Abstract
Reactive oxygen species (ROS) can induce oxidative injury via iron interactions (i.e. Fenton chemistry and hydroxyl radical formation). Our prior work suggested that American ginseng berry extract and ginsenoside Re were highly cardioprotective against oxidant stress. To extend this study, we evaluated the protective effect of protopanaxadiol-type ginsenoside Rb1 (gRb1) on H2O2-induced oxidative injury in cardiomyocytes and explored the ROS-mediated intracellular signaling mechanism. Cultured embryonic chick cardiomyocytes (4–5 day) were used. Cell death was assessed by propidium iodide and lactate dehydrogenase release. Pretreatment with gRb1 (0.01, 0.1, or 1 μM) for 2 h and concurrent treatment with H2O2 (0.5 mM) for 2 h resulted in a dose-dependent reduction of cell death, 36.6 ± 2.9% (n = 12, p < 0.05), 30.5 ± 5.1% (n = 12, p < 0.05) and 28.6 ± 3.1% (n = 12, p < 0.01) respectively, compared to H2O2-exposed cells (48.2 ± 3.3%, n = 12). This cardioprotective effect of gRb1 was associated with attenuated intracellular ROS generation as measured by 6-carboxy-2′, 7′-dichlorodihydrofluorescein diacetate, preserved the mitochondrial membrane potential as determined using JC-1. In the ESR study, gRb1 exhibited the scavenging DPPH and hydroxyl radical activities. Furthermore, our data showed the increased JNK phosphorylation (p-JNK) in H2O2-exposed cells was suppressed by the pretreatment with gRb 1 (1 μM) (p < 0.01). Co-treatment of gRb1 with a specific inhibitor of JNK SP600125 (10 μM) further reduced the p-JNK and enhanced the cell survival after H2O2 exposure. Collectively, our results suggest that gRb1 conferred cardioprotection that was mediated via attenuating ROS and suppressing ROS-induced JNK activation.
Keywords: Ginsenoside Rb1, Oxidative stress, Cardiomyocyte, JNK
INTRODUCTION
Reactive oxygen species (ROS) have been extensively implicated in the pathological processes of the heart, including ischemia and reperfusion injury. Excessive oxidative stress leads to cell death and mitochondrial dysfunction (Hwang and Li, 1993; Bolli, 2007). ROS also act as signaling molecules in various cellular signaling pathways as a model of redox modulation (Penna et al., 2006). There is substantial evidence that JNK, a redox-sensitive stress kinase, is strongly induced by ROS and oxidative stress in cultured cardiac myocytes and intact hearts (Das et al., 2006). JNK-knockout mouse hearts have significantly less necrosis and apoptosis induced by ischemia/reperfusion than those in control mice, suggesting that JNK plays a key role in regulating ischemia/reperfusion injury (Kaiser et al., 2005).
Ginsenoside Rb1 (gRb1, C54H92O23, molecular weight 1,109.26) is the principle active ingredient of Panax ginseng root (Taira et al., 2010; Wang et al., 2011a). Recently, more than 40 ginsenosides have been extracted from different species of ginseng and classified into three major groups based on their chemical structure: protopanaxadiol, protopanaxatriol and oleanolic acid (Fujita et al., 2007; Wang et al., 2008b). gRb1 is a representative of the protopanaxadiol group that has been shown to scavenge free radicals (Lim et al., 1997), inhibit cardiac hypotrophy and attenuate the dilated cardiomyopathy in transgenic mice (Jiang et al., 2007). gRb1 has also been reported to protect against ischemia/reperfusion injury in cardiomyocytes (Guan et al., 2002), endothelial cells (Moriue et al., 2008), brain tissue (Park et al., 2005) and liver tissue (Wang et al., 2008a). We have previously demonstrated that ginsenoside Re protected cardiomyocytes from exogenous oxidation by H2O2 and endogenous oxidation by antimycin A, a mitochondrial electron transport chain complex III inhibitor-induced oxidative injury (Xie et al., 2006). The redox signaling has been suggested to play a role in mediating antioxidant effects in the heart (Zhao et al., 2009). The protective effect of antioxidants is associated with inhibiting ROS-mediated multiple intracellular signaling pathways, including JNK (Chan et al., 2003). A number of antioxidants, such as grape seed proanthocyanidins have been shown to reduce cardiomyocyte death by inhibiting ischemia/reperfusion-induced phosphorylation of JNK (Sato et al., 2001). However, the role of JNK signaling in the protective effect of gRb1 on the H2O2- induced oxidant stress model in cardiomyocytes has not yet been evaluated.
In this study, we used an established chick cardiomyocyte model to examine the hypothesis that gRb1 protects cardiomyocytes from H2O2-induced oxidative injury that is mediated by scavenging ROS, preserving mitochondrial function and suppressing ROS-mediated JNK activation.
MATERIALS AND METHODS
Chick primary cardiomyocyte culture
Embryonic chick ventricle myocytes were isolated from 10-day chick embryos as previously described (Vanden Hoek et al., 1996). In brief, the hearts were removed and the ventricles were minced and enzymatically digested with 0.025% trypsin (Invitrogen). In order to exclude non-cardiomyocytes, cells were preplated for 45 min at 37°C. The resultant cell suspension was centrifuged and then resuspended in the culture medium (54% balanced salt solution, 40% medium 199, 6% heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin). Cardiomyocytes were plated onto 25 mm glass coverslips at a density of 0.7 × 106 and incubated at 37°C. Cardiomyocyte purity was assessed by immunofluorescent staining for alpha-sarcomeric actin (Sigma). All experiments were performed with the 4–5 day cultured cells, by which time synchronously contracting cells could be visualized with viability exceeding 95%.
Measurement of free radical scavenging activity of gRb1 by electron spin resonance (ESR) spectroscopy
The free radical scavenging ability of gRb1 was evaluated in a cell-free system using a Bruker EMX ESR spectrometer (Brurker Biospin) as described previously (Zhao et al., 2005; Chang et al., 2007). ESR spectra were recorded with 1 G modulation amplitude, 10 mW microwave power, 100G sweep widths, 3328 G field set. All measurements were performed in replicates at room temperature. Hydroxyl radicals were generated by Fe2+/H2O2 system and reacted with a spin-trap, 5-diethoxyphosphoryl-5-methyl-1-pyrroline-N-oxide (DMPO, 50 mM) (Oxis International). The formed DMPO-OH adducts were detected by ESR spectroscopy. ERS signal was recorded after 10 μL of FeSO4 (0.1 mM) was mixed with 10 μL of DMPO (50 mM), H2O2 (0.1 mM), EDTA (0.1 mM) and 10% CH3CN or with gRb1 in CH3CN. For DPPH (2,2,- diphenyl-1-picrylhydrazyl) radical, the reaction mixture contained 50 μL of gRb1 dissolved in 50% CH3CN and DPPH (0.5 mM) in CH3CN.
Measurement of intracellular ROS
The 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (6-carboxy-H2DCFDA, 1 μM) probe was used to measure intracellular ROS. It is a non-fluorescent and cell permeable analog that is oxidized to highly fluorescent carboxy-dichlorofluorescein (carboxy-DCF) as measured at the wavelength of excitation 488 nm/emission 520 nm and expressed in arbitrary units (a.u.). This carboxylated form is more permeant than the classic H2DCFDA (Wrona et al., 2005). Increases in DCF fluorescence suggest H2O2 or hydroxylradical generation.
Viability assay
Cell viability was assessed with the exclusion fluorescent dye, propidium iodide (5 μM, Sigma) measured at excitation 540 nm/emission 590 nm using a Nikon TE 2000-U inverted phase/epifluorescent microscope (Photometrics). This dye exhibited no toxicity in control cells even after a ten-hour exposure (Vanden Hoek et al., 1997). At the end of the experiment, all cells on the coverslip were permeabilized with digitonin (300 μM, Sigma). Measurement of propidium iodide (PI) fluorescence was done with an average of 3 random fields on each coverslip at the end of 2-h H2O2 exposure and after 1-h digitonin exposure. Percentage cell death (PI uptake) was expressed as the PI fluorescence relative to the maximal value seen after digitonin exposure (100%). Each experiment was repeated in different batches of cells.
Lactate dehydrogenase (LDH) release assay
The LDH is a stable cytoplasmic enzyme and is released into culture supernatant when cell membranes are damaged. LDH activity was measured using an LDH cytotoxicity assay kit (BioVision) through the oxidation of lactate to pyruvate which then reacts with tetrazolium salt INT to form formazan. The rate of increase in formazan is directly proportional to the LDH activity in the sample. The percentage of LDH released was estimated as a proportion of the LDH released into the medium divided by the total amount of LDH present in medium and within cells lysed with 0.5% Triton X-100. Spectrophotometric values were measured by a microplate reader (Synergy HT, Bio-Tek Instruments) at a wavelength of 490 nm.
Cell morphological observation
Following the treatments, cells were reviewed on a Nikon Inverted Stage microscope (Nikon) using 10× objective. Photomicrographs were taken with a Nikon digital camera, and images were imported into Adobe Photoshop for visualization.
Measurement of mitochondrial membrane potential (ΔΨm)
The ΔΨm was determined using the dual-emission mitochondrion-specific lipophilic, cationic dye, 5,5′,6,6′-tetrachloro-1,1′,3,3′ tetraethylbenzimidazoly-carbocyanine iodide (JC-1) (Gibco-Invitrogen). The punctate red fluorescence (excitation 530 nm/emission 600 nm) represents the potential-dependent aggregate form of JC-1 in the mitochondria of healthy cells (polarized mitochondria). Diffuse green fluorescence (excitation 490 nm, emission 530 nm) represents the monomeric form of JC-1 in the cytosol of unhealthy cells (depolarized mitochondria) (Xu et al., 2011). Cells grown on the coverslip were incubated with JC-1 (10 μg/mL) at 37°C for 15 min and washed with PBS and then mounted on the microscope (Olympus IX71) equipped with an on-stage incubator (20/20 Technologies) for imaging. TRITC and FITC filter sets (Semrock) were used to detect the depolarized and repolarized mitochondria, respectively. Basal images and H2O2-elicited images were collected. Both color channels were overlaid in IPLab software (BD Biosciences) to measure the distribution of both repolarized and depolarized mitochondria in the field (Li et al., 2010).
JNK phosphorylation by western blot analysis
JNK activation was estimated by determining the level of phosphorylated JNK (p-JNK). Cells were harvested following the treatment and protein were extracted in a lysis buffer containing 20 mM Tris (pH 7.5), 137 mM NaCl, 2 mM EDTA, 10% glycerol, 10 mM Sodium pyrophosphate, 50 mM NaF, 1 mM Na3VO4, 200 μM PMSF, and 1% Triton X-100. The supernatants were resolved on a 10% SDS-Page gel and the proteins were transferred to a nitrocellulose membrane. Membranes were probedwith antibodies against p-JNK (1:2000) (Cell Signaling Technology) or α-tubulin (NeoMarkers). Tubulin is used as a loading control (1:3000). Signals were visualized using chemiluminescence reagents. Densitometry analysis was performed using NIH ImageJ 1.42.
Statistical analysis
All values were presented as means ± S.E.M. Multiple comparisons among different treatment groups were analyzed by one-way ANOVA followed by Tukey test as post hoc analysis. P < 0.05 was considered statistically significant.
RESULTS
gRb1 scavenged free radicals determined by ESR spectroscopy
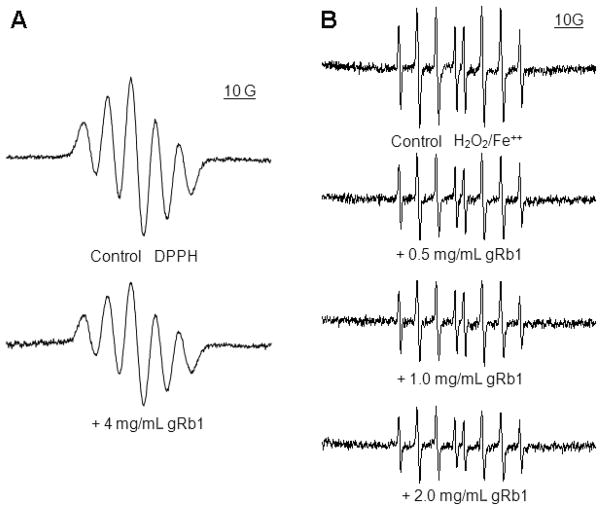
The scavenging DPPH radical activity of gRb1 was tested. The DPPH is a stable free radical donor which has been widely used to determine the free radical scavenging capacity of natural antioxidants (Rapisarda et al., 1999). As shown in Fig. 1A, the ESR signals of DPPH declined after addition of gRb1 (4 mg/mL). We also tested the effect of gRb1 on hydroxyl radical scavenging activity in a Fenton reaction system with DMPO, a spin trapping agent. The typical ESR signals of DMPO-OH adducts was concentration-dependently attenuated when addition of gRb1 0.5, 1 or 2 mg/mL (Fig. 1B). These results indicated that gRb1 possessed free radical scavenging abilities.
Fig. 1.
The scavenging free radical activity of gRb1 in a cell-free chemical system was detected by ESR spectroscopy. (A) The scavenging DPPH radical activity of gRb1. The intensity of DPPH ESR signals was decreased when addition of gRb1 (4 mg/mL). (B) The scavenging hydroxyl radical activity of gRb1. The intensity of ESR spectra of DMPO-OH adducts was attenuated in a dose-response fashion after addition of gRb1 (0.5, 1 and 2 mg/mL) in H2O2/Fe2+ chemical reaction system. Data were recorded as described in the Materials and Methods.
gRb1 attenuated the DCF fluorescence in H2O2-exposed cardiomyocytes
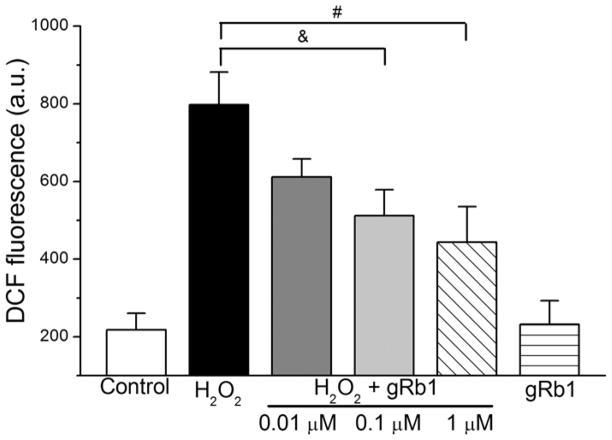
Cells were loaded with 6-carboxy-H2DCFDA and pretreated with gRb1 (0.01, 0.1 or 1 μM; n = 12 per group) for 2 h and concurrent treated with H2O2 (0.5 mM) for 2 h or exposed to H2O2 (0.5 mM) alone for 2 h. Fig. 2 showed that H2O2 exposure caused a significant increase in DCF fluorescence from 218 ± 42 a.u. to 798 ± 84 a.u. Pretreatment with gRb1 (0.01, 0.1, or 1 μM) attenuated the DCF fluorescence to 612 ± 46 a.u. (p < 0.05, n = 12), 512 ± 67 a.u. (p < 0.05, n = 12), 444 ± 91 a.u. (p < 0.01, n = 12), respectively. No significant changes in DCF fluorescence were observed in the cells treated with gRb1 (1 μM) alone compared to control cells. This result suggested that gRb1 pretreatment significantly attenuated intracellular oxidant generation induced by H2O2.
Fig. 2.
Effect of gRb1 on DCF fluorescence in H2O2-exposed cardiomyocytes. Cells were loaded with H2DCFDA (1 μM) and pretreated with gRb1 (0.01, 0.1 or 1 μM) for 2 h and co-treated with H2O2 (0.5 mM) for 2 h or exposed to H2O2 alone for 2 h. The DCF fluorescence was increased by H2O2, but attenuated by pretreatment with gRb1, &p < 0.05, #p < 0.01, vs H2O2 alone. The data are presented as mean ± S.E.M.
gRb1 decreased cell death and LDH release in H2O2-exposed cells
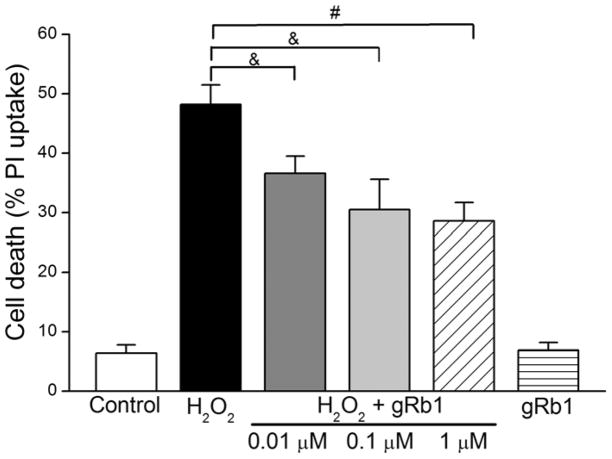
We determined whether the attenuated ROS by gRb1 was correlated with the reduced cell death. As shown in Fig. 3, H2O2 (0.5 mM) increased cell death from 6.4 ± 1.4% in control (n = 12) to 48.2 ± 3.3%. Pretreatment with gRb1 (0.01, 0.1 or 1 μM; n = 12 per group) resulted in a dose-dependent reduction of cell death to 36.6 ± 2.9% (p < 0.05, n = 12), 30.5 ± 5.1% (p < 0.05, n = 12) and 28.6 ± 3.1% (p < 0.01, n = 12), respectively. Similarly, the LDH release significantly decreased in gRb1 (0.01, 0.1 or 1 μM)/H2O2-exposed cells (p < 0.05, p < 0.01 compared to H2O2 alone cells, n = 5 per group, Table I). There was no difference between the control and gRb1 (1 μM) alone cells. These results indicated that gRb1 protected cardiomyocytes against H2O2- induced cell damage.
Fig. 3.
Effect of gRb1 on cell death in H2O2-exposed cardiomyocytes. Cells were loaded with propidium iodide (PI, 5 μM) and pretreated with gRb1 (0.01, 0.1 or 1 μM) for 2 h and co-treated with H2O2 (0.5 mM) for 2 h or exposed to H2O2 alone for 2 h. The cell death was increased by H2O2, which was concentration-dependently reduced by pretreatment with gRb1, &p < 0.05, #p < 0.01 vs H2O2 alone. The results are presented as mean ± S.E.M.
gRb1 ameliorated myocardial morphological alterations afterH2O2-exposure
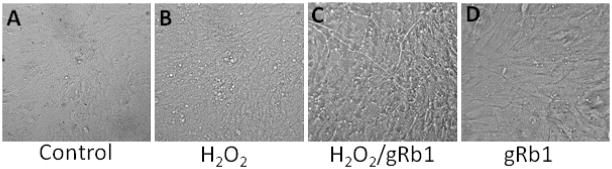
Fig. 4 shows that cell membrane blebs, granule formations and decreased membrane definition were observed in H2O2-exposed cells. Co-treatment of gRb1 (1 μM) significantly ablates these morphological changes, suggesting that gRb1 prevented oxidant-induced alterations in myocardial morphology. No morphological changes were noticed in gRb1 alone cells.
Fig. 4.
Effect of gRb1 on H2O2-induced myocardial morphological alterations. (A) Control cells displayed no blebs. (B) H2O2 (0.5 mM)-exposure resulted in myocardial morphological changes with blebs and granule formation. (C) Pretreatment of gRb1 (1 μM) improved these morphological alterations by H2O2. (D) gRb1 (1 μM) treatment alone had no obvious difference compared to control cells.
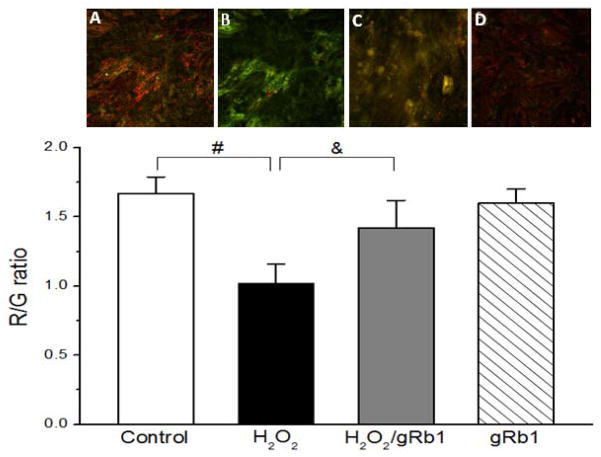
gRb1 attenuated mitochondrial membrane potential (ΔΨm) in H2O2-exposed cells
Cell death is generally associated with loss of ΔΨm and mitochondrial dysfunction (Lai et al., 2003). To test whether H2O2 disrupts ΔΨm, while the gRb1 prevents the loss of ΔΨm, the JC-1, a mitochondrial membrane potential indicator was used. JC-1 accumulates to form aggregates in the polarized mitochondria that emit red (R) fluorescence. With depolarization of ΔΨm, these aggregates dissipate to the cytosol to form monomers that emit green (G) fluorescence (i.e. indicating mitochondrial membrane potential dissipation). As seen in Fig. 5, the control cells (A) showed a polarized ΔΨm with more red and less green JC-1 fluorescence (high R/G ratio). The H2O2 (0.5 mM)-exposed cells (B) displayed a depolarized ΔΨm with high green over red fluorescence (low R/G ratio) compared with control cells (n = 5, p < 0.01). Co- treatment of gRb1 (1 μM) resulted in the changes of fluorescence from green to red and merged to orange. The R/G ratio was partially increased in gRb1/H2O2-exposed cells (C) compared with H2O2 alone cells (n = 5, p < 0.05). There were no obvious changes in treatment of gRb1 alone cells (D) compared to control cells. These results indicate that gRb1 prevented the dispassion of ΔΨm and preserved the mitochondrial function.
Fig. 5.
Effect of gRb1 on mitochondrial membrane potential (ΔΨm) in cardiomyocytes. Cells on the glass-coverslip were incubated with JC-1 (10 μg/mL) at 37°C for 15 min and then washed with PBS twice. The coverslip was mounted on the microscope with an on-stage incubator for imaging. The PLab software was used for analyzing the color changes of JC-1 fluorescence. Control cell (A) image showed the more red/less green JC-1 fluorescence. H2O2 (0.5 mM)-exposed cell (B) image showed low red/higher green JC-1 fluorescence. gRb1 (1 μM)/H2O2 (0.5 mM)-exposed cell (C) image showed the higher red/lower green JC-1 fluorescence. H2O2 decreased the G/R ratio compared with control cells (#p < 0.01). Co-treatment with gRb1 partially increased the R/G ratio compared with H2O2-exposed cells (&p < 0.05). In gRb1 (1 μM)-treated alone cell (D), the R/G ratio had no difference compared with control cells. The data are presented as mean ± S.E.M. of five individual experiments per group.
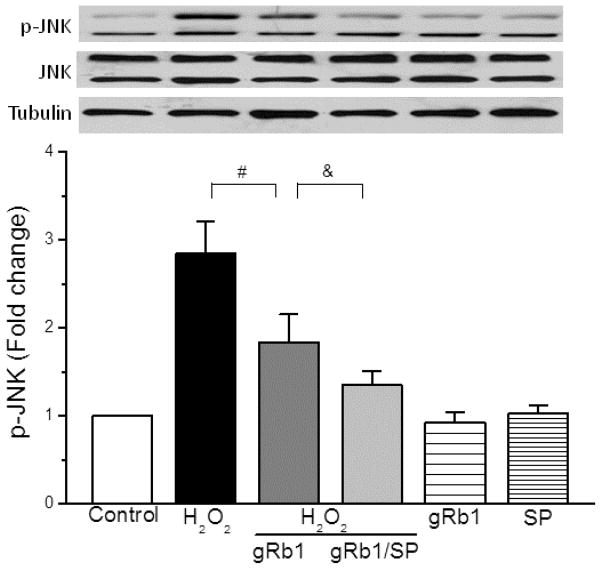
gRb1-induced protection was mediated by JNK phosphorylation
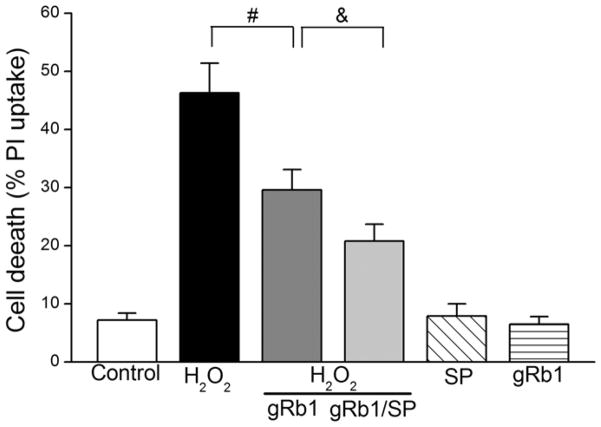
To understand the mechanism that gRb1 protects cardiomyocyte against H2O2-induced cell injury, we examined JNK phosphorylation. Fig. 6 (upper-panel) showed that the p-JNK was increased in H2O2 (0.5 mM)-exposed cells compared to control cells. Pretreatment with gRb1 (1 μM) inhibited the p-JNK and co-treatment of SP600125 (SP, 10 μM), a specific inhibitor of JNK (Bennett et al., 2001), further reduced p-JNK. The SP or gRb1 alone treatment had no effect on JNK phosphorylation. Densitometric analysis (lower-panel) illustrated that the increased p-JNK by H2O2 was attenuated by gRb1 (p < 0.01, n = 5) and co-treatment of SP further attenuated JNK phosphorylation. There were no considerable differences among the control, gRb1 and SP-treated alone cells. Correspondingly, gRb1 reduced H2O2-induced cell death (46.3 ± 5.1% vs 29.6 ± 3.5%, p < 0.01, n = 5) and co-treatment with SP further reduced cell death compared with gRb1/H2O2-exposed cells (29.6 ± 3.5% vs 20.8 ± 2.9%, p < 0.05, n = 8) (Fig. 7). These results suggest that H2O2-induced cell death is mediated by JNK phosphorylation and gRb1 protection exerted through inhibition of JNK activation.
Fig. 6.
Effect of gRb1 on phosphorylation of JNK in H2O2-exposed cardiomyocytes. The cells were pretreated with gRb1 (1 μM) or gRb1/SP600125 (SP, 10 μM) for 2 h prior to the H2O2 and concurrent treated with H2O2 (0.5 mM) for 2 h. The phosphorylation of JNK (p-JNK) was analyzed by western blots. Upper panel: Representative immunoblots of p-JNK in cardiomyocytes. The gRb1 attenuated the increased p-JNK caused by H2O2 (#p < 0.01) that was further attenuated by a JNK inhibitor SP (10 μM), &p < 0.05 vs gRb1/H2O2-treated cells. gRb1 and SP treatment alone had no effect on p-JNK. Lower panel: Densitometry showing the mean ± S.E.M. of four experiments for each condition. α-tubulin: a loading control.
Fig. 7.
Effect of inhibition of JNK phosphorylation on gRb1-cardioprotection. Cells were pretreated with gRb1 (1 μM) or gRb1/SP600125 (SP, 10 μM) for 2 h prior to the H2O2 and concurrent treated with gRb1/SP/H2O2 (0.5 mM) or H2O2 alone for 2 h. The cell death was reduced by pretreatment of gRb1, #p < 0.01 vs H2O2 alone. Co-treatment with SP/gRb1/H2O2 further reduced the cell death, &p < 0.05 vs gRb1/H2O2-treated cells.
DISCUSSION
The present study demonstrates that gRb1 protects cardiomyocytes against H2O2 -induced oxidative injury as evidenced by attenuated ROS generation, preserved mitochondrial membrane potential, decreased LDH release and improved cell survival. These findings further support our previous studies that American ginseng berry extract and ginsenoside Re exhibits antioxidative effects in cardiomyocytes (Shao et al., 2004; Xie et al., 2006) and confirms that ginsenoside Rb1 is a major antioxidant component with antioxidant capacity in the ginseng root (Park et al., 2005; Wang et al., 2008b). In addition, this work suggestes that cardioprotection by gRb1is mediated by the suppression of H2O2-induced JNK activation. Although several intracellular signaling pathways involved in the protective effect of gRb1 have been reported (Zhang et al., 2006; Wang et al., 2008a), this study is the one of the first to demonstrate that gRb1 can protect against the H2O2-induced cell death through the inhibition of JNK activation in cardiomyocytes.
Living cardiomyocytes can produce two major ROS, superoxide (O2·−) and H2O2. Although H2O2 is a non-radical molecule with limited reactivity, it can decompose to generate the highly reactive hydroxyl radicals (OH·) via the Fenton reaction leading to cell damage (Fenton, 1984; Moriue et al., 2008). Moreover, H2O2 can readily traverse membranes reacting with and damaging vital cellular macromolecules, such as proteins, lipids and DNA, thereby disrupting cellular function and integrity (Barbouti et al., 2002). We have previously shown that American ginseng berry extract and ginsenoside Re, a similar compound to Ginsenoside Rb1, attenuated the H2O2-induced oxidant stress in our cardiomyocyte model (Xie et al., 2006). Suppression of ROS by antioxidants might be an effective strategy aimed at preventing oxidative stress-induced cell death. Therefore, the use of antioxidant agents as a way of cardioprotection could be a potential therapy to slow or ameliorate the progress of cardiovascular diseases (Wang et al., 2007). Ginseng, the root of Panax ginseng C.A. Meyer, is one of the most commonly used herbal medicines possessing a wide spectrum of biological and pharmacological activities (Scott et al., 2001; Wang et al., 2011b) that have been attributed to its active ingredients, ginsenosides. Our ESR study showed that gRb1 possesses scavenging ROS properties that may partially contribute to its cardioprotection against H2O2-induced oxidative stress.
Oxidative stress may activate multiple cellular signaling pathways (Kannan and Jain, 2000). Prior studies have shown that gRb1 scavenges ROS, however, little work has been done to test whether gRb1 also affects ROS-mediated death signaling pathways, particularly JNK. The c-Jun N-terminal kinase JNK, or stress-activated protein kinase forms an important subgroup of the mitogen-activated protein kinase (MAPK) superfamily. JNKs are primarily activated by various environmental stresses, including ROS and oxidative stress, UV radiation, and chemotherapeutic agents (Mittler and Berkowitz, 2001; Chan et al., 2003). Of these, ROS and oxidative stress appear to be particularly important (Matsukawa et al., 2004). A body of evidence shows that JNK signaling promotes apoptosis via a mitochondrial-dependent pathway (Lei et al., 2002). Consistent with work by others (Sato et al., 2001), we found that gRb1 pretreatment significantly inhibited the increased JNK phosphorylation induced by H2O2. Our data also showed that the inhibition of phosphorylation of JNK by a specific JNK inhibitor SP600125 further attenuated JNK activation and decreased cell death in gRb1/H2O2-treated cells, suggesting that the JNK signaling pathway mediated gRb1-cardioprotection. These results support a notion that gRb1 not only acts as a ROS scavenger, but significantly attenuates ROS-mediated JNK activation- induced by H2O2 in cardiomyocytes.
Normal mitochondrial electrochemical gradients or intact mitochondrial membrane potential (ΔΨm) is essential for the survival of cardiomyocytes during and after oxidant insult (Honda et al., 2005). Depolarized mitochondrial membrane potential defines mitochondrial dysfunction (Hwang and Li, 1993). Oxidative stress by H2O2 depolarized mitochondrial membrane potential results in cell death. We previously demonstrated that mitochondrial oxidant injury is a significant source of cell death in this cardiomyocyte ischemia/reperfusion model and antioxidants effectively improved cell survival (Vanden Hoek et al., 1996, 2000; Shao et al., 2002). The present study also showed that gRb1 prevented the dissipation of mitochondrial membrane potential and improved the mitochondrial function during lethal oxidant stress, suggesting that gRb1 may play a critical role in attenuating mitochondrial ROS and preserving mitochondrial function. ROS-induced mitochondrial dysfunction has been shown to connect to the redox pathways. A body of evidence has demonstrated that JNK can regulate mitochondrial function by induction of cytochrome c release from mitochondria (Aoki et al., 2002) and modulation of Bax and Bcl-2 (Kharbanda et al., 2000; Aoki et al., 2002). Our data showed that gRb1 inhibited JNK phosphorylation and prevented the disrupted mitochondrial membrane potential and improved mitochondrial function during H2O2-induced oxidant stress, suggesting that gRb1 may play an important role in attenuating mitochondrial ROS and preserving mitochondrial function.
In conclusion, this study showed that gRb1 with its free radical scavenging property attenuated mitochondrial ROS generation protecting cardiomyocytes from H2O2-induced oxidative injury. A probable underlying mechanism of the gRb1-cardioprotection may be mediated by suppression of ROS-induced JNK activation. As a major component of ginseng, ginsenoside Rb1 may be a promising candidate for the prevention and treatment of cardiovascular diseases associated with oxidative stress.
Table 1.
Effect of gRb1 on H2O2-induced lactate dehydrogenase (LDH) release
| Treatment | LDH release (%) |
|---|---|
| Control | 3.7 ± 0.3 |
| H2O2 | 20.8 ± 1.5 |
| H2O2 + gRb1 (0.01 μM) | 15.2 ± 1.1 & |
| H2O2 + gRb1 (0.1 μM) | 13.9 ± 0.5 # |
| H2O2 + gRb1 (1 μM) | 12.9 ± 0.6 # |
| gRb1 (1 μM) | 3.2 ± 0.5 |
The LDH activity was measured using LDH cytotoxicity assay kit. LDH release increased after H2O2 exposure for 2 h and was decreased by pretreatment with gRb1 (0.01, 0.1 and 1 μM).
P < 0.05, H2O2 (0.5 mM) + gRb1 (0.01 μM) vs. H2O2 (0.5 mM);
P < 0.01, H2O2 (0.5 mM) + gRb1 (0.1 μM, 1 μM) vs. H2O2 (0.5 mM); P = NS, gRb1 (1 μM) alone vs. control. Results are expressed as mean ± SEM.
Acknowledgments
This work was supported in part by the NIH/NCCAM grants AT003441, AT004418 and AT005362.
References
- Aoki H, Kang PM, Hampe J, Yoshimura K, Noma T, Matsuzaki M, Izumo S. Direct activation of mitochondrial apoptosis machinery by c-Jun N-terminal kinase in adult cardiac myocytes. J Biol Chem. 2002;277:10244–10250. doi: 10.1074/jbc.M112355200. [DOI] [PubMed] [Google Scholar]
- Barbouti A, Doulias PT, Nousis L, Tenopoulou M, Galaris D. DNA damage and apoptosis in hydrogen peroxide-exposed Jurkat cells: bolus addition versus continuous generation of H(2)O(2) Free Radic Biol Med. 2002;33:691–702. doi: 10.1016/s0891-5849(02)00967-x. [DOI] [PubMed] [Google Scholar]
- Bennett BL, Sasaki DT, Murray BW, O’Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolli R. Preconditioning: a paradigm shift in the biology of myocardial ischemia. Am J Physiol Heart Circ Physiol. 2007;292:H19–H27. doi: 10.1152/ajpheart.00712.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WH, Wu CC, Yu JS. Curcumin inhibits UV irradiation-induced oxidative stress and apoptotic biochemical changes in human epidermoid carcinoma A431 cells. J Cell Biochem. 2003;90:327–338. doi: 10.1002/jcb.10638. [DOI] [PubMed] [Google Scholar]
- Chang WT, Shao ZH, Yin JJ, Mehendale S, Wang CZ, Qin Y, Li J, Chen WJ, Chien CT, Becker LB, Vanden Hoek TL, Yuan CS. Comparative effects of flavonoids on oxidant scavenging and ischemia-reperfusion injury in cardiomyocytes. Eur J Pharmacol. 2007;566:58–66. doi: 10.1016/j.ejphar.2007.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Otani H, Maulik N, Das DK. Redox regulation of angiotensin II preconditioning of the myocardium requires MAP kinase signaling. J Mol Cell Cardiol. 2006;41:248–255. doi: 10.1016/j.yjmcc.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Fenton HJH. Oxidation of tartaric acid in the presence of iron. J Chem Soc. 1984;65:899–910. [Google Scholar]
- Fujita K, Hakuba N, Hata R, Morizane I, Yoshida T, Shudou M, Sakanaka M, Gyo K. Ginsenoside Rb1 protects against damage to the spiral ganglion cells after cochlear ischemia. Neurosci Lett. 2007;415:113–117. doi: 10.1016/j.neulet.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Guan L, Li W, Liu Z. Effect of ginsenoside-Rb1 on cardiomyocyte apoptosis after ischemia and reperfusion in rats. J Huazhong Univ Sci Technolog Med Sci. 2002;22:212–215. doi: 10.1007/BF02828182. [DOI] [PubMed] [Google Scholar]
- Honda HM, Korge P, Weiss JN. Mitochondria and ischemia/reperfusion injury. Ann N Y Acad Sci. 2005;1047:248–258. doi: 10.1196/annals.1341.022. [DOI] [PubMed] [Google Scholar]
- Hwang GY, Li JK. Identification and localization of a serotypic neutralization determinant on the VP2 protein of bluetongue virus 13. Virology. 1993;195:859–862. doi: 10.1006/viro.1993.1445. [DOI] [PubMed] [Google Scholar]
- Jiang QS, Huang XN, Dai ZK, Yang GZ, Zhou QX, Shi JS, Wu Q. Inhibitory effect of ginsenoside Rb1 on cardiac hypertrophy induced by monocrotaline in rat. J Ethnopharmacol. 2007;111:567–572. doi: 10.1016/j.jep.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Kaiser RA, Liang Q, Bueno O, Huang Y, Lackey T, Klevitsky R, Hewett TE, Molkentin JD. Genetic inhibition or activation of JNK1/2 protects the myocardium from ischemia-reperfusion-induced cell death in vivo. . J Biol Chem. 2005;280:32602–32608. doi: 10.1074/jbc.M500684200. [DOI] [PubMed] [Google Scholar]
- Kannan K, Jain SK. Oxidative stress and apoptosis. Pathophysiology. 2000;7:153–163. doi: 10.1016/s0928-4680(00)00053-5. [DOI] [PubMed] [Google Scholar]
- Kharbanda S, Saxena S, Yoshida K, Pandey P, Kaneki M, Wang Q, Cheng K, Chen YN, Campbell A, Sudha T, Yuan ZM, Narula J, Weichselbaum R, Nalin C, Kufe D. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-x(L) in response to DNA damage. J Biol Chem. 2000;275:322–327. doi: 10.1074/jbc.275.1.322. [DOI] [PubMed] [Google Scholar]
- Lai HC, Liu TJ, Ting CT, Sharma PM, Wang PH. Insulin-like growth factor-1 prevents loss of electrochemical gradient in cardiac muscle mitochondria via activation of PI 3 kinase/Akt pathway. Mol Cell Endocrinol. 2003;205:99–106. doi: 10.1016/s0303-7207(03)00200-4. [DOI] [PubMed] [Google Scholar]
- Lei K, Nimnual A, Zong WX, Kennedy NJ, Flavell RA, Thompson CB, Bar-Sagi D, Davis RJ. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH(2)-terminal kinase. Mol Cell Biol. 2002;22:4929–4942. doi: 10.1128/MCB.22.13.4929-4942.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Liu H, Ramachandran S, Waypa GB, Yin JJ, Li CQ, Han M, Huang HH, Sillard WW, Vanden Hoek TL, Shao ZH. Grape seed proanthocyanidins ameliorate Doxorubicin-induced cardiotoxicity. Am J Chin Med. 2010;38:569–584. doi: 10.1142/S0192415X10008068. [DOI] [PubMed] [Google Scholar]
- Lim JH, Wen TC, Matsuda S, Tanaka J, Maeda N, Peng H, Aburaya J, Ishihara K, Sakanaka M. Protection of ischemic hippocampal neurons by ginsenoside Rb1, a main ingredient of ginseng root. Neurosci Res. 1997;28:191–200. doi: 10.1016/s0168-0102(97)00041-2. [DOI] [PubMed] [Google Scholar]
- Matsukawa J, Matsuzawa A, Takeda K, Ichijo H. The ASK1-MAP kinase cascades in mammalian stress response. J Biochem. 2004;136:261–265. doi: 10.1093/jb/mvh134. [DOI] [PubMed] [Google Scholar]
- Mittler R, Berkowitz G. Hydrogen peroxide, a messenger with too many roles? Redox Rep. 2001;6:69–72. doi: 10.1179/135100001101536067. [DOI] [PubMed] [Google Scholar]
- Moriue T, Igarashi J, Yoneda K, Nakai K, Kosaka H, Kubota Y. Sphingosine 1-phosphate attenuates H2O2-induced apoptosis in endothelial cells. Biochem Biophys Res Commun. 2008;368:852–857. doi: 10.1016/j.bbrc.2008.01.155. [DOI] [PubMed] [Google Scholar]
- Park JK, Namgung U, Lee CJ, Park JO, Jin SH, Kwon OB, Ko SR, Kim SW, Kang EJ, Ko JH, Lee SM, Kim DH, Won MH. Calcium-independent CaMKII activity is involved in ginsenoside Rb1-mediated neuronal recovery after hypoxic damage. Life Sci. 2005;76:1013–1025. doi: 10.1016/j.lfs.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Penna C, Rastaldo R, Mancardi D, Raimondo S, Cappello S, Gattullo D, Losano G, Pagliaro P. Post-conditioning induced cardioprotection requires signaling through a redox-sensitive mechanism, mitochondrial ATP-sensitive K+ channel and protein kinase C activation. Basic Res Cardiol. 2006;101:180–189. doi: 10.1007/s00395-006-0584-5. [DOI] [PubMed] [Google Scholar]
- Rapisarda P, Tomaino A, Lo Cascio R, Bonina F, De Pasquale A, Saija A. Antioxidant effectiveness as influenced by phenolic content of fresh orange juices. J Agric Food Chem. 1999;47:4718–4723. doi: 10.1021/jf990111l. [DOI] [PubMed] [Google Scholar]
- Sato M, Bagchi D, Tosaki A, Das DK. Grape seed proanthocyanidin reduces cardiomyocyte apoptosis by inhibiting ischemia/reperfusion-induced activation of JNK-1 and C-JUN. Free Radic Biol Med. 2001;31:729–737. doi: 10.1016/s0891-5849(01)00626-8. [DOI] [PubMed] [Google Scholar]
- Scott GI, Colligan PB, Ren BH, Ren J. Ginsenosides Rb1 and Re decrease cardiac contraction in adult rat ventricular myocytes: role of nitric oxide. Br J Pharmacol. 2001;134:1159–1165. doi: 10.1038/sj.bjp.0704377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao ZH, Vanden Hoek TL, Qin Y, Becker LB, Schumacker PT, Li CQ, Dey L, Barth E, Halpern H, Rosen GM, Yuan CS. Baicalein attenuates oxidant stress in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2002;282:H999–H1006. doi: 10.1152/ajpheart.00163.2001. [DOI] [PubMed] [Google Scholar]
- Shao ZH, Xie JT, Vanden Hoek TL, Mehendale S, Aung H, Li CQ, Qin Y, Schumacker PT, Becker LB, Yuan CS. Antioxidant effects of American ginseng berry extract in cardiomyocytes exposed to acute oxidant stress. Biochim Biophys Acta. 2004;1670:165–171. doi: 10.1016/j.bbagen.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Taira S, Ikeda R, Yokota N, Osaka I, Sakamoto M, Kato M, Sahashi Y. Mass spectrometric imaging of ginsenosides localization in Panax ginseng root. Am J Chin Med. 2010;38:485–493. doi: 10.1142/S0192415X10008007. [DOI] [PubMed] [Google Scholar]
- Vanden Hoek T, Becker LB, Shao ZH, Li CQ, Schumacker PT. Preconditioning in cardiomyocytes protects by attenuating oxidant stress at reperfusion. Circ Res. 2000;86:541–548. doi: 10.1161/01.res.86.5.541. [DOI] [PubMed] [Google Scholar]
- Vanden Hoek TL, Shao Z, Li C, Zak R, Schumacker PT, Becker LB. Reperfusion injury on cardiac myocytes after simulated ischemia. Am J Physiol. 1996;270:H1334–1341. doi: 10.1152/ajpheart.1996.270.4.H1334. [DOI] [PubMed] [Google Scholar]
- Vanden Hoek TL, Shao Z, Li C, Schumacker PT, Becker LB. Mitochondrial electron transport can become a significant source of oxidative injury in cardiomyocytes. J Mol Cell Cardiol. 1997;29:2441–2450. doi: 10.1006/jmcc.1997.0481. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Mehendale SR, Yuan CS. Commonly used antioxidant botanicals: active constituents and their potential role in cardiovascular illness. Am J Chin Med. 2007;35:543–558. doi: 10.1142/S0192415X07005053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Kim KE, Du GJ, Qi LW, Wen XD, Li P, Bauer BA, Bissonnette MB, Musch MW, Chang EB, Yuan CS. Ultra-performance liquid chromatography and time-of-flight mass spectrometry analysis of ginsenoside metabolites in human plasma. Am J Chin Med. 2011a;39:1161–1171. doi: 10.1142/S0192415X11009470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Qi LW, Wang CZ, Li P. Bioactivity enhancement of herbal supplements by intestinal microbiota focusing on ginsenosides. Am J Chin Med. 2011b;39:1103–1115. doi: 10.1142/S0192415X11009433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Qiao L, Li Y, Yang G. Ginsenoside Rb1 attenuates intestinal ischemia-reperfusion- induced liver injury by inhibiting NF-kappaB activation. Exp Mol Med. 2008a;40:686–698. doi: 10.3858/emm.2008.40.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Li M, Wu WK, Tan HM, Geng DF. Ginsenoside Rb1 preconditioning protects against myocardial infarction after regional ischemia and reperfusion by activation of phosphatidylinositol-3-kinase signal transduction. Cardiovasc Drugs Ther. 2008b;22:443–452. doi: 10.1007/s10557-008-6129-4. [DOI] [PubMed] [Google Scholar]
- Wrona M, Patel K, Wardman P. Reactivity of 2′,7′-dichlorodihydrofluorescein and dihydrorhodamine 123 and their oxidized forms toward carbonate, nitrogen dioxide, and hydroxyl radicals. Free Radic Biol Med. 2005;38:262–270. doi: 10.1016/j.freeradbiomed.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Xie JT, Shao ZH, Vanden Hoek TL, Chang WT, Li J, Mehendale S, Wang CZ, Hsu CW, Becker LB, Yin JJ, Yuan CS. Antioxidant effects of ginsenoside Re in cardiomyocytes. Eur J Pharmacol. 2006;532:201–207. doi: 10.1016/j.ejphar.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Xu Z, Chen X, Zhang Q, Chen L, Wang Y. Corydalis yanhusuo W.T. Wang extract inhibits MCF-7 cell proliferation by inducing cell cycle G2/M arrest. Am J Chin Med. 2011;39:579–586. doi: 10.1142/S0192415X11009044. [DOI] [PubMed] [Google Scholar]
- Zhang B, Hata R, Zhu P, Sato K, Wen TC, Yang L, Fujita H, Mitsuda N, Tanaka J, Samukawa K, Maeda N, Sakanaka M. Prevention of ischemic neuronal death by intravenous infusion of a ginseng saponin, ginsenoside Rb(1), that upregulates Bcl-x(L) expression. J Cereb Blood Flow Metab. 2006;26:708–721. doi: 10.1038/sj.jcbfm.9600225. [DOI] [PubMed] [Google Scholar]
- Zhao H, Joseph J, Fales HM, Sokoloski EA, Levine RL, Vasquez-Vivar J, Kalyanaraman B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc Natl Acad Sci U S A. 2005;102:5727–5732. doi: 10.1073/pnas.0501719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Fan GC, Zhang ZG, Bandyopadhyay A, Zhou X, Kranias EG. Protection of peroxiredoxin II on oxidative stress-induced cardiomyocyte death and apoptosis. Basic Res Cardiol. 2009;104:377–389. doi: 10.1007/s00395-008-0764-6. [DOI] [PMC free article] [PubMed] [Google Scholar]