Abstract
Fine root production is the largest component of belowground production and plays substantial roles in the biogeochemical cycles of terrestrial ecosystems. The increasing availability of nitrogen (N) and phosphorus (P) due to human activities is expected to increase aboveground net primary production (ANNP), but the response of fine root production to N and P remains unclear. If roots respond to nutrients as ANNP, fine root production is anticipated to increase with increasing soil N and P. Here, by synthesizing data along the nutrient gradient from 410 natural habitats and from 469 N and/or P addition experiments, we showed that fine root production increased in terrestrial ecosystems with an average increase along the natural N gradient of up to 0.5 per cent with increasing soil N. Fine root production also increased with soil P in natural conditions, particularly at P < 300 mg kg−1. With N, P and combined N + P addition, fine root production increased by a global average of 27, 21 and 40 per cent, respectively. However, its responses differed among ecosystems and soil types. The global average increases in fine root production are lower than those of ANNP, indicating that above- and belowground counterparts are coupled, but production allocation shifts more to aboveground with higher soil nutrients. Our results suggest that the increasing fertilizer use and combined N deposition at present and in the future will stimulate fine root production, together with ANPP, probably providing a significant influence on atmospheric CO2 emissions.
Keywords: carbon cycle, fine roots, net primary production, nitrogen deposition, nitrogen and phosphorus fertilization
1. Introduction
Nitrogen (N) and phosphorus (P) are generally considered as the two elements that limit or co-limit autotroph (plant) growth, net primary productivity (NPP) and other processes in many managed and natural terrestrial ecosystems [1–4]. Over the past several decades, human activities have roughly doubled the amount of N entering the terrestrial environment, primarily through N fertilizer application, fossil fuel combustion and legume cultivation [5]. This trend of increasing N [6,7] is predicted to continue, with another twofold or threefold increase in many regions during the twenty-first century [8,9]. Additionally, transient N increase is also expected owing to climate change-induced increases in soil moisture and temperature [10]. Among ecosystem types, N deposition is anticipated to be higher in the tropical regions than in other ecosystems [9]. Similarly, global P fertilizer use has increased fourfold over the past five decades [11]. The increasing use of N and P fertilizers, together with the formation of reactive N in various combustion processes, will ultimately increase their availability in the biosphere.
A better understanding of how and to what extent N and P affect plant growth is essential to predict the interaction between global carbon (C) and N cycles in terrestrial ecosystems [12]. The changes in N and P inputs have been found to significantly influence nutrient cycling, plant growth and competition, consequently influencing C flux, partitioning and sequestration within ecosystems [4,13]. However, those conclusions are based on aboveground processes; few studies have focused on belowground processes. It is still an open debate whether fine root production, representing a large (33% of total NPP globally [14] and up to 76% in some ecosystems [15]) and relatively unknown portion of the total terrestrial production, changes with increasing nutrient availabilities [16,17]. Nadelhoffer [18] proposed four theoretically possible changes in fine root production in relation to N and argued that it is most likely that increasing N availability would keep production relatively constant, but increase mortality and turnover rates, resulting in a decrease in fine root biomass. However, few studies have tested this prediction. Furthermore, no study, to the best of our knowledge, has provided a framework for understanding the response of fine root production to the range of P availability.
Although previous studies have shown that aboveground NPP increases with soil N and P in natural habitats [19–21] and that N and P additions can increase aboveground NPP [1,22], litterfall [23] and root biomass [18], no studies have tested the N and P effects on fine root production at a global scale. Fine root production, if mirroring the aboveground production (particularly leaf production) in response to soil nutrients [24], is expected to increase with nutrient supply. This idea is supported by the often observed positive correlations between aboveground and belowground production both at local, community and global scales [25,26], suggesting that these two components of total NPP are positively linked and are limited by some common factors. However, according to the optimal partitioning theory [27] and the cost–benefit theory [28], which predict that plants preferentially allocate additional biomass to organs where resources are limiting, increasing nutrient supply could reduce C allocation to fine roots. Consequently, the magnitude of increase in aboveground NPP in response to nutrient supply [19,22] may be partially offset by a concomitant decrease in belowground NPP, mostly in fine root production. Furthermore, because leaves have lower C : N and C : P ratios than roots [29], leaf production is expected to be more sensitive to nutrients than fine root production. Thus, commonly reported (and easily measured) aboveground NPP or leaf production are not necessarily the best measurements to determine the response of fine root production to soil nutrients.
To cope with the heterogeneity of soil resources, root systems can respond to nutrients by altering their morphological and physiological plasticity, including spatially explicit root proliferation [30,31]. Physiological responses often occur first and may trigger new root construction (i.e. morphological responses) and thus increase root production. It is necessary for plants to respond to spatial patches and temporal pulses of nutrients with rapid exploitation and uptake. Given the spatial variation in soil N and P across the world [4,32], the considerable plasticity in fine roots can lead to the prediction that stand-level fine root production will change as soil fertility varies within and among ecosystems; however, this prediction has not been tested at broad geographical scales.
There are two ways to examine the relationships between fine root production and soil nutrients: along a natural soil nutrient gradient and nutrient addition experiments. A comparative study to examine the response of fine root production to soil nutrients in natural habitats can give valuable information on the long-term adaptive response of fine root systems to the local soil nutrient regime. In contrast, nutrient addition experiments can produce information on short-term responses of fine root production to soil nutrients [33]. To reveal the global patterns of soil nutrients on fine root production, we examined the responses of fine root production to nutrients in both natural habitats and nutrient addition experiments across all terrestrial ecosystems.
Evaluation of the responses of fine root production to soil nutrients is complicated by fine root sampling methodology, resulting largely in differences in estimates. The ingrowth core method has been used in a relatively large number of studies to estimate fine root production. Thus, we chose to use only ingrowth coring studies, to minimize methodological biases, to examine how fine root production varies along natural soil N and P gradients. For nutrient addition experiments, since our analysis is conducted on response ratios, we included all studies in which the same method was used to estimate fine root production in the control and treatment sites.
2. Results
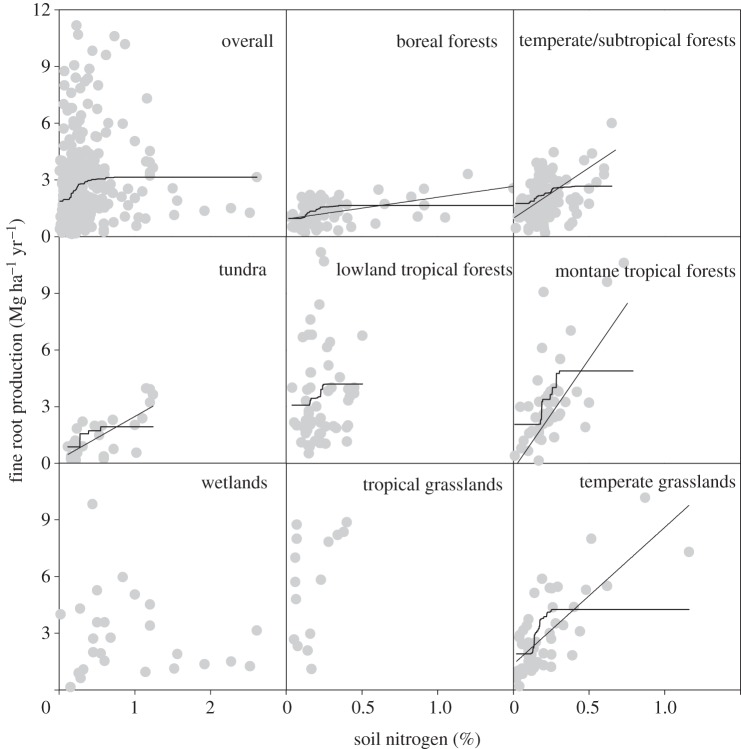
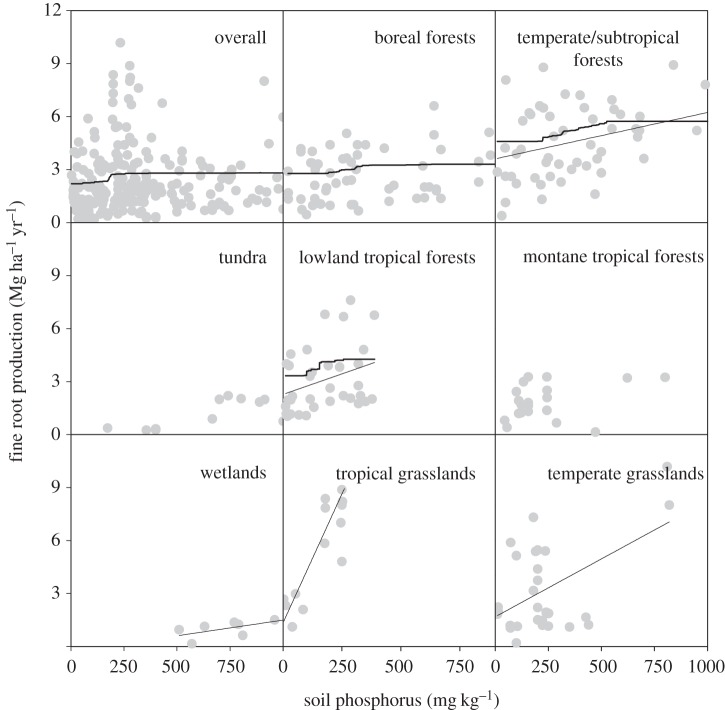
In natural habitats, the boosted regression trees indicated that, after soil and ecosystem types, soil total N and P were the next strongest drivers to fine root production at the global scale, explaining 13.4 per cent of the variation in fine root production (table 1). Soil type and ecosystem type accounted for 28.3 per cent and 19.7 per cent of the variation, respectively. Both soil total N and P showed positive influences on fine root production (figures 1 and 2), accounting for 9.7 per cent and 3.7 per cent influence, respectively (table 1). By contrast, soil available N and P had only approximately 1 per cent influence. At a global scale, the predicted value plots indicated that, when other predictors were held constant, fine root production strongly increased with soil total N up to approximately 0.5 per cent. Fine root production also increased with soil total P up to approximately 300 mg kg−1, and then plateaued or even decreased at sites with high soil total P (figures 1 and 2).
Table 1.
BRT analysis for the influence of ecosystem type, soil type, leaf type (broadleaves versus coniferous) and soil nutrients on fine root production. In the model, predictors with large values in influence mean that they have more explanatory power. Total, all predictors; overall, all ecosystems; forests, all forests (boreal + temperate/subtropical + tropical), grasslands, all grasslands (temperate + tropical/savannah).
| predictor | deviance (%) |
||
|---|---|---|---|
| overall | forests | grasslands | |
| ecosystem type | 19.72 | 14.22 | 1.71 |
| soil type | 28.29 | 23.70 | 41.32 |
| leaf type | 0.05 | 0.87 | — |
| soil chemistry | 22.04 | 28.19 | 15.44 |
| pH | 3.24 | 1.30 | 0 |
| organic matter | 0.08 | 0.22 | 0 |
| total carbon | 3.07 | 6.76 | 0 |
| total nitrogen | 9.70 | 10.60 | 13.61 |
| total phosphorus | 3.72 | 5.39 | 1.05 |
| available nitrogen | 1.41 | 2.41 | 0 |
| available phosphorus | 0.83 | 1.50 | 0 |
| total | 70.11 | 66.98 | 58.47 |
Figure 1.
The relationships between fine root production (Mg ha−1 yr−1) and soil nitrogen concentration (%). Regression lines were plotted with p < 0.05: boreal forests (r2 = 0.200, p < 0.001, n = 76), temperate/subtropical forests (r2 = 0.161, p < 0.001, n = 104), lowland tropical forests (r2 = 0.027, p = 0.218, n = 59), montane tropical forests (r2 = 0.473, p < 0.001, n = 45), temperate grasslands (r2 = 0.454, p < 0.001, n = 51), tropical grasslands/savannah (r2 = 0.226, p = 0.054, n = 17), tundra (r2 = 0.487, p < 0.001, n = 27) and wetlands (r2 = 0.010, p = 0.633, n = 26). The curved lines indicate the predicted values from monotonically fitted models using boosted regression tree (BRT) analysis. Owing to the small sample size, BRT was not performed for tropical grasslands and wetlands.
Figure 2.
The relationships between fine root production (Mg ha−1 yr−1) and soil phosphorus concentration (mg kg−1). Regression lines were plotted with p < 0.05: boreal forests (r2 = 0.029, p = 0.196, n = 59), temperate/subtropical forests (r2 = 0.149, p < 0.01, n = 53), lowland tropical forests (r2 = 0.117, p = 0.04, n = 34), montane tropical forests (r2 = 0.058, p = 0.311, n = 20), temperate grasslands (r2 = 0.159, p = 0.036, n = 28), tropical grasslands/savannah (r2 = 0.752, p < 0.001, n = 14), tundra (r2 = 0.195, p = 0.114, n = 14) and wetlands (r2 = 0.553, p = 0.034, n = 8). The curved lines indicate the predicted values from monotonically fitted models using BRT analysis. Owing to the small dataset size, BRT is not performed in tundra, wetlands, grasslands and montane tropical forests.
The influence of soil nutrient variables on fine root production in natural habitats differed among ecosystem types (see the electronic supplementary material, table S1; figures 1 and 2). Linear regression analysis showed that fine root production increased significantly with soil total N in all ecosystem types, except for lowland tropical forests (F = 1.551, p = 0.218) and wetlands (F = 0.234, p = 0.633; figure 1). The response in tropical grasslands was marginally significant (F = 4.384, p = 0.054). Within the tropical forests, fine root production was more strongly dependent on soil total N in the montane region than in the lowland region.
In natural habitats, soil total P also affected fine root production, but with less influence than soil total N (see the electronic supplementary material, tables S1 and S2). Linear regression analysis indicated significantly positive effects of soil total P on fine root production in temperate/subtropical forests, tropical grasslands, temperate grasslands and wetlands, but not in boreal forests or tundra (figure 2). Within the tropical forests, fine root production responded positively to soil total P in the lowland region, whereas the effect of soil total P on fine root production in the montane region was not significant (F = 1.089, p = 0.311).
Adding the ecosystem types to the soil N and P data increased explanatory power by 47 per cent (see the electronic supplementary material, table S3). An additional 20 per cent of the total variation in fine root production was explained if the qualitative traits of soil and leaf types were also included in the model. The combination of all predictors explained approximately three-quarters of the total variation in fine root production.
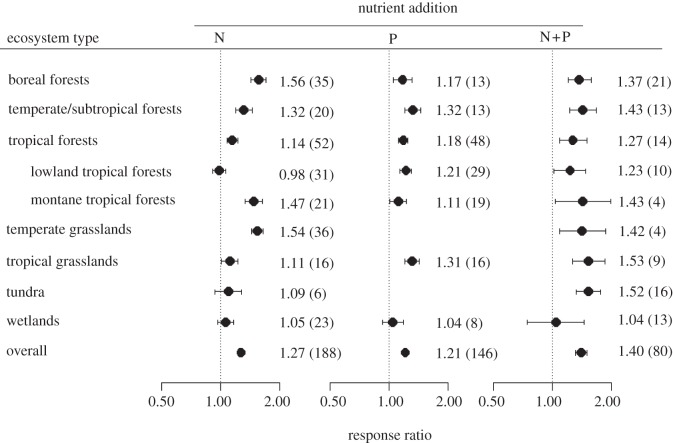
For the nutrient addition experiments, the random effect model with all data pooled showed a significant effect of N addition on fine root production (Q = 289.13, p < 0.001). Fine root production was on average 27 per cent higher in the N-fertilized treatments than in the control (figure 3). The mixed effect model showed that there was a significant effect of ecosystem type (QM = 273.32, p < 0.001). The mean response ratios (RRs) were significantly higher than 1 for boreal forests (RR = 1.56), temperate/subtropical forests (RR = 1.32), tropical forests (RR = 1.14), temperate grasslands (RR = 1.54), but not significantly different from 1 for tropical grasslands (RR = 1.11), tundra (RR = 1.09) or wetlands (RR = 1.05; figure 3). Within the tropical forests, the response in montane tropical forests (RR = 1.47) was greater than that in lowland tropical lowlands (RR = 0.98; p = 0.011).
Figure 3.
Responses of fine root production to fertilizer addition by nitrogen (N), phosphorus (P) and combined N + P in boreal forests, temperate/subtropical forests, tropical forests, temperate grasslands, tropical grasslands, tundra and wetlands. The numbers out- and inside parentheses represent response ratio and the number of observations in each ecosystem, respectively. The dot with error bars shows the mean effect size with the 95% CI.
P-fertilized treatments could also significantly increase fine root production (figure 3). There was also a significant effect of ecosystem type (QM = 20.72, p < 0.01). The response ratios were significantly higher than 1 for boreal forests (RR = 1.17), temperate/subtropical forests (RR = 1.32), tropical forests (RR = 1.18), tropical grasslands (RR = 1.31) and lowland tropical forests (RR = 1.21), but not for wetlands (RR = 1.04).
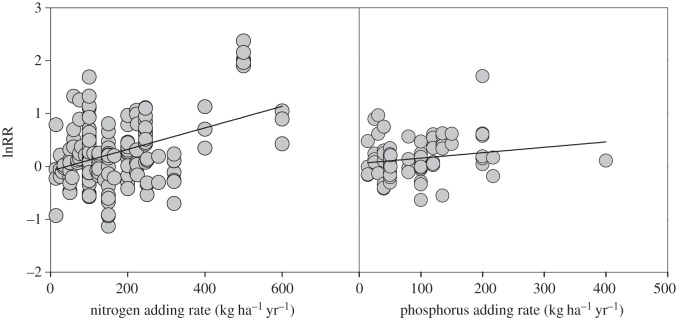
Treatments with combined N and P fertilization increased fine root production on average by 40 per cent (figure 3). The response of fine root production to N + P fertilization did not differ significantly among ecosystem types (p = 0.457). Analysis conducted for each ecosystem type indicated that RR was significantly higher than 1 for all ecosystems except wetlands (figure 3). Based on paired data from the same site, sampling date, fertilization type and rate, the response of fine root production to combined N + P addition was significantly higher than N or P addition alone (see the electronic supplementary material, figure S1). When all data were pooled, the response ratios also increased with the amounts of N and P added, but the slope was lower in N- than P-fertilized treatments (figure 4).
Figure 4.
Natural log response ratios (lnRR) of fine root production to nitrogen (N) or phosphorus (P) addition rates. Regression lines in black were plotted with p < 0.05: N addition experiments (r2 = 0.149, p < 0.001, n = 224), P addition experiments (r2 = 0.034, p = 0.043, n = 124).
Soil type had significant effects of N, P or combined N + P fertilization on fine root production (all p < 0.001 in mixed models; see the electronic supplementary material, table S3 and figure S2). However, the responses differed significantly among the 14 FAO soil types. Even within the same soil type, fine root production might show different responses to different nutrient additions. For example, fine root production in Acrisols responded negatively to N addition but positively to P addition.
Both broadleaved and coniferous ecosystems responded positively to N, P or combined N + P additions (see the electronic supplementary material, figure S3). The increase in fine root production was greater in coniferous species than broadleaved species when treated with N, or combined N + P additions, but their differences in responding to P addition were not significant (QM = 0.585, p = 0.447).
3. Discussion
Based on from data both natural habitats and from nutrient addition experiments, our analysis demonstrated that fine root production increased with both soil N and P, supporting the third hypothesis proposed by Nadelhoffer [18], i.e. fine root production might increase with soil N. Although fine root biomass often declines or is relatively constant with increasing soil nutrients [16,18,23], the positive responses of fine root production to soil nutrients suggest that fine roots turn over faster in nutrient-rich than nutrient-poor soils, as proposed for N by Nadelhoffer [18]. The variation in fine root production with soil nutrients indicated that root systems are highly elastic in response to environments, and they can change their production at the stand level across broad geographical scales.
It is not surprising that fine root production increases with soil nutrients since plant growth is often limited by soil nutrients in natural ‘infertile’ ecosystems [34]. At the plant level, C and nutrient (N and P) cycles are closely linked: because fine roots serve as the organs for absorbing nutrients to support plant growth, more nutrient uptake can lead to greater nutrient concentrations and faster metabolism in fine roots [3,35], resulting in more nutrient translocated to aboveground, higher leaf area index and foliar nutrient concentrations, and consequently greater photosynthesis rates. A high rate of photosynthesis can provide more C to meet the metabolic demands of increased root growth and nutrient uptake. Similarly, at the ecosystem level, more nutrient supply increases leaf production and aboveground NPP [1,22], and in turn, higher aboveground NPP fuels higher belowground growth.
All ecosystems other than wetlands responded positively to soil N both in natural habitats and N addition experiments, adding to the growing evidence of N limitation in global terrestrial ecosystems [3]. Despite water limitation in temperate grasslands, fine root production increased with soil N, consistent with the results from the analysis of aboveground NPP [22,36]. Fine root production responded to soil N in a more complex form in natural wetlands than the linear relationship that was assumed in our model, showing a saturating response where soil total N is greater than 1 per cent (figure 1). This finding may suggest that other factors in N-rich wetlands may be more important in influencing root production.
Fine root production in natural habitats responded positively to soil P in temperate/subtropical and tropical forests, grasslands and wetlands (figure 2). The significant relationships for soil P but not for N in wetlands suggest that wetlands may be more limited by P than by N. By contrast, fine root production in boreal forests and tundra did not change with soil P but with N, indicating that those cold ecosystems are limited more by N than by P. Together with our meta-analysis showing positive effects of P addition in most ecosystems, those results provided evidence that P limitation is not unimportant and plays a role in terrestrial ecosystems.
Although fine root production in tropical forests was positively related to soil nutrients, lowland and montane tropical forests responded differently: along the natural N gradients, fine root production in montane tropical forests was positively associated with soil N but not with P. By contrast, the relationship was significant for P but not for N in lowland tropical forests. The same was true in N and P addition experiments. Our analysis supports the widely held idea that N limits productivity in montane tropical forests [37,38], and the idea that production in lowland tropical forests are more controlled by climate and P availability [4,21,39].
The positive responses of fine root production to both N and P in natural habitats suggest that both nutrients are limited (co-limited), although the extent of such controls may differ in an individual ecosystem. This conclusion was further strengthened by our meta-analysis in which simultaneously adding both N and P gave a much stronger response than either of them alone (see the electronic supplementary material, figure S2), challenging the conventional view that ecosystems are generally limited by one nutrient at a time (Liebig's law of the minimum [40]).
The positive responses of fine root production to soil nutrients in most terrestrial ecosystems, however, do not necessarily refute the optimal partitioning theory [27] or the cost–benefit theory [28]. In nutrient-rich soils, plants may reduce C allocation to belowground root systems, leading to a smaller root/shoot ratio; however, belowground NPP could still be higher with much higher aboveground NPP than in nutrient-poor soils. The same would be true for the relationship between leaf and fine root production. Therefore, although the absolute rates of fine root production and likely belowground NPP may increase with soil nutrients, the proportion of total NPP accounted for by root systems probably decreases with soil nutrients, resulting in a change in C allocation patterns. The average response of fine root production to N (+27%), P (+21%) and combined N + P (40%) additions found in our analysis was lower than that for aboveground NPP (N: +30%, P: +26%, N + P: +57% [1,22]). Our analyses, combined with previous studies for aboveground NPP, support the idea that nutrient addition can reduce the relative C allocation to belowground as predicted by the optimal partitioning theory [27] and the cost–benefit theory [28].
The N addition rate in our meta-data was on average 15.4 g N m−2 yr−1. Anthropogenic N input into terrestrial ecosystems increase by an average rate of 1 g N m−2 yr−1 [5,41]. If the response of root production to N is linear, the 27 per cent increase of fine root production to N addition suggests that the anthropogenic N deposition can increase fine root production by a yearly rate of 1.8 per cent, on average. Since fine roots represent 33 per cent of global annual NPP [14] and the total NPP is 59.9 Gt C yr−1 [26], the yearly 1.8 per cent increase is equivalent to an increase of 0.35 Gt C yr–1 in fine root production, accounting for one-third of the response of global total NPP to N enrichment (0.98 Gt C yr−1) [7,9]. This potential increase in fine root production, coupled with coarse root and aboveground productions, may provide an important terrestrial ecosystem feedback to the future global climate regime.
Aside from soil nutrients and ecosystem type, soil type had strong effects on fine root production and its response to nutrient additions. For instance, P addition had a positive effect, but N addition had a negative effect on fine root production in acrisols, which are clay-rich and associated with humid and tropical climates. By contrast, N addition in andosols, a young soil type in volcanic areas formed in volcanic tephra with poor N but rich P, had a positive effect on fine root production while P addition did not. Ecosystems dominated by different leaf types demonstrated similar responses to nutrients. Compared with soil total nutrients, available N and P had little explanatory power. The most probable reasons were the diverse indices of nutrient availability and difficulties in accurately sampling and assessing nutrient availability, especially P, thereby obscuring a potentially stronger association that may exist. Owing to the close relationship between soil total and available nutrients, our analysis, however, revealed controls of nutrient availability on fine root production.
In summary, this is, to our knowledge, the first attempt to quantitatively evaluate how fine root production responds to soil nutrients at the global scale. Both our analyses based on data from natural habitats and from N and/or P addition experiments showed that fine root production, coupled with the increases in aboveground NPP and litterfall, increased with soil nutrients in most terrestrial ecosystems. Soil N had significant effects on fine root production in montane tropical forests but not in lowland tropical forests. Soil P showed opposite effects, indicating that the production of those two tropical forest types is not driven by the same factors. While the global average increases in fine root production with N, P or combined N + P additions are somewhat similar to but lower than the responses of ANPP, indicating that above- and belowground are coupled, increased production is allocated more to the aboveground with higher soil nutrients. Based on our findings, the increasing use of N and P fertilizers, together with the formation of reactive N from fossil fuel combustion and increasing N-fixation by legumes, at present and in the future will stimulate fine root production, and likely below- and aboveground NPP.
Acknowledgments
The authors thank Dr Wolfgang Viechtbauer, who provided some new codes for us to use metaphor for R. This work was financially supported by an Ontario Post-Doctoral Fellowship from the Ontario Ministry of Research and Innovation, the Natural Science and Engineering Council of Canada (DG283336-09) and Ontario Ministry of Research and Innovation Early Researcher Award program.
References
- 1.Elser J. J., et al. 2007. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 10, 1135–1142 10.1111/j.1461-0248.2007.01113.x (doi:10.1111/j.1461-0248.2007.01113.x) [DOI] [PubMed] [Google Scholar]
- 2.Harpole W. S., et al. 2011. Nutrient co-limitation of primary producer communities. Ecol. Lett. 14, 852–862 10.1111/j.1461-0248.2011.01651.x (doi:10.1111/j.1461-0248.2011.01651.x) [DOI] [PubMed] [Google Scholar]
- 3.Vitousek P. M., Howarth R. W. 1991. Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13, 87–115 10.1007/BF00002772 (doi:10.1007/BF00002772) [DOI] [Google Scholar]
- 4.Vitousek P. M., Porder S., Houlton B. Z., Chadwick O. A. 2010. Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen–phosphorus interactions. Ecol. Appl. 20, 5–15 10.1890/08-0127.1 (doi:10.1890/08-0127.1) [DOI] [PubMed] [Google Scholar]
- 5.Galloway J. N., et al. 2004. Nitrogen cycles: past, present, and future. Biogeochemistry 70, 153–226 10.1007/s10533-004-0370-0 (doi:10.1007/s10533-004-0370-0) [DOI] [Google Scholar]
- 6.Aber J. D., Goodale C. L., Ollinger S. V., Smith M. L., Magill A. H., Martin M. E., Hallett R. A., Stoddard J. L. 2003. Is nitrogen deposition altering the nitrogen status of northeastern forests? Bioscience 53, 375–389 10.1641/0006-3568(2003)053[0375:INDATN]2.0.CO;2 (doi:10.1641/0006-3568(2003)053[0375:INDATN]2.0.CO;2) [DOI] [Google Scholar]
- 7.Vitousek P. M., Aber J. D., Howarth R. W., Likens G. E., Matson P. A., Schindler D. W., Schlesinger W. H., Tilman G. D. 1997. Human alteration of the global nitrogen cycle: sources and consequences. Ecol. Appl. 7, 737–750 [Google Scholar]
- 8.Lamarque J. F., et al. 2005. Assessing future nitrogen deposition and carbon cycle feedback using a multimodel approach: analysis of nitrogen deposition. J. Geophys. Res. 110, D19303. 10.1029/2005JD005825 (doi:10.1029/2005JD005825) [DOI] [Google Scholar]
- 9.Reay D. S., Dentener F., Smith P., Grace J., Feely R. A. 2008. Global nitrogen deposition and carbon sinks. Nat. Geosci. 1, 430–437 10.1038/ngeo230 (doi:10.1038/ngeo230) [DOI] [Google Scholar]
- 10.Rustad L. E., Campbell J. L., Marion G. M., Norby R. J., Mitchell M. J., Hartley A. E., Cornelissen J. H. C., Gurevitch J. 2001. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126, 543–562 10.1007/s004420000544 (doi:10.1007/s004420000544) [DOI] [PubMed] [Google Scholar]
- 11.IFA 2010. International Fertilizer Industry. See www.fertilizer.org/ifa/
- 12.Penuelas J., Sardans J., Rivas-Ubach A., Janssens I. A. 2012. The human-induced imbalance between C, N and P in Earth's life system. Global Change Biol. 18, 3–6 10.1111/j.1365-2486.2011.02568.x (doi:10.1111/j.1365-2486.2011.02568.x) [DOI] [Google Scholar]
- 13.Magnani F., et al. 2007. The human footprint in the carbon cycle of temperate and boreal forests. Nature 447, 848–850 10.1038/nature05847 (doi:10.1038/nature05847) [DOI] [PubMed] [Google Scholar]
- 14.Jackson R. B., Mooney H. A., Schulze E. D. 1997. A global budget for fine root biomass, surface area, and nutrient contents. Proc. Natl Acad. Sci. USA 94, 7362–7366 10.1073/pnas.94.14.7362 (doi:10.1073/pnas.94.14.7362) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gower S. T., Pongracic S., Landsberg J. J. 1996. A global trend in belowground carbon allocation: can we use the relationship at smaller scales? Ecology 77, 1750–1755 10.2307/2265780 (doi:10.2307/2265780) [DOI] [Google Scholar]
- 16.Yuan Z. Y., Chen H. Y. H. 2010. Fine root biomass, production, turnover rates, and nutrient contents in boreal forest ecosystems in relation to species, climate, fertility, and stand age: literature review and meta-analyses. Crit. Rev. Plant Sci. 29, 204–221 10.1080/07352689.2010.483579 (doi:10.1080/07352689.2010.483579) [DOI] [Google Scholar]
- 17.Norby R. J., Jackson R. B. 2000. Root dynamics and global change: seeking an ecosystem perspective. New Phytol. 147, 3–12 10.1046/j.1469-8137.2000.00676.x (doi:10.1046/j.1469-8137.2000.00676.x) [DOI] [Google Scholar]
- 18.Nadelhoffer K. J. 2000. The potential effects of nitrogen deposition on fine-root production in forest ecosystems. New Phytol. 147, 131–139 10.1046/j.1469-8137.2000.00677.x (doi:10.1046/j.1469-8137.2000.00677.x) [DOI] [Google Scholar]
- 19.Reich P. B., Grigal D. F., Aber J. D., Gower S. T. 1997. Nitrogen mineralization and productivity in 50 hardwood and conifer stands on diverse soils. Ecology 78, 335–347 10.1890/0012-9658(1997)078[0335:NMAPIH]2.0.CO;2 (doi:10.1890/0012-9658(1997)078[0335:NMAPIH]2.0.CO;2) [DOI] [Google Scholar]
- 20.Yuan Z. Y., Li L. H., Han X. G., Chen S. P., Wang Z. W., Chen Q. S., Bai W. M. 2006. Nitrogen response efficiency increased monotonically with decreasing soil resource availability: a case study from a semiarid grassland in northern China. Oecologia 148, 564–572 10.1007/s00442-006-0409-0 (doi:10.1007/s00442-006-0409-0) [DOI] [PubMed] [Google Scholar]
- 21.Cleveland C. C., et al. 2011. Relationships among net primary productivity, nutrients and climate in tropical rain forest: a pan-tropical analysis. Ecol. Lett. 14, 939–947 10.1111/j.1461-0248.2011.01658.x (doi:10.1111/j.1461-0248.2011.01658.x) [DOI] [PubMed] [Google Scholar]
- 22.LeBauer D. S., Treseder K. K. 2008. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89, 371–379 10.1890/06-2057.1 (doi:10.1890/06-2057.1) [DOI] [PubMed] [Google Scholar]
- 23.Liu L. L., Greaver T. L. 2010. A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecol. Lett. 13, 819–828 10.1111/j.1461-0248.2010.01482.x (doi:10.1111/j.1461-0248.2010.01482.x) [DOI] [PubMed] [Google Scholar]
- 24.Hendricks J. J., Nadelhoffer K. J., Aber J. D. 1993. Assessing the role of fine roots in carbon and nutrient cycling. Trends Ecol. Evol. 8, 174–178 10.1016/0169-5347(93)90143-D (doi:10.1016/0169-5347(93)90143-D) [DOI] [PubMed] [Google Scholar]
- 25.Nadelhoffer K. J., Raich J. W. 1992. Fine root production estimates and belowground carbon allocation in forest ecosystems. Ecology 73, 1139–1147 10.2307/1940664 (doi:10.2307/1940664) [DOI] [Google Scholar]
- 26.Roy J., Saugier B., Mooney H. A. 2001. Terrestrial global productivity. San Diego, CA: Academic Press [Google Scholar]
- 27.Bloom A. J., Chapin F. S., Mooney H. A. 1985. Resource limitation in plants: an economic analogy. Annu. Rev. Ecol. Syst. 16, 363–392 10.1146/annurev.es.16.110185.002051 (doi:10.1146/annurev.es.16.110185.002051) [DOI] [Google Scholar]
- 28.Eissenstat D. M., Yanai R. D. 1997. The ecology of root lifespan. Adv. Ecol. Res. 27, 1–60 10.1016/S0065-2504(08)60005-7 (doi:10.1016/S0065-2504(08)60005-7) [DOI] [Google Scholar]
- 29.Yuan Z. Y., Chen H. Y. H., Reich P. B. 2011. Global-scale latitudinal patterns of plant fine-root nitrogen and phosphorus. Nat. Commun. 2, 344. 10.1038/ncomms1346 (doi:10.1038/ncomms1346) [DOI] [PubMed] [Google Scholar]
- 30.Hodge A. 2004. The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol. 162, 9–24 10.1111/j.1469-8137.2004.01015.x (doi:10.1111/j.1469-8137.2004.01015.x) [DOI] [Google Scholar]
- 31.Casper B., Cahill J., Jackson R. 2000. Plant competition in spatially heterogeneous environments. In The ecological consequences of environmental heterogeneity (eds Hutchings M. J., John E. A., Stewart A. J. A.), pp. 111–130 Oxford, UK: Blackwell Science [Google Scholar]
- 32.Batjes N. H. 1996. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 47, 151–163 10.1111/j.1365-2389.1996.tb01386.x (doi:10.1111/j.1365-2389.1996.tb01386.x) [DOI] [Google Scholar]
- 33.Chapin F. S., Vitousek P. M., Vancleve K. 1986. The nature of nutritional limitation of plant communities. Am. Nat. 127, 48–58 10.1086/284466 (doi:10.1086/284466) [DOI] [Google Scholar]
- 34.Field C. B., Mooney H. A. 1986. The photosynthesis–nitrogen relationship in wild plants. In On the economy of plant form and function (ed. Givnish T. J.), p. 25 Cambridge, UK: Cambridge University Press [Google Scholar]
- 35.Hendricks J. J., Aber J. D., Nadelhoffer K. J., Hallett R. D. 2000. Nitrogen controls on fine root substrate quality in temperate forest ecosystems. Ecosystems 3, 57–69 10.1007/s100210000010 (doi:10.1007/s100210000010) [DOI] [Google Scholar]
- 36.Yahdjian L., Gherardi L., Sala O. E. 2011. Nitrogen limitation in arid-subhumid ecosystems: a meta-analysis of fertilization studies. J. Arid Environ. 75, 675–680 10.1016/j.jaridenv.2011.03.003 (doi:10.1016/j.jaridenv.2011.03.003) [DOI] [Google Scholar]
- 37.Bruijnzeel L. A., Scatena F. N., Hamilton L. S. 2010. Tropical montane cloud forests: science for conservation and management. Cambridge, UK: Cambridge University Press [Google Scholar]
- 38.Tanner E. V. J., Vitousek P. M., Cuevas E. 1998. Experimental investigation of nutrient limitation of forest growth on wet tropical mountains. Ecology 79, 10–22 10.1890/0012-9658(1998)079[0010:EIONLO]2.0.CO;2 (doi:10.1890/0012-9658(1998)079[0010:EIONLO]2.0.CO;2) [DOI] [Google Scholar]
- 39.Lambers H., Brundrett M. C., Raven J. A., Hopper S. D. 2011. Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant Soil 348, 7–27 10.1007/s11104-011-0977-6 (doi:10.1007/s11104-011-0977-6) [DOI] [Google Scholar]
- 40.Liebig J. 1840. Die organische Chemie in ihrer Anwendung auf Agrikultur und Physiologie. Braunschweig, Germany, Friedrich Vieweg und Sohn Publ. Co [Google Scholar]
- 41.Dentener F., et al. 2006. Nitrogen and sulfur deposition on regional and global scales: a multimodel evaluation. Global Biogeochem. Cycle 20, 1–21 10.1029/2005GB002672 (doi:10.1029/2005GB002672) [DOI] [Google Scholar]