Abstract
Parent–offspring recognition is crucial for offspring survival. At long distances, this recognition is mainly based on vocalizations. Because of maturation-related changes to the structure of vocalizations, parents have to learn successive call versions produced by their offspring throughout ontogeny in order to maintain recognition. However, because of the difficulties involved in following the same individuals over years, it is not clear how long this vocal memory persists. Here, we investigated long-term vocal recognition in goats. We tested responses of mothers to their kids’ calls 7–13 months after weaning. We then compared mothers’ responses to calls of their previous kids with their responses to the same calls at five weeks postpartum. Subjects tended to respond more to their own kids at five weeks postpartum than 11–17 months later, but displayed stronger responses to their previous kids than to familiar kids from other females. Acoustic analyses showed that it is unlikely that mothers were responding to their previous kids simply because they confounded them with the new kids they were currently nursing. Therefore, our results provide evidence for strong, long-term vocal memory capacity in goats. The persistence of offspring vocal recognition beyond weaning could have important roles in kin social relationships and inbreeding avoidance.
Keywords: Capra hircus, individual recognition, long-term memory, mammal, vocal communication
1. Introduction
Long-term recognition of socially important individuals should be essential in species subjected to regular periods of separation. However, because of the difficulties involved in following the same individuals over extended periods, long-term recognition has only been tested in a few species [1–3]. Therefore, little is known about which species display this complex cognitive process and how long the memory of conspecifics can persist. Parent–offspring recognition is crucial in species providing parental care, especially in species living in large groups of unrelated individuals. Long-range recognition relies principally on vocal cues [4]. In mammals, several species have been shown to display unidirectional (recognition of the parents by the offspring only, or of the offspring by the parents only) or mutual (both parents and offspring recognize each other) mother–offspring vocal recognition [5–7]. This ability develops quickly; usually during the first few hours or days following parturition [6]. The direction (unidirectional or mutual) and timing of recognition onset depend on ecological constraints [8].
Very few studies have investigated parent–offspring recognition beyond weaning. Australian sea lions (Neophoca cinerea) and northern fur seal (Callorhinus ursinus) pups remember the calls of their mothers for at least 2–3.8 years after weaning [1,9]. Cotton-top tamarins (Saguinus oedipus) recognize the calls of their relatives (parents, offspring or siblings) for up to 4.6 years after separation [10]. Conversely, recognition of offspring by parents is rendered difficult by maturation-related changes to the structure of vocalizations occurring during ontogeny [5,11]. Subantarctic fur seal (Arctocephalus tropicalis) mothers retain all successive versions of their pups’ calls produced until weaning (seven months old) [12]. This memory of old call versions has been suggested to be an evolutionary by-product of the strong learning experienced by mothers when they nurse their offspring [12]. A question that has rarely been investigated is whether mothers remember the calls of their offspring after weaning. To our knowledge, the only study on this topic found that northern fur seal mothers remember call versions of their pups recorded one year previously (8–12 months after weaning) [1]. In this study, we examined this question in goats, which are highly vocal and characterized by strong mother–kid bonds [13,14].
Mother goats provide exclusive care to their own kid(s). At short distances, this bond relies primarily on olfactory recognition, which develops soon after birth [15]. Longer-range mutual recognition depends on visual and vocal cues [13,16]. Previously, we showed that mutual vocal recognition between mothers and kids exists at one and five weeks postpartum, corresponding to two ecologically and socially distinct periods of kids’ early life [13]. Goats adopt a hider strategy for predator avoidance: during the first weeks of life, goat kids remain hidden most of the time in vegetation, alone or with their sibling(s), in order to avoid detection by predators. At five weeks old, however, goat kids would normally have integrated into social groups [17].
We investigated long-term vocal recognition of kids by their mothers, using playback experiments. We tested mothers’ responses to the calls of their kids recorded at five weeks postpartum, after 11–17 months had passed. At this time, kids had been weaned for 7–13 months, and mothers were nursing new, younger kids. In order to investigate how responses changed with time, we compared the responses of mothers to calls of their previous kids with their responses to the same calls tested during a first series of playbacks, when the kids were five weeks old. As a control, during the second series of playbacks, we also tested mothers’ responses to calls of familiar kids born to other females, and living in the same communal pen. We predicted that if mothers remember the calls of their previous kids, they should react more to these calls than to the calls of familiar individuals. Additionally, we performed an acoustic analysis of the calls of the mothers’ previous kids, the calls of the kids they were nursing during the second series of playbacks and the calls of familiar kids used in the experiments. A comparison between these different categories of calls allowed us to determine whether females were responding to their previous kids' calls simply because they were confounding them with the new kids they were currently nursing. The study of long-term recognition can help us to understand how social relationships are maintained in species living in complex societies.
2. Material and methods
(a). Subjects
We carried out the study at White Post Farm, Nottinghamshire, UK, on nine multiparous pygmy goat mothers between 2009 and 2011. They lived in groups of 3–5 adult females and their kids in a communal indoor pen. All kids in this study had the same father.
(b). Playback treatments
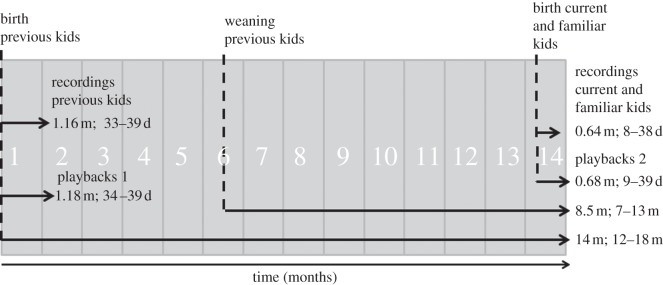
We tested each goat with the contact calls of their own kids (‘previous kids’; eight males and one female, recorded at five weeks old) at two periods: at five weeks postpartum (mean = 36.00 ± 0.65 days old, range = 34–39 days; ‘playbacks 1’), and after 12–18 months had elapsed postpartum (mean = 14.03 ± 0.72 months; 7–13 months after weaning; ‘playbacks 2’; figure 1). The responses of mothers during playbacks 1 were included in the analysis of Briefer & McElligott [13]. They are used here for comparison, in order to investigate how the responses of mothers to calls of their five-week-old kids change with time. These previous analyses showed that mothers responded significantly more strongly to their own kids than to familiar kids from other females at five weeks postpartum [13]. Own kids were raised in the communal pen with their mothers until weaning, which occurred at 5–6 months. Mothers and kids were then moved to different groups, situated at non-visual and non-hearing range from each other. During playbacks 2, mothers were also tested with calls of familiar kids from other females, kept in the communal pen (control; ‘familiar kids’; two males and six females; figure 1). At that time, goats were nursing 10- to 39-day-old (mean ± s.e. = 20.56 ± 3.00 days) single-born or twin kids (‘current kids’; seven males and ten females). The familiar kids used in the playbacks were similar in age to current kids (mean = 20.89 ± 3.82 days old, range = 9–36). For one goat, no other kid was present in the group. The familiar-kid treatment for this goat consisted of calls of a kid (35 days old) raised in the same group as her previous kid. Each treatment consisted of a 30 s sequence of at least 50 per cent different calls interspaced by natural silence intervals (mean = 0.93 ± 0.04 s). All calls in a given sequence were rescaled to the same maximum amplitude.
Figure 1.
Timeline of the experimental design. Birth and weaning events are indicated with vertical dashed lines. Arrows indicate timing of recordings and playbacks with averages and ranges (d, days; m, months). The full experiment, from the birth of previous kids to the second series of playbacks, lasted 14 months on average, depending on the goats (range 12–18 months). ‘Playbacks 1’ correspond to playbacks of previous kid calls at five weeks postpartum. ‘Playbacks 2’ correspond to playbacks of these same calls at 12–18 months postpartum (7–13 months after weaning), and to playbacks of calls from familiar kids, born 9–39 days previously. Goats were nursing new kids at the time of the study (current kids).
(c). Recordings
Previous kids were recorded at five weeks old (mean = 35.44 ± 0.73 days old, range = 33–39 days), and current and familiar kids at 19.65 ± 2.49 days old on average (range = 8–38 days; figure 1). Goats produce low-amplitude closed-mouth contact calls when they are close to each other and higher-amplitude open-mouth contact calls at greater distance [18]. Because our playback experiments mimicked the presence of an individual situated five metres away from the tested subjects, only contact calls emitted with the mouth open were used in this study. We recorded open-mouth contact calls at distances of 1–5 m using a Sennheiser MKH70 directional microphone, connected to a Marantz PMD660 recorder (sampling rate: 44.1 kHz). This was done by separating kids from their mothers (1–10 m) for no more than 5 min, 2–3 times each day [11,13]. Vocalizations were then saved on a computer in waveform audio file (WAV) format at 16-bit amplitude resolution. We used Goldwave v. 5.11, Praat v. 5.0.47 digital signal processing (DSP) package for subsequent analyses and for the preparation of call sequences played back. Calls were visualized on spectrograms in Praat (fast Fourier transform method, window length = 0.01 s, time steps = 1000, frequency steps = 250, Gaussian window shape, dynamic range = 50 dB). Vocalizations with high levels of background noise were not considered.
(d). Playback procedure
We played back call sequences, stored as MP3 files on a secure digital (SD) card at a sampling rate of 44.1 kHz and a bit rate of 224 Kbps, using a Skytronic TEC076 portable system (frequency response: 50 Hz–20 kHz ± 3 dB). Mothers were tested in their home pen. The loudspeaker was situated behind a fence at 4–6 m from the mothers. We played back signals at a natural intensity (81.19 ± 1.51 dB measured at 1 m using an ASL-8851 sound level meter, linear setting [13]). For playbacks 1, mothers were tested when their own kid(s) had been removed and isolated at non-visual and non-hearing range from them [13]. For playbacks 2, mothers were tested in the presence of their current kid(s). This ensured that mothers’ reactions to previous kids' calls were not owing to separation from their current kids. Subjects were tested with the two treatments (playbacks 2: previous kids and familiar kids) on the same day in random order, with 5–10 min intervals that allowed them to return to normal activities [13].
(e). Responses measured
The observer filmed the responses using a Sony DCR-SX50E camcorder at 5–10 m from the animals. During the 30 s of playback, we scored the duration of looks towards the loudspeaker (DurLook), the latency to look (LatLook), the number of calls produced and the latency to call (LatCall) using CowLog v. 1.1 [19]. Because calls produced during the playbacks were sometimes closed-mouth calls and difficult to count by ear from the sound track of the video owing to the low amplitude, the number of calls was scored by observing the strong abdominal contractions linked to vocal production. The number of calls was then divided by the total duration (30 s) to obtain the calling rate (RateCall). For LatLook and LatCall, when the subject did not look in the direction of the loudspeaker or did not call, a latency of 30 s (total playback duration) was attributed.
(f). Acoustic analysis
Playbacks of kid calls elicited no response when goats were pregnant (E. F. Briefer 2010, unpublished observations), indicating that maternal responsiveness is state-dependent [20]. We therefore carried out playbacks when mothers were nursing current kids. Because relatedness enhances vocal similarity [21], mothers could be confusing the vocalizations of their previous kids with those of their current kids. To test if the responses to our playbacks were the result of long-term memory of previous kids' calls, or potential confusion between calls of previous and current kids, we carried out detailed acoustic analyses to compare previous, current and familiar kids' calls (n = 8 calls per kid, 20 kids in total; nine previous kids and 11 current and/or familiar kids). If a mother was nursing twins at the time of the study, only one of these current kids was randomly chosen and included in the analyses.
Goat calls are short, with a clear harmonic structure, and strong frequency and amplitude modulations [11,13]. According to the source-filter theory of voice production [22,23], mammal vocalizations are generated by vibrations of the vocal folds (source, determining the fundamental ‘F0’), and are subsequently filtered by the supralaryngeal vocal tract (filter, producing amplified frequencies called ‘formants’). Using a custom-built program in Praat, we extracted source-related acoustic features (F0 contour) and filter-related acoustic features (formants and energy quartiles; 33 parameters in total). The program batch-processed the editing, the setting of parameters, the analyses and the exporting of output data [24,25]. The vocal parameters that we measured are listed in the electronic supplementary material, table S1, and the analyses are detailed by Briefer & McElligott [11,13].
(g). Statistical analyses
After confirming that our data did not deviate significantly from a normal distribution, we carried out a principal components analysis (PCA; correlation matrix) including the four behavioural responses to previous kids–playbacks 1, previous kids–playback 2 and familiar kids (DurLook, LatLook, RateCall, LatCall), which are likely to be correlated, to create a composite score [26]. The scores of the first principal component (PC1; eigenvalue greater than 1, Kaiser's criterion) were compared using a factorial analysis of variance (ANOVA), including treatment as a fixed factor and mother identity as an error term to account for repeated measurements of the same individuals across treatments. The sex of kids was also included as a fixed factor, to account for differences in the number of female and male kids in the various treatments and for a potential effect of kid sex on the responses of mothers. We then compared the responses to previous kids during playbacks 1 with the responses to these same kids during playbacks 2, as well as the response to previous kids during playbacks 2 with the responses to familiar kids using linear mixed-effects models (LMM; lme function in R [27]). This test allowed us to include the sex of kids as a factor, and to remove it using a standard model simplification procedure. This term was removed if not significant and if the deletion did not cause any significant reduction in goodness of fit. The two models with and without the term, both fitted with maximum log-likelihood method, were compared using a likelihood ratio test. We present the results after model simplification and with restricted-estimate maximum likelihood method. Due to the small sample size (n = 9 goats), we did not to perform Bonferroni corrections for two-by-two comparisons (n = 2 comparisons [28]).
We carried out two sets of analyses on vocal parameters to assess the similarity between the calls of previous, current and familiar kids. Before carrying out these analyses, we checked whether our data (calls of previous, current and/or familiar kids used in the playbacks; eight calls per individual) deviated significantly from a normal distribution and log-transformed them when necessary. Then, we used a PCA to eliminate redundancy caused by the high intercorrelation of the acoustic parameters (n = 33 parameters; see electronic supplementary material, table S1). We retained the PCs of the PCA with eigenvalues greater than 1 (Kaiser's criterion). The first analysis consisted of comparing the similarity between previous and familiar kid calls used in the playbacks with the similarity between previous and current kid calls. To this aim, the scores of PCs extracted from the PCA were averaged for each kid to obtain individual centroids. For each mother, we then calculated Euclidean distances between centroids of the familiar and previous kids used in the playbacks, and between centroids of her previous and current kids. These distances were compared using a two-tailed dependent exact permutation test, because conventional parametric and nonparametric tests are not suitable for analyses in which each individual is included several times in the different pairwise comparisons [29]. The second analysis consisted of a discriminant function analysis (DFA), to quantify the extent to which kids could be classified on the basis of their calls, and to test whether calls of previous kids could be mistaken for calls of current kids. To this aim, we carried out a DFA with one factor (‘kid individual identity’) on the PC scores retained from the PCA. On the basis of the DFA, each PC score (corresponding to a call) was assigned to the appropriate individual (correct classification, CC) or to another individual (incorrect classification) to calculate the percentage of CC. We cross-validated our results by performing a leave-one-out classification. We calculated the CC owing to chance by applying a randomization procedure. The expected level of correct assignment was averaged from DFAs performed on 1000 randomized permutations of the dataset [30]. Then, the CCs obtained for the previous and current kids of each mother were compared with the CC obtained for the familiar kid used in the playbacks with two-tailed Wilcoxon matched-pair tests. Additionally, for each mother, we calculated the number of calls of her previous kid used during the playbacks that were misclassified by the DFA as calls of her current kid included in the analysis, and as calls of the familiar kid used during the playbacks.
Kids were not recorded if they were obviously stressed during the brief isolation at short distances from their mothers. Therefore, the current single-born kid of one mother could not be recorded. Similarly, during playbacks 1, mothers were not tested if their own kids were too stressed during the isolation. As a result, one mother had not been tested during playbacks 1. Statistical analyses were carried out using R v. 2.9.0 [31]. The significance level was set at α = 0.05. All means are given with s.e.
3. Results
(a). Playbacks to mother goats
The PCA performed on behavioural responses to the playbacks generated four PCs. Only PC1 exceeded Kaiser's criterion (PC1 eigenvalue = 3.09). PC1 explained 77.2 per cent of the variance in the responses. Examination of the component loadings revealed that all four behavioural responses loaded highly on PC1. Latencies were negatively correlated with PC1 (LatLook, r = −0.91; LatCall, r = −0.86), whereas the other responses were positively correlated with PC1 (RateCall, r = 0.92; DurLook, r = 0.82). Therefore, more positive PC1 scores corresponded to stronger responses (i.e. goats looked faster and longer at the loudspeaker, and produced more calls, after shorter latencies).
A comparison between the PC1 scores obtained for the three treatments (previous kids–playbacks 1; previous kids–playbacks 2; familiar kids) revealed a general effect of kid sex and playback treatment on mothers’ responses (factorial ANOVA: sex of kids, F1,14 = 10.46, p = 0.006; treatment, F2,14, = 6.30, p = 0.01). For two-by-two comparisons, the effect of kid sex on mothers’ responses was not significant and could be removed from the models (likelihood ratio test: previous kids–playbacks 1 versus previous kids–playbacks 2, 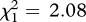 , p = 0.15; previous kids–playbacks 2 versus familiar kids–playbacks 2,
, p = 0.15; previous kids–playbacks 2 versus familiar kids–playbacks 2, 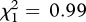 , p = 0.32). The responses of mothers to their previous kids during playbacks 1 tended to be stronger (i.e. more positive PC1 scores) than the responses to these same calls during playbacks 2, but this difference was not significant (LMM: previous kids–playbacks 1 versus previous kids–playbacks 2, t7 = 1.99, p = 0.088; figure 2). However, the responses of mothers to their previous kids during playbacks 2 were significantly stronger (i.e. more positive PC1 scores) than the responses to familiar kids from other females (LMM: previous kids–playbacks 2 versus familiar kids–playbacks 2, t8 = 2.62, p = 0.031; figure 2). Therefore, mothers tended to respond to their five-week-old kids more at five weeks postpartum than 11–17 months later, but they responded more strongly to their kids from the previous year than to familiar kids from other females, suggesting long-term recognition of own kids.
, p = 0.32). The responses of mothers to their previous kids during playbacks 1 tended to be stronger (i.e. more positive PC1 scores) than the responses to these same calls during playbacks 2, but this difference was not significant (LMM: previous kids–playbacks 1 versus previous kids–playbacks 2, t7 = 1.99, p = 0.088; figure 2). However, the responses of mothers to their previous kids during playbacks 2 were significantly stronger (i.e. more positive PC1 scores) than the responses to familiar kids from other females (LMM: previous kids–playbacks 2 versus familiar kids–playbacks 2, t8 = 2.62, p = 0.031; figure 2). Therefore, mothers tended to respond to their five-week-old kids more at five weeks postpartum than 11–17 months later, but they responded more strongly to their kids from the previous year than to familiar kids from other females, suggesting long-term recognition of own kids.
Figure 2.
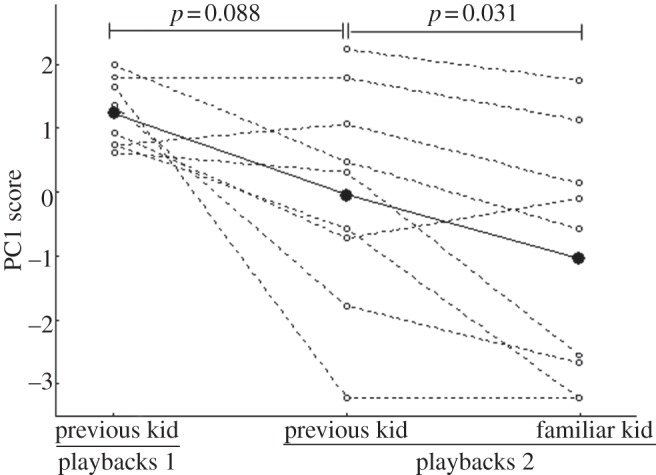
Behavioural responses to the playbacks. Individual PC1 scores (circles) of the PCA carried out on behavioural responses of mothers to calls of their five-week-old kids (previous kids–playbacks 1; n = 8 mothers), of the same calls played back 11–17 months later (previous kids–playbacks 2; n = 9 mothers) and of calls of familiar kids (familiar kids; n = 9 mothers). Black dots indicate the mean for each treatment. More positive scores correspond to stronger responses (i.e. faster response, higher call rate, longer duration of looks towards the loudspeaker). Mothers tended to respond more to their own kids during playbacks 1 than when the same calls were played back during playbacks 2. They also responded more to their previous kids than to familiar kids during playbacks 2 (linear mixed-effects models).
(b). Acoustic analysis of kid calls
For each goat, the calls of her previous kid were more similar to the calls of her current kid (i.e. shorter Euclidean distances; distance between centroids = 5.23 ± 0.69) than to the calls of the familiar kid used in the playbacks (distance between centroids = 6.57 ± 0.73; dependent exact permutation tests: n = 8 previous–current and 9 previous–familiar distances, p = 0.016). However, the cross-validated DFA correctly classified 60.00 ± 5.49 per cent of kid calls, which was higher than the chance level (5.05 ± 0.37, n = 20 kids; permutation tests: 1000 permutations, p = 0.001). For each goat, the percentage of CC of calls obtained for her previous kid (CC = 52.78 ± 9.72%, n = 9 kids) tended to be lower than for the familiar kid (CC = 69.44 ± 6.29%, n = 9 kids) used in the playbacks, but this difference was not significant (Wilcoxon matched-pairs test: Z = 1.68; n = 9; p = 0.093). The CC obtained for each mother's current kid (CC = 64.06 ± 7.26%, n = 8 kids) was not significantly different from the CC obtained for the familiar kid used in the playbacks (Wilcoxon matched-pairs test: Z = 0.81; n = 8; p = 0.42). Furthermore, only one previous kid call (1/64 calls; 1.56%) was misclassified by the DFA for a current kid call. None of the previous kid calls (0/72 calls) were misclassified by the DFA for a familiar kid call. Therefore, despite a higher similarity between a mother's previous and current kids than between her current kid and the familiar kid from another female used in the playbacks, her previous and current kids were still highly distinguishable from each other.
4. Discussion
Studying long-term recognition can provide important information about how relationships between individuals are maintained in species that experience long periods of separation (e.g. during migration or hibernation) or that live in fission–fusion societies. Using playback experiments, we investigated if maternal recognition of offspring calls persists long after weaning in goats. We found that goats tended to respond more to their own kids at five weeks postpartum than 11–17 months later, but displayed stronger responses to calls of their previous kids than to calls of familiar kids from other females. These results suggest that mothers remembered the vocalizations of their weaned kids, despite these calls having been recorded 1.1 years previously. Our acoustic analysis revealed that the calls of mothers’ previous kids were more similar to the calls of the kids they were currently nursing during the second playbacks than to the calls of familiar kids, suggesting a genetic effect on calls (see also [21]). However, the DFA revealed that the calls of their current and previous kids were distinguishable, and therefore it is highly unlikely that mothers would have been confused by the offspring from the different time periods. These results reveal surprising long-term memory for vocalizations in mammals, and suggest that persistence of parent–offspring vocal recognition beyond weaning could be present in many more species than previously believed [1,9].
Vocal recognition of kids by their mothers persisted up to 13 months after weaning (mean = 8.5 ± 0.7 months). Furthermore, mothers remembered versions of their kids’ calls recorded at five weeks postpartum, which they had not heard for 11–17 months (mean = 12.9 ± 0.7 months). Vocal recognition during nursing is crucial for both mothers and offspring. However, it is not clear why mothers would retain the memory of offspring vocalizations after weaning, which occurs in the wild between three and six months postpartum [32]. To achieve this long-term recognition, goats could use the vocal parameters that show consistent individual differences throughout ontogeny, such as the fundamental frequency contour, or the distribution of energy in the call, including the contour of formants [13].
We suggest that benefits for long-term vocal recognition of kid calls might include social association, or inbreeding avoidance between parents and matured offspring [1,9]. Capra spp. live in complex fission–fusion societies, with sexually segregated groups varying in size throughout the day and aggregating in permanent night-camps in the evening [33–36]. Females of these species, like many other mammals [37], do not disperse, and probably associate with their mothers throughout life in stable matriarchal social units [38–40]. At the proximate level, long-term vocal recognition could enable maintenance of social relationships between female kin within these complex societies. Oestrous female goats choose their mates and react very aggressively to the approaches of unwanted males [41,42]. This would be especially important for goats, in which male kids gain matings even in their first year [41]. At the ultimate level, long-term kin recognition may thus help prevent matings between kin, which reduces offspring survival and fertility, before sexually mature offspring males disperse [43]. Goats develop an exclusive and strong bond with their offspring within a few hours following birth, triggered by hormonal and sensory stimulation [14]. This strong bond requires an accurate recognition process based, at long distance, on vocal cues [13]. Therefore, alternatively, long-term vocal recognition could be a by-product of the strong learning process experienced by mothers during nursing [9,12]. This phenomenon might also include costs, such as a decrease in the ability of mothers to retain the calls of their kids as the number of kids they give birth to increases, if the total number of kids they can remember is limited.
Long-term memory of conspecifics should be crucial for maintaining social relationships when extended periods of separation occur, including migration or hibernation, or in species living in fission–fusion societies, such as goats. In addition to the studies on long-term vocal recognition of related individuals [1,9,10], the maintenance of conspecific memory, based on olfactory, visual or vocal cues over long periods of separation, has been shown in only a few species. Male hooded warblers (Wilsonia citrina) maintain a spatial categorization of the songs of their previous adjacent neighbours when returning from migration, after eight months [3]. Ravens (Corvus corax) remember former group members, as well as the relationship valence they had to those individuals for up to 3 years [44]. Belding's ground squirrels (Urocitellus beldingi) recognize kin odours after nine months of hibernation using self-referent phenotype matching [45,46]. Sheep (Ovis aries) remember conspecific faces for up to 2.2 years [2]. Finally, African elephants (Loxodonta africana) have been shown to respond to the vocalizations of a former group member that had left the group 12 years earlier [47]. Conspecific long-term recognition has been poorly documented, because of the difficulties involved in following individuals over extended periods. However, it might in fact be widespread in species characterized by unstable social associations.
We carried out the second series of playbacks when mothers were nursing new kids (‘current kids’), because playbacks of kid calls elicited no response when goats were still pregnant (E. F. Briefer 2010, unpublished observations). Our acoustic analysis revealed that the calls of mothers’ previous kids were more similar to the calls of the kids they were currently nursing than to the calls of familiar kids from other females, suggesting a genetic effect on calls (see also [21]). However, cross-validated DFA classified 60 per cent of kid calls to the correct individual, which is similar to percentages found in a previous study on goat kids (55% of five-week-old kids, n = 23 kids [13]), and to percentages found in other mammals [25,48,49]. We also found that only one call (1.56% of calls) of a previous kid was misclassified by the DFA for a call of a current kid. These results suggest that it is unlikely that mothers were responding more to previous kid calls than to calls of familiar kids during the second playbacks, because they were confusing their previous kids with their current kids.
To conclude, we showed that mother goats remember their kids’ vocalizations for at least one year after weaning. Therefore, even if offspring vocalizations change owing to maturation, mothers probably have long-term memories of successive call versions produced throughout ontogeny. Using domestic animals as models for further research on long-term vocal recognition is far more feasible than the species used to date (e.g. Pinnipeds). Long-term memory of conspecifics is probably widespread in mammals and other species that experience regular and extended periods of social separation. The most probable role of this is in mediating social relationships, but evidence for its existence remains elusive.
Acknowledgements
We are grateful to D. Nussey, B. Pitcher and the referees for helpful comments on the manuscript. E. F. Briefer is funded by a Swiss National Science Foundation fellowship. We acknowledge the financial support of the University of London Central Research Fund. We thank the staff of White Post Farm (http://whitepostfarmcentre.co.uk) for their help and free access to the animals.
References
- 1.Insley S. J. 2000. Long-term vocal recognition in the northern fur seal. Nature 406, 404–405 10.1038/35019064 (doi:10.1038/35019064) [DOI] [PubMed] [Google Scholar]
- 2.Kendrick K. M., da Costa A. P., Leigh A. E., Hinton M. R., Peirce J. W. 2001. Sheep don't forget a face. Nature 414, 165–166 10.1038/35102669 (doi:10.1038/35102669) [DOI] [PubMed] [Google Scholar]
- 3.Godard R. 1991. Long-term memory of individual neighbours in a migratory songbird. Nature 350, 228–229 10.1038/350228a0 (doi:10.1038/350228a0) [DOI] [Google Scholar]
- 4.Aubin T., Jouventin P. 2002. How to vocally identify kin in a crowd: the penguin model. Adv. Study. Behav. 31, 243–277 10.1016/S0065-3454(02)80010-9 (doi:10.1016/S0065-3454(02)80010-9) [DOI] [Google Scholar]
- 5.Torriani M. V. G., Vannoni E., McElligott A. G. 2006. Mother–young recognition in an ungulate hider species: a unidirectional process. Am. Nat. 168, 412–420 10.1086/506971 (doi:10.1086/506971) [DOI] [PubMed] [Google Scholar]
- 6.Sèbe F., Nowak R., Poindron P., Aubin T. 2007. Establishment of vocal communication and discrimination between ewes and their lamb in the first two days after parturition. Dev. Psychobiol. 49, 375–386 10.1002/dev.20218 (doi:10.1002/dev.20218) [DOI] [PubMed] [Google Scholar]
- 7.Knörnschild M., von Helversen O. 2008. Nonmutual vocal mother–pup recognition in the greater sac-winged bat. Anim. Behav. 76, 1001–1009 10.1016/j.anbehav.2008.05.018 (doi:10.1016/j.anbehav.2008.05.018) [DOI] [Google Scholar]
- 8.Charrier I., Mathevon N., Jouventin P. 2001. Mother's voice recognition by seal pups. Nature 412, 873 10.1038/35091136 (doi:10.1038/35091136) [DOI] [PubMed] [Google Scholar]
- 9.Pitcher B., Harcourt R., Charrier I. 2010. The memory remains: long-term vocal recognition in Australian sea lions. Anim. Cogn. 13, 771–776 10.1007/s10071-010-0322-0 (doi:10.1007/s10071-010-0322-0) [DOI] [PubMed] [Google Scholar]
- 10.Matthews S., Snowdon C. T. 2011. Long-term memory for calls of relatives in cotton-top tamarins (Saguinus oedipus). J. Comp. Psychol. 125, 366–369 10.1037/a0023149 (doi:10.1037/a0023149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briefer E., McElligott A. G. 2011. Indicators of age, body size and sex in goat kid calls revealed using the source-filter theory. Appl. Anim. Behav. Sci. 133, 175–185 10.1016/j.applanim.2011.05.012 (doi:10.1016/j.applanim.2011.05.012) [DOI] [Google Scholar]
- 12.Charrier I., Mathevon N., Jouventin P. 2003. Fur seal mothers memorise subsequent versions of developing pups’ calls: adaptation to long-term recognition or evolutionary by-product? Biol. J. Linn. Soc. 80, 305–312 10.1046/j.1095-8312.2003.00239.x (doi:10.1046/j.1095-8312.2003.00239.x) [DOI] [Google Scholar]
- 13.Briefer E., McElligott A. G. 2011. Mutual mother–offspring vocal recognition in an ungulate hider species (Capra hircus). Anim. Cogn. 14, 585–598 10.1007/s10071-011-0396-3 (doi:10.1007/s10071-011-0396-3) [DOI] [PubMed] [Google Scholar]
- 14.Poindron P., Nowak R., Levy F., Porter R. H., Schaal B. 1993. Development of exclusive mother–young bonding in sheep and goats. Oxford Rev. Reprod. B 15, 311–364 [PubMed] [Google Scholar]
- 15.Lickliter R. E., Heron J. R. 1984. Recognition of mother by newborn goats. Appl. Anim. Behav. Sci. 12, 187–192 10.1016/0168-1591(84)90109-6 (doi:10.1016/0168-1591(84)90109-6) [DOI] [Google Scholar]
- 16.Ruiz-Miranda C. R. 1993. Use of pelage pigmentation in the recognition of mothers in a group by 2- to 4-month-old domestic goat kids. Appl. Anim. Behav. Sci. 36, 317–326 10.1016/0168-1591(93)90129-D (doi:10.1016/0168-1591(93)90129-D) [DOI] [Google Scholar]
- 17.McDougall P. 1975. The feral goats of Kielderhead Moor. J. Zool. 126, 215–246 10.1111/j.1469-7998.1975.tb03194.x (doi:10.1111/j.1469-7998.1975.tb03194.x) [DOI] [Google Scholar]
- 18.Ruiz-Miranda C. R., Szymanski M. D., Ingals J. W. 1993. Physical characteristics of the vocalizations of domestic goat does Capra hircus in response to their offspring's cries. Bioacoustics 5, 99–116 10.1080/09524622.1993.9753232 (doi:10.1080/09524622.1993.9753232) [DOI] [Google Scholar]
- 19.Hänninen L., Pastell M. 2009. CowLog: open source software for coding behaviors from digital video. Behav. Res. Meth. 41, 472–476 10.3758/BRM.41.2.472 (doi:10.3758/BRM.41.2.472) [DOI] [PubMed] [Google Scholar]
- 20.Kober M., Trillmich F., Naguib M. 2007. Vocal mother–pup communication in guinea pigs: effects of call familiarity and female reproductive state. Anim. Behav. 73, 917–925 10.1016/j.anbehav.2006.06.020 (doi:10.1016/j.anbehav.2006.06.020) [DOI] [Google Scholar]
- 21.Briefer E. F., McElligott A. G. 2012. Social effects on vocal ontogeny in an ungulate, the goat, Capra hircus. Anim. Behav. 83, 991–1000 10.1016/j.anbehav.2012.01.020 (doi:10.1016/j.anbehav.2012.01.020) [DOI] [Google Scholar]
- 22.Fant G. 1960. Acoustic theory of speech production. The Hague, The Netherlands: Mouton [Google Scholar]
- 23.Titze I. R. 1994. Principles of voice production. Englewood Cliffs, NJ: Prentice Hall [Google Scholar]
- 24.Reby D., McComb K. 2003. Anatomical constraints generate honesty: acoustic cues to age and weight in the roars of red deer stags. Anim. Behav. 65, 519–530 10.1006/anbe.2003.2078 (doi:10.1006/anbe.2003.2078) [DOI] [Google Scholar]
- 25.Charlton B. D., Zhihe Z., Snyder R. J. 2009. Vocal cues to identity and relatedness in giant pandas (Ailuropoda melanoleuca). J. Acoust. Soc. Am. 126, 2721–2732 10.1121/1.3224720 (doi:10.1121/1.3224720) [DOI] [PubMed] [Google Scholar]
- 26.McGregor P. 1992. Playback and studies of animal communication. New York, NY: Plenum Press [Google Scholar]
- 27.Bates D. 2005. Fitting linear mixed models in R. R News 5, 27–30 [Google Scholar]
- 28.Nakagawa S. 2004. A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav. Ecol. 15, 1044–1045 10.1093/beheco/arh107 (doi:10.1093/beheco/arh107) [DOI] [Google Scholar]
- 29.Mundry R. 1999. Testing related samples with missing values: a permutation approach. Anim. Behav. 58, 1143–1153 10.1006/anbe.1999.1246 (doi:10.1006/anbe.1999.1246) [DOI] [PubMed] [Google Scholar]
- 30.McGarigal K. S., Cushman S., Stafford S. 2000. Multivariate statistics for wildlife and ecology research. New York, NY: Springer [Google Scholar]
- 31.R Development Core Team 2009. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 32.Hersher L., Richmond J. B., Moore A. U. 1963. Maternal behaviour in sheep and goats. In Maternal behaviour in mammal (ed. Rheingold H. L.), pp. 203–232 New York, NY: Wiley [Google Scholar]
- 33.Shi J., Dunbar R. I. M., Buckland D., Miller D. 2005. Dynamics of grouping patterns and social segregation in feral goats (Capra hircus) on the Isle of Rum, NW Scotland. Mammalia 69, 185–199 10.1515/mamm.2005.016 (doi:10.1515/mamm.2005.016) [DOI] [Google Scholar]
- 34.O'Brien P. H. 1988. Feral goat social organization: a review and comparative analysis. Appl. Anim. Behav. Sci. 21, 209–221 10.1016/0168-1591(88)90110-4 (doi:10.1016/0168-1591(88)90110-4) [DOI] [Google Scholar]
- 35.Dunbar R. I. M., Shi J. 2008. Sex differences in feeding activity results in sexual segregation of feral goats. Ethology 114, 444–451 10.1111/j.1439-0310.2008.01478.x (doi:10.1111/j.1439-0310.2008.01478.x) [DOI] [Google Scholar]
- 36.Villaret J. C., Bon R. 1995. Social and spatial segregation in alpine ibex (Capra ibex) in Bargy, French Alps. Ethology 101, 291–300 10.1111/j.1439-0310.1995.tb00366.x (doi:10.1111/j.1439-0310.1995.tb00366.x) [DOI] [Google Scholar]
- 37.Clutton-Brock T. H., Lukas D. 2011. The evolution of social philopatry and dispersal in female mammals. Mol. Ecol. 21, 472–492 10.1111/j.1365-294X.2011.05232.x (doi:10.1111/j.1365-294X.2011.05232.x) [DOI] [PubMed] [Google Scholar]
- 38.Villaret J.-C., Bon R. 1998. Sociality and relationships in Alpine ibex. Rev. Ecol. Terre Vie 53, 153–170 [Google Scholar]
- 39.Dunbar R. I. M., Buckland D., Miller D. 1990. Mating strategies of male feral goats: a problem in optimal foraging. Anim. Behav. 40, 653–667 10.1016/S0003-3472(05)80695-5 (doi:10.1016/S0003-3472(05)80695-5) [DOI] [Google Scholar]
- 40.Shackleton D. M., Shank C. C. 1984. A review of the social behavior of feral and wild sheep and goats. J. Anim. Sci. 58, 500–509 [Google Scholar]
- 41.Saunders F. C., McElligott A. G., Safi K., Hayden T. J. 2005. Mating tactics of male feral goats (Capra hircus): risks and benefits. Acta Ethol. 8, 103–110 10.1007/s10211-005-0006-y (doi:10.1007/s10211-005-0006-y) [DOI] [Google Scholar]
- 42.Longpre K. M., Katz L. S. 2011. Estrous female goats use testosterone-dependent cues to assess mates. Horm. Behav. 59, 98–104 10.1016/j.yhbeh.2010.10.014 (doi:10.1016/j.yhbeh.2010.10.014) [DOI] [PubMed] [Google Scholar]
- 43.Pusey A., Wolf M. 1996. Inbreeding avoidance in animals. Trends Ecol. Evol. 11, 201–206 10.1016/0169-5347(96)10028-8 (doi:10.1016/0169-5347(96)10028-8) [DOI] [PubMed] [Google Scholar]
- 44.Boeckle M., Bugnyar T. 2012. Long-term memory for affiliates in ravens. Curr. Biol. 22, 801–806 10.1016/j.cub.2012.03.023 (doi:10.1016/j.cub.2012.03.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mateo J. M. 2010. Self-referent phenotype matching and long-term maintenance of kin recognition. Anim. Behav. 80, 929–935 10.1016/j.anbehav.2010.08.019 (doi:10.1016/j.anbehav.2010.08.019) [DOI] [Google Scholar]
- 46.Mateo J. M., Johnston R. E. 2000. Retention of social recognition after hibernation in Belding's ground squirrels. Anim. Behav. 59, 491–499 10.1006/anbe.1999.1363 (doi:10.1006/anbe.1999.1363) [DOI] [PubMed] [Google Scholar]
- 47.McComb K., Moss C., Sayialel S., Baker L. 2000. Unusually extensive networks of vocal recognition in African elephants. Anim. Behav. 59, 1103–1109 10.1006/anbe.2000.1406 (doi:10.1006/anbe.2000.1406) [DOI] [PubMed] [Google Scholar]
- 48.Charrier I., Aubin T., Mathevon N. 2010. Mother–calf vocal communication in Atlantic walrus: a first field experimental study. Anim. Cogn. 13, 471–482 10.1007/s10071-009-0298-9 (doi:10.1007/s10071-009-0298-9) [DOI] [PubMed] [Google Scholar]
- 49.Soltis J., Leong K., Savage A. 2005. African elephant vocal communication. II. Rumble variation reflects the individual identity and emotional state of callers. Anim. Behav. 70, 589–599 10.1016/j.anbehav.2004.11.016 (doi:10.1016/j.anbehav.2004.11.016) [DOI] [Google Scholar]