Abstract
Neuroendocrine correlates of chronic stress in human infants have not been established. The goal of the present study was to create an animal model of continuous chronic stress using the immature rat to measure basal plasma corticosterone, and secretion of plasma corticosterone in response to an acute stress. This was achieved by modulation of the cage environment for rat pups and their mothers. During postnatal days 2–9, pups were maintained in three groups: (1) handled, (2) not handled and with ample bedding; and (3) not handled with limited bedding. On postnatal day 9, some pups from each group were subjected to acute cold-separation stress and were killed 90, 240, or 360 min later along with unstressed controls. The group not handled and with limited bedding manifested increased plasma corticosterone output even without cold exposure and a sustained increase of plasma corticosterone after cold-separation stress. Plasma corticosterone interanimal variability was increased and body weight was decreased in these pups, typical of a state of chronic stress. The first model of continuous stress in infant rats in which upregulation of hypothalamic-pituitary-adrenal axis is achieved without maternal separation is presented. This paradigm may more closely approximate the human situation of chronically stressed, neglected infants.
Introduction
Exaggerated activity of the hypothalamic-pituitary-adrenal axis (HPA) is a neurobiologic correlate of adult depression and anxiety [1,2]. Depression, abnormal attachments and poor growth are well established consequences of chronic neglect occurring in infancy [3–7]. Little is known, however, about the ontogeny and function of the neuroendocrine system of neglected infants and its contribution to abnormal development, growth failure, or depression [1,2,5,8]. No animal models simulate the highly stressful human condition of early life neglect. Development of such a model should provide a critical tool to study the neuroendocrine changes induced by severe, continuous, early life stress.
The rat infant HPA shares many molecular and biological characteristics with the human HPA, and a body of information is available regarding the development of the stress response and HPA regulation in rat pups [9–13]. Hormonal stress responses in the infant rat differ from those of the adult, particularly in the magnitude of response to specific stimuli [14]. Maternal separation and daily handling in the rat are two paradigms known to alter the development of the stress response and the responses to acute stressors experienced as adults [15–19]; e.g., extended periods of as much as 24 hours of maternal separation after 7 days of age increases plasma corticosterone (CORT) response to novelty [12,16,18,19]. Daily handling of the preweanling rat for even short periods (15 min) results in an HPA system that is hypo responsive to acute stressors and remains so for the duration of the animal’s life [14,15].
Cold-separation is a potent age-specific stressor for infant rat pups. Unlike the adult rat, the infant rat has little fur and immature thermoregulation [11,13,20]. Acute cold-separation stress in previously unstressed 9-day-old pups produces a robust increase in plasma CORT, peaking at 60–90 min, with a subsequent return toward baseline by 2–3 hours [13].
The goal of the present study was to create a model of continuous stress in infant rats and evaluate its effect on plasma CORT, both under basal conditions and in response to an acute age-specific stressor, cold-separation. The continuous stress paradigm consisted of limiting bedding and of not handling pups or mothers during postnatal days 2–9. Bedding type and volume are important components of the dams’ nesting environment, and severely limiting the amount of available bedding constitutes a continuous stressor for the dam and her pups. This novel paradigm was examined in comparison to the established models of handling and nonhandling [17].
Materials and Methods
Animals and Experimental Groups
Animal Studies were approved by the Childrens Hospital Los Angeles institutional animal care and use committee under National Institutes of Health (NIH) guidelines. One hundred thirty-six infant rats were studied. They were born in our NIH-approved facility to timed-pregnancy Sprague-Dawley-derived rats (Zivic-Miller, Zelienople, PA), All cages were kept in a single, quiet, uncrowded room maintained at 20°–22°C with laminar air flow. Except for experimental stressors (altered bedding or handling), rats were maintained under stress-free conditions, with a 12-h light/12-h dark cycle (lights on at 0700 h), and with unlimited access to lab chow and water.
Delivery was verified at 12-hour intervals, and the day of birth was considered day 0. Pups of both sexes from all litters were mixed and culled to 12 pups per litter on day 2. Three experimental bedding and handling groups were defined.
Handled pups
Pups were removed from bedded cages daily from postnatal day 2 to 9 and placed on a warming pad for 15 min as a group. The warming pad was used to prevent a decrease in body temperature. The pups were then returned to the home cage. After handling on postnatal day 8, pups were left undisturbed until the morning of day 9. Bedding for both this group and the nonhandled group consisted of Sani-Chips from Vermont hardwoods. Bedding volume was about 0.33 cubic feet per cage for both handled and nonhandled litters.
Nonhandled pups
Pups were left completely undisturbed with mothers in bedded cages from postnatal day 2 to 9. Bedding in cages containing nonhandled animals was not changed, because changing the bedding constitutes a handling procedure [15,21,22].
No handling and no bedding (NHNB)
On postnatal day 2, pups were placed with mothers in cages with a small-gauge wire mesh bottom (to allow passage of excreta) raised 2.5 cm from the cage floor. The only bedding material consisted of one to two paper towels, approximately 0.09 cubic feet. The towels were shredded by dams to provide a nest area. Paper towels were not replaced because dams uniformly kept their nest area free of excreta. Cages were left completely undisturbed until postnatal day 9.
Cold-separation Stress and Time Course of Plasma CORT
On postnatal day 9, pups from each experimental group were either kept under stress-free conditions until they were killed or were subjected to an acute cold-separation stress. Pups exposed to cold-separation were separated from their mothers, weighed as a group, and placed in individual compartments in a cold room (4°C). Each pup was exposed to cold for the maximal time it could tolerate (30–40 min). which was defined by the development of rigor and no movement on stimulation, and was associated with an average core temperature of 9.8°C [13], Although the time to development of rigor was weight-dependent, actual core temperature was weight-independent [13]. Cold-stressed pups were rewarmed as a group on a warming pad. They were kept normothermic until they were killed.
Separate groups of pups were decapitated before the onset of this cold stress or 90, 240, or (in nonhandled and NHNB groups), 360 min after its termination, and trunk blood was collected. The choice of timepoints was based on previous data indicating that peak CORT occurs between 60 and 90 min [13]. Four to 10 pups were cold-exposed per timepoint from each of the three experimental groups (mean 6.6 ± 0.9 pups per group).
For each treatment group, the remaining pups that were not cold-exposed were left in home cages with their dams in the same quiet room in which they had been reared. At times concurrent with the killing of cold-exposed pups, groups of 4 to 18 “control” pups (mean 7.5 ± 1.3 pups per group per timepoint) were rapidly removed from home cages with minimal disturbance to remaining pups or their dams, taken to another room and killed within 45 s of disturbance.
All experiments were started between 0730 and 0900 h to limit the effects of diurnal variability on basal and stress-induced plasma CORT [11,23]. Plasma CORT levels were determined by radioimmunoassay (ICN. Irvine, CA) as previously described [24]. Assay sensitivity was 0.05 ng/ml; interassay variability was determined by two dilutions of adult rat plasma and averaged 15% The term “basal” plasma CORT describes levels of animals that were not cold-exposed and were killed within 45 seconds of disturbance.
Statistical Analysis
Groups were compared by the non parametric Mann-Whitney U test. All data are mean ± SEM except when indicated otherwise. Variance was measured as the sum of squared deviations of observations from their mean.
Results
Basal Plasma CORT
Plasma CORT of NHNB pups not exposed to cold was 2.21 ± 0.39 µg/dl at 0730 versus 1.98 ± 0.22 and 1.97 ± 0.20 µg/dl for nonhandled and handled groups, respectively (Fig 1a). At the 240-min timepoint, mean plasma CORT of cold-exposed NHNB animals (3.49 ± 0.67) was significantly higher than that of nonhandled animals (1.67 ± 0.36) (P < 0.05), and handled animals (1.06 ± 0.16) (P < 0.01). Total plasma CORT output in 240 min, determined as the integrated area under the curve (AUC), was greater in the NHNB group in comparison with nonhandled and handled groups (784.5 vs. 552.1 and 350.6 µg): 142% that of the nonhandled group and 224% that of the handled group (Fig 1b).
Figure 1.
(a) Plasma corticosterone (CORT) of the Three experimental groups under basal (undisturbed, and without cold stress) conditions. NHNB, nonhandled with no access to bedding. Values are mean ± SEM. The handled group was not examined at 360 min. (b) Integrated area under the curve representative of total CORT output over time. *P < 0.05 for (1) nonhandled vs. NHNB at 240 min, (2) handled time 0 vs. handled at 240 min; and (3) NHNB time 0 vs. NHNB at 240 min. **P < 0.01 for handled vs. NHNB at 240 min.
Plasma CORT Induction by Acute Stress
Plasma CORT response to cold-separation stress differed among the three experimental groups. In both handled and nonhandled groups, cold induced a robust, well-defined increase in plasma CORT by 90 minutes after cold exposure (Fig 2a and b). In the handled group, plasma CORT levels subsequently decreased slightly, from 3.82 ± 0.58 at 90 min to 3.46 ± 0.39 at 240 min (Fig 2a), whereas plasma CORT in the nonhandled group increased slightly, from 3.89 ± 0.43 at 90 min to 4.06 ± 0.53 at 240 min (Fig 2b). Neither of these changes achieved statistical significance. The increased plasma CORT persisted in nonhandled pups at 360 min after stress termination.
Figure 2.
(a, b. and c) Cold-separation stress-induced plasma corticosterone (CORT) values as compared with those in nonstressed “controls” in handled animals (a), nonhandled animals (b), and (c) chronically stressed (nonhandled with no access to bedding, NHNB) experimental groups. Controls: no exposure to cold. (Scale is increased in 2c.) The magnitude of the stress response between groups attained significance only for the NHNB group versus the nonhandled group at 360 min (P < 0.05). *P < 0.05. **P < 0.01.
In contrast, plasma CORT of NHNB pups was not significantly different from baseline by 90 min after cold-stress termination (2.69 ± 0.49 vs. 2.71 ± 0.64). By 240 min after cold-stress termination, increase in plasma CORT in NHNB was observed (4.25 ± 1.94), with large variability among animals. At 360 min after cold-stress termination, a very significant increase in plasma CORT was evident in the NHNB group as compared with the 360-min NHNB nonstressed controls (8.42 ± 1.16 vs. 2.07 ± 0.47, P < 0.01) (Fig 2c). The plasma CORT value in NHNB at 360 min after cold stress was also significantly higher than that of the nonhandled cold-stressed group (8.42 ± 1.16 vs. 4.33 ± 0.76, P < 0.05).
Within-Group Individual Variability in Plasma CORT
Variance of plasma CORT among animals in a group depended on the experimental paradigm. Handling resulted in the least variance in plasma CORT levels, between pups. Chronically stressed (NHNB) pups had the greatest individual variation in plasma CORT, whether or not they were cold exposed (Fig 3).
Figure 3.
Interanimal variability of plasma corticosterone (CORT) in the three experimental groups: Basal (no cold-separation stress) state (a), after cold-separation stress (b).
Weight Differences
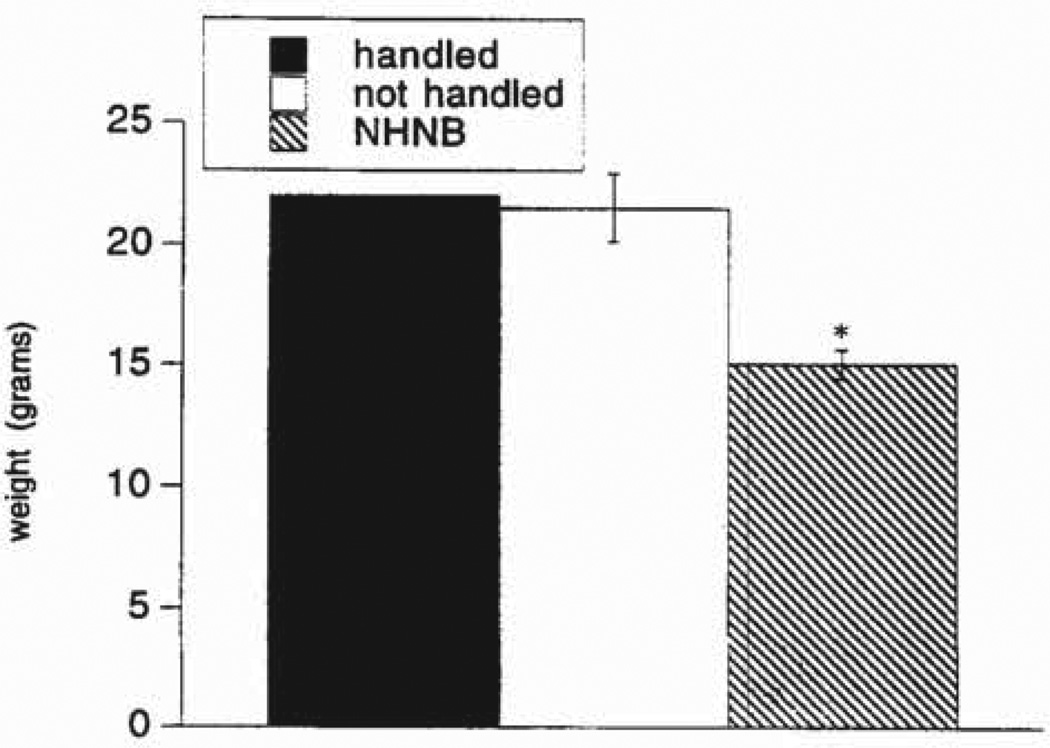
Mean weight of chronically stressed (NHNB) pups was 15.1 ± 0.6 gr. The mean weight of the nonhandled rat pups (21.5 ± 1.41 gr) was significantly different (P < 0.05). The mean pup weight of the single weighed group of handled animals was 22.0 (Fig 4).
Figure 4.
Group weights of handled, nonhandled, and chronically stressed pups. A single group of handled pups was weighed; therefore, no SEM is shown. *P < 0.05.
Behavioral Alterations
The present study was not designed to evaluate behavior. However, NHNB dams were far more restless and aggressive than those of the other two groups. Nesting behaviors were not obviously altered, as all dams were observed to make maximal use of available paper towels for nesting.
Discussion
Early life stresses in infants and young children, such as chronic neglect and abuse, are associated with long-term effects on intellectual development, motor skills, and socialization [4,25,26]. Rigorous studies of the neuroendocrine systems of such children have not been performed. The central nervous system mechanisms coordinating the hormonal stress response are complex. Both limbic and hypothalamic neurotransmission, mediated by corticotropin-releasing hormone (CRH), regulate peripheral hormone output, i.e., adrenocorticotropin hormone (ACTH) and CORT.
Animal models provide a critical tool for delineating the effects of chronic stress on the neuroendocrine system regulating the stress response. This neuroendocrine system in infant rats is similar to that of humans; e.g., CRH is identical in both species. The stress response of infant rats is age dependent [11,13,16,27]. Even during the first postnatal week, the HPA system of infant rats is fully capable of responding to specific stressors, such as maternal separation and cold [11,13,19.28]. The first two weeks of postnatal life in the infant rat are a critical period, during which HPA axis components involved in the response to stress are susceptible to both short and long term modulation [15,29].
Maternal separation and handling are two paradigms known to modulate the development of the stress response. By 7 days of life, the developing rat HPA exhibits several effects of maternal separation. Maternal deprivation for 2 hours or more on postnatal day 7 increases CORT secretion by pups during the deprivation period. Deprived pups also release more CORT in response to exogenous ACTH [18]. The longer the separation, the greater the magnitude of this response [30].
Handling of the rat pup alters HPA responsiveness in adulthood. These adult rats secrete less ACTH and CORT after a variety of stressors. Plasma CORT levels return to baseline faster than those of nonhandled animals after discontinuation of the stressor, presumably related to alterations in the negative feedback system [14,18].
Neither brief daily handling nor a single episode of maternal separation approximates the human situation of the chronic severe stress of neglect. The goal of the current model was to avoid separation of the infant and mother yet create a continuously stressful environment. Furthermore, animal models of acute stress must be age specific. Cold-separation is a powerful acute stressor in infant rats because of rapidly induced thermal loss. Moreover, it has been fully defined and is an easily reproducible stress paradigm in the infant [11,13].
Our study demonstrates that altering the cage environment by not handling and limiting betiding (NHNB) results in four key features of a chronic stress paradigm: increased “basal” CORT secretion, an altered response to an acute stressor, increased interanimal variability, and poor weight gain. These features are the hallmarks of tin up-regulated HPA.
Chronically stressed (NHNB) pups had an increased basal output of CORT. Furthermore, these pups had a “spontaneous” increase in plasma CORT between the morning and afternoon samples on postnatal day 9 even without exposure to cold-separation stress. The likely explanation for this increase is an enhanced sensitivity of the rats to the minimal interference caused by removing sibs from the cage. These minimal stresses were not sufficient to alter plasma CORT in either handled or nonhandled pups. The current design did not permit us to distinguish whether increased pup stress was secondary to abnormal maternal response to the environment. This is the focus of current studies.
The pattern of plasma CORT response in NHNB rats after acute cold-separation stress differed substantially from the other groups (Fig 2). The apparent delay in CORT response of NHNB animals to an acute physical stressor may reflect their higher basal CORT secretion over time [14].
Six hours after termination of cold-separation stress, plasma CORT of NHNB animals had not reached a plateau (Fig 2c). This sustained increase in plasma CORT is consistent with a less effective termination mechanism, i.e., a decreased sensitivity to negative feedback at the level of the pituitary, hypothalamus, or extrahypothalamic regions [17]. Such an abnormal negative feedback mechanism may explain both the alterations in basal CORT secretion and the abnormal termination of the acute stress response.
If chronically stressed (NHNB) pups have a diminished negative feedback mechanism, a potential site of the dysfunction is at the level of the hippocampus [31,32]. Hippocampal glucocorticoid receptors are considered essential for the termination of CORT secretion [31], Indeed, handled animals that have rapid termination of the stress response have been shown to have permanently increased glucocorticoid receptor levels in the hippocampus [17].
Pups in the NHNB paradigm had much greater interanimal variability in both basal and stress-induced plasma CORT (Fig 3). Individual variation in the response to stressful stimuli is a well-established phenomenon that has been shown to correlate with the degree of chronic stress [8,33]. Krieger and Good, for instance, documented significantly greater cortisol secretion rates as well as greater individual variance among children with growth failure due to both psychological and nutritional deprivation than in controls with growth failure alone [8].
NHNB pups were also significantly underweight. The increased basal plasma CORT output of these pups should result in increased levels of circulating CORT, This may be one of the factors contributing to their decreased weight because CORT induces catabolism [34,35]. Alternatively, if CRH production is increased, anorexia might result [36]. Dams may have had altered milk production or disturbed feeding and stroking behaviors, all of which could have contributed to poor weight gain [37].
The implications of this paradigm are threefold. First, it provides the first reproducible model of continuous stress in infant rats; i.e. the physical environment of the maternal-infant dyad alters the development of the HPA axis and HPA reactivity to novel stressors [16,38]. Second, this model achieves upregulation of the HPA without maternal separation and may more closely approximate the human situation of the chronically stressed, neglected infant. Third, the model produces an infant rat with increased CORT secretion that may be useful for studying the long term effects of CORT on, for example, limbic neuronal survival [39].
Acknowledgments
This work was supported in part by NINDS Grant No. NS28912 (TZB). We thank Dr. Leslie P. Weiner for ongoing support and encouragement.
References
- 1.Gold P, Goodwin F, Chrousos C. Clinical and biochemical manifestations of depression. II. Relation to the neurobiology of stress. N Engl J Med. 1988;319:413–420. doi: 10.1056/NEJM198808183190706. [DOI] [PubMed] [Google Scholar]
- 2.Owens M, Nemeroff C. The role of CRF in the pathophysiology of affective and anxiety disorders: Laboratory and clinical studies. In: Chadwick DJ, Marsh J, Ackrill K, editors. Corticotropin Releasing Factor. Chichester: John Wiley and Sons; 1993. pp. 296–316. [DOI] [PubMed] [Google Scholar]
- 3.Money J. The syndrome of abuse dwarfism (psychosocial dwarfism or reversible hyposomatotropism) Am J Dis Child. 1977;131:508–513. doi: 10.1001/archpedi.1977.02120180022002. [DOI] [PubMed] [Google Scholar]
- 4.Dietrich KN, Starr RH, Weisfeld GE. Infant maltreatment: Caretaker-infant interaction and developmental consequences at different levels of parenting failure. Pediatrics. 1983;72:532–540. [PubMed] [Google Scholar]
- 5.Powell GF, Bettes BA. Infantile depression, nonorganic failure to thrive, and DSM III-R: A different perspective. Child Psychiatry Hum Dev. 1992;22:185–198. doi: 10.1007/BF00705891. [DOI] [PubMed] [Google Scholar]
- 6.Weston JA, Colloton M, Halsey S, et al. A legacy of violence in nonorganic failure to thrive. Child Abuse Negl. 1993;17:709–714. doi: 10.1016/s0145-2134(08)80002-6. [DOI] [PubMed] [Google Scholar]
- 7.Pearce JW, Pezzot-Pearce TD. Attachment theory and its implications for psychotherapy with maltreated children. Child Abuse Negl. 1994;18:245–238. doi: 10.1016/0145-2134(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 8.Krieger I, Good MH. Adrenocortical and thyroid function in the deprivation syndrome. Am J Dis Child. 1970;120:95–102. [PubMed] [Google Scholar]
- 9.Walker C-D, Sapolsky RM, Meaney MJ, Vale WW, Rivier CL. Increased pituitary sensitivity to glucocorticoid feedback during the stress nonresponsive period in the neonatal rat. Endocrinology. 1986;119:1816–1821. doi: 10.1210/endo-119-4-1816. [DOI] [PubMed] [Google Scholar]
- 10.Stanton ME, Wallstrom J, Levine S. Maternal contact inhibits pituitary-adrenal stress responses in preweanling rats. Dev Psychobiol. 1987;20:131–145. doi: 10.1002/dev.420200204. [DOI] [PubMed] [Google Scholar]
- 11.Walker C-D, Scribner KA, Cascio CS, Dallman MF. The pituitary-adrenocortical system of neonatal rats is responsive to stress throughout development in a time-dependent and stressor-specific fashion. Endocrinology. 1991;128:1385–1395. doi: 10.1210/endo-128-3-1385. [DOI] [PubMed] [Google Scholar]
- 12.Rosenfeld P, Wetmore JB, Levine S. Effects of repeated maternal separations on the adrenocortical response to stress of preweanling rats. Physiol Behav. 1992;52:787–791. doi: 10.1016/0031-9384(92)90415-x. [DOI] [PubMed] [Google Scholar]
- 13.Yi S-J, Baram TZ. Corticotropin-releasing hormone mediates the response to cold stress in the neonatal rat without compensatory enhancement of the peptide’s gene expression. Endocrinology. 1994;135:2364–2368. doi: 10.1210/endo.135.6.7988418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meaney MJ, Bhatnagar S, Diorio J, et al. Molecular basis for the development of individual differences in the hypothalamic-pituitary-adrenal stress response. Cell Mol Neurobiol. 1993;13:321–346. doi: 10.1007/BF00711576. [DOI] [PubMed] [Google Scholar]
- 15.Hess JL, Denenberg VH, Zarrow M, Pfeifer WD. Modification of the corticosterone response curve as a function of handling in infancy. Physiol Behav. 1969;4:109–111. [Google Scholar]
- 16.Stanton ME, Gutierrez YR, Levine S. Maternal deprivation potentiates pituitary-adrenal stress responses in infant rats. Behav Neurosci. 1988;102:692–700. doi: 10.1037//0735-7044.102.5.692. [DOI] [PubMed] [Google Scholar]
- 17.Meaney MJ, Aitken DH, Viau V, Sharma S, Sarrieau A. Postnatal handling alters adrenocortical negative feedback sensitivity and hippocampal type II glucocorticoid receptor binding in the rat. Neuroendocrinology. 1989;50:597–604. doi: 10.1159/000125287. [DOI] [PubMed] [Google Scholar]
- 18.Levine S, Huchton DM, Wiener SG, Rosenfeld P. Time course of the effect of maternal deprivation on the hypothalamic-pituitary-adrenal axis in the infant rat. Dev Psychobiol. 1992;24:547–558. doi: 10.1002/dev.420240803. [DOI] [PubMed] [Google Scholar]
- 19.Avishai-Eliner S, Yi S-J, Newth CJL, Baram TZ. Effects of maternal and sibling deprivation on basal and stress induced hypothalamic-pituitary-adrenal components in the infant rat. Neurosci Lett. 1995;192:49–52. doi: 10.1016/0304-3940(95)11606-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conklin P, Heggeness F. Maturation of temperature homeostasis in the rat. Am J Physiol. 1971;220:333–336. doi: 10.1152/ajplegacy.1971.220.2.333. [DOI] [PubMed] [Google Scholar]
- 21.Zarrow M, Campbell P, Denenberg V. Handling in infancy: Increased levels of the hypothalamic corticotropin releasing factor (CRF) following exposure to a novel situation. Proc Soc Exp Biol Med. 1972;141:356–358. doi: 10.3181/00379727-141-36776. [DOI] [PubMed] [Google Scholar]
- 22.Viau V, Sharma S, Plotsky PM, Meaney MJ. Increased plasma ACTH responses to stress in nonhandled rats require basal levels of corticosterone and are associated with increased levels of ACTH secretogogues in the median eminence. J Neurosci. 1993;13:1097–1105. doi: 10.1523/JNEUROSCI.13-03-01097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dallman M, Akana S, Cascio C, Darlington D, Jacobson L, Levin N. Regulation of ACTH secretion: Variations on a theme of B. Recent Prog Horm Res. 1987;43:113–173. doi: 10.1016/b978-0-12-571143-2.50010-1. [DOI] [PubMed] [Google Scholar]
- 24.Baram T, Schultz L. Fetal and maternal levels of corticosterone and ACTH after pharmacological adrenalectomy. Life Sci. 1990;47:485–489. doi: 10.1016/0024-3205(90)90607-s. [DOI] [PubMed] [Google Scholar]
- 25.Culp RE, Heide J, Richardson MT. Maltreated children’s developmental scores: treatment versus nontreatment. Child Abuse Negl. 1987;11:29–34. doi: 10.1016/0145-2134(87)90030-5. [DOI] [PubMed] [Google Scholar]
- 26.Kristiansson B, Fällström SP. Growth at the age of 4 years subsequent to early failure to thrive. Child Abuse Negl. 1987;11:35–40. doi: 10.1016/0145-2134(87)90031-7. [DOI] [PubMed] [Google Scholar]
- 27.Smotherman WP. Mother-infant interaction and the modulation of pituitary-adrenal activity in rat pups after early stimulation. Dev Psychobiol. 1983;16:169–176. doi: 10.1002/dev.420160303. [DOI] [PubMed] [Google Scholar]
- 28.Walker C-D, Perrin M, Vale W, Rivier C. Ontogeny of the stress response in the rat: Role of the pituitary and the hypothalamus. Endocrinology. 1986;118:1445–1451. doi: 10.1210/endo-118-4-1445. [DOI] [PubMed] [Google Scholar]
- 29.Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- 30.Kuhn C, Paul J, Schanberg S. Endocrine responses to mother-infant separation in developing rats. Dev Psychobiol. 1990;23:395–410. doi: 10.1002/dev.420230503. [DOI] [PubMed] [Google Scholar]
- 31.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenal axis. Endocr Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 32.LaRocque S, O’Donnell D, Gianoulakis C, Seckl J, Meaney M. Postnatal handling in the rat alters hippocampal glucocorticoid receptor gene expression. Soc Neurosci Abstr. 1992;18:479. [Google Scholar]
- 33.Lewis M. Individual differences in response to stress. Pediatrics. 1992;90:487–490. [PubMed] [Google Scholar]
- 34.King B, Zansler C, Tatford A, III, Neville K, Sam H, Kass J, Dallman M. Level of corticosterone replacement determines body weight gain in adrenalectomized rats with VMH lesions. Physiol Behav. 1993;54:1187–1190. doi: 10.1016/0031-9384(93)90346-h. [DOI] [PubMed] [Google Scholar]
- 35.Santana P, Akana S, Hanson E, Strack A, Sebastian R, Dallman M. Aldosterone and dexamethasone both stimulate energy acquisition whereas only the glucocorticoid alters energy storage. Endocrinology. 1995;136:2214–2222. doi: 10.1210/endo.136.5.7720670. [DOI] [PubMed] [Google Scholar]
- 36.Krahn D, Gosnell B, Majchrzak M. The anorectic effects of CRH and restraint stress decrease with repeated exposures. Biol Psychiatry. 1990;27:1094–1102. doi: 10.1016/0006-3223(90)90046-5. [DOI] [PubMed] [Google Scholar]
- 37.Suchecki D, Mozaffarian D, Gross G, Rosenfeld P, Levine S. Effects of maternal deprivation on the ACTH stress response in the infant rat. Neuroendocrinology. 1993;57:204–212. doi: 10.1159/000126361. [DOI] [PubMed] [Google Scholar]
- 38.Hofer M. The role of nutrition in the physiological and behavioral effects of early maternal separation on infant rats. Psychosomat Med. 1973;35:350–359. doi: 10.1097/00006842-197307000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Sapolsky R. Glucocorticoid toxicity in the hippocampus: Reversal by supplementation with brain fuels. J Neurosci. 1986;6:2240–2244. doi: 10.1523/JNEUROSCI.06-08-02240.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]