Abstract
By living in social groups with potential competitors, animals forgo monopolizing access to resources. Consequently, debate continues over how selection might favour sociality among competitors. For example, several models exist to account for the evolution of shared reproduction in groups. The ‘concession model’ hypothesizes that dominant reproducers benefit from the presence of subordinates, and hence tolerate some reproduction by subordinates. This mutual benefit to both dominants and subordinates may provide a foundation for the formation of social groups in which multiple members reproduce—a necessary step in the evolution of cooperation. To date, however, the concession model has received virtually no support in vertebrates. Instead, the vast majority of vertebrate data support ‘limited control models’, which posit that dominant reproducers are simply unable to prevent subordinates from reproducing. Here we present the most comprehensive evidence to date in support of the concession model in a vertebrate. We examined natural variation in the number of adult males in gelada (Theropithecus gelada) reproductive units to assess the extent of reproductive skew in multi-male units. Dominant (‘leader’) males in units that also had subordinate (‘follower’) males had a 30 per cent longer tenure than leaders in units that did not have followers, mainly because followers actively defended the group against potential immigrants. Follower males also obtained a small amount of reproduction in the unit, which may have functioned as a concession in return for defending the unit. These results suggest that dominants and subordinates may engage in mutually beneficial reproductive transactions, thus favouring male–male tolerance and cooperation.
Keywords: reproductive transaction, reproductive skew, cooperation, paternity, gelada
1. Introduction
In most mammals, male reproductive success is limited by access to mates [1]. It thus follows that males can increase their access to females by increasing the ratio of females to males in their reproductive group. Indeed, polygynous single-male groups are likely to be the ancestral mammalian social system, representing a majority of extant mammalian mating systems [2–6]. Nevertheless, many species of primates live in multi-male groups, where males compete for reproductive access to a limited number of females. Such competition often leads to highly variable (i.e. skewed) male reproductive success, with dominant individuals obtaining the majority (but not all) of the reproduction [7,8]. Thus, the presence of subordinate males has obvious reproductive costs to the dominant male.
The reproductive competition between dominant and subordinate individuals has been the focus of much theoretical and empirical research and debate over the past several decades [9,10]. Reproductive skew models attempting to explain dominants’ apparent ‘tolerance’ of subordinate rivals have typically fallen into two broad models, which differ in their assumptions about the relative power of dominants and subordinates: the limited control model and the concession model. Limited control models (also known as compromise [11] and tug-of-war models [12]) assume that a male's ability to monopolize reproduction within his group depends on social density, synchrony of female cycling (e.g. priority of access [13]) and the number of other males in the group. In these models, reproductive skew is the result of a costly competition between dominant and subordinate males for reproduction that negatively affects the dominant's fitness and, in some cases, overall group productivity.
In contrast, concession (also known as transactional [11]) models assume that the dominant male is able to control all reproduction in his group, but nonetheless tolerates some mating by subordinates because he benefits from their presence [14,15]. In other words, despite the reproduction ceded to the subordinate as a ‘staying incentive’, the dominant has a net gain in reproductive success.
Although concession models are mathematically viable (see [14] for review), most studies of reproductive skew in male mammals have provided support only for limited control models [11,16]. To date, only three studies have provided some support for concession models in male mammals [17–19]. Males in each of these species, however, habitually live in multi-male groups, suggesting that dominant males cannot prevent other males from immigrating into their group. Furthermore, for two of these species, lions (Panthera leo) and chimpanzees (Pan troglodytes), some degree of male cooperation is arguably obligatory [20,21]. As a result, data from these species cannot address variation in whether males live in single-male or multi-male groups, or whether dominant males derive net benefits or incur net costs when other males are present.
On the other hand, two studies have considered the costs and benefits that male mammals derive from living in single-male groups, and they have reported conflicting results. The first, on mountain gorillas (Gorilla beringei beringei), indicated that dominant males incurred only costs and no measurable benefits from the presence of a subordinate male, providing support for the limited control model [22]. Subordinate male gorillas apparently have few alternative options other than remaining as a subordinate in a group with a dominant male, so that dominant males would not need to cede reproductive opportunities to subordinates as a staying incentive (as predicted by the ‘unified model of reproductive skew’ [23]). In contrast, the second study, on red-fronted lemurs (Eulemur fulvus rufus), found that dominant males were less likely to be evicted by immigrant males when living in groups with more males as compared with groups with fewer males [24]. Although subordinate males sired 29 per cent of the offspring in this population, dominants benefited via an increase in tenure [24,25]. Unfortunately, single-male groups in this population were rare (4/45 group-years) and unstable (all single-male groups were taken over within a year [24]), complicating any definitive comparison of concession and limited control models.
In this study, we examine the causes and consequences of male reproductive skew in the gelada—a gregarious primate species that regularly forms both single- and multi-male groups, and has high male replacement rates, high sexual dimorphism and a female-biased adult sex ratio. Gelada reproductive ‘units’—social groups in which reproductively active individuals reside—are composed of one dominant (‘leader’) male and 1–12 females who are likely to be closely related to each other [26,27]. Geladas forage in very large (more than 1100 individuals) semi-stable aggregations composed of multiple units, forming a fluid, multi-level society [28–30]. In addition to potential cuckoldry from males in neighbouring units, leader males face frequent aggressive challenges from unattached males living in all-male ‘bachelor’ groups. Following a successful challenge (hereafter, ‘takeover’), a bachelor male replaces the leader male in the unit and gains reproductive access to the females [31].
In approximately one-third of all units, the leader male co-resides with one or more subordinate males (‘followers’), to form a multi-male unit [31,32]. There are two routes to becoming a follower among gelada males. First, a former leader or follower male can remain in the unit as an ‘old follower’ after a takeover. Second, some takeovers involve multiple bachelor males in which one male eventually becomes the unambiguous leader and one or more of the remaining bachelors remain in the unit as ‘new followers’ [31]. Furthermore, follower males are not restricted to the unit that they initially join; they may leave a unit to join a different unit or to (re)join a bachelor group [31].
The presence of followers in only some units suggests that leader males with followers are either unable to exclude follower males from their units or unwilling to do so, despite the potential cost of increased reproductive competition. From a leader male's perspective, tolerating a follower is adaptive only if it increases the leader's net reproductive output compared with what he would achieve by excluding a follower male. From a follower male's perspective, joining a unit as a follower is only adaptive if it increases his own reproductive output compared with what he would achieve by joining a different unit or returning to a bachelor group.
We took advantage of natural variation in the number of males in gelada reproductive units to examine the applicability of the two reproductive skew models in the evolution of multi-male groups. We first used a combination of behavioural observations and molecular genetic paternity analysis to measure male reproductive success within single- and multi-male units. We then drew on longitudinal behavioural data to investigate whether leader males might be tolerating the presence of followers. Specifically, under limited control models, follower reproduction would result from the limited ability of the leader male to exclude follower males. Consequently, the best competitors would be males who are able to completely exclude other males and are leaders of single-male units. These males should score well on other indicators of competitive ability such as unit size and tenure length. Conversely, under concession models, leader males benefit from the presence of followers through territory or group defence [33]. Thus, under concession models, leaders in multi-male units should have longer tenures and/or larger units (i.e. more females) than leader males in single-male units. Finally, we looked for evidence of staying incentives—whether reproduction by followers was associated with longer follower tenure.
2. Methods
(a). Study sample and population
Study subjects were members of a population of wild geladas living in the Simien Mountains National Park, Ethiopia. Data for this study were collected during a 60-month period from January 2006 to January 2011 as part of the University of Michigan Gelada Research Project. Subjects included 45 leader males, 28 follower males and 134 females in 21 reproductive units.
A team of four observers conducted a weekly census of all individuals, identified all leader and follower males, noted the presence and identity of females in all units, recorded the individuals involved in unit takeovers, noted the days after a takeover that males entered units and noted all births of new infants.
(b). Behavioural data collection
To determine whether follower males directly participated in unit defence against bachelors, we collected data on chases involving known unit males and bachelors from January 2008 to April 2011. We attempted to collect all occurrences [34] of chases, but we did miss some occurrences. However, we have evidence to suggest that our records represented an unbiased sample of chases, because we were not more likely to identify a male from a multi-male unit in a chase than a male from a single-male unit. For each chase, we recorded the identity of the unit male(s) and whether the participant was a leader or a follower. To assess whether extra males in the unit deterred potential intruders, we calculated the rate of chases for single- and multi-male units, while controlling for the number of females present in the unit.
(c). Genetic analysis
From January 2009 to April 2011, we obtained multiple faecal samples from 78 offspring with known birth dates. We also obtained multiple corresponding faecal samples for 64 of 65 known mothers (11 mothers had multiple offspring included in the analyses) and 52 potential fathers, including all leaders and followers of the units in which the offspring were born. One mother disappeared before we could collect a sample. All samples were collected using methods described by Alberts et al. [35], with the exception that our samples were collected in RNAlater (Applied Biosystems/Ambion, Austin, TX). We extracted DNA from the faecal samples using the QIAamp DNA Stool Mini Kit (Qiagen, Valencia, CA), with slight modifications as described by Buchan et al. [36]. These extractions yielded sufficient genetic material to genotype 76/78 sampled offspring, 52/52 potential fathers and 64/65 known mothers.
We genotyped samples using PCR at 20 human-derived MapPairs microsatellite loci (AGAT006, AGAT007, D1s548, D2s122, D2s1399, D3s1766, D3s1768, D5s111, D5s1457, D6s1960, D6s291, D6s311, D6s501, D7s817, D8s1106, D11s2002, D14s306, D17s791, D18536, D18s851), which were found to be variable in this gelada population (average=6.05 alleles/locus). We performed PCR reactions at Duke University using Qiagen multiplex PCR kits with three to six loci run in a single PCR reaction. One primer of each pair was labelled with a 5′ fluorescent dye to facilitate visualization. PCR products were separated via capillary electrophoresis on an ABI 3730 automated DNA Analyzer at the Duke Institute for Genome Sciences and Policy DNA Sequencing Facility Core. We used GeneMapper v. 3.5 software to assign microsatellite genotypes for each locus.
We assigned paternity using maximum-likelihood analysis with the program CERVUS v. 3.0 [37]. We assumed a pool of 52 candidate fathers (which we assumed represented 70% of the total possible candidate fathers), 91.5 per cent loci typed, a minimum of 12 loci typed and a 1.5 per cent genotyping error (estimated genotyping error based on number of mismatches between known mother–offspring pairs was 0.95%). The confidence levels associated with paternity assignments were obtained by simulating parentage for 100 000 offspring based on allele frequencies derived from the study population. Since we had the genotypes of most known mothers (75/76 offspring), we used these genotypes in the simulation to more accurately assess paternity. Paternity was assigned with 95 per cent confidence for the 75 mother–father–offspring trios that we were able to completely genotype. For all mother–father–offspring trios, there were only 35 mismatches of the 2680 alleles compared (1.3%), which is similar to our genotyping error rate estimated above for mother–offspring pairs alone. For the one offspring for which we did not have a sample from the known mother, the father was assigned with 95 per cent confidence using CERVUS and also by performing exclusion analysis; the assigned father was the only male in the population with no mismatches at 18 loci compared.
We calculated Queller and Goodnight's genetic estimate of relatedness [38] using the analysis program GenAlEx [39]. We calculated pairwise relatedness, R, for all sampled adults in the population (n = 114 females, 94 males).
(d). Tenure analysis
All statistical analyses were conducted with R statistical software [40]. Survival analysis of leader tenure length (n = 29) was carried out using the R package ‘Survival’ [41]. We used generalized linear mixed models (GLMM), calculated using the function ‘lmer’ of the R package ‘lme4’ [42], to test the effect of unit size and presence of a follower on the probability of a takeover (n = 47 possible takeover events; see the electronic supplementary material for how these data were censored). Models were fitted using binomial error structure and logit link. Because different units appeared with different frequencies in our overall dataset, we entered the unit ID as a categorical random effect in the model. The number of females in a unit (a continuous variable) and the presence or absence of a follower (a binary variable) were entered as fixed effects, and the occurrence or lack of a takeover was entered as the binary dependent variable. We then used a likelihood ratio test to compare a model containing number of females in a unit and presence/absence of a follower male as additive effects against our null model, which contained only the random effect, unit ID. Finally, we computed all possible models that could be built with the two predictor variables and ranked them using Akaike's information criterion (AIC).
We calculated follower tenure for 22 followers in 12 multi-male units for whom we knew their exact day of unit entry and either date of exit or the date the study period had ended (see the electronic supplementary material for information on how these data were censored). Follower tenure was calculated as the amount of time a follower spent with a particular leader male. We calculated a linear mixed model (LMM) to determine the effect of paternity success (‘sired an offspring as a follower’ and ‘did not sire an offspring as a follower’) and follower type (‘old’ versus ‘new’) on follower tenure in the unit. Unit, leader ID and follower ID were entered as random effects, while follower type, paternity success and number of females in the unit were entered as fixed effects. All possible models were compared using AIC. Significance of each predictor in the best model (i.e. lowest AIC) was calculated using a Markov chain Monte Carlo simulation of 10 000 iterations.
We estimated the net gain in offspring sired for leaders in multi-male units compared with leaders in single-male units using the following formula:
We used the average values of tenure, number of females and percentage of offspring sired by the leader for multi-male units and single-male units. We calculated the average interbirth interval as the number of surviving offspring per female-year observed (i.e. number of mature females times number of years that we had followed that unit) for units with or without followers.
3. Results
(a). Paternity and kinship
In single-male units, leader males sired 100 per cent (47/47) of all offspring conceived during their tenure. In multi-male units, leader males sired 83 per cent (24/29) of all offspring conceived during their tenure. All genotyped offspring were assigned a father within their unit. Thus, we observed no cases of extra-unit paternity. All five offspring not fathered by the leader male were sired by a follower male in the unit. Two offspring were sired by old followers and three were sired by new followers. All offspring sired by followers were conceived after the male dominance hierarchy within the unit had stabilized (i.e. there was only one leader male)—a process that takes up to 90 days [43]. Followers who sired offspring did not appear larger or older than followers who did not sire offspring, but we lacked exact age and size data. In no cases of follower paternity was the mother potentially a daughter of the current leader male. Furthermore, followers sired offspring in units of all sizes (specifically, units of four, six, eight and ten females). The majority of the follower-sired offspring were conceived within the first year of the follower's tenure (four of five). On average, leaders and followers in our population were unrelated to each other (R = 0.05 ± 0.03, n = 25 leader–follower dyads; see the electronic supplementary material, figure S1), suggesting that any tolerance shown by leader males towards followers was unlikely to have been the result of shared kinship.
(b). Leader tenure length and group defence
Survival analysis of tenure lengths for 29 males with known start dates and known or right-censored end dates (i.e. either we observed the date on which they experienced a takeover or the study period ended and they were still the leader) revealed a highly linear relationship between tenure length and takeover probability. We found a constant probability of takeover across a male's tenure (figure 1), indicating that inexperienced leaders were just as likely to succumb to takeovers as experienced leaders. We next examined the distributions of our left-censored tenures (those that had begun before the onset of observations; n = 18), and compared them with our complete (n = 11) and right-censored tenures (n = 18). We found that they were very similar (see the electronic supplementary material, figure S2). This suggested that including the left-censored values along with the complete and right-censored tenures in further analyses was warranted. Consequently, we modelled sources of variance in takeovers (below) using all the data.
Figure 1.
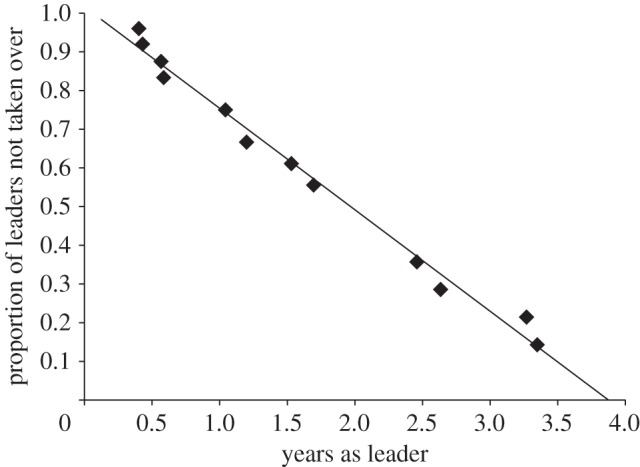
Survival curve showing the relationship between tenure length and probability of a takeover. The linear curve indicates a constant probability of takeover across a male's tenure.
Using the occurrence of takeover as the dependent variable, the GLMM in which number of females and presence of a follower were incorporated as predictor variables was a significantly better fit to the data than the null model (p < 0.05). When all possible models that could be built with these two predictor variables were compared using AIC, the best model was the purely additive model that included both predictors with no interactions (table 1). The number of females in a unit was positively correlated with the probability of a takeover [31,44], and, more importantly, single-male units were more likely to be taken over than multi-male units. Multi-male units had more females (mean = 6.25, range = 2–10 females, s.e.m. = 0.47) than single-male units (mean = 5.07, range = 1–10 females, s.e.m. = 0.38), but the presence of a follower decreased the likelihood of a takeover at all unit sizes (figure 2). Multi-male units were taken over at a pooled rate of 0.27 takeovers per unit per year (seven takeovers pooled across 25.65 unit-years of observation; i.e. 3.70 year average tenure), while single-male units were taken over at a pooled rate of 0.35 takeovers per unit per year (20 takeovers pooled across 56.96 unit-years of observation; i.e. 2.86 year average tenure).
Table 1.
Results of best-fit GLMM describing the relationships among unit types and probability of takeover.
| best model | AIC | χ2 | d.f. | p-value (χ2) |
| no. of females + presence of follower | 72.402 | 8.9314 | 2 | 0.01150* |
| fixed effects | estimate | s.e. | Z | p-value (z) |
| no. of females | 0.4116 | 0.2007 | 2.051 | 0.0403* |
| presence of follower | −2.0217 | 0.8492 | −2.381 | 0.0173* |
Figure 2.
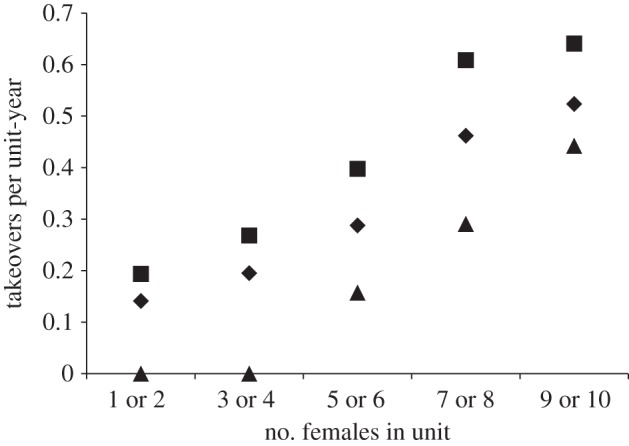
Number of females in unit versus takeover rate per unit-year observed. Unit size was positively correlated with takeover rate. Single-male units (units without followers) were taken over more frequently than multi-male units (units with followers), regardless of unit size. Diamonds, all units; squares, single-male unit; triangles, multi-male unit.
We then calculated the average number of surviving offspring per female in single- and multi-male units. There was a trend in which females in multi-male units produced more surviving offspring per female-year observed than those in single-male units. Specifically, multi-male units had 81 surviving offspring in 217 female-years, resulting in 0.37 surviving offspring per female-year, while single-male units had 63 surviving offspring in 227 female-years, resulting in 0.28 infants per female-year (i.e. average female interbirth interval in multi-male units = 2.67 years; in single-male units = 3.60 years; interbirth intervals calculated as the inverse of the number of surviving offspring per female-year followed). We then estimated the net benefit to leaders of multi-male units compared with single-male units (see §2 for formula) and found that leaders in multi-male units had, on average, three more surviving offspring during their tenure than leaders in single-male units (multi-male unit leaders = 7.2 surviving offspring, single-male unit leaders = 4.0 surviving offspring). Using these parameters, we calculated the per cent of subordinate reproduction at which the leaders of multi-male units would have fewer surviving offspring than the leaders of single-male units. We found that even if there was equal sharing (i.e. leader and follower(s) each sired 50% of the offspring in the unit), the leader males of multi-male units would still have higher reproductive success (4.3 surviving offspring) than leaders of single-male units (4.0 surviving offspring). These data support the prediction of the concession model of reproductive skew stipulating that subordinates receive the minimum share of reproduction compatible with group stability [14].
Unit males (i.e. leaders or followers) were involved in 118 competitive chases (a measure of unit defence [31]) with bachelor males. Bachelor males showed a trend towards chasing males in single-male units more often than males in multi-male units (average chases per female per day observed for: single-male units = 0.26, multi-male units = 0.18; 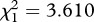 , p = 0.0574). In 38 per cent (45/118) of the chases, the unit male belonged to a multi-male unit. In 20 per cent (9/45) of the chases, the follower male was either the sole participant from the unit or he participated with one or more other unit males in the chase. Thus, follower males actively defended their unit from the threat of a takeover by bachelor males.
, p = 0.0574). In 38 per cent (45/118) of the chases, the unit male belonged to a multi-male unit. In 20 per cent (9/45) of the chases, the follower male was either the sole participant from the unit or he participated with one or more other unit males in the chase. Thus, follower males actively defended their unit from the threat of a takeover by bachelor males.
(c). New follower entries
New followers typically entered units during the 90-day ‘chaotic’ period following a takeover (19/22 new follower entries occurred during this period). Half of the new followers (11/22) entered the unit within a week of the takeover event. All three of the new followers who entered a unit 90 days after the initial takeover event left the unit within 90 days of entering and failed to sire offspring in the unit.
(d). Follower tenure
The tenure length of follower males was predicted significantly better by the LMM with number of females, follower type and follower paternity success as predictor variables than by the null model, which included only random effects (p < 0.05). When all possible models that could be built with these three predictor variables were compared, the best model was the purely additive model that included only follower type and follower paternity success, and did not include number of females as a predictor. Followers that sired offspring during their time as a follower had a longer tenure than followers that did not sire offspring (did sire=960 days, did not sire=309 days), and this was significant in our model (p < 0.01; figure 3) even when controlling for follower type, which also predicted tenure (mean tenure of old followers = 584 days; mean tenure of new followers=239 days; p < 0.01).
Figure 3.
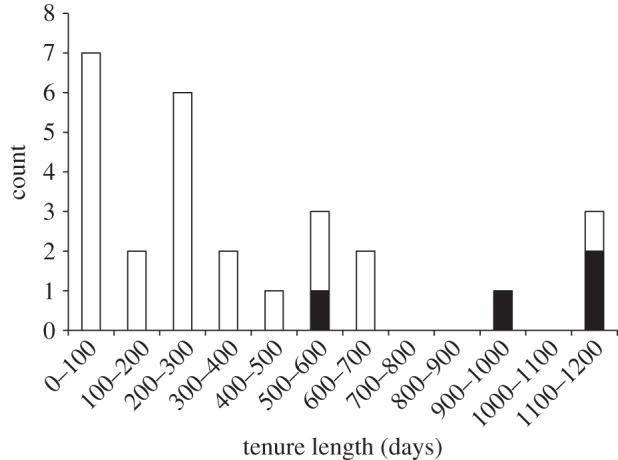
Histogram of follower tenure. Followers who sired offspring (black bars) had longer tenures than followers who did not sire offspring (white bars).
4. Discussion
(a). Effects of followers on paternity
All offspring in this study were sired by males within a unit, indicating that unit males (leaders and followers) were effective in preventing extra-unit males from reproducing despite considerable spatial overlap between units [30]. Yet, within multi-male units, leader males in units with followers did not sire all offspring born during their tenure; they sired 83 per cent of offspring. Although their immediate reproductive output was diminished, leader males nevertheless benefited from the presence of followers in at least two ways. First, multi-male units were slightly larger than single-male units. This pattern could result because males in larger units were less able to exclude followers, or because the presence of a follower allowed a leader to take over a larger unit, or both. Whatever the mechanism, the presence of a follower was associated with a leader's access to a larger number of females. Second, in accordance with a previous study, in units of all sizes, leaders in multi-male units were less susceptible to takeovers than leaders in single-male units [31]. As a result, the presence of a follower was correlated with an increase of approximately 30 per cent in the duration of a leader male's tenure. Behavioural data indicate that followers actively defended the unit from takeover by participating in competitive chases against bachelors, which could contribute to longer leader male tenures. Additionally, we found that bachelors targeted multi-male units less frequently than single-male units, supporting the hypothesis that the presence of an additional male acted as a deterrent to bachelors’ intent on taking over a unit. An additional male in the group increases the male-to-female sex ratio, which has also been shown to affect the probability of male immigration in other species [45–49]. Taken together, these observations show that leader males clearly benefitted from the presence of a follower male.
(b). Concession versus limited control models of reproductive skew
Our data on reproductive skew in geladas represent the best evidence to date for a concession model of reproductive skew in a wild mammal. Leader males that tolerated followers had a longer tenure and access to more females than leader males that did not tolerate followers because the followers actively defended the unit. Follower males had limited but positive reproductive success. One likely interpretation of these results is that leaders tolerated some reproduction by follower males (i.e. made ‘concessions’) as a staying incentive (see below for alternative explanations). Importantly, follower males that did not successfully reproduce within their group were more likely to leave, as has been found in other species [45,46,50]. Taken together, these results suggest that, in geladas, leader and follower males are potentially engaged in a reproductive transaction that benefits both parties.
We cannot yet rule out the possibility that individual variation in competitive ability affects whether leaders are able to exclude followers—a result that is predicted by the limited control model. However, our results do not support other predictions of this model. First, we have no evidence that within-group competition significantly decreased the dominant males’ reproductive output. In fact, multi-male units produced more offspring per female-year than single-male units, suggesting that males in multi-male units are actually more productive than males in single-male units. Second, leaders of single-male units fathered all offspring regardless of unit size, suggesting that some leader males have the ability to successfully monopolize reproduction within a unit by preventing extra-group males from mating with group females. Furthermore, follower males sired offspring in multi-male units irrespective of unit size. Finally, all of the old and new follower males reproduced after the 90-day ‘chaotic’ period [43] following the initial takeover event, indicating that followers did not rely on the group instability that follows a takeover in order to achieve reproductive success.
Overall, our data suggest that the benefits of tolerating a follower (increased tenure and reproductive access to more females) may outweigh the costs (shared reproduction). Followers benefit by siring a small percentage of offspring within the unit. Furthermore, old followers may have an additional benefit by providing care to their offspring (sired when they were the leader of the unit). New followers, like old followers, obtain only a small amount of reproduction, but unlike old followers they lack the opportunity to provide for existing offspring. However, they are likely to fare better as a new follower in a unit than as a non-reproductive male in a bachelor group. Furthermore, it is possible that new followers eventually develop into effective leader males in another unit. Thus, while some followers may be particularly successful at fathering offspring, it is unlikely that the follower strategy represents an evolutionarily stable alternative mating strategy because leaders sire approximately five times (83%/17%) as many offspring as the followers during their tenure. For the follower strategy to be as successful as the leader strategy, the average follower would need to have a tenure five times as long as the average leader tenure (3.07 year average tenure; 27 takeovers pooled across 82.61 unit-years of observation), which is years longer than the lifespan of wild adult gelada males [31]. In part, this may explain why multi-male gelada units comprise only one-third of the population, despite the fact that the majority of reproductively active geladas (i.e. leaders, females and old followers) fare better in such units. Females may also fare better in multi-male units, and may even encourage the presence of followers [51] because females suffer a high risk of infanticide or male-induced pregnancy termination if their leader is replaced [52,53]. By encouraging and/or tolerating the presence of followers, females may increase the likelihood of paternal investment by the follower [54] while decreasing the likelihood of a takeover.
It is possible that selection may favour the acceptance of subordinate males into units, but not the sharing of reproduction. Stronger support for the concession would require evidence that leader males benefit (i.e. have higher reproductive output) not only from accepting follower(s) into the unit, but also from allowing them to reproduce. Our data strongly support the first part of the model, as we found that leader males gained a net reproductive benefit if they had a follower in their group. However, our data represent only indirect evidence for the second part (that leader males had higher reproductive success if they allowed followers to reproduce); we found that follower males remained in groups longer if they succeeded in reproducing. A larger dataset will be required to directly compare the success of leader males in groups where followers did and did not reproduce. In both concession models and limited control models, theory predicts that there will be conflict between the dominant and subordinate on the exact proportion of reproduction gained by the subordinate [55]. Nevertheless, our data suggest that selection favours leader males that do not consistently exclude followers. These data are important to our understanding of the evolution of sociality among reproductive competitors. In fact, recent models have begun to focus on the evolution of sociality in non-cooperative breeders [56]. Tolerance and cooperation are prerequisites to the evolution of sociality among reproductive competitors. We found that dominant males can benefit by living with a reproductive subordinate in their group, demonstrating a benefit of forming multi-male groups in a predominantly single-male system—an important step in the evolution of sociality.
Acknowledgements
This research was approved by the University Committee on Use and Care of Animals (UCUCA no. 09554) at the University of Michigan and the University of Pennsylvania Institutional Animal Care and Use Committee (IACUC no. 802996), and adhered to the laws and guidelines of Ethiopia.
We thank the Ethiopian Wildlife Conservation Authority (EWCA) along with the wardens and staff of the Simien Mountains National Park for the opportunity to conduct research on geladas. We are grateful to D. Cheney, R. Seyfarth and two anonymous reviewers for their insightful comments on earlier drafts of this manuscript. We also thank J. Beehner and the members of the University of Michigan Gelada Research Project (in particular, Ambaye Fanta and Eshetie Jajaw) for assistance in data collection. We additionally thank J. Tung, A. Burrell, C. Fitzpatrick, P. Chiyo, S. Morrow, J. Stroud and J. Gordon for their help and guidance in the genetics lab, and Duke University for logistical support. This research was funded by the National Science Foundation (BCS-0715179, BCS-0962118 and the Graduate Research Fellowship Programme), the Leakey Foundation, the National Geographic Society (no. 8100–06), the University of Pennsylvania and the University of Michigan.
References
- 1.Clutton-Brock T., Parker G. 1992. Potential reproductive rates and the operation of sexual selection. Q. Rev. Biol. 67, 437–456 10.1086/417793 (doi:10.1086/417793) [DOI] [Google Scholar]
- 2.Orians G. H. 1969. On the evolution of mating systems in birds and mammals. Am. Nat. 103, 589–603 10.1086/282628 (doi:10.1086/282628) [DOI] [Google Scholar]
- 3.Emlen S. T., Oring L. W. 1977. Ecology, sexual selection, and the evolution of mating systems. Science 197, 215–223 10.1126/science.327542 (doi:10.1126/science.327542) [DOI] [PubMed] [Google Scholar]
- 4.Kleiman D. G. 1977. Monogamy in mammals. Q. Rev. Biol. 52, 39–69 10.1086/409721 (doi:10.1086/409721) [DOI] [PubMed] [Google Scholar]
- 5.Rutberg A. T. 1983. The evolution of monogamy in primates. J. Theor. Biol. 104, 93–112 10.1016/0022-5193(83)90403-4 (doi:10.1016/0022-5193(83)90403-4) [DOI] [PubMed] [Google Scholar]
- 6.Clutton-Brock T. H. 1989. Review lecture: mammalian mating systems. Proc. R. Soc. Lond. B 236, 339–372 10.1098/rspb.1989.0027 (doi:10.1098/rspb.1989.0027) [DOI] [PubMed] [Google Scholar]
- 7.Cowlishaw G., Dunbar R. 1991. Dominance rank and mating success in male primates. Anim. Behav. 41, 1045–1056 10.1016/S0003-3472(05)80642-6 (doi:10.1016/S0003-3472(05)80642-6) [DOI] [Google Scholar]
- 8.Hager R., Jones C. B. (eds) 2009. Reproductive skew in vertebrates: proximate and ultimate causes, 1st edn Cambridge, UK: Cambridge University Press [Google Scholar]
- 9.Port M., Kappeler P. M. 2010. The utility of reproductive skew models in the study of male primates, a critical evaluation. Evol. Anthropol. 19, 46–56 10.1002/evan.20243 (doi:10.1002/evan.20243) [DOI] [Google Scholar]
- 10.Nonacs P., Hager R. 2010. The past, present and future of reproductive skew theory and experiments. Biol. Rev. 86, 271–298 10.1111/j.1469-185X.2010.00144.x (doi:10.1111/j.1469-185X.2010.00144.x) [DOI] [PubMed] [Google Scholar]
- 11.Clutton-Brock T. 1998. Reproductive skew, concessions and limited control. Trends Ecol. Evol. 13, 288–292 10.1016/S0169-5347(98)01402-5 (doi:10.1016/S0169-5347(98)01402-5) [DOI] [PubMed] [Google Scholar]
- 12.Reeve H. K., Emlen S. T., Keller L. 1998. Reproductive sharing in animal societies: reproductive incentives or incomplete control by dominant breeders. Behav. Ecol. 9, 267–278 10.1093/beheco/9.3.267 (doi:10.1093/beheco/9.3.267) [DOI] [Google Scholar]
- 13.Altmann S. 1962. A field study of the sociology of rhesus monkeys, Macaca mulatta. Ann. NY Acad. Sci. 102, 338–435 10.1111/j.1749-6632.1962.tb13650.x (doi:10.1111/j.1749-6632.1962.tb13650.x) [DOI] [PubMed] [Google Scholar]
- 14.Johnstone R. A. 2000. Models of reproductive skew: a review and synthesis (Invited Article). Ethology 106, 5–26 10.1046/j.1439-0310.2000.00529.x (doi:10.1046/j.1439-0310.2000.00529.x) [DOI] [Google Scholar]
- 15.Keller L., Reeve H. K. 1994. Partitioning of reproduction in animal societies. Trends Ecol. Evol. 9, 98–102 10.1016/0169-5347(94)90204-6 (doi:10.1016/0169-5347(94)90204-6) [DOI] [PubMed] [Google Scholar]
- 16.Ostner J., Nunn C. L., Schülke O. 2008. Female reproductive synchrony predicts skewed paternity across primates. Behav. Ecol. 19, 1150–1158 10.1093/beheco/arn093 (doi:10.1093/beheco/arn093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Packer C., Gilbert D., Pusey A. E., O'Brien S. 1991. A molecular genetic analysis of kinship and cooperation in African lions. Nature 351, 562–565 10.1038/351562a0 (doi:10.1038/351562a0) [DOI] [Google Scholar]
- 18.Duffy K. G., Wrangham R. W., Silk J. B. 2007. Male chimpanzees exchange political support for mating opportunities. Curr. Biol. 17, R586–R587 10.1016/j.cub.2007.06.001 (doi:10.1016/j.cub.2007.06.001) [DOI] [PubMed] [Google Scholar]
- 19.Henzi S., Clarke P., van Schaik C., Pradhan G., Barrett L. 2010. Infanticide and reproductive restraint in a polygynous social mammal. Proc. Natl Acad. Sci. 107, 2130–2135 10.1073/pnas.0913294107 (doi:10.1073/pnas.0913294107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bygott J., Bertram B., Hanby J. 1979. Male lions in large coalitions gain reproductive advantages. Nature 282, 839–841 10.1038/282839a0 (doi:10.1038/282839a0) [DOI] [Google Scholar]
- 21.Muller M. N., Mitani J. C. 2005. Conflict and cooperation in wild chimpanzees, pp. 275–331 New York, NY: Academic Press [Google Scholar]
- 22.Bradley B., Robbins M., Williamson E., Steklis H., Steklis N., Eckhardt N., Boesch C., Vigilant L. 2005. Mountain gorilla tug-of-war: silverbacks have limited control over reproduction in multimale groups. Proc. Natl Acad. Sci. USA 102, 9418–9423 10.1073/pnas.0502019102 (doi:10.1073/pnas.0502019102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen S.-F., Kern Reeve H. 2010. Reproductive skew theory unified: the general bordered tug-of-war model. J. Theor. Biol. 263, 1–12 10.1016/j.jtbi.2009.11.009 (doi:10.1016/j.jtbi.2009.11.009) [DOI] [PubMed] [Google Scholar]
- 24.Port M., Johnstone R. A., Kappeler P. M. 2010. Costs and benefits of multi-male associations in redfronted lemurs (Eulemur fulvus rufus). Biol. Lett. 6, 620–622 10.1098/rsbl.2010.0091 (doi:10.1098/rsbl.2010.0091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kappeler P. M., Port M. 2008. Mutual tolerance or reproductive competition? Patterns of reproductive skew among male redfronted lemurs (Eulemur fulvus rufus). Behav. Ecol. Sociobiol. 62, 1477–1488 10.1007/s00265-008-0577-5 (doi:10.1007/s00265-008-0577-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunbar R. I. M. 1979. Structure of gelada baboon reproductive units. I. Stability of social relationships. Behaviour 69, 72–87 10.1163/156853979X00403 (doi:10.1163/156853979X00403) [DOI] [Google Scholar]
- 27.Dunbar R. I. M. 1980. Determinants and evolutionary consequences of dominance among female gelada baboons. Behav. Ecol. Sociobiol. 7, 253–265 10.1007/BF00300665 (doi:10.1007/BF00300665) [DOI] [Google Scholar]
- 28.Dunbar R. I. M., Dunbar E. P. 1975. Social dynamics of gelada baboons. In Contributions to primatology (eds Kuhn H., Luckett W. P., Noback C. R., Schultz A. H., Starck D., Szalay F. S.), pp. 1–157 Basel, Switzerland: Karger; [PubMed] [Google Scholar]
- 29.Kawai M., Ohsawa H., Mori U., Dunbar R. I. M. 1983. Social organization of gelada baboons: social units and definitions. Primates 24, 13–24 10.1007/BF02381450 (doi:10.1007/BF02381450) [DOI] [Google Scholar]
- 30.Snyder-Mackler N., Beehner J., Bergman T. In press Defining higher levels in the multilevel societies of geladas (Theropithecus gelada). Int. J. Primatol. (doi:10.1007/s10764-012-9584-5) [Google Scholar]
- 31.Dunbar R. I. M. 1984. Reproductive decisions: an economic analysis of gelada baboon social strategies. Princeton, NJ: Princeton University Press [Google Scholar]
- 32.Dunbar R. I. M. 1993. Social organization of the gelada. In Theropithecus: the rise and fall of a genus (ed. Jablonski N. G.), pp. 425–439 Cambridge, UK: Cambridge University Press [Google Scholar]
- 33.Port M., Kappeler P. M., Johnstone R. A. 2011. Communal defense of territories and the evolution of sociality. Am. Nat. 178, 787–800 10.1086/662672 (doi:10.1086/662672) [DOI] [PubMed] [Google Scholar]
- 34.Altmann J. 1974. Observational study of behavior: sampling methods. Behaviour 49, 227–267 10.1163/156853974X00534 (doi:10.1163/156853974X00534) [DOI] [PubMed] [Google Scholar]
- 35.Alberts S. C., Buchan J. C., Altmann J. 2006. Sexual selection in wild baboons: from mating opportunities to paternity success. Anim. Behav. 72, 1177–1196 10.1016/j.anbehav.2006.05.001 (doi:10.1016/j.anbehav.2006.05.001) [DOI] [Google Scholar]
- 36.Buchan J. C., Alberts S. C., Silk J. B., Altmann J. 2003. True paternal care in a multi-male primate society. Nature 425, 179–181 10.1038/nature01866 (doi:10.1038/nature01866) [DOI] [PubMed] [Google Scholar]
- 37.Kalinowski S. T., Taper M. L., Marshall T. C. 2007. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 16, 1099–1106 10.1111/j.1365-294X.2007.03089.x (doi:10.1111/j.1365-294X.2007.03089.x) [DOI] [PubMed] [Google Scholar]
- 38.Queller D. C., Goodnight K. F. 1989. Estimating relatedness using genetic markers. Evolution 43, 258–275 10.2307/2409206 (doi:10.2307/2409206) [DOI] [PubMed] [Google Scholar]
- 39.Peakall R., Smouse P. 2006. genalex 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Not. 288–295 10.1111/j.1471-8286.2005.01155.x (doi:10.1111/j.1471-8286.2005.01155.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.R Development Core Team 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 41.Therneau T. 2009. Survival: survival analysis, including penalised likelihood. R package v. 2.35–8. See http://cran.r-project.org/web/packages/survival/survival.pdf.
- 42.Sarkar D. & Bates D. 2009. lme4: Linear mixed-effects models using S4 classes. R package v. 0.999375–25. See http://cran.r-project.org/package=lme4.
- 43.le Roux A., Bergman T. J. 2012. Indirect rival assessment in a social primate, Theropithecus gelada. Anim. Behav. 83, 249–255 10.1016/j.anbehav.2011.10.034 (doi:10.1016/j.anbehav.2011.10.034) [DOI] [Google Scholar]
- 44.Bergman T. J., Ho L., Beehner J. C. 2009. Chest color and social status in male geladas (Theropithecus gelada). Int. J. Primatol. 30, 791–806 10.1007/s10764-009-9374-x (doi:10.1007/s10764-009-9374-x) [DOI] [Google Scholar]
- 45.Alberts S. C., Altmann J. 1995. Balancing costs and opportunities: dispersal in male baboons. Am. Nat. 145, 279–306 10.1086/285740 (doi:10.1086/285740) [DOI] [Google Scholar]
- 46.Henzi S., Lycett J., Weingrill T. 1998. Mate guarding and risk assessment by male mountain baboons during inter-troop encounters. Anim. Behav. 55, 1421–1428. 10.1006/anbe.1997.0716 (doi:10.1006/anbe.1997.0716) [DOI] [PubMed] [Google Scholar]
- 47.Jack K. M., Fedigan L. 2004. Male dispersal patterns in white-faced capuchins, Cebus capucinus. Anim. Behav. 67, 771–782 10.1016/j.anbehav.2003.06.015 (doi:10.1016/j.anbehav.2003.06.015) [DOI] [Google Scholar]
- 48.Jarnemo A. 2011. Male red deer (Cervus elaphus) dispersal during the breeding season. J. Ethol. 29, 329–336 10.1007/s10164-010-0262-9 (doi:10.1007/s10164-010-0262-9) [DOI] [Google Scholar]
- 49.Parga J. A., Lessnau R. G. 2008. Dispersal among male ring-tailed lemurs (Lemur catta) on St. Catherines Island. Am. J. Primatol. 70, 650–660 10.1002/ajp.20542 (doi:10.1002/ajp.20542) [DOI] [PubMed] [Google Scholar]
- 50.Alberts S. C., Watts H. E., Altmann J. 2003. Queuing and queue-jumping: long-term patterns of reproductive skew in male savannah baboons, Papio cynocephalus. Anim. Behav. 65, 821–840 10.1006/anbe.2003.2106 (doi:10.1006/anbe.2003.2106) [DOI] [Google Scholar]
- 51.Cant M., Reeve H. 2002. Female control of the distribution of paternity in cooperative breeders. Am. Nat. 160, 602–611 10.1086/342820 (doi:10.1086/342820) [DOI] [PubMed] [Google Scholar]
- 52.Beehner J. C., Bergman T. J. 2008. Infant mortality following male takeovers in wild geladas. Am. J. Primatol. 70, 1152–1159 10.1002/ajp.20614 (doi:10.1002/ajp.20614) [DOI] [PubMed] [Google Scholar]
- 53.Roberts E. K., Lu A., Bergman T. J., Beehner J. C. 2012. A Bruce effect in wild geladas. Science 335, 1222–1225 10.1126/science.1213600 (doi:10.1126/science.1213600) [DOI] [PubMed] [Google Scholar]
- 54.Moscovice L., Di Fiore A., Crockford C., Kitchen D., Wittig R., Seyfarth R., Cheney D. 2010. Hedging their bets? Male and female chacma baboons form friendships based on likelihood of paternity. Anim. Behav. 79, 1007–1015 10.1016/j.anbehav.2010.01.013 (doi:10.1016/j.anbehav.2010.01.013) [DOI] [Google Scholar]
- 55.Reeve H. K., Shen S.-F. 2006. A missing model in reproductive skew theory: the bordered tug-of-war. Proc. Natl Acad. Sci. USA 103, 8430–8434 10.1073/pnas.0603005103 (doi:10.1073/pnas.0603005103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Por M., Kapp P. M., Johnst R. A. 2011. Communal defense of territories and the evolution of sociality. Am. Nat. 178, 787–800 10.1086/662672 (doi:10.1086/662672) [DOI] [PubMed] [Google Scholar]


