. However, this elevation remained in analyses restricted to women. In addition to sexual orientation, other unmeasured confounding factors associated with lower CD28 expression may be preferentially enriched in our HIV-infected participants including injection drug use, CMV, and other coinfections. Because these factors were not comprehensively assessed in both populations, we cannot exclude the possibility that part of the expansion in CD28− T cells observed during treated HIV infection may be mediated at least in part by factors other than HIV infection.
Although virally suppressive ART improves CD28 expression in both CD4+ and CD8+ T-cell subsets, expression remains abnormally low even after 3 years, with younger HIV-infected individuals having an expression profile similar to that of older uninfected adults. This finding emphasizes the importance of further research on senescence in HIV-infected populations who will be living to older ages through the use of effective antiretroviral therapies, and at risk for age-associated morbidities.
Notes
Acknowledgments. We thank the ACTG sites and study participants for their time and effort; Frontier Science Foundation for data management; and Matthew McKenna for programming support. We gratefully acknowledge the contributing protocol chairs: G. Robbins and R. Shafer for ACTG 384; M. Fischl for ACTG 388; A. Landay and M. Lederman for ACTG A5014; R. Gulick for A5095; S. Riddler and R. Haubrich for A5142; and C. Benson for A5001.
Financial support. This work was supported by the ACTG funded by the National Institute of Allergy and Infectious Diseases (grant numbers AI 68636, AI 68634, AI 38858, and AI 38855) and the National Institutes of Health (grant number K24 AI069994).
Potential conflicts of interest. All authors: No reported conflicts.
The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Effros R, Fletcher C, Gebo K, et al. Workshop on HIV infection and aging: what is known and future directions. Clin Infect Dis. 2008;47:542–53. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenschow D, Walunas T, Bluestone J. CD28/B7 system of T-cell costimulation. Annu Rev Immunol. 1996;14:233–58. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 3.Effros RB, Allsopp R, Chiu CP, et al. Shortened telomeres in the expanded CD28−CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS. 1996;10:F17–22. doi: 10.1097/00002030-199607000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Cao W, Jamieson B, Hultin L, Hultin P, Effros R, Detels R. Premature aging of T-cells is associated with faster HIV-1 disease progression. J Acquir Immune Defic Syndr. 2009;50:137–47. doi: 10.1097/QAI.0b013e3181926c28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hunt P, Sinclair E, Epling L, et al. T-cell senescence and proliferation defects persist in treated HIV-infected individuals maintaining viral suppression and are associated with poor CD4+ T-cell recovery. Program and abstracts of the 17th Conference on Retroviruses and Opportunistic Infections; 16–19 February 2010; San Francisco, California. Abstract 316. [Google Scholar]
- 6.Valdez H, Connick E, Smith K, et al. Limited immune restoration after 3 years’ suppression of HIV-1 replication in patients with moderately advanced disease. AIDS. 2002;16:1859–66. doi: 10.1097/00002030-200209270-00002. [DOI] [PubMed] [Google Scholar]
- 7.Lange C, Lederman M, Madero J, et al. Impact of suppression of viral replication by highly active antiretroviral therapy on immune function and phenotype in chronic HIV-1 infection. J Acquir Immune Defic Syndr. 2002;30:33–40. doi: 10.1097/00042560-200205010-00005. [DOI] [PubMed] [Google Scholar]
- 8.Desai S, Ronquillo R, Usuga X, et al. Immune senescence, activation, and abnormal T-cell homeostasis despite effective HAART, a hallmark of early aging in HIV disease. Program and abstracts of the 16th Conference on Retroviruses and Opportunistic Infections; 8–11 February 2009; Montreal, Canada. Abstract 381. [Google Scholar]
- 9.Kaplan R, Sinclair E, Landay AL, et al. T-cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis. 2011;203:452–63. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smurzynski M, Collier AC, Koletar S, et al. AIDS Clinical Trials Group Longitudinal Linked Randomized Trials (ALLRT): rationale, design, and baseline characteristics. HIV Clin Trials. 2008;9:268–81. doi: 10.1310/hct0904-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robbins GK, De Gruttola V, Shafer RW, et al. Comparison of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349:2293–303. doi: 10.1056/NEJMoa030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malhotra U, Bosch RJ, Chan E, et al. Association of T-cell proliferative responses and phenotype with virus control in chronic progressive HIV-1 disease. J Infect Dis. 2004;189:515–9. doi: 10.1086/381038. [DOI] [PubMed] [Google Scholar]
- 13.Landay AL, Spritzler J, Kessler H, et al. Immune reconstitution is comparable in antiretroviral-naive subjects after 1 year of successful therapy with a nucleoside reverse-transcriptase inhibitor- or protease inhibitor-containing antiretroviral regimen. J Infect Dis. 2003;188:1444–54. doi: 10.1086/379041. [DOI] [PubMed] [Google Scholar]
- 14.Gulick RM, Ribaudo HJ, Shikuma CM, et al. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med. 2004;350:1850–61. doi: 10.1056/NEJMoa031772. [DOI] [PubMed] [Google Scholar]
- 15.Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095–106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalayjian R, Landay A, Pollar R, et al. Age-related immune dysfunction in health and in human immunodeficiency virus (HIV) disease: association of age and HIV infection with naive CD8+ cell depletion, reduced expression of CD28 on CD8+ cells, and reduced thymic volumes. J Infect Dis. 2003;187:1924–33. doi: 10.1086/375372. [DOI] [PubMed] [Google Scholar]
- 17.Volberding P, Demeter L, Bosch R, et al. Antiretroviral therapy in acute and recent HIV infection: a prospective multicenter stratified trial of intentionally interrupted treatment. AIDS. 2009;23:1987–95. doi: 10.1097/QAD.0b013e32832eb285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robbins GK, Spritzler J, Chan E, et al. Incomplete reconstitution of T-cell subsets on combination antiretroviral therapy in the AIDS Clinical Trials Group Protocol 384. Clin Infect Dis. 2009;48:350–61. doi: 10.1086/595888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin J, Epel E, Cheon J, et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J Immunol Methods. 2010;352:71–80. doi: 10.1016/j.jim.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vivar N, Ruffin N, Sammicheli S, Hejdeman B, Rethi B, Chiodi F. Survival and proliferation of CD28− T-cells during HIV-1 infection relate to the amplitude of viral replication. J Infect Dis. 2011;203:1658–67. doi: 10.1093/infdis/jir156. [DOI] [PubMed] [Google Scholar]
- 21.Fish E. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8:737–44. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meier A, Chang J, Chan E, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15:955–9. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovacs A, Al-Harthi L, Christensen S, Mack W, Cohen M, Landay A. CD8+ T-cell activation in women coinfected with human immunodeficiency virus type 1 and hepatitis C virus. J Infect Dis. 2008;197:1402–7. doi: 10.1086/587696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paravisini A, Cianchetta MS, Garcia R, et al. Pattern of CD28 expression on CD8+ T-cells in a group of coinfected and monoinfected HIV and HCV patients. J Allergy Clin Immunol. 2007;119:S180. [Google Scholar]
- 25.Giubilato S, Liuzzo G, Brugaletta S, et al. Expansion of CD4+CD28null T-lymphocytes in diabetic patients: exploring new pathogenetic mechanisms of increased cardiovascular risk in diabetes mellitus. Eur Heart J. 2011;32:1214–26. doi: 10.1093/eurheartj/ehq499. [DOI] [PubMed] [Google Scholar]
- 26.Jonasson L, Tompa A, Wikby A. Expansion of peripheral CD8+ T cells in patients with coronary artery disease: relation to cytomegalovirus infection. J Intern Med. 2003;254:472–8. doi: 10.1046/j.1365-2796.2003.01217.x. [DOI] [PubMed] [Google Scholar]
- 27.Betjes M, Huisman M, Weimar W, Litjens N. Expansion of cytolytic CD4+CD28− T cells in end-stage renal disease. Kidney Int. 2008;74:760–7. doi: 10.1038/ki.2008.301. [DOI] [PubMed] [Google Scholar]
- 28.Giorgi JV, Majchrowicz MA, Johnson TD, Hultin P, Matud J, Detels R. Immunologic effects of combined protease inhibitor and reverse transcriptase inhibitor therapy in previously treated chronic HIV-1 infection. AIDS. 1998;12:1833–44. doi: 10.1097/00002030-199814000-00015. [DOI] [PubMed] [Google Scholar]