Abstract
In contrast to birds, bats are possibly limited in their capacity to use body fat as an energy source for long migrations. Here, we studied the fuel choice of migratory Pipistrellus nathusii (approximate weight: 8 g) by analysing the stable carbon isotope ratio (δ13CV-PDB) of breath and potential energy sources. Breath δ13CV-PDB was intermediate between δ13CV-PDB of insect prey and adipocyte triacylglycerols, suggesting a mixed-fuel use of P. nathusii during autumn migration. To clarify the origin of oxidized fatty acids, we performed feeding experiments with captive P. nathusii. After an insect diet, bat breath was enriched in 13C relative to the bulk and fat portion of insects, but not deviating from the non-fat portion of insects, suggesting that bats oxidized exogenous proteins and carbohydrates, but not exogenous fatty acids. A feeding experiment with 13C-labelled substrates confirmed these findings. In conclusion, migratory P. nathusii oxidized dietary proteins directly from insects captured en route in combination with endogenous fatty acids from adipocytes, and replenished their body reserves by routing dietary fatty acids to their body reserves.
Keywords: chiroptera, energetics, migration, fuel choice, vertebrate flight
1. Introduction
Migratory birds and bats are known to move over long distances between a summer and winter habitat [1,2]. Although both taxa use aerial locomotion as an efficient mode of transport, migratory ranges differ greatly between birds and bats [3–5]. Many migratory birds cover long distances of many thousands of kilometres during their seasonal journeys [6], whereas only a few bats cover a few thousands of kilometres [3,7,8]. Along their journey, both taxa face high metabolic rates during sustained flights [9]. Consequently, migration is preceded by many physiological changes, such as hyperphagia and fat deposition (birds: [10–12]; bats: [13–15]). Triacylglycerols (TAGs) from adipocytes are the optimal energy source for powering endurance flights because of their high energy density [16,17]. Indeed, fatty acids originating from adipocyte TAG are the primary fuel for most migratory birds [12,18–20]. In contrast, even highly aerobic mammals depend more on intra-muscular fuels than on adipocyte fuel when exercising over long periods [21,22], which raises the question of whether bats are able to power endurance exercise solely via oxidation of fatty acids from adipocyte TAG. Also, bats may be constrained in the use of TAG during autumn migration, because they have to save adipocyte TAG for surviving the many months of hibernation. As an additional supply of energy, bats could hunt insects and oxidize exogenous nutrients directly [23,24], as recent observations of hunting in migratory bats suggest [25]. The additional use of exogenous nutrients would enable bats to save adipocyte TAG for hibernation. Yet excessive hunting during migration may impede bats from covering long distances, since foraging requires energetically costly and time-consuming aerial manoeuvres [26,27].
We studied the metabolic substrate use in migratory Pipistrellus nathusii. This insectivorous bat (approximate weight: 8 g) migrates seasonally from its northeastern range in Germany, Poland, Belarus, Fennoscandia, the Baltic States and Russia to the southwest of Europe where it hibernates in overground roosts such as in trees or buildings [28]. Earlier studies confirmed that banded bats cover up to approximately 2000 km (one way) along their annual migration [7,29], which is one of the longest recorded migratory movements for a bat worldwide [3]. To determine whether P. nathusii oxidize 13C-enriched proteins from insects that they hunt en route, or whether they oxidize 13C-depleted endogenous fatty acids from adipocyte TAG, we determined the source of oxidized substrates in migratory P. nathusii by measuring the stable carbon isotope ratios (δ13CV-PDB) of exhaled breath; breath δ13CV-PDB matches closely δ13CV-PDB of the pool of oxidized substrates [30,31].
We hypothesized that migratory P. nathusii oxidize insect proteins or a mixture of exogenous insect proteins and endogenous fatty acids, because sedentary insectivorous bats are known to oxidize dietary nutrients or endogenous fatty acids from adipocytes according to whether or not they have fed recently [24]. Accordingly, we predicted that δ13CV-PDB of exhaled breath should be similar to δ13CV-PDB of insects captured in the same habitat as bats. Alternatively, if migratory bats oxidize additionally or exclusively 13C-depleted fatty acids from adipocyte TAG, then δ13CV-PDB of exhaled breath should be lower than δ13CV-PDB of potential insect prey. To disentangle whether oxidized fatty acids originate from endogenous (adipose TAG from body reserves) or exogenous (insect fat) sources, we performed two feeding experiments. First, we measured δ13CV-PDB of breath in resting P. nathusii after having fed several mealworms (larval stages of Tenebrio molitor, Coleoptera). We predicted that breath δ13CV-PDB should be higher than δ13CV-PDB of bulk mealworms and the fat portion of mealworms, but similar to the δ13CV-PDB of the non-fat portion of mealworms, if bats oxidize exogenous proteins but not fatty acids. Second, we measured δ13CV-PDB in breath collected from P. nathusii after having fed on a dose of 13C-labelled amino acids (glycine) or 13C-labelled fatty acids (palmitic acid). We calculated the cumulative oxidation of both substrates [32], and predicted that migratory P. nathusii oxidize the exogenous amino acid but not the fatty acid. Our experiments may shed new light on the physiological abilities and constraints of insectivorous bats when performing long-distance migration.
2. Material and methods
Fieldwork was conducted at Pape Ornithological Station in Pape, Latvia (56°09′ N 21°03′ E) between 18 August and 5 September 2011. Migratory bats were captured between 21.00 and 2.00 h or dawn using a Helgoland funnel trap as described by Petersons [7].
(a). Experiment I: metabolic substrate use of migratory Pipistrellus nathusii
Immediately after capture, we mechanically restrained P. nathusii by wrapping gauze bandage around their bodies. Then, bats were transferred singly into a plastic container (0.2 l volume; LockLock, iSi Deutschland GmbH, Solingen, Germany) that could be hermetically sealed. The container was equipped with an inlet through which CO2-free air entered at a flow rate of 700 ml min−1 [31]. At the opposite side of the container, we attached a needle (0.9 gauge; Braun, Melsungen, Germany). Once we closed the container with a bat inside, we flushed the container for 1 min with CO2-free air. Afterwards, we stopped the flushing and allowed CO2 to accumulate for 1.5 min. Then, we pierced the Teflon membrane of a Vacutainer (Labco, Buckinghamshire, UK) with the needle so that the air from inside the container (including the bat's breath) was sucked into the Vacutainer. Accordingly, about 2.5–3 min passed between the capture of the flying bat and the collection of the breath sample. In theory, stress-related changes in metabolic fuel choice could have biased our results, because catecholamines and glucocorticoids respond quickly to stressors. However, plasma glucocorticoids have been shown to remain constant within a 3 min period following a stressor [33], and a previous model suggested that the hysteresis effect of a bat's body bicarbonate pool is sufficiently slow to make stable carbon isotope ratios of breath representative of what happened a few minutes before [24]. After breath collection, animals were either brought to the station for further experiments or released. Vacutainers were shipped to the stable isotope laboratory of the Leibniz Institute for Zoo and Wildlife Research (IZW) where the stable carbon isotope ratio of CO2 was analysed using a blind protocol within a maximum period of three weeks as described in earlier studies [24,27]. Samples were analysed together with a laboratory standard gas that we previously calibrated with the international 13C reference materials NBS 19 and L-SVEC. Ratios of 13C and 12C were expressed relative to the international standard (Vienna-PeeDee Belemnite) using the δ notation in parts per mille (‰): δ13CV-PDB = (Rsample/Rstandard − 1) × 103, where R is the ratio of heavy and light carbon isotopes (13C/12C) in the sample and the standard. Precision was always better than ± 0.14‰ (1 s.d.).
For endurance migration, insectivorous bats can use two major energy sources: 13C-enriched proteins (from captured insects) and 13C-depleted TAG (from captured insects or body reserves) [34,35]. Breath δ13CV-PDB of fasting animals is on average approximately 3‰ lower than that of animals feeding on a protein diet, because fasting animals oxidize 13C-depleted fatty acids [31]. To compare breath δ13CV-PDB with that of potential energy sources, we captured insects at the same time and at the same place where we captured bats. Insects were identified according to order and then morphotype. For shipment to the IZW, we stored insects in ethanol-filled plastic vials. In the laboratory, we collected two subsamples from each morphotype. We extracted fat from one of these samples by washing it with a 2 : 1 chloroform–methanol solution for 24 h. Afterwards, extracted and non-extracted samples were dried until constant mass in an oven at 50°C. We then filled about 0.35 mg of dried samples in tin capsules. Loaded capsules were analysed as described in earlier studies [24,27]. Precision for bulk δ13CV-PDB measurement was better than ±0.10‰ (1 s.d.). To determine the δ13C of body fat in bats, we obtained fresh carcases of P. nathusii from a project on the impact of windfarm facilities on European bat populations. All of these bats were killed by wind turbines in Germany between August and September. In a separate study, we modelled the geographical origin of these bats based on stable hydrogen isotope ratios of fur keratin and found that all most likely to have originated from the Baltic countries or Russia [36]. Thus, we used samples from these bats instead of sacrificing P. nathusii at our study site in Latvia. We dissected the bat carcases and obtained white adipose tissues from the inter-scapular region for stable isotope analysis (n = 6). Fat samples were treated and analysed as described earlier.
During most nights, we captured bats during two periods (evening: 21.30–23.00 h; morning: 2.00–5.30 h). Consequently, we first tested whether breath δ13CV-PDB of bats captured in the evening and in the morning differed by using an unpaired Student's t-test with Welch correction. We then performed separate analyses for each activity period. In particular, we asked whether migratory P. nathusii captured during a given activity period oxidized exogenous nutrients (i.e. ingested insects) by comparing δ13CV-PDB of bat breath with δ13CV-PDB of local insects using a Mann–Whitney U-test. Additionally, we tested whether bats oxidize endogenous TAG by comparing δ13CV-PDB of bat breath with δ13CV-PDB of body fat using a Mann–Whitney U-test. All statistical tests were two-tailed, assuming an alpha-value of 5 per cent and using InStat v. 3 (GraphPad Software, Inc., San Diego, CA). We present values as mean ± 1 s.d. if not indicated otherwise.
(b). Experiment II: Pipistrellus nathusii feeding on mealworms
We fed eight P. nathussi (four males/four females) mealworms to see at what plateau breath δ13CV-PDB would level off in relation to dietary δ13CV-PDB when bats have access to exogenous nutrients. Bats were captured 1 day prior to the experiment and kept in a wooden box over 24 h. We used the same experimental set-up as previously described [24,27] to collect breath samples. From each bat, we collected a breath sample 100 min after the bat had started to feed on mealworms, because we expected breath δ13CV-PDB to converge on δ13CV-PDB of the oxidized substrate over this period in a bat of this size [24,31]. Before feeding and after taking the breath sample, we recorded the body mass of bats with an electronic balance (accuracy 0.01 g; Voltcraft, Germany). All bats were released at the site of capture. Breath samples were shipped to the stable isotope laboratory of the IZW and then analysed as described earlier.
We collected 50 mealworms to obtain reference values of δ13CV-PDB from ingested insects. We extracted fat from mealworms using the same protocol as described earlier for free-ranging insects. We conducted stable isotope analyses for the bulk mealworm, the fat portion and the non-fat portion of the mealworm as described earlier. We tested whether mean breath δ13CV-PDB differed from δ13CV-PDB of bulk mealworms, the fat portion of mealworms and the non-fat portion of mealworms based on one-sample t-tests using Instat.
(c). Experiment III: Pipistrellus nathusii feeding on 13C-labelled substrates
During the course of our fieldwork, we captured 17 P. nathussi (5 males/12 females) using the Helgoland funnel trap and transferred these bats in pairs to our feeding experiment with labelled substrates. We fed 10 bats each with 5 mg 13C-labelled glycine (C1-glycine; Euriso-Top GmbH, Saarbrücken, Germany) and seven bats each with 5 mg 13C-labelled palmitic acid (C1-palmitic acid; Euriso-Top GmbH) to assess whether migratory bats oxidize either of the substrates, and, if so, how much of the orally fed dosage would be used for oxidation over the experimental period. Breath collection followed the procedure described for experiment II with the difference that breath samples were collected at times 0, 5, 10, 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 75 and 90 min. Stable isotope ratios of breath were analysed as described earlier.
We converted the delta notation of measurements into atom per cent according to Slater et al. [37] and followed McCue et al. [32] in calculating the cumulative amount of oxidized substrate based on excess atom fraction of 13C, xE(13C), and extrapolated resting metabolic rates ( ). xE(13C) was calculated as the difference between atom fraction of 13C, x(13C) in exhaled breath of fed and unfed animals. We used measurements of basal metabolic rate and thermal conductance in a closely related species (i.e. 6.6 g P. pipistrellus [9]) as the best estimate for
). xE(13C) was calculated as the difference between atom fraction of 13C, x(13C) in exhaled breath of fed and unfed animals. We used measurements of basal metabolic rate and thermal conductance in a closely related species (i.e. 6.6 g P. pipistrellus [9]) as the best estimate for  of P. nathusii. We converted published oxygen consumption rates of 0.202 ml O2 min−1 into
of P. nathusii. We converted published oxygen consumption rates of 0.202 ml O2 min−1 into  assuming a respiratory quotient
assuming a respiratory quotient 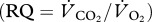 of 0.8 for protein digestion
of 0.8 for protein digestion 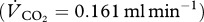 and an RQ of 0.7 for fat digestion
and an RQ of 0.7 for fat digestion 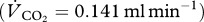 . To account for increased thermoregulatory costs in bats outside the thermo-neutral zone at 20°C, we multiplied these values by 1.4. We calculated the instantaneous rate of label oxidation (T; μg min−1) following the Fick equation modified after equation (7) of McCue et al. [32] for substrate labelled with a single 13C per molecule. We used a bicarbonate retention factor of 86 per cent because previous studies in various mammal taxa consistently showed that bicarbonate retention was almost identical across taxa [38–41]. Any deviation between assumed and true bicarbonate retention factors may not affect the relative difference in our estimates of compound-specific oxidation rates because this constant applies to both calculations in the same way. Further, we have refrained from controlling for the loss of 13C in urea, because the suggested stoichiometric correction [32] may not accurately reflect the situation in mammals. The mentioned correction requires the following equation for urea-producing mammals: k = [(C − 2N) × 22.4]/M, where k is the volume of CO2 (ml) produced for each milligram of tracer oxidized, 22.4 is the volume (l) of one mole, C and N the number of carbon and nitrogen atoms in the specific tracer molecule, respectively, and M the mole mass of the tracer molecule. The factor 2 controls for the fact that twice the number of C are required to excrete a given number of N (this number reads 1.2 in the original equation for uric acid excreting birds [32]). In the present experiment, we have used glycine (2 C and 1 N), yielding a k-factor of 0 ml for the volume of CO2 produced for each milligram of oxidized glycine. This contrasts with the fact that we were able to trace 13C in the exhaled breath of bats after they fed on glycine. Therefore, we assumed that all carbon atoms of the 13C-labelled substrates leave the body as CO2 and not as urea. Accordingly, we have modified the above equation to: k = [C × 22.4]/M. On this basis of assumption and the equations provided in McCue et al. [32], we then calculated the cumulative oxidation of 13C-labelled substrate (% of fed dose) over the 90 min experimental period.
. To account for increased thermoregulatory costs in bats outside the thermo-neutral zone at 20°C, we multiplied these values by 1.4. We calculated the instantaneous rate of label oxidation (T; μg min−1) following the Fick equation modified after equation (7) of McCue et al. [32] for substrate labelled with a single 13C per molecule. We used a bicarbonate retention factor of 86 per cent because previous studies in various mammal taxa consistently showed that bicarbonate retention was almost identical across taxa [38–41]. Any deviation between assumed and true bicarbonate retention factors may not affect the relative difference in our estimates of compound-specific oxidation rates because this constant applies to both calculations in the same way. Further, we have refrained from controlling for the loss of 13C in urea, because the suggested stoichiometric correction [32] may not accurately reflect the situation in mammals. The mentioned correction requires the following equation for urea-producing mammals: k = [(C − 2N) × 22.4]/M, where k is the volume of CO2 (ml) produced for each milligram of tracer oxidized, 22.4 is the volume (l) of one mole, C and N the number of carbon and nitrogen atoms in the specific tracer molecule, respectively, and M the mole mass of the tracer molecule. The factor 2 controls for the fact that twice the number of C are required to excrete a given number of N (this number reads 1.2 in the original equation for uric acid excreting birds [32]). In the present experiment, we have used glycine (2 C and 1 N), yielding a k-factor of 0 ml for the volume of CO2 produced for each milligram of oxidized glycine. This contrasts with the fact that we were able to trace 13C in the exhaled breath of bats after they fed on glycine. Therefore, we assumed that all carbon atoms of the 13C-labelled substrates leave the body as CO2 and not as urea. Accordingly, we have modified the above equation to: k = [C × 22.4]/M. On this basis of assumption and the equations provided in McCue et al. [32], we then calculated the cumulative oxidation of 13C-labelled substrate (% of fed dose) over the 90 min experimental period.
In both experiments, we did not obtain sufficient CO2 for stable isotope analysis from three individuals. Thus, we performed Friedman tests only with data of the remaining animals to test whether the excess atom fraction changes over time when bats are fed either glycine or palmitic acid. We compared the rate of oxidation using a Wilcoxon matched-pairs test with Instat, assuming an alpha-value of 5 per cent.
3. Results
(a). Experiment I: metabolic substrate use of migratory Pipistrellus nathusii
We captured migratory P. nathusii during two activity peaks at night: in the late evening (21.30 until 23.00 h) and in the early morning hours (2.00 until 5.30 h; figure 1). Bats captured between 21.30 and 23.00 h weighed on average 7.6 ± 0.6 g and had an average breath δ13CV-PDB of −29.8 ± 1.1‰ (n = 20). Body mass of bats captured between 2.00 and 5.30 h averaged 7.8 ± 0.8 g, which was not significantly different from those captured earlier (Student's t-test: t45 = 1.18, p = 0.246). However, δ13CV-PDB of breath collected from bats between 2.00 and 5.30 h was significantly lower than in those bats captured between 21.30 and 23.00 h (δ13CV-PDB = −31.4 ± 2.4‰, n = 27; t25 = 2.7, p = 0.013). All bats defaecated insect fragments when we collected breath samples or when we kept bats for a short period in separate linen bags.
Figure 1.
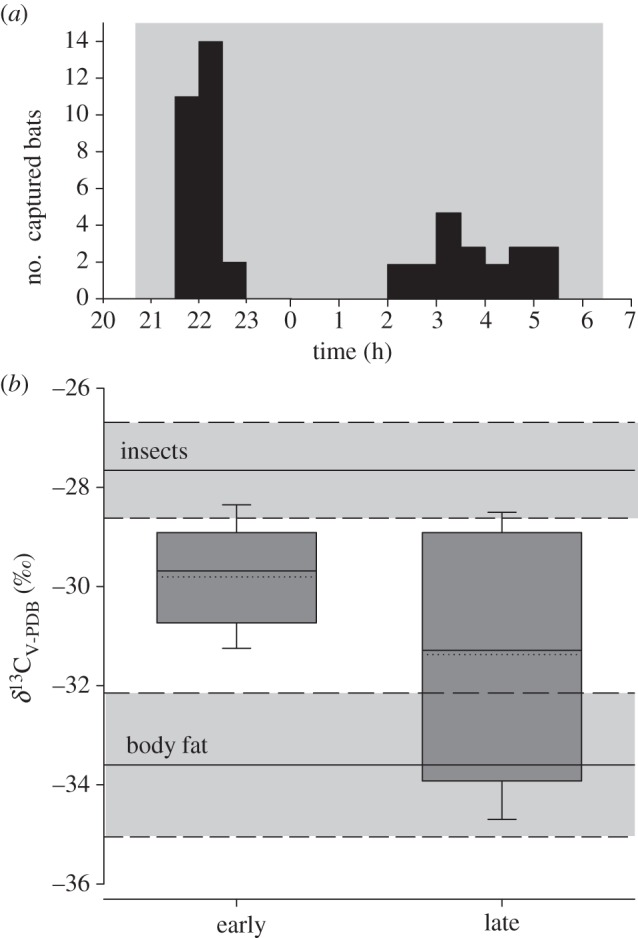
(a) Distribution of capture events in migratory Pipistrellus nathusii (grey-shaded area indicates the onset and end of night) and (b) stable carbon isotope ratios of exhaled breath (δ13CV-PDB; ‰) in bats that were captured during the evening peak (21.30 until 23.00 h) and the morning peak (2.00 until 5.30 h). Box margins indicate the 25 and 75 percentiles, whiskers the 5 and 95 percentiles, solid lines within the boxes the median and the dotted lines within the boxes the mean values. Horizontal grey boxes describe the δ13CV-PDB of potential energy sources, i.e. insects and body fat (solid horizontal line = mean value; dashed horizontal line = ±1 s.d.).
We captured nocturnal insects at our study site that belonged to three taxonomic groups: Diptera, Lepidoptera and Heteroptera. The large majority of all insects were nocturnal moths, and therefore we performed pair-wise δ13CV-PDB comparisons only in Lepidopterans (n = 9 morphotypes). In moths, δ13CV-PDB of the non-fat portion of organic tissues was more enriched in 13C by 1.5‰ (−26.1 ± 1.8‰) compared with the total organic tissue (−27.7 ± 1.0‰; paired Student's t-test: t8 = 4.4, p = 0.0023). As the best proxy for the isotopic composition of the alternative endogenous fuel source in migratory P. nathusii, we measured δ13CV-PDB of fat obtained from fresh bat carcases at wind turbines [36]. Fat reserves of these migratory P. nathusii were depleted in 13C (−33.6 ± 1.5‰) in relation to potential insect prey at our study site in Latvia.
Breath δ13CV-PDB was lower than δ13CV-PDB of potential insect prey (both bulk or fat portion of samples), irrespective of whether bats were captured early or late in the night (bats captured in the evening versus insects: U = 13, U′ = 230, n1 = 27, n2 = 9, p < 0.0001; bats captured in the morning versus insects: U = 9, U′ = 171, n1 = 20, n2 = 9, p < 0.0001). The mean difference between δ13CV-PDB of bulk insects and breath δ13CV-PDB from bats captured in the evening equalled 2.2‰, and between δ13CV-PDB of bulk insects and breath δ13CV-PDB from bats captured in the morning equalled 3.7‰. Breath δ13CV-PDB of bats that were captured early in the night was higher than δ13CV-PDB of body fat (U = 2, U′ = 160, n1 = 27, n2 = 6, p < 0.0001). However, breath δ13CV-PDB of bats captured in the morning was not significantly different from δ13CV-PDB of body fat (U = 28, U′ = 92, n1 = 20, n2 = 6, p = 0.0536).
(b). Experiment II: Pipistrellus nathusii feeding on mealworms
Before we started feeding, bats weighed 6.6 ± 0.6 g (n = 8 individuals). On average, we fed 9.3 ± 2.5 mealworms to each bat. Individual bats gained 0.5 ± 0.2 g body mass until the end of the experiment; an increase that is equivalent to more than 7 per cent of their initial body mass. At t = 100 min, δ13CV-PDB of bats averaged −25.4 ± 1.5‰ (figure 2), which significantly deviated from the δ13C of bulk mealworms (U = 1, U′ = 63, n1 = 8, n2 = 8, p < 0.003) and the fat portion of mealworm (U = 0, U′ = 48, n1 = 8, n2 = 6, p < 0.001), but not from δ13CV-PDB of the non-fat portion of mealworm (U = 22, U′ = 42, n1 = 8, n2 = 8, p = 0.328; figure 2).
Figure 2.
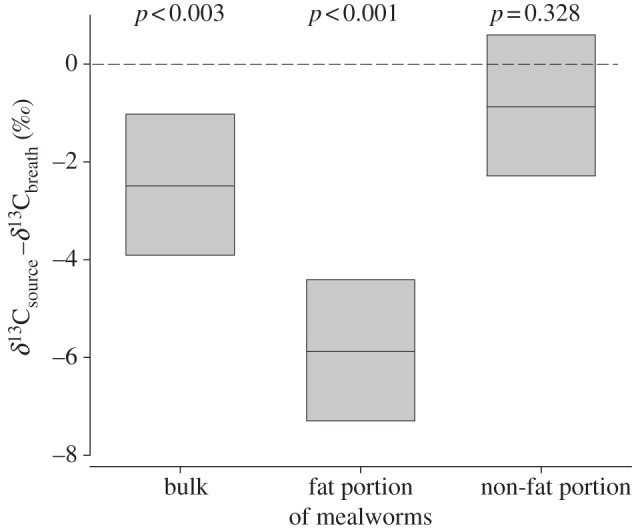
Difference in stable carbon isotope ratios between available nutrients (δ13Csource; ‰) and exhaled breath (δ13CV-PDB; ‰) of eight Pipistrellus nathusii that had repeatedly fed on mealworms over 100 min. Box margins indicate the 25 and 75 percentiles, and the solid lines within the boxes the median. The dashed line indicates when exhaled breath and nutrient have the same δ13C value. p-values indicate significant differences between nutrient δ13CV-PDB and breath δ13CV-PDB.
(c). Experiment III: Pipistrellus nathusii feeding on 13C-labelled substrates
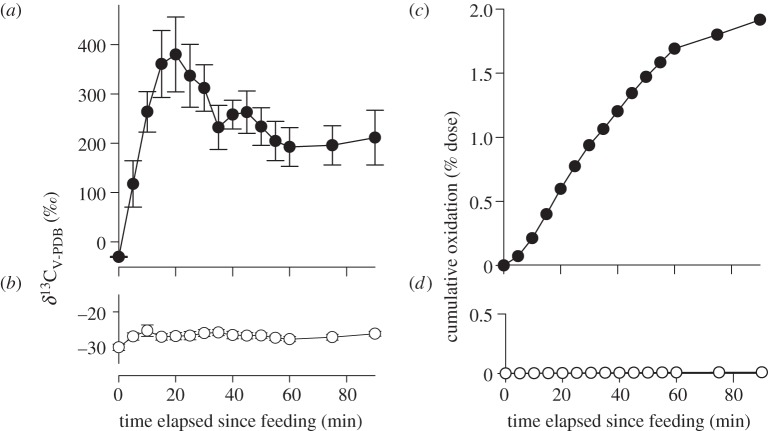
δ13CV-PDB of unfed bats averaged −30.5 ± 2.8‰. A Friedman test showed that δ13CV-PDB did not change over the course of the experimental period when bats were fed 13C-labelled palmitic acid (k = 7, l = 15, F = 17.6, p = 0.225; figure 3b), whereas the atom fraction changed when bats were fed 13C-labelled glycine (k = 4, l = 15, F = 27.4, p = 0.0172; figure 3a). In bats fed 13C-labelled glycine, δ13CV-PDB increased after a few minutes following the feeding event (figure 3a). Peak enrichments averaged 380.1 ± 75.8‰ at t = 20 min in bats fed 13C-labelled glycine. Afterwards, breath δ13CV-PDB decreased to lower values of about 200‰ for the rest of the experimental period (figure 3a). Oxidation rates were always lower in bats fed 13C-labelled palmitic acid (0.084 ± 0.016 nmol min−1) than in bats fed 13C-labelled glycine (18.2 ± 4.6 nmol min−1; Wilcoxon matched pairs test: T+ = 105, T− = 0; n = 14 pairs, p < 0.001). Within the experimental time, P. nathusii oxidized about 2 per cent of the ingested dose of 13C-labelled glycine, whereas it oxidized only about 0.01 per cent of the ingested dose of 13C-labelled palmitic acid (figure 3c,d).
Figure 3.
Stable carbon isotope ratios in exhaled breath (mean δ13CV-PDB ± s.e.; ‰) in migratory Pipistrellus nathusii fed (a) 13C-labelled glycine (solid circles; n = 10) and (b) 13C-labelled palmitic acid (open circles; n = 7). (c,d) δ13CV-PDB of exhaled breath was used to calculate the cumulative oxidation (% dose) of both substrates.
4. Discussion
To evaluate whether insectivorous bats face energetic constraints during migration, we studied what oxidative fuels P. nathusii choose to power their autumn migration. We found that P. nathusii used a combination of exogenous insect proteins and endogenous fatty acids derived from TAGs of adipocyte tissue to fuel the journeys to their wintering habitats. Our observation is based on three findings: first, we documented that all P. nathusii defaecated insect fragments after being captured during migration, indicating that they hunted insects en route and suggesting that exogenous nutrients were available as an oxidative fuel to bats. Second, we found that the stable carbon isotope ratio (δ13CV-PDB) was lower in exhaled breath of recently captured P. nathusii than in local insects but higher than in fat deposits typical for P. nathusii. These intermediate δ13CV-PDB values of breath samples indicated a mixed use of 13C-enriched insect proteins and 13C-depleted TAGs. During the early morning hours (between 2.00 and 5.30 h), δ13CV-PDB of bat breath converged on the isotopic composition of adipocyte TAG, suggesting that oxidation of fatty acids from adipocytes became more important later at night. Third, we demonstrated in feeding experiments that P. nathusii oxidized the protein but not the fat portion of an insect diet, or the amino acids and not the fatty acids in experiments with 13C-labelled substrates. In contrast to migratory P. nathusii, non-migratory insectivorous bats, such as Noctilio albiventris, oxidized both the non-fat and the fat portion of recently ingested mealworms [24].
The finding that migratory P. nathusii use a mixed-fuel strategy (combined use of exogenous amino acids and endogenous fatty acids as an oxidative fuel) for autumn migration is novel, and most relevant for our understanding of the ecology and conservation of migratory insectivorous bats. First of all, migratory insectivorous bats seem to depend on continuous supplies of insects along their migratory route and not only at stopover sites. Indeed, it is unclear whether bats need to make stopovers for refuelling given their ability to hunt insects while migrating towards their wintering habitats. Second, fat deposits are essential for migratory insectivorous bats, not only for hibernation but also for powering the strenuous migratory flight, at least partly. Oxidation of fatty acids from adipocyte TAG seems to become increasingly important as an energy supply with decreasing insect abundance during the second half of a night (this study), and possibly also during cold nights when air-borne insects are scarce. An excessive depletion of fat stores may even hamper insectivorous bats from continuing their migration or to save sufficient fat deposits for surviving several months of hibernation. Thus, a high availability of nocturnal insects, and consequently an uninterrupted sequence of intact ecosystems, seems to be crucial for the successful migration of insectivorous bats. Consequently, conservation efforts in support of migratory bats should not necessarily focus on one or a few stopover sites, as is the case in conservation plans supporting migratory birds, but rather on intact and continuous migratory corridors.
Our findings are also interesting from a physiological perspective. Migratory P. nathusii routed exogenous TAG (presumably) to adipose tissues instead of oxidizing them immediately. This nutrient routing seems to be highly efficient given that dietary fatty acids were not oxidized at all in our feeding experiment with 13C-labelled substrate. Thus, aerial refuelling has two aspects in P. nathusii: the immediate oxidation of exogenous proteins and carbohydrates as a power supply, and the routing of exogenous fatty acids to adipose tissue for later use as an energy supply for either migration (when insects get scarce) or for hibernation. Interestingly, bats oxidized glycine at a lower rate than house sparrows (Passer domesticus [32]). The underlying cause for this difference is yet unclear, but given the many differences between the studied vespertilionid bat and passerine bird (e.g. in body mass, phylogeny, origin, feeding habit, migration status), this difference is not surprising. In summary, aerial refuelling seems to represent a highly efficient migration strategy for insectivorous bats to minimize time spent along the migratory route. A similar strategy has already been suggested for migratory nectar-feeding bats, such as Leptonycteris curasoae, that benefit from nectar provided by agave and cactus plants along a migratory corridor from the southern United States to Mexico [42]. The efficacy of such an aerial refuelling strategy is even increased in bats when migrants use torpor during daytime rest, a strategy that has been recently defined for Chiroptera as ‘torpor-assisted migration’ [43].
Migratory birds and bats of the Northern Hemisphere respond physiologically to the same adverse environmental conditions, namely reduced food availability and decreasing ambient temperatures. Insectivorous birds that migrate at night cannot forage for insects because of the limited ability to use vision at night to locate and hunt insects. Also, they are incapable of extended periods of torpor, let alone hibernation [3]. Therefore, migratory birds are probably forced to move towards areas with a sufficient and continuous supply of food and acceptable climatic conditions. In contrast, insectivorous bats of the temperate zone do not have to take the risk of an extended migration, because they are capable of surviving the cold winter by hibernation. This seems to be a better migratory strategy for P. nathusii than continuing to more southerly wintering habitats. Possibly, a mixed-fuel strategy in autumn could power even longer migrations. Yet it is uncertain whether bats could also benefit from a mixed-fuel strategy when migrating backwards to their breeding habitats in spring, given that insect food may be not as abundant in spring as in autumn. Aerial refuelling by feeding on insects en route may also keep migratory bats from becoming dehydrated, a physiological constraint that limits the migratory performance of birds [44].
We conclude that insectivorous migratory bats, such as P. nathusii, use a mixed-fuel strategy to power autumn migration. This strategy contrasts with the TAG-fuel strategy of most temperate zone birds [10–12]. The mixed-fuel strategy allows bats to oxidize nutrients immediately en route and to refuel their fat deposits at the same time. Insectivorous bats of the temperate zone have found a unique way of supplying their migration with energy by oxidizing simultaneously exogenous proteins and endogenous fatty acids derived from adipocyte TAG.
Acknowledgements
We thank Ilze Brila, Alma Vītola, Morics Mūrnieks, Ilze Čakare, Normunds Kukārs, Kristaps Sokolovskis, Viesturs Vintulis and Ineta Kalniņa for help during the fieldwork. Donāts Spalis is acknowledged for technical support constructing and maintaining the Helgoland trap at Pape. We also thank Marshall McCue for discussing the mathematical approaches in his model paper, and two anonymous reviewers for providing constructive and helpful comments. The project was supported by the Baltic-German University Liaison Office with funds from the German Academic Exchange Service (DAAD). The capture of bats was conducted under the permit no. 29/2011 to the Institute of Biology, University of Latvia.
References
- 1.Baker R. R. 1978. The evolutionary ecology of animal migration. London, UK: Hodder and Stoughton [Google Scholar]
- 2.Griffin D. R. 1970. Migrations and homing of bats. In Bat biology and conservation (ed. Wimsatt W. A.), pp. 233–264 New York, NY: Academic Press [Google Scholar]
- 3.Fleming T. H., Eby P. 2003. Ecology of bat migration. In Bat ecology (eds Kunz T. H., Fenton M. B.), pp. 156–208 Chicago, IL: University of Chicago Press [Google Scholar]
- 4.McGuire L. P., Guglielmo C. G. 2009. What can birds tell us about the migration physiology of bats? J. Mammal. 90, 1290–1297 10.1644/09-MAMM-S-084R.1 (doi:10.1644/09-MAMM-S-084R.1) [DOI] [Google Scholar]
- 5.Popa-Lisseanu A., Voigt C. C. 2009. Bats on the move. J. Mammal. 90, 1283–1289 10.1644/09-MAMM-S-130R2.1 (doi:10.1644/09-MAMM-S-130R2.1) [DOI] [Google Scholar]
- 6.Bairlein F., Norris D. R., Nagel R., Bulte M., Voigt C. C., Fox J. W., Jussel D., Schmaljohann H. 2012. Cross-hemisphere migration of a 25-gram songbird. Biol. Lett. 10.1098/rsbl.2011.1223 (doi:10.1098/rsbl.2011.1223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersons G. 2004. Seasonal migrations of north-eastern populations of Nathusius’ bat Pipistrellus nathusii (Chiroptera). Myotis 41, 29–56 [Google Scholar]
- 8.Hedenström A. 2009. Optimal migration strategies in bats. J. Mammal. 90, 1298–1309 10.1644/09-MAMM-S-075R2.1 (doi:10.1644/09-MAMM-S-075R2.1) [DOI] [Google Scholar]
- 9.Speakman J. R., Thomas D. 2003. Physiological ecology. In Bat ecology (eds Kunz T. H., Fenton M. B.), pp. 430–490 Chicago, IL: University of Chicago Press [Google Scholar]
- 10.Bairlein F. 1990. Nutrition and food selection in migratory birds. In Bird migration: physiology and ecophysiology (ed. Gwinner E.), pp. 198–213 Berlin, Germany: Springer [Google Scholar]
- 11.Bairlein F., Gwinner E. 1994. Nutritional mechanisms and temporal control of migratory energy accumulation in birds. Ann. Rev. Nutr. 14, 187–215 10.1146/annurev.nu.14.070194.001155 (doi:10.1146/annurev.nu.14.070194.001155) [DOI] [PubMed] [Google Scholar]
- 12.McWilliams S. R., Guglielmo C., Pierce B., Klaassen M. 2004. Flying, fasting and feeding in birds during migration: a nutritional and physiological ecology perspective. J. Avian Biol. 35, 377–393 10.1111/j.0908-8857.2004.03378.x (doi:10.1111/j.0908-8857.2004.03378.x) [DOI] [Google Scholar]
- 13.Krulin G. S., Sealander J. A. 1972. Annual lipid cycle of the gray bat, Myotis grisescens. Comp. Biochem. Physiol. A 42, 537–549 10.1016/0300-9629(72)90132-6 (doi:10.1016/0300-9629(72)90132-6) [DOI] [PubMed] [Google Scholar]
- 14.O'Shea T. J. 1976. Fat content in migratory central Arizona Brazilian free-tailed bats, Tadarida brasiliensis (Molossidae). Southwest Nat. 21, 321–326 10.2307/3669717 (doi:10.2307/3669717) [DOI] [Google Scholar]
- 15.Pagles J. F. 1975. Temperature regulation, body weight and changes in total body fat of the free-tailed bat, Tadarida brasiliensis cynocephala (Le Conte). Comp. Biochem. Physiol. A 50, 237–246 10.1016/0300-9629(75)90005-5 (doi:10.1016/0300-9629(75)90005-5) [DOI] [PubMed] [Google Scholar]
- 16.Schmidt-Nielsen K. 1997. Animal physiology: adaptation and environment. Cambridge, UK: Cambridge University Press [Google Scholar]
- 17.Weber J. M. 1988. Design of exogenous fuel supply systems: adaptive strategies for endurance locomotion . Can. J. Zool. 66, 1116–1121 10.1139/z88-163 (doi:10.1139/z88-163) [DOI] [Google Scholar]
- 18.Jenni L., Jenni-Eiermann S. 1998. Fuel supply and metabolic constraints in migrating birds. J. Avian Biol. 29, 521–528 10.2307/3677171 (doi:10.2307/3677171) [DOI] [Google Scholar]
- 19.Jenni-Eiermann S., Jenni L., Kvist A., Lindstrom A., Piersma T., Visser G. H. 2002. Fuel use and metabolic response to endurance exercise: a wind tunnel study of a long distance migrant shorebird. J. Exp. Biol. 205, 2453–2460 [DOI] [PubMed] [Google Scholar]
- 20.Price E. R., Krokfors A., Guglielmo C. G. 2008. Selective mobilization of fatty acids from adipose tissue in migratory birds. J. Exp. Biol. 211, 29–34 10.1242/jeb.009340 (doi:10.1242/jeb.009340) [DOI] [PubMed] [Google Scholar]
- 21.Weber J.-M. 2009. The physiology of long-distance migration: extending the limits of endurance metabolism. J. Exp. Biol. 212, 593–597 10.1242/jeb.015024 (doi:10.1242/jeb.015024) [DOI] [PubMed] [Google Scholar]
- 22.Weber J.-M. 2011. Metabolic fuels: regulating fluxes to select mix. J. Exp. Biol. 214, 286–294 10.1242/jeb.047050 (doi:10.1242/jeb.047050) [DOI] [PubMed] [Google Scholar]
- 23.Voigt C. C., Speakman J. R. 2007. Nectar-feeding bats fuel their high metabolism directly with exogenous carbohydrates. Funct. Ecol. 21, 913–921 10.1111/j.1365-2435.2007.01321.x (doi:10.1111/j.1365-2435.2007.01321.x) [DOI] [Google Scholar]
- 24.Voigt C. C., Sörgel K., Dechmann D. K. N. 2010. Refuelling while flying: foraging bats combust food rapidly and directly to fuel flight. Ecology 91, 2908–2917 10.1890/09-2232.1 (doi:10.1890/09-2232.1) [DOI] [PubMed] [Google Scholar]
- 25.Ahlén I., Baagøe H. J., Bach L. 2009. Behavior of Scandinavian bats during migration and foraging at sea. J. Mammal 90, 1318–1323 10.1644/09-MAMM-S-223R.1 (doi:10.1644/09-MAMM-S-223R.1) [DOI] [Google Scholar]
- 26.Voigt C. C., Holderied M. W. 2012. High manoeuvring costs force narrow-winged molossid bats to forage in open space. J. Comp. Physiol. B 182, 415–424 10.1007/s00360-011-0627-6 (doi:10.1007/s00360-011-0627-6) [DOI] [PubMed] [Google Scholar]
- 27.Voigt C. C., Schuller B. M., Greif S., Siemers B. M. 2010. Perch-hunting in insectivorous Rhinolophus bats is related to the high energy costs of manoeuvring in flight . J. Comp. Physiol. B 180, 1079–1088 10.1007/s00360-010-0466-x (doi:10.1007/s00360-010-0466-x) [DOI] [PubMed] [Google Scholar]
- 28.Dietz C., von Helverson O., Nill D. 2009. Handbook of bats of Europe and Northwest Africa. London, UK: A & C Black [Google Scholar]
- 29.Hutterer R., Ivanova T., Meyer-Cords C., Rodrigues L. 2005. Bat migrations in Europe: a review of banding data and literature. Naturschutz und Biologische Vielfalt, vol. 28, pp. 1–162. Bonn, Germany: Federal Agency for Nature Conservation
- 30.Schoeller D. A., Brown C., Nakamura K., Nakagawa A., Mazzeo R. S., Brooks G. A., Budinger T. F. 1984. Influence of metabolic fuel on the 13C/12C ratio of breath CO2. Biomed. Mass Spectrom. 11, 557–561 10.1002/bms.1200111103 (doi:10.1002/bms.1200111103) [DOI] [PubMed] [Google Scholar]
- 31.Voigt C. C., Baier L., Speakman J. R., Siemers B. M. 2008. Stable carbon isotopes in exhaled breath as tracers for dietary information in birds and mammals . J. Exp. Biol. 211, 2233–2238 10.1242/jeb.018523 (doi:10.1242/jeb.018523) [DOI] [PubMed] [Google Scholar]
- 32.McCue M. D., Sivan O., McWilliams S. R., Pinshow B. 2010. Tracking the oxidative kinetics of carbohydrates, amino acids and fatty acids in the house sparrow using exhaled 13CO2. J. Exp. Biol. 213, 782–789 10.1242/jeb.039842 (doi:10.1242/jeb.039842) [DOI] [PubMed] [Google Scholar]
- 33.Greiner S., Stefanski V., Dehnhard M., Voigt C. C. 2010. Plasma testosterone levels decrease after activation of skin immune system in a free-ranging mammal. Gen. Comp. Endocrinol. 168, 466–473 10.1016/j.ygcen.2010.06.008 (doi:10.1016/j.ygcen.2010.06.008) [DOI] [PubMed] [Google Scholar]
- 34.DeNiro M. J., Epstein S. 1977. Mechanism of carbon isotope fractionation associated with lipid synthesis. Science 197, 261–263 10.1126/science.327543 (doi:10.1126/science.327543) [DOI] [PubMed] [Google Scholar]
- 35.DeNiro M. J., Epstein S. 1978. Influence of diet on the distribution of carbon isotopes in animals . Geochim. Cosmochim. Acta 42, 495–506 10.1016/0016-7037(78)90199-0 (doi:10.1016/0016-7037(78)90199-0) [DOI] [Google Scholar]
- 36.Voigt C. C., Popa-Lisseanu A. G., Niermann I., Kramer-Schadt S. In press The catchment area of killed bats at German wind turbines: a plea for international regulations. Biol. Conserv. 10.1016/j.biocon.2012.04.027 (doi:10.1016/j.biocon.2012.04.027) [DOI] [Google Scholar]
- 37.Slater C., Preston T., Weaver L. T. 2001. Stable isotopes and the international system of units. Rapid Commun. Mass Spectrom. 15, 1270–1273 10.1002/rcm.328 (doi:10.1002/rcm.328) [DOI] [PubMed] [Google Scholar]
- 38.Hoerr R. A., Yu Y.-M., Wagner D. A., Burke J. F., Young V. R. 1989. Recovery of 13C in breath from NaH13CO3 infused by gut and vein: effect of feeding. Am. J. Physiol. 257, E426–E438 [DOI] [PubMed] [Google Scholar]
- 39.Tomera J. F., Goetz P. G., Rand W. M., Brunengraber H. 1982. Underestimation of metabolic rates owing to reincorporation of 14CO2 in the perfused rat liver. Biochem. J. 208, 231–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wykes L. J., House J. D., Ball R. O., Pencharz P. B. 1994. Amino acid profile and aromatic amino acid concentration in total parental nutrition: effect on growth, protein metabolism and aromatic amino acid metabolism in the neonatal piglet. Clin. Sci. (Lond.) 87, 75–84 [DOI] [PubMed] [Google Scholar]
- 41.Clugston G. A., Garlick P. J. 1983. Recovery of infused [14C]bicarbonate as respiratory 14CO2 in man. Clin. Sci. (Lond.) 64, 231–233 [DOI] [PubMed] [Google Scholar]
- 42.Fleming T. H., Nuñez R. A., da Silveira Lobo Sternberg L. 1993. Seasonal changes in the diets of migrant and non-migrant nectarivorous bats as revealed by carbon stable isotope analysis. Oecologia 94, 72–75 10.1007/BF00317304 (doi:10.1007/BF00317304) [DOI] [PubMed] [Google Scholar]
- 43.McGuire L. P., Guglielmo C. G., Mackenzie S. A., Taylor P. D. 2011. Migratory stopover in the long-distance migrant silver-haired bat, Lasionycteris noctivagans. J. Anim. Ecol. 81, 377–385 10.1111/j.1365-2656.2011.01912.x (doi:10.1111/j.1365-2656.2011.01912.x)x [DOI] [PubMed] [Google Scholar]
- 44.Klaassen M. 1996. Metabolic constraints on long-distance migration in birds. J. Exp. Biol. 199, 57–64 [DOI] [PubMed] [Google Scholar]