Abstract
The Step study of a recombinant adenovirus serotype 5 (Ad5)–based human immunodeficiency virus type 1 (HIV-1) vaccine revealed an increased risk of HIV-1 acquisition in vaccinees who were Ad5 seropositive at baseline. We therefore investigated whether preexisting Ad seropositivity to 7 different Ad serotypes was associated with increased risk of HIV-1 infection in 3 HIV-1 vaccine efficacy trials. In a case-control study involving 1570 adults enrolled in the VAX003 and VAX004 trials of a recombinant protein subunit HIV-1 vaccine and in the Step study, we observed that preexisting seropositivity to multiple Ad serotypes was not intrinsically associated with increased risk of HIV-1 acquisition.
Recombinant adenoviruses are commonly used as vectors to deliver antigens and to stimulate immune responses in humans [1]. The Step study of the Merck recombinant adenovirus serotype 5 (MRKAd5) human immunodeficiency virus type 1 (HIV-1) gag/pol/nef vaccine revealed a 2.2-fold increased risk of HIV-1 acquisition among baseline adenovirus serotype 5 (Ad5) seropositive vaccinees compared to placebo recipients [2]. In post-hoc analyses, this risk appeared to be greatest in baseline Ad5 seropositive vaccinees who were uncircumcised. These findings raised a concern that preexisting Ad seropositivity might intrinsically enhance susceptibility to HIV-1 infection, particularly in recipients of Ad-based vaccines. However, a recent case-control study of 2 cohorts of individuals at elevated risk of HIV-1 infection did not show an association between Ad5 serostatus and incident HIV-1 infection [3].
It remains to be determined whether preexisting immunity to non-Ad5 serotypes is associated with enhanced HIV-1 acquisition, either among unvaccinated subjects or those who received experimental Ad-based or non-Ad-based HIV-1 vaccines. Moreover, the potential association of Ad5 seropositivity with HIV-1 acquisition risk following immunization with non-Ad5 HIV-1 vaccines remains unknown. Here, we show in a large case-control study that there is no association between preexisting seropositivity to 7 Ad serotypes and acquisition of HIV-1 infection among 1570 adults enrolled in 3 HIV-1 vaccine efficacy trials: the VAX003 [4] and VAX004 trials [5] of a recombinant HIV-1 envelope glycoprotein subunit (rgp120) vaccine and the Step study of the MRKAd5 HIV-1 gag/pol/nef vaccine [2].
METHODS
Study Populations
Baseline serum samples for this case-control study were obtained from 1570 adults enrolled in 1 of 3 HIV-1 vaccine efficacy trials: (1) VAX003, a randomized, double-blind, placebo-controlled efficacy trial of AIDSVAX B/E, a bivalent rgp120/alum adjuvant HIV-1 vaccine, in 2546 injection drug users in Bangkok, Thailand [4]; (2) VAX004, a randomized, double-blind, placebo-controlled efficacy trial of AIDSVAX B/B, also a bivalent rgp120/alum adjuvant vaccine, in 5403 men who have sex with men and women at high risk for heterosexual transmission of HIV-1 in the United States [5]; and (3) the Step study (HVTN 502/Merck 023), a randomized, double-blind, placebo-controlled, test-of-concept trial of the MRKAd5 vaccine among 3000 subjects at high risk of HIV-1 acquisition in North America, the Caribbean, South America, and Australia [2]. Detailed descriptions of the 3 vaccine trial designs are presented elsewhere [2, 4–5]. All samples were collected with local Institutional Review Board (IRB) approvals.
All subjects in the above trials who became infected with HIV-1 were included in our analysis as cases (229 in VAX003, 359 in VAX004, and 81 in Step). Subjects who remained uninfected with HIV-1 were selected from the VAX003 and VAX004 studies and included as controls at 1:1 to cases and were matched by demographics and vaccine status. Subjects who remained uninfected with HIV-1 were selected from the Step study as controls at 4:1 to cases and were matched by vaccine status, circumcision status, region, and baseline Ad5 titer, as previously described [6].
Adenovirus Neutralizing Antibody Assays
Preexisting Ad seropositivity was measured for serotypes 1, 2, 5, 6, 26, 35, and 48; that is, a diverse selection of Ads with high and low seroprevalence from a variety of Ad subfamilies [1]. Seropositivity was determined by luciferase-based virus neutralization assays as described [7], utilizing vectors provided by Merck Research Laboratories (gift from Dr Danilo Casimiro) or Beth Israel Deaconess Medical Center (BIDMC). Positive neutralizing antibody (NAb) titers were defined as titers ≥18, which represents the standard cutoff utilized in recent seroprevalence and vaccine studies [7–9]. Ad neutralization assays were approved by the BIDMC IRB.
Statistical Analysis
Baseline Ad seropositivity was compared between cases and controls in 2 × 2 contingency tables. Data was analyzed with 2-sided Fisher exact tests for categorical variables. The primary analysis was performed comparing preexisting seropositivity to Ad serotypes 1, 2, 5, 6, 26, 35, and 48 among cases and controls in a combined analysis of all 3 trials. P values were not adjusted for multiple comparisons for the primary analysis to preserve statistical power. Odds ratios with 95% confidence intervals (CIs) were also calculated. Subgroup analyses were performed comparing Ad seropositivity and HIV-1 acquisition by vaccine/placebo status in each individual vaccine trial and among circumcised/uncircumcised subjects in the Step study. The association between preexisting Ad5 seropositivity and HIV-1 acquisition in the Step study was not addressed in this study, since the controls were matched to cases by baseline Ad5 titer, and the results of this analysis are available elsewhere [2]. For the subanalysis by individual vaccine trial, P < .001 were considered statistically significant after Bonferroni correction for multiple comparisons. For subanalysis by circumcision status, P < .002 were considered statistically significant after Bonferroni correction. All calculations were performed using GraphPad Prism 4 software.
For power calculations, we chose 3 Ads with different global seroprevalence rates: Ad5 (72%), Ad26 (40%), and Ad35 (13%) [9]. Cases and controls were selected as described above. This study has 80% power to detect a 10% increase in the relative risk (RR) of Ad5 seropositivity associated with cases (7% absolute increase). By comparison, a previous study of the association of Ad5 serostatus and incident HIV-1 infection was powered to detect a 20% increase in Ad5 seropositivity associated with cases [3]. For Ad26, this study has 80% power to detect an 18% increase in RR (7% absolute increase). For Ad35, this study has 80% power to detect a 40% increase in RR (5% absolute increase).
RESULTS
Baseline NAb titers to Ad serotypes 1, 2, 5, 6, 26, 35, and 48 were determined for 669 HIV-1–infected subjects (cases) and 901 HIV-1–uninfected subjects (controls) in a combined analysis of the VAX003, VAX004, and Step studies (Table 1). Ad seroprevalence among all subjects ranged from rare (Ad35, 9%) to common (Ad2, 87%) and varied between cases and controls by no more than 5% for any serotype. In the primary analysis of all subjects in the 3 studies, there was no statistically significant association between preexisting Ad seropositivity and HIV-1 acquisition for any of the 7 serotypes tested (P ≥ .05 for all comparisons, not adjusted for multiple comparisons to preserve statistical power). Odds ratios ranged from 1.0 to 1.3 and the 95% CI crossed 1.0 for all serotypes. The largest difference in seroprevalence was observed for Ad1 (74% for HIV-1–infected cases vs 69% for HIV-1–uninfected controls, P = .05), but there was no pattern to Ad1 seroprevalence when analyzed across individual trials.
Table 1.
Subjects with Preexisting Adenovirus Seropositivity by HIV-1 Status in the Combined Case-Control Analysis of the VAX003, VAX004, and Step Studies
| Serotypea | HIV-1 Infected (n = 669) | HIV-1 Uninfected (n = 901) | OR (95% CI) | P Valueb |
|---|---|---|---|---|
| Adenovirus 35c | 60 (9%) | 74 (8%) | 1.1 (0.8–1.6) | .65 |
| Adenovirus 48d | 101 (15%) | 117 (13%) | 1.2 (0.9–1.6) | .24 |
| Adenovirus 26e | 209 (31%) | 251 (28%) | 1.2 (0.9–1.5) | .15 |
| Adenovirus 5f | 333 (57%) | 306 (53%) | 1.2 (0.9–1.5) | .22 |
| Adenovirus 6 | 393 (59%) | 525 (58%) | 1.0 (0.8–1.3) | .88 |
| Adenovirus 1 | 495 (74%) | 625 (69%) | 1.3 (1.0–1.6) | .05 |
| Adenovirus 2 | 586 (88%) | 781 (87%) | 1.1 (0.8–1.5) | .65 |
Data are no. (%) of subjects, unless otherwise noted.
Abbreviations: CI, confidence interval; HIV-1, human immunodeficiency virus type 1; OR, odds ratio.
a Sorted in order of prevalence.
b Two-sided P values from Fischer exact test.
c One case and 4 controls are missing data.
d Two cases are missing data.
e One case is missing data.
f Step samples excluded; cases n = 587, controls n = 577.
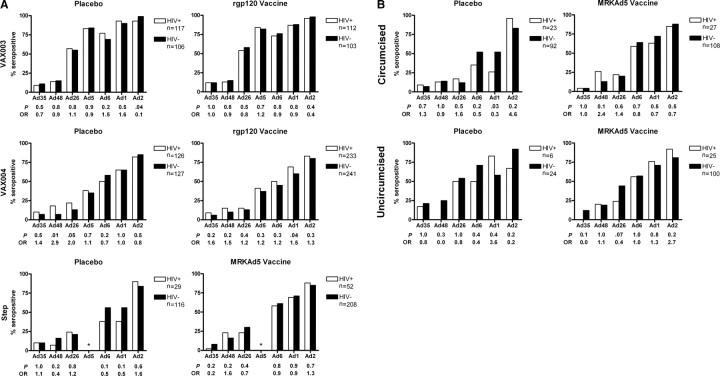
We next analyzed data individually for each trial by vaccine/placebo status. Ad seroprevalence was generally higher in the VAX003 study (N = 438), which was conducted in Thailand, as compared with the VAX004 (N = 727) or Step (N = 405) studies, which were conducted primarily in North and South America (Figure 1A). Ad35 and Ad48 seroprevalence remained relatively low and Ad26 seroprevalence proved intermediate across all studies, as published elsewhere [8, 9]. In subgroup analyses, there was no statistically significant association between baseline seropositivity to any of the 7 Ad serotypes and HIV-1 acquisition among subjects who received the protein subunit rgp120 vaccine in either the VAX003 or VAX004 studies. Similarly, there was no association between baseline seropositivity to the 6 non-Ad5 serotypes and HIV-1 acquisition in subjects who received the MRKAd5 vaccine in the Step study. There was also no association among placebo recipients. P values for these subgroup comparisons were all ≥.01 (P < .001 required for significance). The association between baseline Ad5 seropositivity and HIV-1 acquisition in the Step study was not analyzed here because this variable was included in selecting the control subjects (see “Methods”), and this association has been reported previously [2]. Ad48 seroprevalence appeared slightly different between cases and controls among placebo recipients in the VAX004 study (P = .01), but this trend was not statistically significant after adjusting for multiple comparisons, and an opposite trend was observed in placebo recipients in both the Step and VAX003 studies (Figure 1A).
Figure 1.
Percent of subjects with preexisting adenovirus seropositivity by HIV-1 status and subgroup. P, 2-sided Fischer exact test; OR, odds ratio. A, Subjects are divided by vaccine/placebo status and individual vaccine trial. For the VAX004 study, Ad48 data are missing for 1 HIV-1–infected, vaccinated subject. For the Step study, Ad35 data are missing for 3 HIV-1-uninfected, unvaccinated subjects, and 1 HIV-1–infected, vaccinated subject. *Ad5 data not analyzed in the Step study as this variable was included in selecting control subjects. B, Subjects are divided by vaccine/placebo and circumcision status in the Step study. Among circumcised subjects, Ad35 data are missing for 3 HIV-1-uninfected placebo recipients and 1 HIV-1–uninfected vaccinated subject.
Finally, we analyzed the potential association between preexisting Ad seropositivity to the 6 non-Ad5 serotypes and HIV-1 acquisition in the Step study as stratified by vaccine status and circumcision status (Figure 1B). Ad seroprevalence was generally higher in uncircumcised subjects than circumcised subjects, likely reflecting confounding geographic variables. Nevertheless, we found no significant association between Ad seropositivity and HIV-1 acquisition risk in all subgroups, including among uncircumcised MRKAd5 vaccinees (P ≥ .03 for all comparisons; P < .002 required for significance).
DISCUSSION
Adenoviruses have been developed in recent years as vectors for both vaccination and gene therapy [1, 10]. In 2007, vaccinations were prematurely terminated in the Step study because the recombinant Ad5 vector expressing HIV-1 gag/pol/nef (MRKAd5) was ineffective at preventing HIV-1 infection [2]. In addition, subgroup analyses demonstrated a trend towards increased HIV-1 acquisition among subjects with preexisting Ad5 seropositivity who received the vaccine, particularly in individuals who were both uncircumcised and Ad5 seropositive at baseline [2, 11]. These findings provoked a comprehensive effort in the HIV-1 vaccine field to examine in detail the interaction of preexisting Ad seropositivity and HIV-1 acquisition. One question that has arisen is whether Ad seropositivity is intrinsically associated with increased HIV-1 acquisition.
We performed a case-control study of 1570 subjects at high risk for HIV-1 infection and enrolled in 3 independent HIV-1 vaccine efficacy trials. We observed no significant association between preexisting seropositivity to Ads 1, 2, 5, 6, 26, 35, and 48 and HIV-1 acquisition. Our study excluded an assessment of the impact of Ad5 seropositivity on HIV-1 acquisition in the Step study, which has been reported previously [2]. To the best of our knowledge, this is the largest cohort of subjects analyzed for the potential association between preexisting Ad seropositivity and HIV-1 acquisition, and the first study to evaluate non-Ad5 serotypes.
Our findings confirm and extend a recent case-control study of baseline Ad5 seropositivity and HIV-1 acquisition among persons enrolled in the Multicenter AIDS Cohort Study and HPTN 039, a trial of herpes simplex virus type 2 suppression in adults in the United States, South America, and Africa [3]. The authors of this previous study reported that the relative risk of incident HIV-1 infection among baseline Ad5 seropositive adults was 1.1 (95% CI, .8–1.5; P = .57) and was no higher than that among baseline Ad5-seronegative adults (1.0, 95% CI, .4–2.3; P = .99). Our study shows similar findings for Ad5 seropositivity in a larger number of subjects, including among subjects who received the non-Ad-based HIV-1 vaccine rgp120 in the VAX003 and VAX004 studies. These results demonstrate that Ad5 seropositivity does not intrinsically increase HIV-1 acquisition risk nor is it a surrogate marker for HIV-1 risk.
Our study also examined the potential association between preexisting Ad seropositivity for non-Ad5 serotypes and HIV-1 acquisition among subjects who received the MRKAd5 vaccine or the rgp120 vaccine. One of the questions raised by the Step study was whether subjects with baseline seropositivity to non-Ad5 serotypes might also have had increased HIV-1 acquisition risk after MRKAd5 vaccination, perhaps through the expansion of cross-reactive Ad-specific CD4+ cells at mucosal sites [12–15]. Importantly, our study demonstrated no association between preexisting Ad seropositivity to 6 diverse non-Ad5 serotypes and HIV-1 acquisition among recipients of MRKAd5. This was also true in the subgroup of uncircumcised MRKAd5 vaccinees, the group with the highest increased risk of HIV-1 infection in the Step study [2]. These data suggest that cross-reactive Ad-specific immunity to non-Ad5 serotypes did not contribute to the increased HIV-1 acquisition observed in the Step study. Finally, we observed that there was no relationship between preexisting Ad seropositivity to all 7 serotypes and HIV-1 acquisition among recipients of the protein subunit HIV-1 vaccine rgp120.
In summary, we demonstrate in a large case-control study that preexisting seropositivity to multiple Ad serotypes did not intrinsically enhance the risk of HIV-1 infection. Moreover, preexisting Ad seropositivity did not enhance HIV-1 acquisition risk in individuals who received an Ad-based vaccine from a heterologous serotype or an unrelated recombinant protein subunit vaccine. Although our study did not address the potential risks of utilizing Ad vectors in individuals with homologous Ad seropositivity, our data clarify important questions in the HIV-1 vaccine field and support further clinical evaluation of Ad-based vaccines with non-Ad5 serotypes.
Notes
Acknowledgments. We thank P. Abbink, A. Bett, D. Casimiro, R. Dilan, S. King, L. Maxfield, and M. Robertson for generous advice, assistance, and reagents.
Financial support. This work was supported by the US National Institutes of Health grants AI07387 (K. E. S.), AI066924 (D. H. B.), and AI078526 (D. H. B.); the Bill and Melinda Gates Foundation; and the Ragon Institute of MGH, MIT and Harvard.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Barouch DH. Novel adenovirus vector-based vaccines for HIV-1. Curr Opin HIV AIDS. 2010;5:386–90. doi: 10.1097/COH.0b013e32833cfe4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–93. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curlin ME, Cassis-Ghavami F, Magaret AS, et al. Serological immunity to adenovirus serotype 5 is not associated with risk of HIV infection: a case-control study. AIDS. 2011;25:153–8. doi: 10.1097/QAD.0b013e328342115c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pitisuttithum P, Gilbert P, Gurwith M, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–71. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 5.Flynn NM, Forthal DN, Harro CD, et al. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–65. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 6.McElrath MJ, De Rosa SC, Moodie Z, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sprangers MC, Lakhai W, Koudstaal W, et al. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J Clin Microbiol. 2003;41:5046–52. doi: 10.1128/JCM.41.11.5046-5052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbink P, Lemckert AA, Ewald BA, et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol. 2007;81:4654–63. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barouch DH, Kik SV, Weverling GJ, et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29:5203–9. doi: 10.1016/j.vaccine.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rollier CS, Reyes-Sandoval A, Cottingham MG, Ewer K, Hill AV. Viral vectors as vaccine platforms: deployment in sight. Curr Opin Immunol. 2011;23:377–82. doi: 10.1016/j.coi.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Gray G, Buchbinder S, Duerr A. Overview of STEP and Phambili trial results: two phase IIb test-of-concept studies investigating the efficacy of MRK adenovirus type 5 gag/pol/nef subtype B HIV vaccine. Curr Opin HIV AIDS. 2010;5:357–61. doi: 10.1097/COH.0b013e32833d2d2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts DM, Nanda A, Havenga MJ, et al. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441:239–43. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- 13.Robb ML. Failure of the Merck HIV vaccine: an uncertain step forward. Lancet. 2008;372:1857–8. doi: 10.1016/S0140-6736(08)61593-7. [DOI] [PubMed] [Google Scholar]
- 14.O'Brien KL, Liu J, King SL, et al. Adenovirus-specific immunity after immunization with an Ad5 HIV-1 vaccine candidate in humans. Nat Med. 2009;15:873–5. doi: 10.1038/nm.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutnick NA, Carnathan DG, Dubey S, et al. Baseline Ad5 serostatus does not predict Ad5-HIV vaccine-induced expansion of Ad-specific CD4+ T-cells. Nat Med. 2009;15:876–8. doi: 10.1038/nm.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]