Abstract
Coral reefs are threatened by increasing levels of coral disease and the functional loss of obligate algal symbionts (bleaching). Levels of immunity relate directly to susceptibility to these threats; however, our understanding of fundamental aspects of coral immunology is lacking. We show that three melanin-synthesis pathway components (mono-phenoloxidase, ortho-diphenoloxidase (tyrosinase-type pathway) and para-diphenoloxidase (laccase-type pathway)) are present in both their active (phenoloxidase, PO) and inactive (prophenoloxidase, PPO) forms across a diverse range of 22 species of healthy Indo-Pacific anthozoans. We also demonstrate transglutaminase activity of the coagulation cascade for, to our knowledge, the first time in a coral. Melanin-synthesis enzyme activities varied among taxa, although they were generally lowest in the coral family Acroporidae and highest in the Poritidae and Oculinidae. Inactive tyrosinase-type activity (PPO) and active laccase-type activity (PO) correlate with taxonomic patterns in disease resistance, whereas the converse pattern in activity levels correlates with bleaching resistance. Overall, we demonstrate the presence of several melanin-synthesis pathways in Indo-Pacific corals, co-regulation among some pathway components, and highlight their potential roles in coral health.
Keywords: coral, phenoloxidase, melanin, coagulation, disease, bleaching
1. Introduction
Reef-building corals are declining globally [1], largely as a result of increased anthropogenic stressors that promote health-related threats to coral persistence, such as disease and the breakdown of coral–Symbiodinium symbiosis known as bleaching [2]. As key responses in invertebrate innate immunity, the proteolytic cascades of melanin synthesis and coagulation significantly contribute to maintaining organism health [3,4]. Melanin synthesis provides cytotoxic defence, a protective barrier and structural support [5], and the coagulation pathway induces clot formation to seal wounds and prevent infection [6]. Although these immune pathways have been investigated within numerous invertebrates [5,6], their presence in reef-building corals has only recently been explored [7–9]. As health-related disturbances continue to contribute to global coral reef declines [10], unravelling the components of coral immunity is a research priority.
(a). The melanin-synthesis pathway
Phenoloxidases (POs) activate melanin-synthesis and are stored as zymogens (prophenoloxidases; PPOs) [11]. Multiple PPOs have been identified within some invertebrates [12], suggesting that several melanin-synthesis pathway components and/or distinct pathways exist. On the basis of their ability to metabolize different phenolic substrates (e.g. mono-phenols, para-diphenols and ortho-diphenols) [13], POs (and their PPOs) fall into two categories: tyrosinase-type and laccase-type, representing distinct melanin-synthesis pathways [12]. Within arthropods, tyrosinase-type activity is involved in disease resistance via cytotoxic defence, encapsulation and phagocytosis [14], whereas the laccase-type is involved in sclerotization (cuticle tanning) [15]. However, the presence and possible functions of multiple melanin-synthesis pathways remains poorly defined within most invertebrates.
The use of various POs is best described for insects, whereby melanin synthesis occurs via the hydroxylation of mono-phenol substrates (e.g. tyrosine or tyramine) to diphenol substrates (e.g. l-1,3-dihydroxyphenylalanine, l-DOPA) by a tyrosinase-type PO demonstrating mono-phenoloxidase activity (figure 1; [11,12]). Diphenoloxidases oxidize ortho-diphenols (e.g. dopamine) to chromatic compounds and quinones (o-diphenoloxidase or catecholase activity), from which melanin is produced non-enzymatically [11,12]. Therefore, tyrosinase-type POs can be detected by their specificity to both mono-phenols (e.g. tyrosine) and o-diphenols (e.g. DOPA-based substrates) [13], and represent different components of the same melanin-synthesis pathway [11,12]. Conversely, laccase-type PO(s) (referred to simply as laccase henceforth) may be involved in a separate melanin-synthesis pathway. Laccases oxidize both para-diphenols and o-diphenols but not mono-phenols [16], and may be involved in the oxidation of dopamine-derived N-acyldopamines to corresponding quinones (figure 1; [12]).
Figure 1.
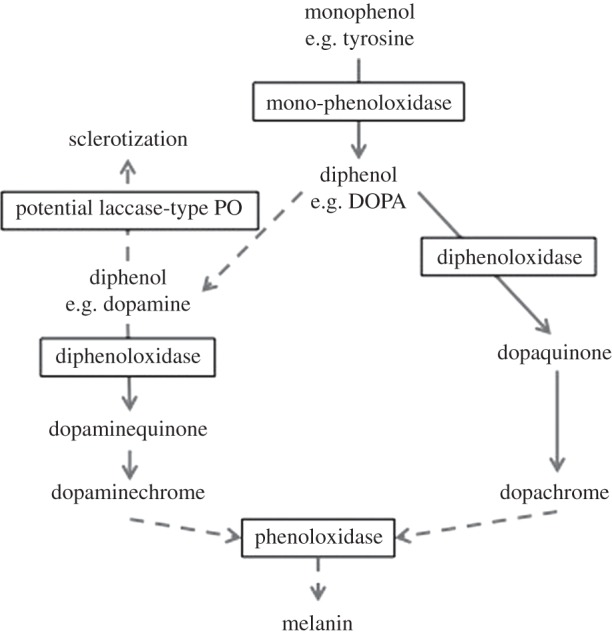
Insect melanin synthesis. Dashed arrows indicate multiple steps (simplified and adapted from earlier studies [11,12]).
(b). The coagulation pathway
To seal wounds and prevent infection and fluid loss, transglutaminases catalyse plasma protein cross-linking during coagulation to form an insoluble gel that can be hardened into a clot (reviewed by Cerenius & Soderhall [4]). Although wound-healing observations have been made for a variety of anthozoans [17–19], transglutaminase activity has not been investigated.
The primary aims of this study were to determine whether: (i) multiple components of the melanin-synthesis cascade(s) and the coagulation pathway are present within Indo-Pacific anthozoans, (ii) baseline activities of different melanin-synthesis pathway components are correlated, and (iii) potential variation in baseline activities of these pathways relates to coral disease and/or bleaching susceptibility. The presence and relative activities of PO and PPOs were investigated in healthy samples of 18 hard corals, three soft corals and a zoanthid using substrate-specificity assays. In addition, an exploratory investigation into the presence of transglutaminase activity was conducted on one hard coral species.
2. Methods
Tissue samples were collected (from Orpheus Island) and processed for biochemistry as per Palmer et al. [8] from 18 scleractinian species, three alcyonacean species and a zoanthid: Pocillopora damicornis and Seriatopora hystrix (family (F): Pocilloporidae); Acropora hyacinthus, A. tenuis, A. millepora and Montipora digitata (F: Acroporidae); Merulina ampliata and Hydnophora sp. (F: Merulinidae); Lobophyllia hemprichii and Symphyllia sp. (F: Mussidae); Fungia sp. (F: Fungiidae); Diploastrea heliopora, Platygyra sinensis and Goniastrea aspera (F: Faviidae); Porites massive sp. and P. cylindrica (F: Poritidae); Physogyra sp. (F: Euphyllidae); Galaxea sp. (F: Oculinidae); Sarcophyton, Lobophyton and Sinularia (F: Alcyoniidae); and Palythoa sp. (F: Sphenopidae).
(a). Enzyme assays
Dose–response curves using pooled samples of Porites massive sp., were used to confirm the presence of melanin-synthesis pathway components within an Indo-Pacific hard coral (see the electronic supplementary material). Activities of three types of phenoloxidase were determined in triplicate for each of the five samples of each species, using 20 µl of coral sample, 40 µl of phosphate buffer (50 mmol l−1 pH 7.5), 25 µl of ddH2O, and 30 µl of 10 mmol l−1 substrate tyramine (Fluka 93810) for mono-phenoloxidase activity; dopamine hydrochloride (Sigma-Aldrich H8502) and l-DOPA (3(3,4-Dihydroxyphenyl)-l-alanine; Fluka 37830) for o-diphenoloxidase activity; and hydroquinone (Sigma H9003) for p-diphenoloxidase (laccase) activity [16]. PPO activity was calculated for each coral and each substrate by repeating the assays using 25 µl of trypsin (0.1 mg ml−1 Sigma) instead of ddH2O, to convert PPO to its active and measureable form PO, as per Mydlarz & Palmer [7]. Controls for substrate auto-oxidation were included where the sample extract was replaced with ddH2O. Independent effects of samples were tested (and not detected) in the absence of substrate, and no colour change was detected using boiled sampled extracts (data not shown). Absorbance at 490 nm was recorded every 5 min for 45 min using a Spectramax M2 (Molecular Devices) spectrophotometer, and activity determined from the change in absorbance for the linear portion of the reaction curve. Enzyme activities were standardized to sample total protein as determined using the Bradford Quick-start assay (Bio-Rad).
Transglutaminase activity was determined in triplicate using 5–25 μl of sample extracts pooled from five colonies of the massive coral Porites sp. ddH2O (45–25 μl) was added to each well to a total of 50 μl. Subsequently, 20 μl of z-Gln-Gly (Sigma C6154) and 70 μl of buffer (19.5% of 1 M Tris-acetate with 0.2 M EDTA, 4.5% 0.1 M CaCl2 and 72.5% ddH2O) with 2.8% 25 mmol l−1 N,N-dimethyl-p-phenylenediamine (DMPDA) (Sigma C7750) were added to each well. Tests for DMPDA auto-oxidation and colour change of sample extracts showed no change over time (data not shown). Transglutaminase activity was measured as the change in absorbance, at 278 nm, over the linear portion of the reaction curve (20 min), owing to the oxidation of DMPDA. Inhibition of enzyme activity was tested using 10 mmol l−1 of iodoacetamide (Sigma I1149). Additionally, the influence of pH on PO assays using A. millepora was also tested (see the electronic supplementary material).
(b). Statistics
Data were log-transformed to meet the parametric assumptions of residual normality and homoscedasticity. To discern the relative contribution of taxonomic level to overall variance in enzyme activities, a generalized linear-mixed model (GLMM) by maximum likelihood was conducted on PO and PPO data for each of the four substrates with both family and species as random factors using R (http://www.R-project.org). PO and PPO activities for each substrate were compared among coral species and families using MANOVA, and Tukey's honestly significant difference (HSD) post hoc tests were conducted on the univariate analyses in R. To test the relationships among PO and PPO activities among substrates, correlations were conducted in Sigma Plot. Linear regression ANOVA analyses (Sigma Plot) were used to investigate the relationship between PO and PPO activities with each substrate, and with disease and bleaching susceptibility data at the family level. Disease and bleaching susceptibility data were obtained from Page & Willis [20] and Marshall & Baird [21], respectively, and transformed into family ranks as per Palmer et al. [8]. Enzyme activity in the presence and absence of inhibitors was compared using a t-test for transglutaminase and using a Mann–Whitney U-test for PO, as parametric assumptions were not met.
3. Results
(a). The melanin-synthesis pathway
PO and PPO activities were detected within all 22 species for each of the four substrates, with the exception of Fungia sp. using tyramine (figures 2 and 3, respectively). The majority of variance in both PO and PPO activity for all substrates, except tyramine, was explained by family rather than by species (tables 1 and 2, respectively). Both mean PO and PPO activity varied significantly among substrates (F3,420 = 43.37, p < 0.001; F3,420 = 29.87, p < 0.001, respectively), with dopamine (o-diphenoloxidase) yielding significantly higher activities than all other substrates, and tyramine (mono-phenoloxidase) and hydroquinone (p-diphenoloxidase) the lowest (Tukey's HSD p < 0.05).
Figure 2.
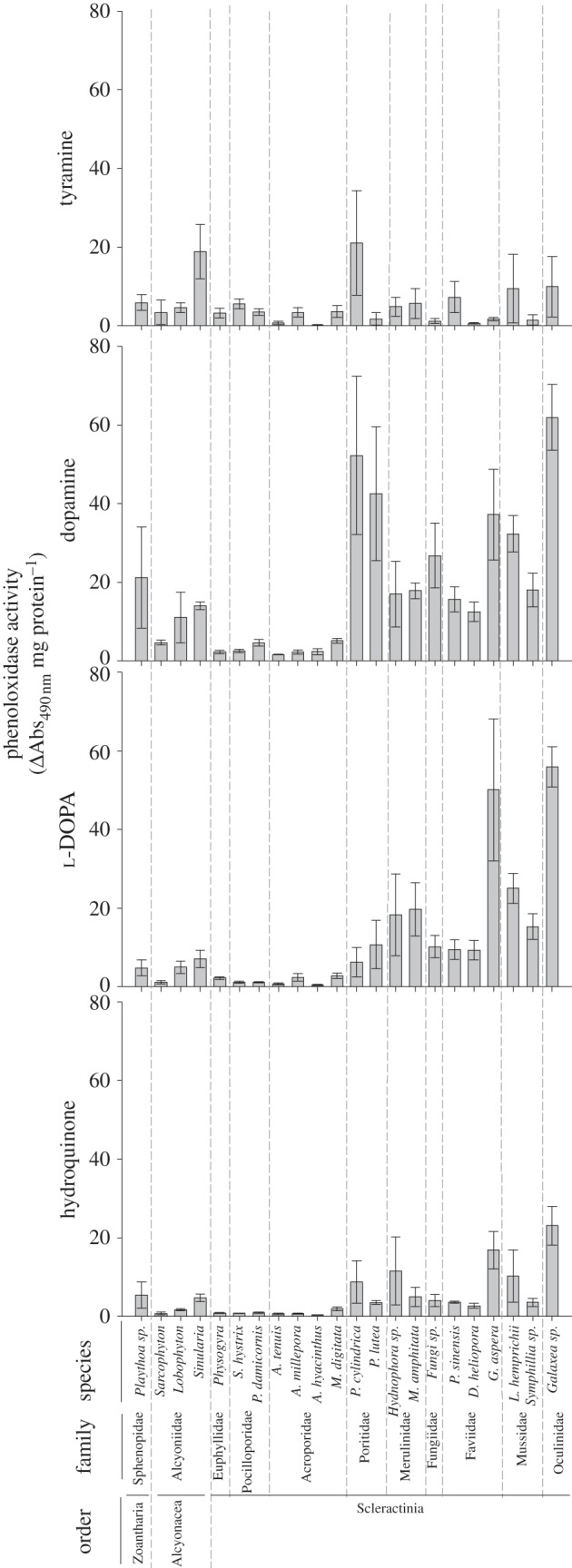
Mean phenoloxidase activities (±s.e.) for 22 species of anthozoans using tyramine, dopamine, l-DOPA and hydroquinone as substrates.
Figure 3.
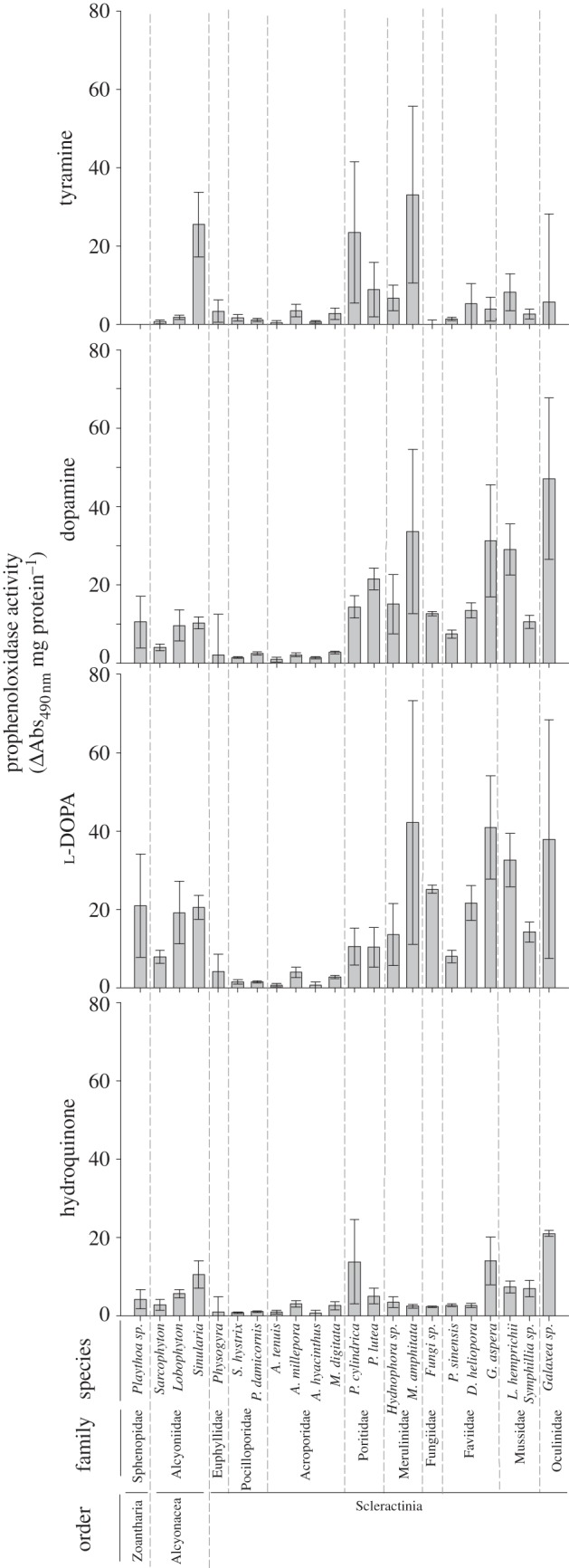
Mean prophenoloxidase activities (±s.e.) for the 22 anthozoans using tyramine, dopamine, l-DOPA and hydroquinone as substrates.
Table 1.
A summary of generalized linear-mixed model (GLMM) analysis examining the contributions of family and species random effects to the variance in PO activity. (Italicized numbers indicate the highest relative contribution to the overall variance per substrate.)
| substrate | species (n = 22) |
family (n = 11) |
residual | s.d. | ||
|---|---|---|---|---|---|---|
| variance | s.d. | variance | s.d. | |||
| tyramine | 0.039 | 0.197 | 0.002 | 0.041 | 0.160 | 0.400 |
| dopamine | 0.023 | 0.151 | 0.152 | 0.390 | 0.084 | 0.291 |
| l-DOPA | 0.046 | 0.214 | 0.130 | 0.361 | 0.074 | 0.271 |
| hydroquinone | 0.032 | 0.179 | 0.052 | 0.228 | 0.065 | 0.254 |
Table 2.
A summary of GLMM analysis examining the relative contribution of family and species random effects to the variance in PPO activity. (Italicized numbers indicate the highest relative contribution to the overall variance per substrate.)
| substrate | species (n = 22) |
family (n = 11) |
residual | s.d. | ||
|---|---|---|---|---|---|---|
| variance | s.d. | variance | s.d. | |||
| tyramine | 0.059 | 0.242 | 0 | 0 | 0.190 | 0.436 |
| dopamine | 0.026 | 0.161 | 0.115 | 0.340 | 0.109 | 0.330 |
| l-DOPA | 0.037 | 0.192 | 0.110 | 0.332 | 0.085 | 0.292 |
| hydroquinone | 0.032 | 0.180 | 0.043 | 0.208 | 0.057 | 0.238 |
(i). Activity of melanin-synthesis pathway components among taxa
PO activities for each substrate varied significantly among species and families (table 3); however, trends in activities among taxa were broadly consistent for the three substrates (figure 2). Although at the family level, Pocilloporidae and Euphyllidae had activities statistically similar to Acroporidae for each of the three substrates (table 4), the acroporid species A. hyacinthus and A. tenuis had the lowest PO activities for each substrate. Galaxea sp. (Oculinidae) and Faviidae species had the highest PO activities, although with much greater variability (figure 2; table 4). Although P. cylindrica had the highest PO activity, with the greatest fold difference (greater than 100-fold) in absorbance among species for any of the substrates, this difference was not statistically significant because of high variance (Tukey's HSD p > 0.05; figure 2). Instead, the 18-fold change in absorbance for Sinularia sp., was mainly responsible for the significant variation among species (figure 2). o-diphenoloxidase activity with l-DOPA was generally lower than with dopamine; however, the trends among species remained broadly consistent (figure 2), although enzyme activities of S. hystrix and P. damicornis (Pocilloporidae) were as low as those of the Acroporidae with l-DOPA (Tukey's HSD p < 0.05; figure 2). p-diphenoloxidase activity (laccase) was generally lower than o-diphenoloxidase activities with both dopamine and l-DOPA, although again, trends in activity levels among species remained consistent (figure 2). All four acroporids had among the lowest levels of p-diphenoloxidase activity, in addition to Physogyra sp., and two Alcyoniidae: Sarcophyton sp. and Lobophyton sp. (Tukey's HSD p < 0.05; figure 2).
Table 3.
A summary table of the MANOVA results of PO and PPO activities among both species and families.
| substrate | species d.f. = 11,84 |
family d.f. =10,84 |
||
|---|---|---|---|---|
| F | p | F | p | |
| PO | ||||
| whole model | 2.39 | <0.001 | 5.73 | <0.001 |
| tyramine | 2.50 | 0.009 | 2.12 | 0.032 |
| dopamine | 2.27 | 0.018 | 19.80 | <0.001 |
| l-DOPA | 3.97 | <0.001 | 22.39 | <0.001 |
| hydroquinone | 12.74 | <0.001 | 11.52 | <0.001 |
| PPO | ||||
| whole model | 1.91 | <0.001 | 5.23 | <0.001 |
| tyramine | 2.31 | 0.016 | 2.94 | 0.003 |
| dopamine | 2.15 | 0.025 | 13.56 | <0.001 |
| l-DOPA | 3.20 | 0.001 | 18.61 | <0.001 |
| hydroquinone | 3.49 | <0.001 | 11.25 | <0.001 |
Table 4.
A summary table of Tukey's HSD post hoc comparisons for PO activities for three substrates. (Letters a–f denote families of similar PO activity; statistical differences in activity (p < 0.05) separate letters. There was no significant difference among family tyramine.)
| family | dopamine | l-DOPA | hydroquinone |
|---|---|---|---|
| Sphenopidae | bcd | abcd | ab |
| Alcyoniidae | abc | abc | ab |
| Euphyllidae | a | ab | a |
| Pocilloporidae | ab | a | a |
| Acroporidae | a | a | a |
| Poritidae | de | abcde | ab |
| Merulinidae | cd | cde | ab |
| Fungiidae | cd | bcde | ab |
| Faviidae | cd | def | b |
| Mussidae | cd | e | ab |
| Oculinidae | e | f | c |
Overall trends in levels of PPO activity were similar to those of PO activity across the four substrates, with A. hyacinthus and A. tenuis (Acroporidae) generally having the lowest activities and Galaxea sp. (Oculinidae) and Faviidae species the highest, although with much greater variability (figure 3 and table 5). PPO activities also varied significantly among families (table 3); however, distinct from the species level trends, Pocilloporidae had the lowest activity, followed by Acroporidae (table 5). No mono-PPO activity (tyramine) was detected in Fungia sp., and the zoanthid Palythoa sp., had a change in absorbance of just 0.04-fold (lower than that of A. tenuis and A. hyacinthus). Sinularia sp., had the highest activity (Tukey's HSD p < 0.05; figure 3), at levels 600-fold higher than those recorded for Palythoa sp. Consistent with PO activity, PPO activity with dopamine was higher than with the other o-diphenol l-DOPA, but interspecific trends were maintained. However, at the family level, rankings varied with substrate (table 5). For example, for dopamine, Poritidae had significantly higher PO activity than Acroporidae and Pocilloporidae, and equivalent activity to Oculinidae, but for l-DOPA, Poritidae had significantly lower activity than Oculinidae. With hydroquinone substrate (figure 3), A. hyacinthus, A. tenuis and S. hystrix had the lowest p-diprophenoloxidase activities, whereas Galaxea sp., P. cylindrica, G. aspera and Sinularia sp. had significantly higher activities (Tukey's HSD p < 0.05; figure 3).
Table 5.
A summary table of Tukey's HSD post hoc comparisons for PPO activities for each substrate. (Letters a–d denote families of similar PPO activity; statistical differences in activity (p < 0.05) separate letters. There was no significant difference among family tyramine.)
| family | dopamine | l-DOPA | hydroquinone |
|---|---|---|---|
| Sphenopidae | ab | cde | abc |
| Alcyoniidae | ab | cde | bc |
| Euphyllidae | a | abc | a |
| Pocilloporidae | a | a | a |
| Acroporidae | a | ab | ab |
| Poritidae | b | bcd | bc |
| Merulinidae | bc | cde | abc |
| Fungiidae | bc | de | abe |
| Faviidae | bc | de | abc |
| Mussidae | bc | de | cd |
| Oculinidae | c | e | d |
(ii). Relationships between melanin-synthesis pathway components
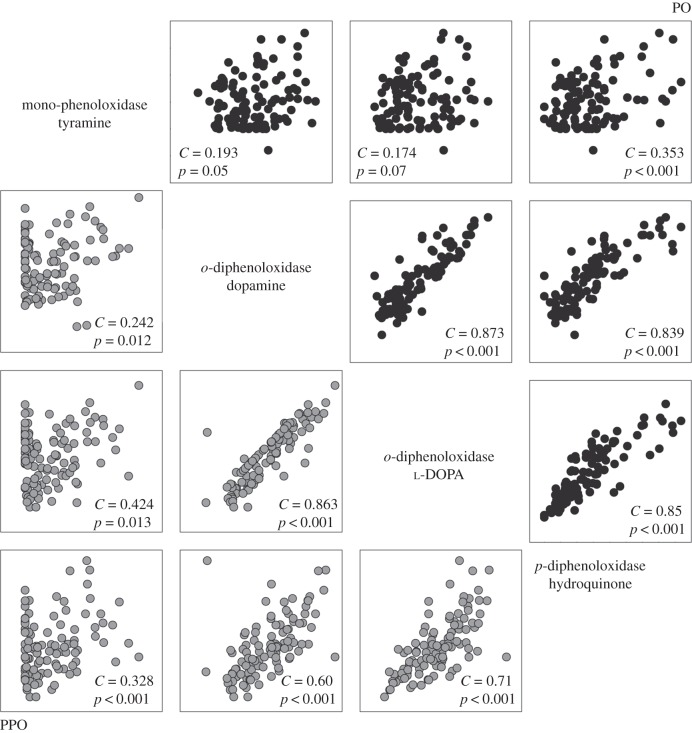
There was a positive correlation between all substrates for both PO and PPO activities (figure 4). The strongest correlation for both PO and PPO activities was between the two o-diphenol substrates, dopamine and l-DOPA (C = 0.87, p < 0.001; C = 0.86, p < 0.001). There was no significant correlation between tyramine (mono-phenoloxidase) PO activity and o-diphenoloxidase activity (tyrosinase-type pathway), with either substrate; however, mono-phenoloxidase (tyramine) activity was a significant predictor of p-diphenoloxidase (C = 0.35, p < 0.001). Inactive PPO forms of mono-phenoloxidase and o-diphenoloxidase activity (tyrosinase-type pathway) were significantly correlated (figure 4). However, the strongest correlations were for PO between hydroquinone and both dopamine and l-DOPA (C = 0.84–0.85; p < 0.001, respectively), which, although weaker, were maintained for PPO, indicating a direct relationship between laccase- and tyrosinase-type activity (figure 4).
Figure 4.
The relationship between melanin-synthesis pathway components for both PO and PPO activities. The four substrates, along the diagonal of the array, form the y-axes, with PO activity on the x-axes of the graphs in black and PPO activity on the x-axes of the graphs (grey).
(iii). Relationships between melanin-synthesis pathway components and disease and bleaching susceptibility
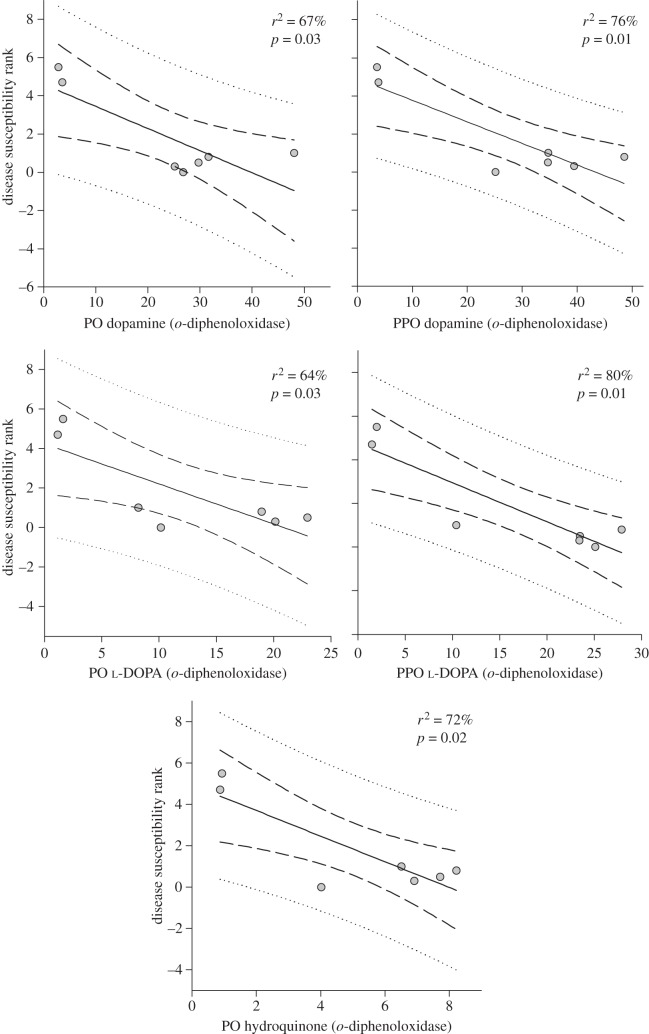
Activities of several of the melanin-synthesis pathway components significantly explained the disease susceptibility rank of seven coral families (figure 5). PPO activity with l-DOPA substrate (tyrosinase-type pathway) explained 80 per cent of the variation in coral family disease susceptibility rank (r2 = 0.80, F1,6 = 20.54; p = 0.01), followed by PPO activity with dopamine (r2 = 0.76, F1,6 = 15.66; p = 0.01). PO activity with hydroquinone (laccase; r2 = 0.72, F1,6 = 13.11; p = 0.02) explained more of the variation in disease rank among families than PO activity with either dopamine (r2 = 0.67, F1,6 = 10.1; p = 0.03) or l-DOPA (r2 = 0.64, F1,6 = 8.74; p = 0.03; figure 5). Neither PPO nor PO activity with tyramine, or PPO activity with hydroquinone significantly explained any variation in disease susceptibility rank.
Figure 5.
The relationship between disease susceptibility rank (as per Palmer et al. [8]) and melanin-synthesis pathway component activity. The solid line indicates the 1st order regression, the dashed lines are the 95% confidence interval and the dotted lines are the 95% prediction interval.
The variation in bleaching mortality index among eight coral families was significantly explained by just two melanin-synthesis pathway components. PO activity with dopamine (tyrosinase-type pathway) explained 61 per cent of the variation (r2 = 0.61, F1,7 = 9.35; p = 0.02), whereas PPO with hydroquinone (laccase) explained 54 per cent (r2 = 0.54, F1,7 = 6.92; p = 0.04; figure 6).
Figure 6.
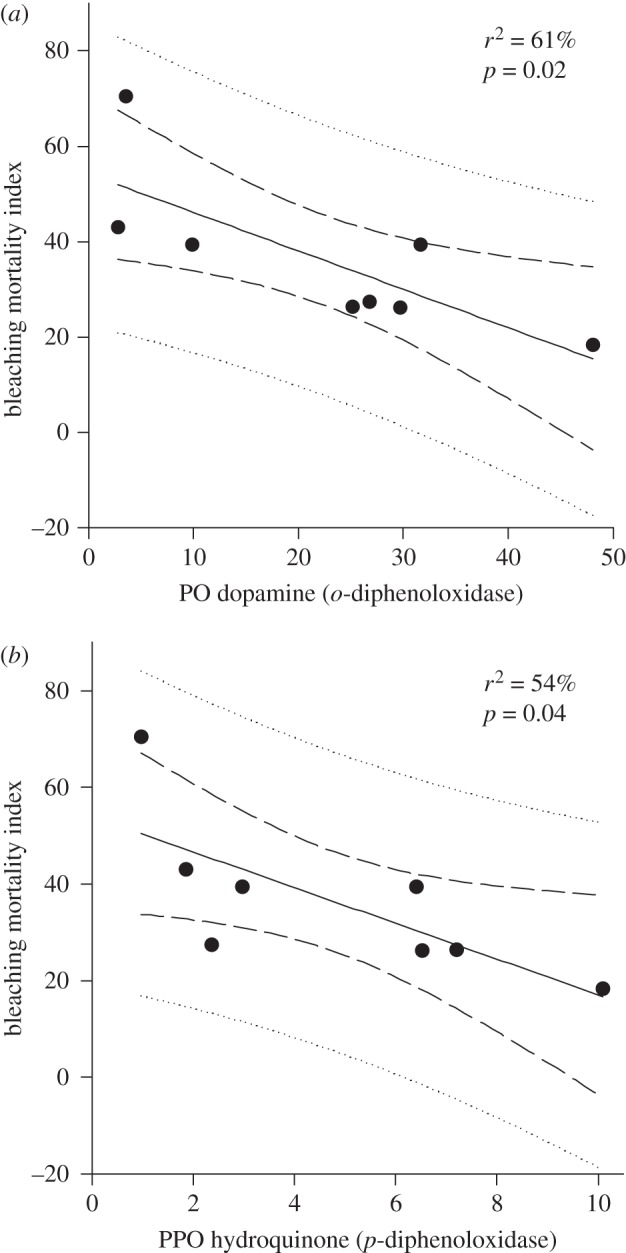
The relationship between both (a) PO with dopamine substrate and (b) PPO with hydroquinone and bleaching mortality index (as per Palmer et al. [8]). The solid line indicates the 1st order regression, the dashed lines are the 95% confidence interval and the dotted lines are the 95% prediction interval.
(b). The coagulation pathway
Transglutaminase activity was demonstrated within a massive species of the hard coral genus Porites. Transglutaminase activity increased significantly with increasing sample extract volume, demonstrating a dose-dependent response (figure 7; r2 = 0.97, F2,4 = 36.05, p = 0.03). Transglutaminase activity was significantly inhibited, by approximately 50 per cent, with the addition of iodoacetamide (figure 7; t4 = 2.846, p = 0.047).
Figure 7.
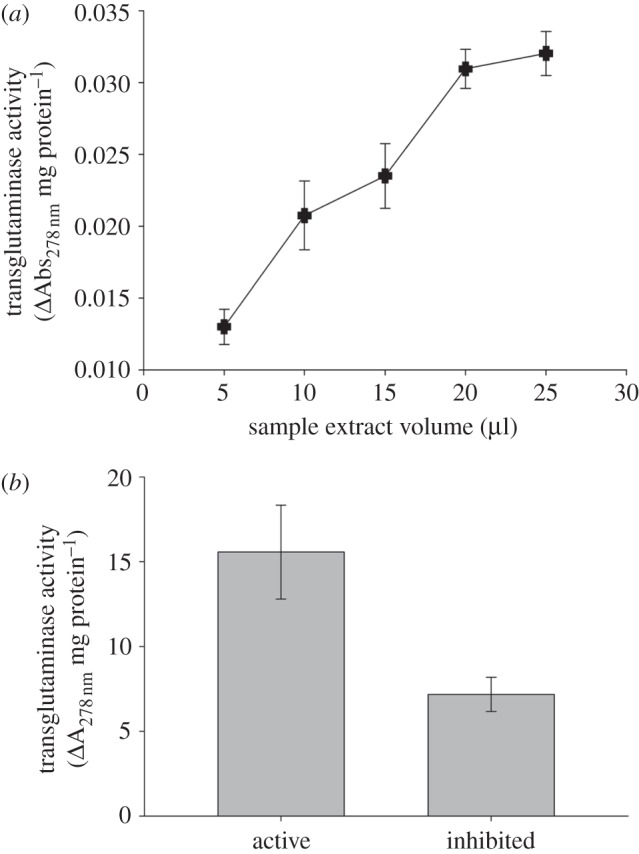
Transglutaminase activity of pooled samples of a Porites massive sp. showing: (a) dose-dependent response (r2 = 0.97, F2,4 = 36.05, p = 0.03); and (b) comparative activity with and without the inhibitor (±s.e.; t4 = 2.846, p = 0.047).
4. Discussion
Three melanin-synthesis pathway components were documented in both their active (PO) and inactive (PPO) forms within a suite of Indo-Pacific anthozoans. This result indicates the presence of different stages of two melanin-synthesis pathways in anthozoans: the tyrosinase-type and laccase pathways, consistent with Caribbean corals [7]. Transglutaminase activity was also found within Porites, the one coral species assayed, demonstrating the presence of the coagulation pathway. Correlations between enzyme activities and disease and bleaching susceptibility suggest that anthozoans may use melanin pathways differently than documented for other invertebrates [22].
(a). Comparison of melanin-synthesis pathway components among taxa
Variation in PO and PPO activities among taxa indicates differences in baseline levels of immunity and different uses of the immune repertoire [16]. Mono-phenoloxidase activity, the precursor to both tyrosinase-type and laccase pathways (figure 1; [12]), was present in all species and varied the least among them. However, activities of the tyrosinase-type and laccase POs and PPOs varied significantly among taxa, indicating functional differences of these pathways [13,23].
For the majority of species, tyrosinase-type activity (o-diphenol substrates) was the highest of the three POs and PPOs, indicating greater baseline activity of this pathway than laccase. This substrate propensity for o-diphenols is consistent with previous reports from other invertebrates [12], including Caribbean corals [7], and indicates the use of the more cytotoxic tyrosinase-type melanin-synthesis pathway over the laccase pathway under healthy conditions [15].
The lower activity of laccase than the tyrosinase-type enzymes suggests that its presence is less crucial to the maintenance of tissue integrity and disease resistance, assuming equivalent effectiveness per unit activity of each enzyme. Although within other invertebrates, melanin from the laccase pathway is advantageous for cuticle hardening [13,15], melanin deposited as a hardened layer would be deleterious in anthozoans, as corals rely on light penetrating their tissues to maintain obligate photosynthetic endosymbionts [24]. However, the presence of ultraviolet (UV) and visible-light-absorbing melanin may be advantageous during times of temperature stress [8]. Laccase activity within anthozoans is therefore likely to be involved in clot stabilization [19] or another aspect of immunity, as hypothesized for the Pacific oyster, which also lacks an integument [22].
Consistent with arthropod substrate specificity, differences in enzyme activities with the two o-diphenol substrates indicate a propensity for dopamine over l-DOPA [12,25]. This disparity was particularly notable for Poritidae, where o-diphenoloxidase activity with dopamine was up to eight times higher than with l-DOPA. This indicates that these two substrates may be used differently and highlights the need to investigate phenoloxidase substrate-specificity of study organisms.
(b). Relationships between melanin-synthesis pathway components
The strong relationship between active PO and inactive PPO for all substrates demonstrates that the activity of one is indicative of the other, particularly using the o-diphenol substrate dopamine. The relationship among melanin-synthesis pathway components indicates potential co-regulation, for example between tyramine and hydroquinone PO activity, but not between tyramine and the o-diphenols. This indicates a direct relationship between mono-phenoloxidase and laccase. The lack of correlation with tyramine and the o-diphenols suggests that the tyrosinase-type pathway is not dependent upon mono-phenoloxidase for activation. As the tyrosinase-type melanin-synthesis pathway is highly cytotoxic and probably involved more specifically in immune defence [3,23], rather than clot stabilization or maintenance [15], it may have tighter controls on activation and different triggers [26] to the laccase pathway.
(c). Relationships between melanin-synthesis pathway components and disease and bleaching susceptibility
Activity of the stored (PPO) tyrosinase-type pathway precursor explained the majority of variation in disease susceptibility among coral families. This indicated a fitness advantage associated with higher constituent levels of this enzyme, potentially as a strong immediate antimicrobial assault that could be activated in response to infection [3]. However, the PO that activates the less cytotoxic laccase pathway explained more of the variation in disease susceptibility than the tyrosinase-type POs. This indicates that coral laccase melanin-synthesis may be involved in infection resistance and the tyrosinase-type in infection elimination, providing different but complimentary functions.
Melanin-synthesis pathway activity also related to coral bleaching-induced mortality. Active tyrosinase-type enzymes (PO) explained the most variation among families, suggesting that the resultant melanin may be used for absorbance of UV and photosynthetically active radiation [8]. The negative relationship between bleaching susceptibility and stored enzymes of the less cytotoxic laccase pathway at the family level suggests that melanin production from this pathway may also enhance bleaching resistance, potentially by protecting against light-enhanced damage without the extreme cytotoxicity of the tyrosinase-type pathway.
(d). The coagulation pathway
Detection of transglutaminase activity in a massive Porites coral species demonstrates the presence of a coagulation pathway component [4] for, to our knowledge, the first time in anthozoans. While only a preliminary result, the identification of an additional innate immune component within corals significantly contributes to the emerging field of coral immunology. Future studies into the regulation of this coagulation pathway component during wound healing of corals would be particularly insightful.
5. Conclusion
We show several melanin-synthesis pathway components and a coagulation enzyme for the first time, to our knowledge, within Indo-Pacific corals. Co-regulation among some melanin-synthesis pathway components, and not others, suggests differing functional roles. Additionally, the relationship between some melanin-synthesis enzymes and both disease susceptibility and bleaching mortality provides further support for their role in both pathogen resistance and tolerance of environmental insults.
Acknowledgments
The authors thank David Abrego and Patricia Warner for assistance with sample collection, and two anonymous reviewers and Prof. Ken Wilson for suggestions that significantly improved the manuscript. Research funding was provided by a Natural Environment Research Council (NERC) grant to J.C.B. (NE/E006949/1) and an Australian Research Council (ARC) Discovery Project grant to B.L.W.
References
- 1.Hoegh-Guldberg O., Bruno J. F. 2010. The impact of climate change on the world's marine ecosystems. Science 328, 1523–1528 10.1126/science.1189930 (doi:10.1126/science.1189930) [DOI] [PubMed] [Google Scholar]
- 2.Suggett D. J., Smith D. J. 2011. Interpreting the sign of coral bleaching as friend versus foe. Glob. Change Biol. 17, 45–55 10.1111/j.1365-2486.2009.02155.x (doi:10.1111/j.1365-2486.2009.02155.x) [DOI] [Google Scholar]
- 3.Cerenius L., Lee B. L., Söderhäll K. 2008. The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol. 29, 263–271 10.1016/j.it.2008.02.009 (doi:10.1016/j.it.2008.02.009) [DOI] [PubMed] [Google Scholar]
- 4.Cerenius L., Soderhall K. 2011. Coagulation in invertebrates. J. Innate Immunity 3, 3–8 10.1159/000322066 (doi:10.1159/000322066) [DOI] [PubMed] [Google Scholar]
- 5.Söderhäll K., Smith V. J. 1986. The prophenoloxidase activating system: the biochemistry of its activation and role in arthropod cellular immunity, with special reference to crustaceans. In Immunity in invertebrates (ed. Brehélin M.), pp. 208–223 Berlin, Germany: Springer [Google Scholar]
- 6.Theopold U., Schmidt O., Soderhall K., Dushay M. S. 2004. Coagulation in arthropods: defence, wound closure and healing. Trends Immunol. 25, 289–294 10.1016/j.it.2004.03.004 (doi:10.1016/j.it.2004.03.004) [DOI] [PubMed] [Google Scholar]
- 7.Mydlarz L. D., Palmer C. V. 2011. The presence of multiple phenoloxidases in Caribbean reef-building corals. Comp. Biochem. Physiol. 159, 372–378 10.1016/j.cbpa.2011.03.029 (doi:10.1016/j.cbpa.2011.03.029) [DOI] [PubMed] [Google Scholar]
- 8.Palmer C. V., Bythell J. C., Willis B. L. 2010. Immunity parameters of reef corals underpin bleaching and disease susceptibility. Fed. Am. Soc. Exp. Biol. 24, 1935–1946 10.1096/fj.09-152447 (doi:10.1096/fj.09-152447) [DOI] [PubMed] [Google Scholar]
- 9.Palmer C. V., Mydlarz L. D., Willis B. L. 2008. Evidence of an inflammatory-like response in non-normally pigmented tissues of two scleractinian corals. Proc. R. Soc. B 275, 2687–2693 10.1098/rspb.2008.0335 (doi:10.1098/rspb.2008.0335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harvell C. D., Jordan E., Merkel S., Raymundo L., Rosenberg E., Smith G., Weil E., Willis B. L. 2007. Coral disease: environmental change and the balance between coral and microbes. Oceanography 20, 58–81 [Google Scholar]
- 11.Nappi A. J., Christensen B. M. 2005. Melanogenesis and associated cytotoxic reactions: applications to insect innate immunity. Insect Biochem. Mol. Biol. 35, 443–459 10.1016/j.ibmb.2005.01.014 (doi:10.1016/j.ibmb.2005.01.014) [DOI] [PubMed] [Google Scholar]
- 12.Sugumaran M. 2002. Comparative biochemistry of eumelanogenesis and the protective roles of phenoloxidase and melanin in insects. Pigment Cell Res. 15, 2–9 10.1034/j.1600-0749.2002.00056.x (doi:10.1034/j.1600-0749.2002.00056.x) [DOI] [PubMed] [Google Scholar]
- 13.Yatsu J., Asano T. 2009. Cuticle laccase of the silkworm, Bombyx mori: purification, gene identification and presence of its inactive precursor in the cuticle. Insect Biochem. Mol. Biol. 39, 254–262 10.1016/j.ibmb.2008.12.005 (doi:10.1016/j.ibmb.2008.12.005) [DOI] [PubMed] [Google Scholar]
- 14.Iwanaga S., Lee B. L. 2005. Recent advances in the innate immunity of invertebrate animals. J. Biochem. Mol. Biol. 38, 128–150 10.5483/BMBRep.2005.38.2.128 (doi:10.5483/BMBRep.2005.38.2.128) [DOI] [PubMed] [Google Scholar]
- 15.Dittmer N. T., Gorman M. J., Kanost M. R. 2009. Characterization of endogenous and recombinant forms of laccase-2, a multicopper oxidase from the tobacco hornworm, Manduca sexta. Insect Biochem. Mol. Biol. 39, 596–606 10.1016/j.ibmb.2009.06.006 (doi:10.1016/j.ibmb.2009.06.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan P. J., Deaton L. E. 2005. Characterization of phenoloxidase from Crassostrea virginica hemocytes and the effect of Perkinsus marinus on phenoloxidase activity in the hemolymph of Crassostrea virginica and Geukensia demissa. J. Shellfish Res. 24, 477–482 [Google Scholar]
- 17.Meszaros A., Bigger C. 1999. Qualitative and quantitative study of wound healing processes in the Coelenterate, Plexaurella fusifera: spatial, temporal, and environmental (light attenuation) influences. J. Invert. Pathol. 73, 321–331 10.1006/jipa.1999.4851 (doi:10.1006/jipa.1999.4851) [DOI] [PubMed] [Google Scholar]
- 18.Olano C. T., Bigger C. H. 2000. Phagocytic activities of the gorgonian coral Swiftia exserta. J. Invert. Pathol. 76, 176–184 10.1006/jipa.2000.4974 (doi:10.1006/jipa.2000.4974) [DOI] [PubMed] [Google Scholar]
- 19.Palmer C. V., Traylor-Knowles N. G., Willis B. L., Bythell J. C. 2011. Corals use similar immune cells and wound-healing processes as those of higher organisms. PLoS ONE 6, e23992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page C. A., Willis B. L. 2008. Epidemiology of skeletal eroding band on the Great Barrier Reef and the role of injury in the initiation of this widespread coral disease. Coral Reefs 27, 257–272 10.1007/s00338-007-0317-8 (doi:10.1007/s00338-007-0317-8) [DOI] [Google Scholar]
- 21.Marshall P. A., Baird A. H. 2000. Bleaching of corals on the Great Barrier Reef: differential susceptibilities among taxa. Coral Reefs 19, 155–163 10.1007/s003380000086 (doi:10.1007/s003380000086) [DOI] [Google Scholar]
- 22.Luna-Acosta A., Rosenfeld E., Amari M., Fruitier-Arnaudin I., Bustamante P., Thomas-Guyon H. 2010. First evidence of laccase activity in the Pacific oyster Crassostrea gigas. Fish Shellfish Immunol. 4, 719–726 [DOI] [PubMed] [Google Scholar]
- 23.Bidla G., Hauling T., Dushay M. S., Theopold U. 2008. Activation of insect phenoloxidase after injury: endogenous versus foreign elicitors. J. Innate Immun. 1, 301–308 10.1159/000168009 (doi:10.1159/000168009) [DOI] [PubMed] [Google Scholar]
- 24.Bythell J. C. 1988. A total nitrogen and carbon budget for the elkhorn coral Acropora palmata (Lamarck). In Proc. 6th Int Coral Reef Symp., Townsville, Australia, vol. 2, pp. 535–540. [Google Scholar]
- 25.Hall M., Scott T., Sugumaran M., Soderhall K., Law J. H. 1995. Proenzyme of M. sexta phenoloxidase purification, activation, substrate specificity of the active enzyme and molecular cloning. Proc. Natl Acad. Sci. USA 92, 7764–7768 10.1073/pnas.92.17.7764 (doi:10.1073/pnas.92.17.7764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cerenius L., Kawabata S. I., Lee B. L., Nonaka M., Soderhall K. 2010. Proteolytic cascades and their involvement in invertebrate immunity. Trends Biochem. Sci. 35, 575–583 10.1016/j.tibs.2010.04.006 (doi:10.1016/j.tibs.2010.04.006) [DOI] [PubMed] [Google Scholar]