Abstract
Iron deficiency anemia in early life alters the development and functioning of the dopamine neurotransmitter system, but data regarding the specific effects of brain iron loss on dopamine D2 receptor regulation are lacking. Cell culture and animal models were employed in this study to determine whether D2 receptor expression is altered when cellular iron levels are depleted. Endogenous D2 receptor-expressing PC12 cells exposed to increasing concentrations of the iron chelator desferrioxamine (25–100 μmol/L) exhibited dose-dependent decreases in total D2 receptor protein concentrations (20–65%), but there were minimal effects on D2 receptor mRNA levels. When iron-deficient cells were repleted with ferric ammonium citrate for 24 h, D2 receptor protein densities were similar to control. Dietary iron deficiency for 6 wk in weanling rats also reduced regional iron concentrations by nearly 50% in the ventral midbrain and caudate but did not affect D2 receptor mRNA levels in the ventral midbrain. Iron deficiency significantly reduced membrane D2 receptor protein levels by >70% in caudate, whereas cytosolic concentrations showed only 25% losses. D2 receptor protein densities and regional iron concentrations were restored within 2 wk of dietary iron repletion. These results support the concept that D2 receptor gene expression is not significantly changed by iron deficiency, whereas dopamine receptor trafficking is affected and is likely related to known dopamine system alterations in iron deficiency.
Introduction
Neural functioning and behavioral development are affected by iron deficiency in early life (1), which results in lethargy, reduced cognitive performance, decreased attention as well as abnormalities in auditory evoke potentials responses, spontaneous motor activity, and sleep patterns that may not improve after iron therapy (1–6). Deficits in iron metabolism in the central nervous system have also been linked to Restless Legs Syndrome (RLS).5 Individuals diagnosed with RLS display diurnal-based deficiencies in motor control and the severity of symptoms increases during the evening hours. Support for the role of iron in RLS comes from a study in which infusion of iron dextran improved RLS symptoms and elevated substantia nigra and prefrontal cortex iron to normal levels (7). RLS is also linked to changes in the dopamine neurotransmitter system, because enhancement of dopamine activity reduces RLS symptoms (8,9), whereas dopamine receptor antagonists exacerbate these symptoms (10,11).
Rodent and cell culture model examinations indicate that the dopamine system is particularly sensitive to dietary iron deficiency. In vivo microdialysis studies using postweaning iron-deficient rats and mice show that extracellular dopamine concentrations are consistently elevated in the striatum compared with controls (12–14). Postweaning iron-deficient animal models also have deficits in intracellular dopamine concentrations, but the amount of dopamine loss varies by brain region, illustrating the heterogenous effect of iron deficiency on the brain (15–17). Moreover, recent evidence shows that the response of iron-deficient rats to striatal infusion of 3,4-dihyroxy-l-phenylalanine, the precursor of dopamine, is blunted and delayed compared with control diet-fed rats (18). The expression and functioning of the dopamine transporter, the main site of dopamine reuptake into the cell, is also altered in striatal synaptosomes from iron-deficient rats (19) in an iron-chelated cell culture model (20) and in vivo (18). Together, these data provide evidence that decreased reuptake of dopamine by the dopamine transporter is associated with elevated extracellular striatal dopamine levels when iron is depleted. The exploration of the dopamine system after iron deficiency also includes studies that examine dopamine D1 and D2 receptor densities (16,19,21). These studies consistently show decreased D2 receptor levels, but not necessarily D1 receptor levels, in striatum.
Through pharmacological manipulations in mice and rats, it has been hypothesized that a signal transduction pathway links the D2 receptor and the dopamine transporter. Specifically, administration of the D2 agonist quinpirole results in an enhancement of dopamine transport velocity in striatal synaptosomes (22), whereas the D2 receptor antagonist raclopride decreases dopamine transport (23). Recent evidence suggests that this specific pathway is altered in iron deficiency (18). Using in vivo microdialysis, these authors showed that infusion of quinpirole into striatum increases dopamine transport velocity in rats fed control diet, whereas the D2 receptor agonist did not affect uptake in iron-deficient rats. These effects are consistent with a reduced D2 receptor levels (16,19,21) and possible alterations in the signal transduction pathway(s). To date, the mechanism(s) of effect of neuronal iron deficiency on dopamine receptor metabolism or gene expression remains unexplored.
In the current report, we examined the effect of iron depletion on D2 receptor protein and gene expression levels in a cell culture model of iron deficiency and in ventral midbrain (substantia nigra and ventral tegmentum) and caudate putamen from iron-deficient rats. These studies will provide insights into the potential mechanisms by which iron influences D2 receptor functioning and expression, thereby leading to alterations in dopamine neurotransmission.
Materials and Methods
Pheochromocytoma cell culture.
PC12 cells, a pheochromocytoma cell line, were grown on Collagen IV Cellware (BD Biosciences) and maintained in RPMI 1640 medium (Gibco) supplemented with 10 mmol/L HEPES, 4.5 g/L glucose, 10% equine serum (HyClone), 5% defined fetal bovine serum (HyClone), 2 mmol/L glutamine (Sigma-Aldrich), and 100 kU/L penicillin-streptomycin (HyClone) at 37°C in a water-saturated atmosphere containing 7% CO2. For all cell culture experiments, cells were treated with 25, 50, or 100 μmol/L desferrioxamine (DFO) for 24 h or 50 μmol/L DFO for 24 h followed by 24-h treatment with 50 mg/L ferrous ammonium citrate (FAC) for repletion studies.
Western blot analysis.
All conditions for cell treatments, cell collection, and western blotting have been previously described (20,24) and were uniform over all experiments. Membranes were probed with either a monoclonal rabbit anti-D2 receptor amino-terminal antibody (Research Diagnostics) or a mouse anti-transferrin receptor (TfR) monoclonal antibody (Zymed Laboratories) in blocking solution (1:1000) for 12 h at 4°C. Membranes were also probed for actin immunoreactivity (rabbit anti-actin antibody, 1:500, Santa Cruz Biotechnology) using assay conditions similar to those described above. Protein band densities were quantified by scanning exposed film (Epson Perfection 636U digital scanner, Epson America) into Adobe Photoshop (version 7.0) and band integrative densities were determined with NIH Image software. To enable comparisons of different blots with variable exposure times, results from experimental groups were normalized to the mean of control lanes from the same gel. Data are expressed as percent of control values.
PC12 mRNA expression.
For cell RNA analysis, PC12 cells were washed 3 times with ice-cold PBS (9.1 mmol/L Na2HPO4, 1.7 mmol/L NaH2PO4, 150 mmol/L NaCl, pH 7.4) and exposed to 25, 50, and 100 μmol/L DFO for 24 h. The cells were harvested in PBS using vigorous trituration and collected by centrifugation. Total RNA was isolated using a RNeasy Mini kit (Qiagen), quantified spectrophotometrically, and examined for integrity by electrophoresis (1.25% agarose-0.67 mol/L formaldehyde gel) using ethidium bromide staining. We used quantitative real-time PCR analysis to determine D2 receptor mRNA expression. Triplicate aliquots of RNA were analyzed using the Perkin Elmer/Applied Biosystems Division 7700 Sequence Detector. The sequences for the primers were as follows: D2 receptor forward primer, 5′-CTT CAC ATG GCT GGG CTA TGT-3′, reverse primer, 5′-GCG GAA CTC GAT GTT GAA GG- 3′; TfR forward primer, 5′-CAT CTC CAT CTG ACC CTC AC-3′, reverse primer, 5′-GTC TTT GGC TTC TGG TCT CA-3′; divalent metal transporter 1 (DMT1) + iron responsive element forward primer, 5′-GGA GGG ATT CCT GAA CCT AA-3′, reverse primer, 5′-AGG TGA GGA TAG GGA TGA GG-3′; ferritin H forward primer, 5′-ACT GAT GAA GCT GCA GAA CC-3′, reverse primer, 5′-GTG GGG ATC ATT CTT GTC AG-3′. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (forward primer, 5′- GGA GTC TAC TGG CGT CTT CA-3′, reverse primer, 5′-ATG CCA AAG TTG TCA TGG AT-3′) and β-actin (forward primer, 5′-GAA GTA CCC CAT TGA ACA CG-3′, reverse primer, 5′- GAG GCA TAC AGG GAC AAC AC-3′) were used as controls. The primers were designed using the Perkin Elmer/Applied Biosciences Primer Express software based on mRNA sequences reported in the National Center for Biotechnology Information public database. Each value for mRNA was normalized relative to GAPDH expression.
Animals, dietary treatments, and iron assessment.
Male 21-d-old Sprague-Dawley rats (Harlan Sprague Dawley) were randomly divided into 3 dietary treatment groups as follows: control (50 μg/g diet, 6 wk), iron deficient (3 μg/g diet, 6 wk), and iron repleted (3 μg/g diet for 4 wk followed by 50 μg/g diet for 2 wk). The diets were prepared according to the AIN-93G diet (24) with cornstarch as the sole source of carbohydrate and ferric citrate as the source of iron. Mean dietary iron concentrations were 48 ± 2 μg/g for the control rats and 3.6 ± 0.9 μg/g for the iron-deficient diets, respectively, as determined using atomic absorption spectrophotometry (Perkin Elmer AAnalyst 600, Perkin Elmer). All animals were housed under controlled environmental conditions with a 12-h-light:12-h-dark cycle (lights on 0600–1800; 25 ± 1°C) and consumed food and distilled water ad libitum. Hematology and liver and spleen non-heme iron concentrations were measured according to methods previously described (17,25). The Pennsylvania State University Institutional Animal Care and Use Committee approved all animal procedures used in this study.
Brain D2 receptor protein and mRNA expression and iron determinations.
For D2 receptor protein determinations in brain tissue, rats were killed by decapitation between 0900 and 1100 and brains were dissected for ventral midbrain (substantia nigra and ventral tegmentum) and caudate putamen. We chose these 2 regions because the ventral midbrain contains dopamine cell bodies and the caudate is the terminal field of the nigrostriatal dopamine system. Brain regions were homogenized on ice in TBS (10 mmol/L Tris-HCl, 150 mmol/L NaCl, 1 mmol/L EDTA, pH 7.4) supplemented with protease inhibitors (Roche Diagnostics). We performed protein extraction, SDS-PAGE, and immunoblotting on brain region homogenates according to the methods described above. Total iron concentrations from brain region homogenates were determined according to our standard laboratory method using ultra-pure nitric acid (Mallinckrot Baker) digestion and analysis with atomic absorption spectrophotometry (26). For tissue RNA analysis, ventral midbrain tissue was rapidly dissected, immediately placed in RNAlater (Ambion), and stored at −20°C. RNA was isolated using the ToTALLY RNA kit (Ambion) according to the manufacturer's instructions, quantified spectrophotometrically, and examined for integrity by electrophoresis using ethidium bromide staining. Quantitative real-time PCR analysis, as described above, was used to determine mRNA expression in brain tissue.
Membrane-cytosol fractionation of brain homogenates.
Caudate from each rat was weighed and homogenized in 10 volumes cold TBS (10 mmol/L Tris-HCl, 150 mmol/L NaCl, 1 mmol/L EDTA, pH 7.4) supplemented with protease inhibitors (Roche Diagnostics). A 250-μL aliquot was centrifuged at 500 × g for 2 min at 4°C to clear cell debris. The pellets were discarded and the supernatants centrifuged at 20,000 × g for 30 min at 4°C. The supernatant (cytosolic fraction) was decanted and immediately stored on ice. The pellet (membrane fraction) was resuspended in 750 μL TBS and centrifuged at 20,000 × g for 30 min at 4°C and repeated 3 times. Membrane fractions were then solubilized in 200 μL of cold radio immunoprecipitation assay buffer (25 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 1% TritonX-100, 1% sodium deoxycholate, and 0.1% SDS) supplemented with protease inhibitors for 60 min at 4°C with constant shaking. Solubilized extracts were centrifuged (16,000 × g; 10 min, 4°C) and protein content of the supernatants was assessed using the BCA protein assay (Pierce) with bovine serum albumin as the standard. SDS-PAGE and immunoblotting of each fraction were performed according to the methods described above.
Data analysis.
Data in graphs are represented as means ± SEM. Groups were compared by single-factor ANOVA (SAS). In the presence of significant F-values, individual comparisons between means were made using a Tukey's test (for multiple comparisons) or a Dunnett's test (for comparison of experimental groups to control group). Regression analyses were performed to analyze relationships between cellular iron status and D2 receptor protein and mRNA levels. Significance was set at P < 0.05.
Results
Total D2 receptor protein concentrations in PC12 cells are reduced with iron chelation.
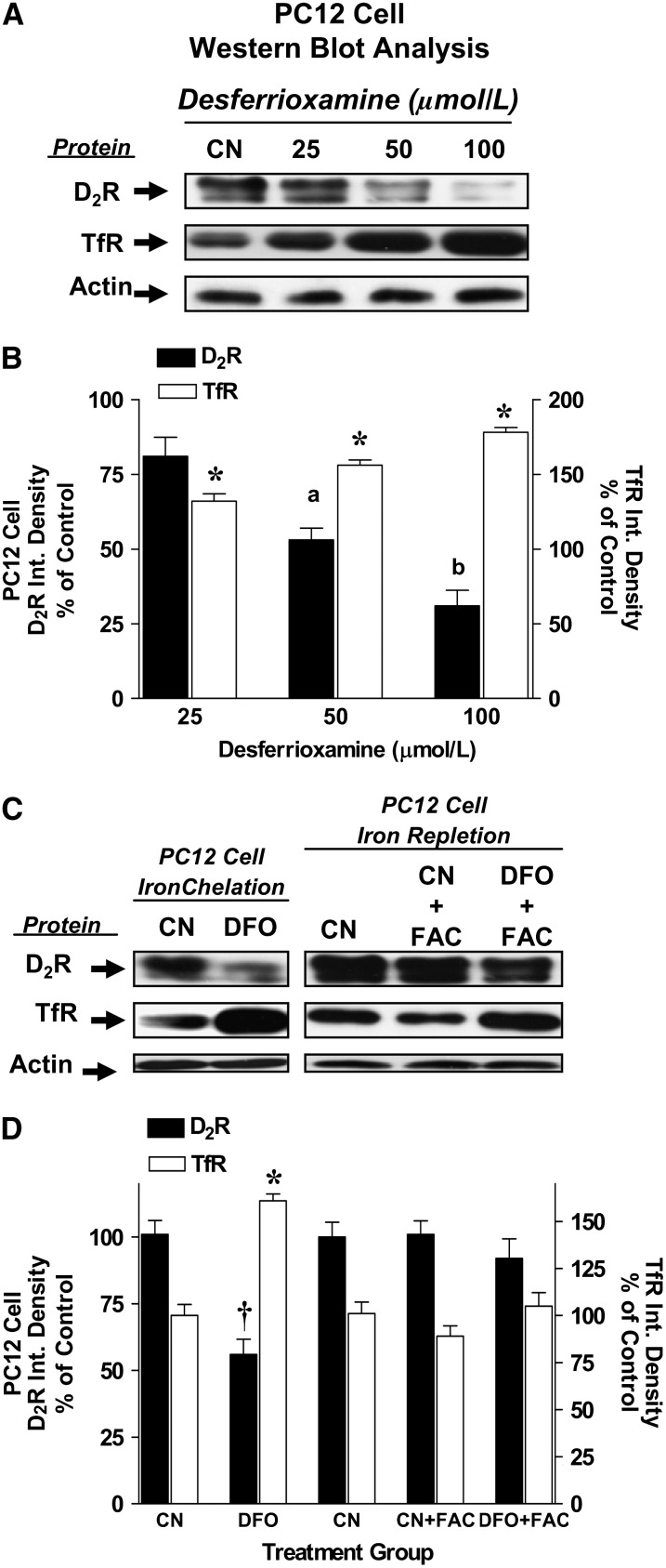
We utilized PC12 cell culture models to examine the effects of iron chelation on D2 receptor expression. Exposure of PC12 cells to DFO for 24 h resulted in a concentration-dependent decrease in D2 receptor protein densities (Fig. 1A,B). D2 receptor densities were 81 ± 6.3% (P = 0.07), 53 ± 4.0% (P < 0.05), and 31 ± 5.2% (P < 0.05) of controls with DFO treatments of 25, 50, and 100 μmol/L, respectively. To monitor the response of PC12 cells to chelation of the labile iron pool, we measured TfR protein concentrations and mRNA levels of several iron-related proteins as biomarkers for cellular iron deficiency. There were dramatic, dose-dependent increases in TfR band intensities in DFO-treated cells, indicating that the severity of iron chelation is enhanced as the cells are exposed to increasing concentrations of DFO (Fig. 1A,B). TfR protein levels were higher than control at all doses of DFO (P < 0.05). Real-time PCR analyses showed a 31% elevation in TfR mRNA levels, a 37% reduction in ferritin H mRNA levels, and a 27% reduction in DMT1 (+IRE) within 24 h of 200 μmol/L DFO treatment (P < 0.05 for all; data not shown). Further experiments measuring the labile iron pool with Phen-Green GSK fluorescence showed that DFO treatment for 24 h reduced the chelatable iron pool by >20% (P < 0.05, data not shown).
FIGURE 1 .
Dopamine D2 receptor and TfR protein concentrations in iron-chelated and iron-repleted PC12 cells. (A) Representative ECL photograph of immunoblotted D2 receptor, TfR, and actin in PC12 cells exposed to 0, 25, 50, and 100 μmol/L DFO for 24 h. CN, Vehicle control. (B) Integrated densities for D2 receptor and TfR after 24 h DFO exposure. Data are mean percent of CN ± SEM, n = 3. *Different from CN, P < 0.001. Means without a common letter differ, P < 0.05. (C) Representative ECL photographs of immunoblotted D2 receptor, TfR, and actin from vehicle- or DFO-treated PC12 cells exposed to FAC for 24 h. Control cells were treated identically excluding the addition of DFO and FAC. (D) Integrated densities for D2 receptor and TfR after iron repletion. Data are percent of CN means ± SEM, n = 3. †,*Different from CN, P < 0.05.
Repletion of iron-chelated PC12 cells (50 μmol/L DFO, 24 h) with FAC (50 mg/L) caused D2 receptor protein levels to return to control levels within 24 h of being repleted (Fig. 1C,D). Restorations in intracellular iron concentrations after FAC were demonstrated by the return of TfR densities to control levels. D2 receptor protein concentrations did not change in control cells supplemented with FAC (50 mg/L, 24 h), indicating that iron did not affect D2 receptor protein expression levels (Fig. 1C,D). In addition, iron supplementation did not change TfR levels. The doses of DFO and FAC used in these experiments did not affect total cellular protein concentrations and overall cell viability (trypan blue exclusion assays).
Moderate changes in PC12 cell D2 receptor gene expression after iron chelation.
The endogenous expression of the D2 receptor protein in our PC12 cell culture model allowed us to examine whether DFO-induced reductions in the D2 receptor may be the result of alterations in the synthesis of the protein. Real-time PCR analysis of mRNA extracted from PC12 cells exposed to increasing concentrations of DFO showed D2 receptor mRNA expression levels were 106 ± 8%, 95 ± 8%, and 87 ± 7% of control after a 24 h treatment with 25, 50, and 100 μmol/L DFO, respectively (data not shown). Treatment with 200 μmol/L DFO for 24 h reduced D2 receptor mRNA levels to 59 ± 7% of control (P < 0.05), but D1 receptor mRNA expression did not change (data not shown). To monitor the effects of iron chelation on overall PC12 gene expression, the mRNA levels of GAPDH, β-actin, and Thy-1, an iron-responsive glycopeptide important to synaptic efficacy, were also measured in cell culture model. DFO (25–100 μmol/L) did not affect the expression levels of GAPDH or β-actin. Thy-1 mRNA levels, however, decreased by 38% in PC12 cells exposed to 200 μmol/L DFO for 24 h (P < 0.05).
Iron deprivation and repletion in 65-d-old rats.
Male rats fed an iron-deficient diet from weaning (postnatal d 21) developed anemia with significantly lower hemoglobin, hematocrit, and serum iron levels than control diet-fed rats (P < 0.05 for all). Cellular iron stores in the liver and the spleen were depleted in iron-deficient rats (P < 0.05). Rats in the iron-repleted group were fed an iron-deficient diet for 4 wk followed by a control diet for 2 wk. After 2 wk of feeding a control diet, hemoglobin, hematocrit, plasma iron, liver iron, and spleen iron levels did not differ from rats fed the control diet for 6 wk (Table 1).
TABLE 1.
Hematology and tissue non-heme iron measurements from control, iron-deficient, and iron-repleted rats1
| Control | Iron deficient | Repleted | |
|---|---|---|---|
| Body weight, g | 273 ± 3 | 197 ± 5* | 259 ± 5* |
| Hemoglobin, g/L | 145 ± 3 | 49 ± 2* | 142 ± 3 |
| Hematocrit, % | 0.45 ± 0.01 | 0.20 ± 0.005* | 0.45 ± 0.005 |
| Serum iron, μmol/L | 33 ± 8 | 12 ± 4* | 29 ± 3 |
| TIBC,2μmol/L | 83 ± 7 | 133 ± 15* | 79 ± 12 |
| Tf saturation,3% | 40 ± 7 | 9.2 ± 4* | 37 ± 10 |
| Liver iron, μmol/g | 5.8 ± 0.2 | 1.3 ± 0.4* | 7.4 ± 1 |
| Spleen iron, μmol/g | 12 ± 1 | 2.8 ± 0.2* | 14 ± 1 |
Values are means ± SEM, n = 6. *Different from control, P < 0.05.
TIBC, total iron-binding capacity.
Tf, Transferrin protein.
Iron levels but not D2 receptor mRNA expression are altered in ventral midbrain in iron-deficient rats.
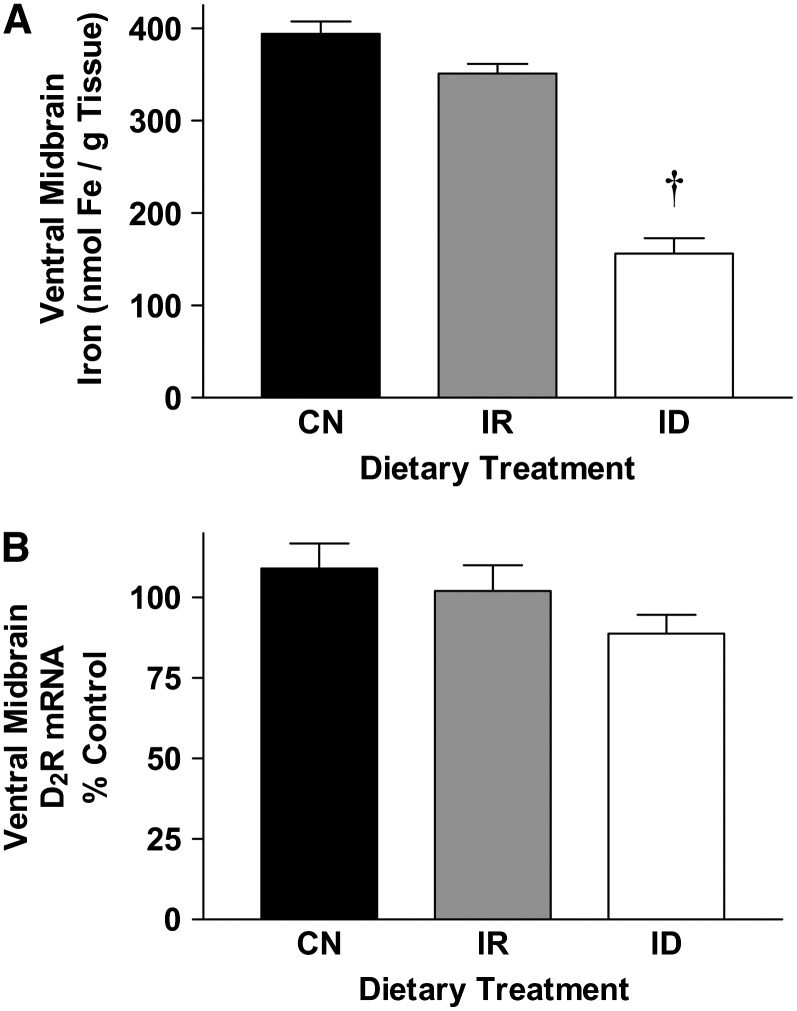
Dietary iron deficiency for 6 wk resulted in large reductions in ventral midbrain iron concentrations (P < 0.05; Fig. 2A). D2 receptor mRNA levels tended to be reduced slightly (P = 0.07; Fig. 2B). In iron-replete rats, ventral midbrain iron levels were restored to control levels, and D2 receptor protein and mRNA levels were unchanged (Fig. 2A,B). Although D2 receptor mRNA levels in iron-deficient rats did not differ from controls, regression analysis of individual rat data revealed a relationship between ventral midbrain D2 receptor mRNA and ventral midbrain iron concentrations through the influence of a small number of observations in the control and iron repletion groups (r2 = 0.412; P < 0.05). Dietary treatment and regional iron changes did not affect β-actin and GAPDH mRNA levels, suggesting that losses in iron concentrations did not globally alter the transcriptional activity within the ventral midbrain (data not shown).
FIGURE 2 .
Iron and D2 receptor mRNA levels in the ventral midbrain from control (CN), iron-repleted (IR), and iron-deficient (ID) rats. (A) Iron concentration in ventral midbrain from CN, IR, and ID rats. Values are means ± SEM, n = 6. †Different from CN, P < 0.05. (B) Real-time-PCR analysis of ventral midbrain D2 receptor mRNA levels in CN, IR, and ID rats. Values are percent of CN means ± SEM, n = 6.
D2 receptor protein levels are reduced in the ventral midbrain in iron-deficient rats.
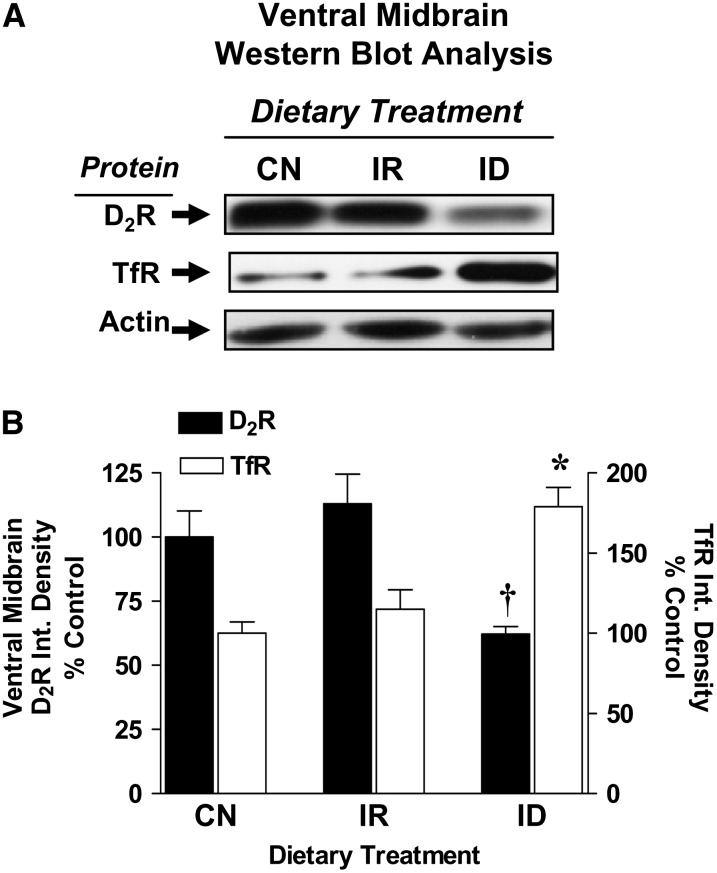
The effects of dietary iron depletion on D2 receptor protein expression patterns were examined in the ventral midbrain, which contains dopamine neuron cell bodies. In iron-deficient rats with 65% reductions in ventral midbrain iron, ventral midbrain D2 receptor protein densities were also significantly lower than rats fed the control diet (Fig. 3A,B; P < 0.05). Cellular responses to regional iron losses were demonstrated by the large elevations in TfR protein levels in the ventral midbrain of iron-deficient rats (P < 0.05; Fig. 3A,B). In iron-repleted rats (Fig. 2A), both D2 receptor and TfR protein densities were similar to control diet-fed rats (Fig. 3A,B). D2 receptor protein densities and total iron content in the ventral midbrain were correlated (r2 = 0.519; P < 0.05).
FIGURE 3 .
D2 receptor protein levels in ventral mibrain from control (CN), iron-repleted (IR), and iron-deficient (ID) rats. (A) Representative ECL photographs of immunoblotted D2 receptor, TfR, and actin in ventral midbrain from CN, IR, and ID rats. (B) Integrated densities for D2 receptor and TfR after iron depletion and iron repletion. Data are mean percent of CN ± SEM, n = 6. †,*Different from CN, P < 0.05.
Iron and D2 receptor protein levels are reduced in the caudate from iron-deficient rats.
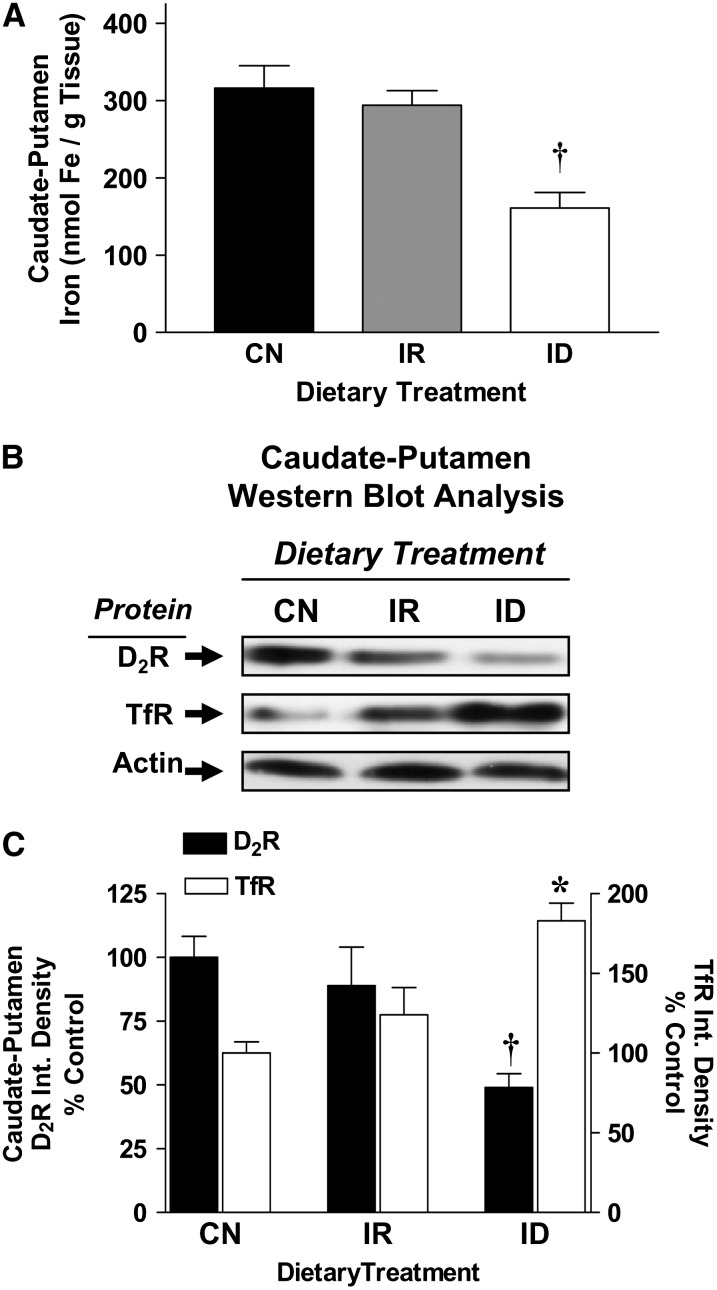
Iron and D2 receptor protein densities were measured in the caudate putamen to demonstrate the impact of an iron-deficient diet on D2 receptor expression patterns in one of the terminal fields of the dopamine system (Fig. 4). Iron concentrations and D2 receptor protein densities in the caudate putamen were both reduced by ∼50% in rats fed an iron-deficient diet (Fig. 4A–C; P < 0.05 for both). Reductions in caudate iron levels were accompanied by large elevations in caudate TfR densities in iron-deficient rats (P < 0.05; Fig. 4B,C). Iron, D2 receptor, and TfR levels in iron-replete rats were similar to levels found in rats fed the control diet (Fig. 4A–C). Regression analysis of individual rat data revealed a direct linear relationship between D2 receptor protein and iron levels in the caudate putamen (r2 = 0.584; P < 0.05).
FIGURE 4 .
Iron content and D2 receptor protein levels in caudate-putamen from control (CN), iron-repleted (IR), and iron-deficient (ID) rats. (A) Mean caudate-putamen iron concentrations in CN, IR, and ID rats. Data are means ± SEM, n = 6. †Different from CN. (B) Representative ECL photographs of immunoblotted D2 receptor, TfR, and actin in CN, IR, and ID rats. (C) Mean integrated densities for caudate D2 receptor and TfR in CN, IR, and ID rats. Data are mean percent of CN ± SEM, n = 6. †,*Different from CN, P < 0.05.
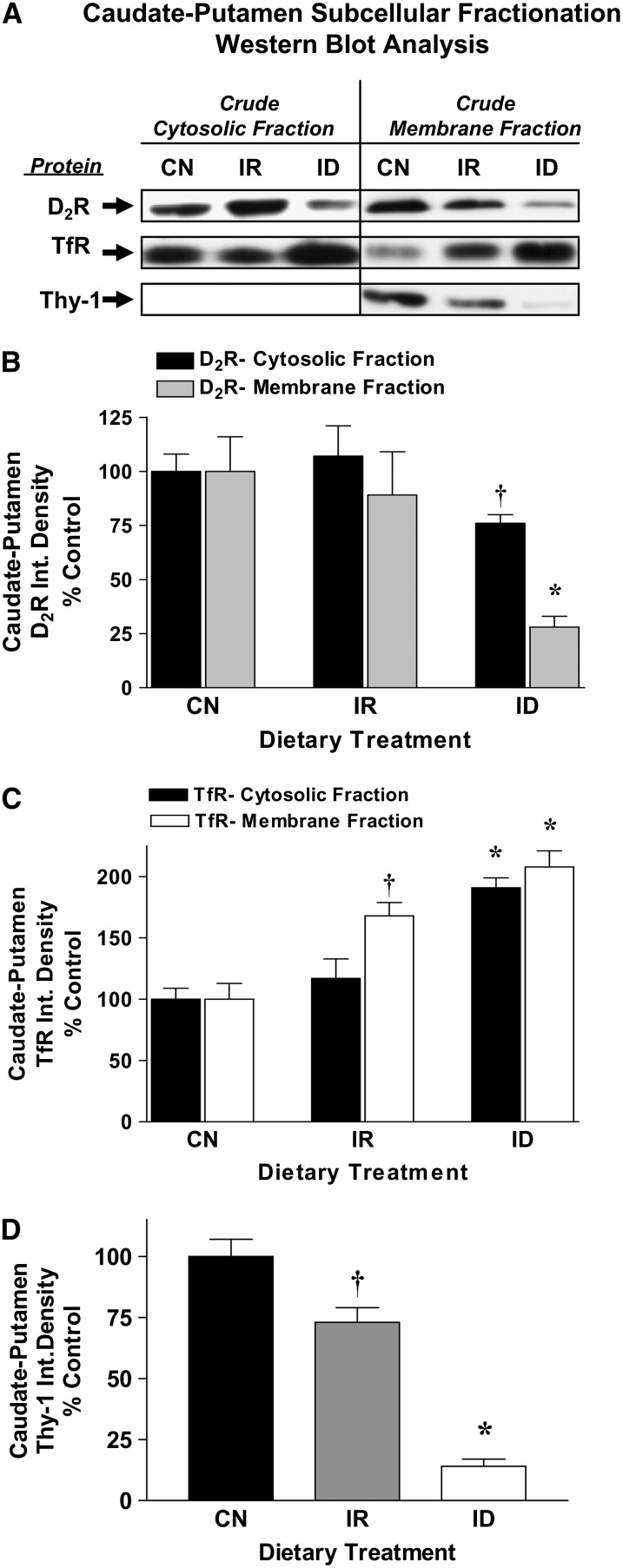
D2 receptor densities in caudate putamen membrane fractions are reduced with iron deficiency.
Subcellular fractionation studies were conducted to examine the effects of iron deficiency on D2 receptor partitioning between the membrane and intracellular (cytosolic) spaces. D2 receptor levels in iron-deficient rats were significantly lower in both crude cytosolic and membrane fractions from the caudate putamen. The losses of D2 receptor in iron-deficient rats were far greater in the crude membrane fraction (P < 0.05; Fig. 5A,B) compared with the cytosolic fraction (P < 0.05; Fig. 5A,B). In iron-repleted rats, D2 receptor densities did not differ from control preparations in either subcellular fraction (Fig. 5A,B). Large elevations in caudate putamen TfR densities were detected in both cytosolic and membrane fractions prepared from iron-deficient rats (Fig. 5A,C). Although cytosolic TfR densities normalized to control levels in iron-repleted rats (Fig. 5A,C), TfR densities remained elevated in caudate putamen membrane fractions (168 ± 11.0% of control; P < 0.05; Fig. 5A,C). Membrane Thy-1 densities were lower in iron-deficient rats (P < 0.05) and remained altered in iron-repleted rats (P < 0.05; Fig. 5A,D). Thy-1 immunoreactivity was not detected in any of the cytosolic fractions examined in these experiments, consistent with its location on the cell membrane.
FIGURE 5 .
The distribution of D2 receptor, TfR, and Thy-1 proteins in the caudate putamen from control (CN), iron-repleted (IR), and iron-deficient (ID) rats. (A) Representative ECL photographs of immunoblotted membrane and cytosolic fractions of D2 receptor, TfR, and Thy-1 proteins in CN, IR, and ID animals. (B) Mean integrated densities for membrane and cytosolic D2 receptor from CN, IR, and ID rats. Data are mean percent of CN ± SEM, n = 6. †Different from CN, P < 0.05; *P < 0.01. (C) Mean integrated densities for membrane and cytosolic TfR from CN, IR, and ID rats. Data are percent of CN means ± SEM, n = 6. †Different from CN, P < 0.05; *P < 0.01. (D) Mean integrated densities for membrane and cytosolic Thy-1 from CN, IR, and ID rats. Data are mean percent of CN ± SEM, n = 6. †Different from CN, P < 0.05; * Different from CN, P < 0.01.
Discussion
The present experiments were designed to determine the influence of cellular iron deficiency on D2 receptor expression patterns in cell culture and in dopamine-containing regions of the brain. Abnormalities in D2 receptor expression in iron deficiency have strong implications regarding human functioning, as a number of aspects of learning, decision making, addiction, and movement can all be altered either directly, or indirectly, when this dopamine receptor subtype loses functionality [for recent review see (27)]. Deficits in brain iron and dopamine signaling are also described in RLS, where patients show reduced symptoms after enhancement of dopamine activity (8,9) and an exacerbation in symptoms in response to dopamine receptor antagonists (10,11).
To examine the relationship between iron depletion and the D2 receptor at the cellular level, we produced an iron deficiency in PC12 cells. PC12 cells were used to model iron deficiency, because they express the dopamine transporter, D2 receptors, D1 receptors, and many of the signal transduction components responsible for regulation of the dopamine system. The dopamine transporter expressed in these cells is also responsive to iron chelation in a similar fashion as this transporter is affected in the iron-deficient brain. The cell culture studies presented here revealed that 24-h DFO treatment decreased the cellular levels of the D2 receptor in a dose-dependent manner without affecting expression of housekeeping proteins or changing cell viability. There were the appropriate changes in iron TfR protein expression and transferrin, ferritin H, and DMT1 gene expression to demonstrate that these cells were iron deficient. In fact, fluorescence assays showed that DFO reduces the iron pool by >20%. Addition of ferric ammonium citrate to iron-chelated cells caused D2 receptor levels to return to normal levels, again demonstrating that these changes in the D2 receptor were a result of iron chelation. Because DFO chelates several other metals, including copper, cobalt, and zinc, these metals could also play a role in changing D2 receptor levels. We believe that this is not the case, because DFO has a much higher affinity for iron with binding constants of 1031 for ferric iron, 1014 for copper (28), 1011 for cobalt (28), and 1011 for zinc (28–30).
Whereas D2 receptor protein levels were robustly reduced in iron-chelated PC12 cells and the ventral midbrain of iron-deficient rats, mRNA levels were only modestly affected. At the highest concentration of DFO (100 μmol/L), D2 receptor mRNA levels were reduced by 13% and protein levels decreased by 69%. Similarly, D2 receptor mRNA and protein levels were reduced by 11% and 65%, respectively, in ventral midbrain. These data suggest that the decrease in D2 receptor protein levels in iron-chelated PC12 cells and in the iron-deficient ventral midbrain may not be due to a decrease in transcription. The D2 receptor gene is transcribed from a TATA-less promoter that has an initiator-like sequence and several putative Sp1/Sp3 and Ap1 transcriptional binding sites (31). Previous work shows that iron chelation alters the levels of these transcription factors but apparently this did not significantly influence mRNA levels in the current paradigm (28,32). Microarray analysis of DFO-treated PC12 cells also did not reveal any effect of iron chelation on these transcription factors (J. Beard and E. Unger, unpublished data).
In the caudate of iron-deficient rats, D2 receptor protein expression is significantly reduced. We explored the possibility that there was an increase in rate of degradation of this protein due to changes in trafficking (33). Previous studies support the hypothesis that cellular iron deprivation alters the turnover of monoamine-related proteins (20,34). In those studies, iron chelation in neuroblastoma or PC12 cell cultures enhanced the rate of degradation of the dopamine transporter and norepinephrine transporter due to increased movement of the transporters to the lysosomal vesicle pool. Similar, but not identical, regulation of D2 receptor functionality occurs through trafficking mediated by dopamine receptor multiplex proteins (35). Clardy et al. (36) recently showed that a number of these dopamine receptor-associated proteins are altered by iron deficiency in early life. Our own real-time PCR data also demonstrate that the mRNA of D2 receptor multiplex proteins, including glycogen synthase kinase 3β, β arrestin 2, protein phosphatase 2A, and regulator of G protein signaling 2 are changed in PC12 cells treated with DFO (J. Beard, unpublished data). We did not explore the direct role of these regulatory proteins in the movement of the D2 receptor from the cell membrane, although iron deficiency clearly altered membrane D2 receptor levels far more than cytosolic levels (37,38). In addition, the important synaptic gylcopeptide, Thy-1, is dramatically decreased in striatum of iron-deficient rats, in PC12 cells treated with DFO, and in autopsy brains from RLS patients who have evidence of brain iron deficiency (39). The loss of Thy-1, or dopamine- and cyclic-AMP-regulated phosphoproteins, could affect D2 receptor recycling on the membrane and thus decrease the half-life of the receptor (35).
The influence of iron status on dopamine is not restricted to just alterations with the dopamine transporter and the D2 receptor, because there are associated changes in intracellular and extracellular dopamine concentrations in iron-deficient rodents (15,40,41), tyrosine hydroxylase activity in cell culture and rodent models (15,41), and phosphorylation of related proteins through protein kinase C (PKC)-mediated pathways in cells (42). D2 receptor trafficking is increased with activation of PKC (43). PKC isoforms are known to be sensitive to changes in cellular iron status, because iron-chelated neuro2A cells have time-dependent alterations in the PKC isoforms α, β1, δ, and ɛ that may reflect a direct effect of iron on PKC or an indirect effect through signal transduction pathways (20). This recent report also demonstrates that application of the PKC inhibitor staurosporine to PC12 cells prevents the reduction in dopamine uptake after iron chelation, providing further support for a possible PKC involvement in D2 receptor trafficking. Future studies are planned to explore the specific effects of PKC activation on downstream signaling deficits that result from iron chelation.
Overall, the decline in D2 receptor protein levels in the current study is consistent with previous reports of reductions in D2 receptor levels in the iron-deficient state (16,19,21). In this report, we did not investigate changes in the presynaptic compared with the postsynaptic D2 receptors in iron-deficient rats. Presynaptic D2 auto-receptors regulate reuptake of dopamine through signal transduction pathway(s). Pharmacological studies have revealed that administration of the D2 agonist quinpirole enhances dopamine transport velocity in striatal synaptosomes (22), whereas the D2 receptor antagonist raclopride decreases dopamine transport (23). Recent evidence also shows that infusion of quinpirole into striatum increases dopamine transport velocity in rats fed a control diet, whereas the D2 receptor agonist did not affect uptake in iron-deficient rats. Together, these data suggest that the D2 receptor, the signal transduction pathway(s), and the dopamine transporter is altered in iron deficiency (18). To date, the mechanism(s) of effect of neuronal iron deficiency on pre- vs. postsynaptic dopamine receptor metabolism or gene expression remains unexplored.
The importance of these observations to nutrition is based on the estimates that as many as 30–50% of the world's infants and children, and > 50% of reproductive-age women, have iron deficiency (44). Changes in brain iron status are related to a number of deficits in brain functioning, of which dopamine metabolism is only one (3). In summary, the results of this study show that in both a cell culture model and a rodent model of iron deficiency, the D2 receptor is affected by iron status. Whether changes in the D2 receptor are a direct effect of iron loss or a result of changes in dopamine signaling (possibly related to dopamine transporter deficiencies, altered PKC signaling, etc.) has yet to be elucidated. The persistence of these effects from a developmental perspective has already been demonstrated in rodent models (45,46). The efficacy of iron treatment in RLS to improve brain iron content and dopamine agonist treatment to alter dopamine metabolism is consistent with a relationship between brain iron status and clinical symptoms (7). Future studies will focus on the availability of iron to neurons and whether transients of iron flux in the brain are important to neural functioning.
Supported by USPHS grant nos. NS 35088, HD 39386, and AG 21190.
Author disclosures: E. L. Unger, J. A. Wiesinger, L. Hao, and J. L. Beard, no conflicts of interest.
Abbreviations used: DFO, desferrioxamine; DMT1, divalent metal transporter 1; FAC, ferrous ammonium citrate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PKC, protein kinase C; RLS, Restless Legs Syndrome; TfR, transferrin receptor.
References
- 1.Beard J. Iron deficiency alters brain development and functioning. J Nutr. 2003;133:S1468–72. [DOI] [PubMed] [Google Scholar]
- 2.Burden MJ, Westerlund AJ, Armony-Sivan R, Nelson CA, Jacobson SW, Lozoff B, Angelilli ML, Jacobson JL. An event-related potential study of attention and recognition memory in infants with iron-deficiency anemia. Pediatrics. 2007;120:e336–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lozoff B, De Andraca I, Castillo M, Smith JB, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics. 2003;112:846–54. [PubMed] [Google Scholar]
- 5.Lozoff B, Klein NK, Nelson EC, McClish DK, Manuel M, Chacon ME. Behavior of infants with iron-deficiency anemia. Child Dev. 1998;69:24–36. [PubMed] [Google Scholar]
- 6.Pollitt E. Early iron deficiency anemia and later mental retardation. Am J Clin Nutr. 1999;69:4–5. [DOI] [PubMed] [Google Scholar]
- 7.Earley CJ, Heckler D, Allen RP. The treatment of restless legs syndrome with intravenous iron dextran. Sleep Med. 2004;5:231–5. [DOI] [PubMed] [Google Scholar]
- 8.Brodeur C, Montplaisir J, Godbout R, Marinier R. Treatment of restless legs syndrome and periodic movements during sleep with L-dopa: a double-blind, controlled study. Neurology. 1988;38:1845–8. [DOI] [PubMed] [Google Scholar]
- 9.Trenkwalder C, Stiasny K, Pollmacher T, Wetter T, Schwarz J, Kohnen R, Kazenwadel J, Kruger HP, Ramm S, et al. L-dopa therapy of uremic and idiopathic restless legs syndrome: a double-blind, crossover trial. Sleep. 1995;18:681–8. [DOI] [PubMed] [Google Scholar]
- 10.Montplaisir J, Lorrain D, Godbout R. Restless legs syndrome and periodic leg movements in sleep: the primary role of dopaminergic mechanism. Eur Neurol. 1991;31:41–3. [DOI] [PubMed] [Google Scholar]
- 11.Winkelmann J, Schadrack J, Wetter TC, Zieglgansberger W, Trenkwalder C. Opioid and dopamine antagonist drug challenges in untreated restless legs syndrome patients. Sleep Med. 2001;2:57–61. [DOI] [PubMed] [Google Scholar]
- 12.Beard JL, Chen Q, Connor J, Jones BC. Altered monamine metabolism in caudate-putamen of iron-deficient rats. Pharmacol Biochem Behav. 1994;48:621–4. [DOI] [PubMed] [Google Scholar]
- 13.Nelson C, Erikson K, Pinero DJ, Beard JL. In vivo dopamine metabolism is altered in iron-deficient anemic rats. J Nutr. 1997;127:2282–8. [DOI] [PubMed] [Google Scholar]
- 14.Chen NH, Reith ME. Monoamine interactions measured by microdialysis in the ventral tegmental area of rats treated systemically with (+/−)-8-hydroxy-2-(di-n-propylamino)tetralin. J Neurochem. 1995;64:1585–97. [DOI] [PubMed] [Google Scholar]
- 15.Beard JL, Unger EL, Bianco LE, Paul T, Rundle SE, Jones BC. Early postnatal iron repletion overcomes lasting effects of gestational iron deficiency in rats. J Nutr. 2007;137:1176–82. [DOI] [PubMed] [Google Scholar]
- 16.Erikson KM, Jones BC, Hess EJ, Zhang Q, Beard JL. Iron deficiency decreases dopamine D1 and D2 receptors in rat brain. Pharmacol Biochem Behav. 2001;69:409–18. [DOI] [PubMed] [Google Scholar]
- 17.Pinero DJ, Li NQ, Connor JR, Beard JL. Variations in dietary iron alter brain iron metabolism in developing rats. J Nutr. 2000;130:254–63. [DOI] [PubMed] [Google Scholar]
- 18.Bianco LE, Wiesinger J, Earley CJ, Jones BC, Beard JL. Iron deficiency alters dopamine uptake and response to L-DOPA injection in Sprague-Dawley rats. J Neurochem. 2008;106:205–15. [DOI] [PubMed] [Google Scholar]
- 19.Erikson KM, Jones BC, Beard JL. Iron deficiency alters dopamine transporter functioning in rat striatum. J Nutr. 2000;130:2831–7. [DOI] [PubMed] [Google Scholar]
- 20.Wiesinger JA, Buwen JP, Cifelli CJ, Unger EL, Jones BC, Beard JL. Down-regulation of dopamine transporter by iron chelation in vitro is mediated by altered trafficking, not synthesis. J Neurochem. 2007;100:167–79. [DOI] [PubMed] [Google Scholar]
- 21.Youdim MB, Ben-Shachar D, Ashkenazi R, Yehuda S. Brain iron and dopamine receptor function. Adv Biochem Psychopharmacol. 1983;37:309–21. [PubMed] [Google Scholar]
- 22.Meiergerd SM, Patterson TA, Schenk JO. D2 receptors may modulate the function of the striatal transporter for dopamine: kinetic evidence from studies in vitro and in vivo. J Neurochem. 1993;61:764–7. [DOI] [PubMed] [Google Scholar]
- 23.Cass WA, Gerhardt GA. Direct in vivo evidence that D2 dopamine receptors can modulate dopamine uptake. Neurosci Lett. 1994;176:259–63. [DOI] [PubMed] [Google Scholar]
- 24.Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51. [DOI] [PubMed] [Google Scholar]
- 25.Cook GA, King MT, Veech RL. Changes in liver inorganic pyrophosphate content during ethanol metabolism. Adv Exp Med Biol. 1980;132:433–40. [DOI] [PubMed] [Google Scholar]
- 26.Erikson KM, Pinero DJ, Connor JR, Beard JL. Regional brain iron, ferritin and transferrin concentrations during iron deficiency and iron repletion in developing rats. J Nutr. 1997;127:2030–8. [DOI] [PubMed] [Google Scholar]
- 27.Waddington JL, O'Tuathaigh C, O'Sullivan G, Tomiyama K, Koshikawa N, Croke DT. Phenotypic studies on dopamine receptor subtype and associated signal transduction mutants: insights and challenges from 10 years at the psychopharmacology-molecular biology interface. Psychopharmacology (Berl). 2005;181:611–38. [DOI] [PubMed] [Google Scholar]
- 28.Kramer-Stickland K, Edmonds A, Bair WB III, Bowden GT. Inhibitory effects of deferoxamine on UVB-induced AP-1 transactivation. Carcinogenesis. 1999;20:2137–42. [DOI] [PubMed] [Google Scholar]
- 29.Dayani PN, Bishop MC, Black K, Zeltzer PM. Desferoxamine (DFO)–mediated iron chelation: rationale for a novel approach to therapy for brain cancer. J Neurooncol. 2004;67:367–77. [DOI] [PubMed] [Google Scholar]
- 30.Richardson DR. Potential of iron chelators as effective antiproliferative agents. Can J Physiol Pharmacol. 1997;75:1164–80. [PubMed] [Google Scholar]
- 31.Yajima S, Lee SH, Minowa T, Mouradian MM. Sp family transcription factors regulate expression of rat D2 dopamine receptor gene. DNA Cell Biol. 1998;17:471–9. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz IG, de la Torre P, Diaz T, Esteban E, Morillas JD, Munoz-Yague T, Solis-Herruzo JA. Sp family of transcription factors is involved in iron-induced collagen alpha1(I) gene expression. DNA Cell Biol. 2000;19:167–78. [DOI] [PubMed] [Google Scholar]
- 33.Miranda M, Wu CC, Sorkina T, Korstjens DR, Sorkin A. Enhanced ubiquitylation and accelerated degradation of the dopamine transporter mediated by protein kinase C. J Biol Chem. 2005;280:35617–24. [DOI] [PubMed] [Google Scholar]
- 34.Beard JL, Felt B, Schallert T, Burhans M, Connor JR, Georgieff MK. Moderate iron deficiency in infancy: biology and behavior in young rats. Behav Brain Res. 2006;170:224–32. [DOI] [PubMed] [Google Scholar]
- 35.Kabbani N, Levenson R. A proteomic approach to receptor signaling: molecular mechanisms and therapeutic implications derived from discovery of the dopamine D2 receptor signalplex. Eur J Pharmacol. 2007;572:83–93. [DOI] [PubMed] [Google Scholar]
- 36.Clardy SL, Wang X, Zhao W, Liu W, Chase GA, Beard JL, True Felt B, Connor JR. Acute and chronic effects of developmental iron deficiency on mRNA expression patterns in the brain. J Neural Transm Suppl. 2006;173–96. [DOI] [PubMed]
- 37.Hartman DS, Lanau F. Diversity of dopamine receptors: new molecular and pharmacological developments. Pol J Pharmacol. 1997;49:191–9. [PubMed] [Google Scholar]
- 38.Lachowicz JE, Sibley DR. Molecular characteristics of mammalian dopamine receptors. Pharmacol Toxicol. 1997;81:105–13. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Wiesinger J, Beard J, Felt B, Menzies S, Earley C, Allen R, Connor J. Thy1 expression in the brain is affected by iron and is decreased in Restless Legs Syndrome. J Neurol Sci. 2004;220:59–66. [DOI] [PubMed] [Google Scholar]
- 40.Beard JL, Erikson KM, Jones BC. Neurobehavioral analysis of developmental iron deficiency in rats. Behav Brain Res. 2002;134:517–24. [DOI] [PubMed] [Google Scholar]
- 41.Unger EL, Paul T, Murray-Kolb LE, Felt B, Jones BC, Beard JL. Early iron deficiency alters sensorimotor development and brain monoamines in rats. J Nutr. 2007;137:118–24. [DOI] [PubMed] [Google Scholar]
- 42.Alcantara O, Obeid L, Hannun Y, Ponka P, Boldt DH. Regulation of protein kinase C (PKC) expression by iron: effect of different iron compounds on PKC-beta and PKC-alpha gene expression and role of the 5′-flanking region of the PKC-beta gene in the response to ferric transferrin. Blood. 1994;84:3510–7. [PubMed] [Google Scholar]
- 43.Namkung Y, Sibley DR. Protein kinase C mediates phosphorylation, desensitization, and trafficking of the D2 dopamine receptor. J Biol Chem. 2004;279:49533–41. [DOI] [PubMed] [Google Scholar]
- 44.WHO. Iron deficiency anemia: assessment, prevention, and control. A guide for program managers. WHO/NHD/013; United Nations University, Geneva Swizerland 2001.
- 45.Beard J, Erikson KM, Jones BC. Neonatal iron deficiency results in irreversible changes in dopamine function in rats. J Nutr. 2003;133:1174–9. [DOI] [PubMed] [Google Scholar]
- 46.Pinero D, Jones B, Beard J. Variations in dietary iron alter behavior in developing rats. J Nutr. 2001;131:311–8. [DOI] [PubMed] [Google Scholar]