Abstract
Convergent morphologies are thought to indicate functional similarity, arising because of a limited number of evolutionary or developmental pathways. Extant taxa displaying convergent morphologies are used as analogues to assess function in extinct taxa with similar characteristics. However, functional studies of extant taxa have shown that functional similarity can arise from differing morphologies, calling into question the paradigm that form and function are closely related. We test the hypothesis that convergent skeletal morphology indicates functional similarity in the fossil record using ornithischian dinosaurs. The rare transition from bipedality to quadrupedality occurred at least three times independently in this clade, resulting in a suite of convergent osteological characteristics. We use homology rather than analogy to provide an independent line of evidence about function, reconstructing soft tissues using the extant phylogenetic bracket and applying biomechanical concepts to produce qualitative assessments of muscle leverage. We also optimize character changes to investigate the sequence of character acquisition. Different lineages of quadrupedal ornithischian dinosaur stood and walked differently from each other, falsifying the hypothesis that osteological convergence indicates functional similarity. The acquisition of features correlated with quadrupedalism generally occurs in the same order in each clade, suggesting underlying developmental mechanisms that act as evolutionary constraints.
Keywords: convergence, function, Ornithischia, Dinosauria, quadrupedality
1. Introduction
A central tenet of palaeobiology is that function can be inferred from the structure of hard parts that usually constitute the only direct evidence of extinct organismal morphology [1–3]. This assumption is based on comparisons with the anatomy of potentially analogous extant taxa and the application of biomechanical principles that assume physical constants and mechanical properties are invariant through time [1]. One outcome of this concept is that the occurrence of convergent skeletal morphology in different taxa also implies functional similarity. In such cases, it is frequently assumed that these skeletal features evolved independently, with the convergence in form and function deriving from selective pressures that channel morphological change in response to the same mechanical factors, or in the context of options among a limited number of biologically viable developmental pathways [2].
By contrast, biomechanical works on extant taxa have shown that different skeletal morphologies can produce the same function, with the corollary that convergent skeletal morphology does not necessarily indicate functional similarity [4,5]. These studies, which place greater emphasis on the need to account for myology and neurological control, present a major problem to those functional morphologists working on extinct taxa, as they imply that any functional study relying on skeletal morphology alone is flawed, and unlikely to lead to reliable conclusions.
The aim of this work is to use stance in ornithischian dinosaurs to test the hypothesis that skeletal convergence alone cannot be used to imply functional similarity in extinct taxa. Ornithischian dinosaurs provide an appropriate model to test this hypothesis because their phylogenetic relationships are well understood, allowing robust identification of convergence, and they have character-rich skeletons [6]. Moreover, ornithischian dinosaurs were primitively bipedal, but quadrupedality evolved at least three times independently within the clade. Secondary quadrupedality is exceptionally rare among tetrapods, and this clade provides a unique opportunity to test whether the convergent acquisition of skeletal characters associated with quadrupedality in closely related but distinct lineages were associated with the same changes in locomotor function. While it is uncontroversial that thyreophorans, ceratopsids and hadrosaurs were quadrupedal (figure 1), details of their stance (how they held their limbs during standing) and gait (how fast they could move) are debated, owing to different interpretations of their skeletal anatomical function [7–10]. In order to assess stance and locomotor patterns, evidence beyond comparative osteology is required.
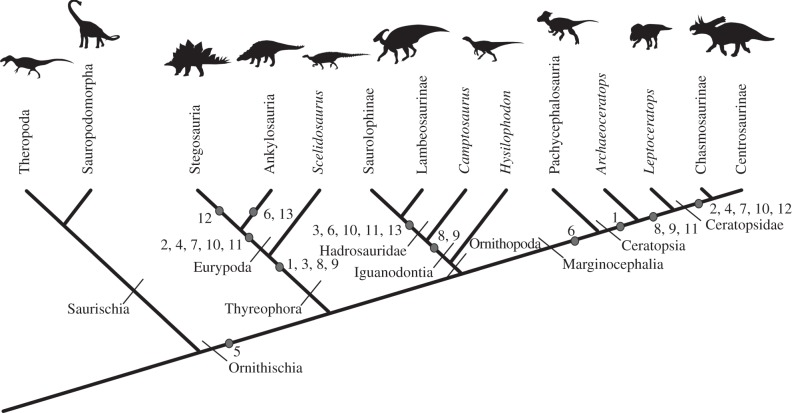
Figure 1.
Dinosaurian relationships with optimized character state changes. 1, incipient enlargement of the preacetabular iliac process; 2, full enlargement of the preacetabular iliac process; 3, incipient development of the supratrochanteric crest; 4, full development of the supratrochanteric crest; 5, retroversion of the pubis; 6, loss of the postpubis; 7, shortening of the ischium; 8, humeral head restricted to the caudal surface of the humerus; 9, slightly enlarged deltopectoral crest; 10, fully enlarged deltopectoral crest that occupies half the length of the humerus; 11, cranial movement of the origin of deltoideus scapularis on the scapula blade; 12, origin of deltoideus clavicularis on acromial process moved caudally; 13, acromial process projects laterally. These characters and their relevance to stance are discussed in detail in the text.
Phylogenetic studies use homology as the primary criterion for identifying synapomorphies. The extant phylogenetic bracket (EPB) approach provides a phylogenetic framework within which homologous osteological correlates can be used to produce well-constrained reconstructions of soft-tissue characters [11,12]. Here, rather than rely on an analogous approach to appraising function, we use features inferred from homology to provide a more rigorous assessment. The EPB of ornithischian dinosaurs was used to produce locomotor muscle reconstructions. These were then coupled with biomechanical concepts [13] to provide, to our knowledge, the first ever assessments of limb posture during stance in all major clades of ornithischians. We also optimized the acquisition of osteological features correlated with quadrupedality (figure 1) to address the hypothesis that the order of character acquisition was the same in each clade, to determine whether an underlying constraint might have been involved in the evolution of quadrupedalism.
We demonstrate that a previously unrealized diversity of locomotor styles was employed by ornithischians, despite some striking convergence in skeletal anatomy, showing that morphological convergence cannot be used as a robust predictor of functional similarity. In general, ornithischians might have been constrained to acquire the osteological features necessary for quadrupedalism in the same evolutionary sequence.
2. Material and methods
The osteology of approximately 200 specimens (90 taxa) of extinct and extant archosaurs was examined (see the electronic supplementary material, table S4). A reconstruction of the musculature of the basalmost ornithischians [14] carried out using the EPB was used as a starting point for reconstructing the stepwise changes that occurred along each of the three ornithischian lineages in which quadrupedality evolved. The forelimbs, hind limbs, girdles, and representative vertebrae from each specimen were examined, photographed, and measured.
It is uncontroversial that the most primitive ornithischians were bipedal [14–16]. Ankylosaurs, stegosaurs and ceratopsids are widely accepted as being obligate quadrupeds [17–19] and to our knowledge quadrupedal stance in these taxa has never been questioned seriously in the literature. Although some earlier works considered hadrosaurs to be bipedal [20,21], recent studies have concluded that they were facultative [22] or obligate [23] quadrupeds. These taxa were considered herein as quadrupeds a priori. Some non-hadrosaurian ornithopods may also have been facultative quadrupeds [24–26] and the stance of some neoceratopsids is debated [27,28], but we did not determine stance in these taxa a priori.
The pectoral and pelvic osteology of many quadrupedal ornithischians differ markedly from that of basal, bipedal ornithischians and a key aspect of data collection was the identification of homologous surfaces for muscle attachment, homologous intermuscular lines and muscle scars [29,30]. Musculoskeletal changes were optimized onto recently published phylogenies of each lineage [6,31–39] and a muscle reconstruction for all taxa examined was produced. Changes in musculoskeletal anatomy were then linked to changes in muscle function inferred by plotting the lines of action onto reconstructed fore- and hind limbs. Function was related to stance using biomechanical concepts of limb bone loading developed by Hutchinson & Gatesy [13]. Osteological changes were optimized onto the phylogenies of each lineage [6,31–39] as a series of discrete, qualitative characters optimized in MacClade [40].
3. Results
Muscle reconstructions for exemplar taxa representing important stages in the transition from bipedality to quadrupedality in Thyreophora, Ornithopoda and Ceratopsia are provided in the electronic supplementary material; reconstructions for other taxa are available from S.C.R.M. Only those muscles whose function is considered to have significantly changed relative to their function in primitive bipedal taxa will be discussed. Justification for muscle homologies and the levels of inference can be found in Maidment & Barrett [14].
(a). Pelvic musculature
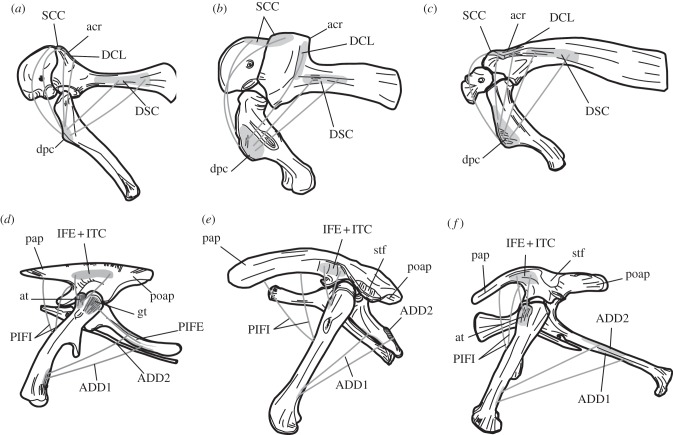
Thyreophorans and ceratopsians display remarkable osteological convergence, indicating that each clade independently underwent a series of similar myological transformations that occurred in the same order during the transition to quadrupedalism. The preacetabular process of the ilium, which in basal ornithischians is dorsoventrally deep and transversely compressed, is elongated cranially and broadened ventrally, so that the area of origin for the puboischiofemoralis internus (PIFI) 1 & 2 [14] is greatly enlarged (figures 1 and 2e, pap). This occurred incipiently in the basal thyreophoran Scelidosaurus and the neoceratopsian Archaeoceratops and is fully developed in ankylosaurs, stegosaurs and ceratopsids. The dorsal margin of the ilium is folded laterally producing a large supratrochanteric flange (figures 1 and 2e, stf), a feature seen incipiently in the basal thyreophoran Scelidosaurus, but developed to a much greater degree in stegosaurs, ankylosaurs and ceratopsids. In basal ornithischians, the iliofemoralis externus (IFE) and the iliotrochantericus caudalis (ITC) originated on the lateral ilium dorsal to the acetabulum [14] (figure 2d) and lateral folding of the dorsal ilium resulted in a great reduction or possibly loss of this muscle complex (figure 2e). The puboischiofemoralis externus (PIFE) 1 & 2 were probably greatly reduced by the retroversion of the pubis in the most basal ornithischians [14] (figures 1 and 2d), and this trend continued in ankylosaurs and ceratopsids with the loss of the postpubis, the area of origin of PIFE [29]. Finally, the ischium provided the origin of the adductors (ADD 1 & 2) [29], and in thyreophorans and ceratopsids, it is shortened relative to the length of the ilium (figures 1 and 2e; electronic supplementary material, table S2), moving the origin of these muscles dorsally and closer to their insertion on the femur.
Figure 2.
(a–c) Ornithischian pectoral girdles and forelimbs and (d–f) pelvic girdles and hind limbs in left lateral view. (a,d) reconstructed generalized bipedal basal ornithischian, after [14]; (b,e) Stegosaurus armatus, quadrupedal, based on various specimens (see the electronic supplementary material, table S4); (c,f) Lambeosaurus lambei, quadrupedal, based on ROM (Royal Ontario Museum, Toronto, Canada) 1218. Labels in uppercase denote musculature: ADD 1 & 2, adductor 1 & 2; DCL, deltoideus clavicularis; DSC, deltoideus scapularis; IFE, iliofemoralis externus; ITC, iliotrochantericus caudalis; PEC, pectoralis; PIFE, puboischiofemoralis externus 1 & 2; PIFI 1 & 2 puboischiofemoralis internus 1 & 2; SCC, supracoracoideus; those in lowercase indicate osteology: acr, acromial process; at, anterior trochanter; dpc, deltopectoral crest; gt, greater trochanter; pap, preacetabular process; poap, postacetabular process; stf, supratrochanteric flange. Not to scale.
Hadrosaurs possess few of these adaptations for quadrupedality. The slender preacetabular process suggests that PIFI was not enlarged relative to the condition in basal ornithischians, and only a small supratrochanteric flange was developed, so that IFE and ITC were unreduced (figures 1 and 2f). The ischium is longer than the ilium, resulting in caudal movement of the origin of ADD relative to the condition in basal ornithischians (cf. figure 2d,f).
(b). Pectoral musculature
Changes in pectoral girdle and forelimb osteology are strongly convergent in quadrupedal ornithischians. The humeral head of all quadrupeds is restricted to the caudal shaft (figure 1), suggesting that the humerus was habitually retracted and could not be extended to produce a straight forelimb [7]. In all taxa, the deltopectoral crest, the insertion area for the deltoideus clavicularis (DCL), the deltoideus scapularis (DSC), the pectoralis (PEC) and the supracoracoideus (SCC), increased greatly in size to occupy at least half the humeral shaft (figure 1). Muscle scars indicate that DSC moved cranially on the scapula blade in all quadrupedal ornithischians (figures 1 and 2a–c). In ceratopsids and stegosaurs, the origin of DCL, the acromial process, [7,14] moved caudally relative to the glenoid (figures 1 and 2b), although the morphologies that achieved this in stegosaurs and ceratopsids were rather different (figure 2). In ankylosaurs and hadrosaurs (figures 1 and 2c), the area of origin was increased in size relative to that of basal taxa and the acromial process projected laterally in both groups.
4. Discussion
(a). Hind limb function
Myological changes along the ceratopsian and thyreophoran lineages allow important functional inferences to be made. (i) PIFI was a femoral protractor in basal ornithischians [14]. The enlargement of the preacetabular process would have increased the moment arm for protraction and the greatly increased surface area available for attachment suggests that this muscle also exhibited a substantial size increase. (ii) IFE and ITC abducted the femur in basal ornithischians [14]. The development of the supratrochanteric flange reduced the area of origin of IFE and ITC, suggesting that these muscles were reduced or lost. (iii) PIFE was probably greatly reduced and its moment arm for femoral protraction had already been lost in basal ornithischians [14]. Loss of this muscle group represents the culmination of a trend of reduction that started with retroversion of the pubis. (iv) In basal ornithischians, ADD retracted and adducted the femur [14]. The movement of the origin of ADD cranially and dorsally resulted in a reduced moment arm for femoral retraction, and an increased moment arm for adduction.
During stance, a biped must place the standing foot underneath the centre of mass (CoM). The ground reaction force (GRF) acts vertically from the foot on the upwards to the CoM. Since the femur is angled obliquely so that the distal end is more medially placed than the proximal end, it is loaded by an adduction moment around the hip by the GRF. Abductor muscles are needed to control this adduction during locomotion [13]. In quadrupedal taxa, the forelimbs are able to contribute to balance, so that the standing hind foot can be placed lateral to the CoM without toppling. The distal end of the femur is placed slightly lateral to the proximal end, resulting in loading of the femur by an abduction moment around the hip by the GRF. Adductor muscles are therefore needed to control the outward collapse of the femur during locomotion [13]. The reduction or loss of abductor musculature in quadrupedal thyreophorans and ceratopsids suggests that they were placing their feet lateral to CoM during locomotion (figure 3b,f); abductive collapse of the femur was resisted by the increased moment arm for adduction by ADD.
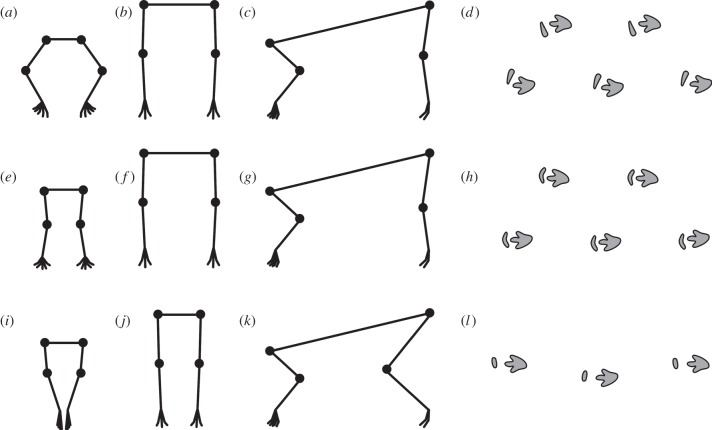
Figure 3.
Stance reconstruction and predicted trackways in quadrupedal ornithischians. (a–d) Stegosaurs and ceratopsids; (e–h) ankylosaurs; (i–l) hadrosaurs. (a,e,i) Forelimbs in cranial view; (b,f,j) hind limbs in cranial view; (c,g,k) fore- and hind limbs in lateral view, cranial is to the left; (d,h,l) predicted trackways. Not to scale.
PIFI was larger and had a larger moment arm in ceratopsids and thyreophorans than in hadrosaurs. The causes for this increase are difficult to elucidate: active during swing phase, the muscle may have been used for adduction and femoral long axis rotation as well as protraction. PIFI is the major femoral protractor in alligators [26] but its homologues are less important for protraction in birds, which greatly decrease the femoral excursion during the step cycle. The large size of PIFI in ceratopsids and thyreophorans could be explained by larger femoral excursions in these taxa than in hadrosaurs. However, since the exact configuration and degree of movement of the hip remain unknown [41], this hypothesis is difficult to test, and it is inappropriate to draw conclusions.
Hadrosaurs exhibit few of the quadrupedal adaptations present in ceratopsids and thyreophorans and appear to have retained many of the features of bipedality despite their quadrupedal stance, such as placing the feet on the midline during locomotion (figure 3j,k).
(b). Forelimb function
In basal ornithischians, DCL functioned as a humeral abductor, while DSC retracted the humerus [14]. In stegosaurs and ceratopsids, insertion of DCL and DSC on the lateral deltopectoral crest would have been caudal to the centre of rotation (CoR) for protraction/retraction, even when the humerus was maximally protracted (figure 2b). Cranial migration of DSC and caudal migration of DCL resulted in a weak moment arm for humeral retraction in both muscles. The positioning of the origin of DCL caudal to the CoR for abduction/adduction resulted in a weaker moment arm for abduction than in basal ornithischians (figure 2b). Both DCL and DSC originate medial to the CoR for medial/lateral rotation and insert lateral to it, so the main function of the deltoids was probably lateral rotation of the humerus.
Much debate has focused on the stance of ceratopsid forelimbs [7–10], but recent studies have concluded that the forelimbs were held flexed during stance with the maximum humeral protraction achievable being approximately 40° from a horizontal plane parallel to the ground when the scapula blade is angled parallel to the vertebral column. The elbow was slightly abducted, forming an angle of around 30° with the sagittal plane [9,10]. This posture is supported by the observation that weight-bearing, hoof-like ungual phalanges are only found on the medial three digits of the manus (Chasmosaurus ROM (Royal Ontario Museum, Toronto, Canada) 843; Vagaceratops, CMN (Canadian Museum of Nature, Ottawa, Canada) 41357 [14]). If the elbow was aligned parasagittally, each of the digits would bear weight equally and all would be expected to have hoof-like unguals.
The stegosaurian pectoral girdle is very similar to that of ceratopsids. The humeral head is restricted to the caudal shaft, suggesting that protraction of the humerus past the vertical would have been impossible, and that the forelimb was flexed to a similar degree. Hoof-like unguals are restricted to the medial two digits [17], suggesting that the elbows were abducted to a similar degree to those of ceratopsids (figure 3a,c).
Since the manus was placed ventral to the glenoid in stegosaurs and ceratopsians, the GRF applies an abductor moment at the shoulder, causing the elbow to splay outwards in a ‘press-up’ position. This abduction could be controlled either by adductor muscles originating medially, or by inward elbow rotation. Ceratopsids and stegosaurs appear to have employed both methods to control abduction: the elongate deltopectoral crest provides a large surface area for attachment of PEC to adduct the humerus, and DCL and DSC laterally rotated the humerus, causing movement of the elbow medially [13].
The musculature of ankylosaurian scapulae implies that their forelimbs operated differently from ceratopsians and stegosaurs during locomotion. In basal ornithischians, DCL originated medial to the CoR for abduction/adduction, and inserted lateral to it, so it functioned as a humeral abductor [14]. Ankylosaurs increased the surface area for origin of DCL by folding the acromial process laterally (ankylosaurids) or generating a laterally projecting process (nodosaurids), but the line of action has not changed significantly relative to basal ornithischians. Both ankylosaur clades independently acquire a buttress around which DCL can act, leading to a larger moment arm for abduction of the humerus. As in ceratopsids and stegosaurs, the humeral head is restricted to the caudal surface of the humerus, suggesting that protraction of the bone past the vertical would have been impossible and that the forelimb was habitually flexed (figure 3g).
Two interpretations are possible to explain the increased moment arm of the abductor musculature in ankylosaurs. (i) Ankylosaurs placed their feet on the midline level with the CoM during locomotion, so that the GRF loaded the humerus with an adductive moment around the glenoid. Large abductors would be required to control the adduction of the humerus [13]. This is unlikely because ankylosaurs had extremely wide bodies [42] and placing their feet on the midline would have made them very unstable. Trackways [43] (see the electronic supplementary material, figure S15A) suggest that ankylosaurs placed their feet lateral to the midline during locomotion. (ii) The elbow was held in tucked in, nearly parallel to the sagittal plane and did not abduct during locomotion (figure 3e). In this scenario, the distal end of the humerus would have been located slightly medial to the proximal end, and the GRF would have adducted the humerus. The feet would have splayed outwards and been placed lateral to the glenoid, so that the manus would fall craniolateral to the pes during locomotion. This stance has been independently suggested for Euoplocephalus [44]. Weight would be evenly distributed among all digits of the manus, and this is supported by available data: although the manus of ankylosaurs is poorly known, all digits appear to bear hoof-shaped, weight-bearing ungual phalanges (Sauropelta, AMNH 3035).
Despite the morphological changes observed on the line to quadrupedal hadrosaurs, the moment arms of the key pectoral muscles do not change substantially from those of basal ornithischians. DCL wrapped around the laterally folded acromial process, similar to ankylosaurids. The origin of DSC moved cranially, and the humeral head was restricted to the caudal shaft, as in other quadrupedal taxa. As in basal taxa, the main function of DCL was as a humeral abductor [14]. DSC also abducted the humerus; the cranial shift of its origin suggests an increased moment arm for abduction and a decreased moment arm for retraction. The presence of large abductors in hadrosaurs suggests the humerus was loaded by the GRF to collapse in adduction in a stance position. Hadrosaurs therefore either placed the manus on the midline during locomotion (figure 3i), or placed their feet lateral to the manus with the elbows tucked in, as inferred for ankylosaurs. Examination of hadrosaur tracks [45] (see the electronic supplementary material, figure S15C) suggests that the former is likely to be correct; hadrosaurs had relatively narrow bodies and placed their hind limbs on the midline during locomotion (see above; figure 3l); tracks suggest the manus was placed cranial to the pes on the midline also.
(c). Morphological convergence as a predictor of function
The EPB has been used to reconstruct soft tissues, and in combination with biomechanical approaches, to provide an independent line of evidence regarding stance in ornithischian dinosaurs. This methodology shows that ornithischians display a disparate array of locomotor styles that are only partially predicted by skeletal convergence. The acromial process of the scapula has been modified to project laterally in both ankylosaurs and hadrosaurs yet the forelimbs were not used in the same way during locomotion. Conversely, the acromial process is rectangular and large in stegosaurs but small and triangular, more similar to the basal ornithischian condition in ceratopsids, but stance in these taxa is similar. All quadrupedal taxa display a convergent increase in the size of the deltopectoral crest of the humerus and the olecranon process of the ulna, but forelimb stance was not the same in all groups. Iliac morphological convergence is a more robust predictor of function, with the transversely broad ilium and elongate preacetabular process indicating a wide-gauge stance and a columnar hind limb in all taxa in which they occur. Skeletal morphology alone is therefore not a particularly good predictor of function in ornithischians.
(d). Order of character acquisition
Figure 1 summarizes the key results of osteological character optimization. Those osteological changes that are identified as being related to the changes in muscle function discussed above are presented. Many characters were acquired in a similar order in all quadrupedal ornithischian clades; for example, characters 8 and 9, related to morphological changes in the humerus, appear to be prerequisites for quadrupedality, occurring early on in each lineage, and prior to major hind limb changes. Characters 12 and 13, related to morphological changes in the scapula, never occur before changes to the hind limb and humerus have taken place. This suggests that the order of character acquisition during the evolution of quadrupedality was constrained in ornithischians, perhaps by the bipedal bauplan of their ancestors. Several characters always appear together; for example, characters 8 and 9 (humerus morphology), and characters 2, 4 and 7 (pelvis morphology). This indicates that these characters are parts of complexes or modules that may be related developmentally [46] and has implications for their use as independent characters in phylogenetic analyses.
5. Conclusions
Studies of extant taxa have shown that a variety of different morphologies can produce the same functional outcome [4,5], implicitly suggesting that convergent morphology is not a good predictor of functional similarity. This presents a major problem for palaeobiologists, who seek to understand the life history of extinct organisms, usually based on preserved hard-part morphology alone. Using quadrupedal ornithischian dinosaurs as our extinct model organism and stance as the function that we are attempting to examine, we find that convergent skeletal morphology alone does not indicate functional similarity in quadrupedal ornithischians. It is only possible to determine function using additional information from soft tissue as reconstructed using the EPB. We therefore advocate an approach whereby function and functional similarity is determined by the use of both analogy (convergent skeletal morphology) and homology (reconstruction of soft tissue characteristics).
Acknowledgements
Thanks to the numerous curators who allowed access to specimens in their care, and Karl Bates (University of Liverpool) for discussion. S.C.R.M. is funded by Natural Environment Research Council grant no. NE/G001898/1 awarded to P.M.B. The comments of Sarah Werning (University of California, Berkeley) and an anonymous reviewer helped to improve this manuscript.
References
- 1.Rudwick M. J. S. 1964. The inference of function from structure in fossils. Br. J. Phil. Sci. 15, 27–40 10.1093/bjps/XV.57.27 (doi:10.1093/bjps/XV.57.27) [DOI] [Google Scholar]
- 2.Gould S. J. 1970. Evolutionary paleontology and the science of form. Earth-Sci. Rev. 6, 77–119 10.1016/0012-8252(70)90027-9 (doi:10.1016/0012-8252(70)90027-9) [DOI] [Google Scholar]
- 3.Hickman C. S. 1988. Analysis of form and function in fossils. Am. Zool. 28, 775–793 [Google Scholar]
- 4.Lauder G. V. 1995. On the inference of function from structure. In Functional morphology in vertebrate paleontology (ed. Thomason J. J.), pp. 1–9 Cambridge, UK: Cambridge University Press [Google Scholar]
- 5.Wainwright P. C., Alfaro M. E., Bolnick D. I., Hulsey C. D. 2005. Many-to-one mapping of form to function: a general principle in organismal design? Integr. Comp. Biol. 45, 256–262 10.1093/icb/45.2.256 (doi:10.1093/icb/45.2.256) [DOI] [PubMed] [Google Scholar]
- 6.Butler R. J., Upchurch P., Norman D. B. 2008. The phylogeny of the ornithischian dinosaurs. J. Syst. Palaeontol. 6, 1–40 10.1017/S1477201907002271 (doi:10.1017/S1477201907002271) [DOI] [Google Scholar]
- 7.Russell L. S. 1935. Musculature and functions in Ceratopsia. National Museum Can. Bull. (Geology) 77, 39–48 [Google Scholar]
- 8.Paul G. S., Christiansen P. 2000. Forelimb posture in neoceratopsian dinosaurs: implications for gait and locomotion. Paleobiology 26, 450–465 (doi:10.1666/0094-8373(2000)026<0450:FPINDI>2.0.CO;2) [DOI] [Google Scholar]
- 9.Thompson S., Holmes R. B. 2007. Forelimb stance and step cycle in Chasmosaurus irvinensis (Dinosauria: Neoceratopsia). Palaeontol. Electron. 10, 17 [Google Scholar]
- 10.Rega E., Holmes R. B., Tirabaso A. 2010. Habitual locomotor behaviour inferred from manual pathology in two Late Cretaceous chasmosaurine ceratopsid dinosaurs, Chamsosaurus irvinensis (CMN41357) and Chasmosaurus belli (ROM 843). In New perspectives on horned dinosaurs: the Royal Tyrrell Museum Ceratopsian Symposium (eds Ryan M. J., Chinnery-Allgeier B. J., Eberth D. A.), pp. 340–354 Bloomington, IN: Indiana University Press [Google Scholar]
- 11.Bryant H. N., Russell A. P. 1992. The role of phylogenetic analysis in the inference of unpreserved attributes of extinct taxa. Phil. Trans. R. Soc. Lond. B 337, 405–418 10.1098/rstb.1992.0117 (doi:10.1098/rstb.1992.0117) [DOI] [Google Scholar]
- 12.Witmer L. M. 1995. The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils. In Functional morphology in vertebrate palaeontology (ed. Thomason J. J.), pp. 19–33 Cambridge, UK: Cambridge University Press [Google Scholar]
- 13.Hutchinson J. R., Gatesy S. M. 2000. Adductors, abductors, and the evolution of archosaur locomotion. Paleobiology 26, 734–751 (doi:10.1666/0094-8373(2000)026<0734:AAATEO>2.0.CO;2) [DOI] [Google Scholar]
- 14.Maidment S. C. R., Barrett P. M. 2011. The locomotor musculature of basal ornithischian dinosaurs. J. Vertebr. Paleontol. 31, 1265–1291 10.1080/02724634.2011.606857 (doi:10.1080/02724634.2011.606857) [DOI] [Google Scholar]
- 15.Sereno P. C. 1991. Lesothosaurus, ‘fabrosaurids’, and the early evolution of Ornithischia. J. Vertebr. Paleontol. 11, 168–197 10.1080/02724634.1991.10011386 (doi:10.1080/02724634.1991.10011386) [DOI] [Google Scholar]
- 16.Butler R. J. 2010. The anatomy of the basal ornithischian dinosaur Eocursor parvus from the Lower Elliot Formation (Late Triassic) of South Africa. Zool. J. Linnean Soc. Lond. 160, 648–684 10.1111/j.1096-3642.2009.00631.x (doi:10.1111/j.1096-3642.2009.00631.x) [DOI] [Google Scholar]
- 17.Galton P. M., Upchurch P. 2004. Stegosauria. In The dinosauria (eds Weishampel D. B., Dodson P., Osmólska H.), pp. 343–362, 2nd edn Berkeley, CA: University of California Press [Google Scholar]
- 18.Vickaryous M. K., Maryańska T., Weishampel D. B. 2004. Ankylosauria. In The dinosauria (eds Weishampel D. B., Dodson P., Osmólska H.), pp. 363–392, 2nd edn Berkeley, CA: University of California Press [Google Scholar]
- 19.Dodson P., Forster C. A., Sampson S. D. 2004. Ceratopsidae. In The dinosauria (eds Weishampel D. B., Dodson P., Osmólska H.), pp. 494–516, 2nd edn Berkeley, CA: University of California Press [Google Scholar]
- 20.Ostrom J. H. 1964. A reconsideration of the paleoecology of hadrosaurian dinosaurs. Am. J. Sci. 262, 975–997 10.2475/ajs.262.8.975 (doi:10.2475/ajs.262.8.975) [DOI] [Google Scholar]
- 21.Galton P. M. 1970. The posture of hadrosaurian dinosaurs. J. Palaeontol. 44, 464–473 [Google Scholar]
- 22.Horner J. R., Weishampel D. B., Forster C. A. 2004. Hadrosauridae. In The dinosauria (eds Weishampel D. B., Dodson P., Osmólska H.), pp. 438–463, 2nd edn Berkeley, CA: University of California Press [Google Scholar]
- 23.Dilkes D. W. 2001. An ontogenetic perspective on locomotion in the Late Cretaceous dinosaur Maiasaura peeblesorum (Ornithischia: Hadrosauridae). Can. J. Earth Sci. 38, 1205–1227 10.1139/e01-016 (doi:10.1139/e01-016) [DOI] [Google Scholar]
- 24.Norman D. B. 1980. On the ornithischian dinosaur iguanodon bernissartensis from the Lower Cretaceous of Bernissart (Belgium). Mémoires de l'Institut Royal des Sciences Naturelles de Belgique 178, 1–103 [Google Scholar]
- 25.Wright J. L. 1999. Ichnological evidence for the use of the forelimb in iguanodontid locomotion. In Cretaceous fossil vertebrates (ed. Unwin D. M.), pp. 209–219 London, UK: The Palaeontological Association [Google Scholar]
- 26.Carpenter K., Wilson Y. 2008. A new species of Camptosaurus (Ornithopoda: Dinosauria) from the Morrison Formation (Upper Jurassic) of Dinosaur National Monument, Utah, and a biomechanical analysis of its forelimb. Ann. Carnegie Mus. 76, 227–265 10.2992/0097-4463(2008)76[227:ANSOCO]2.0.CO;2 (doi:10.2992/0097-4463(2008)76[227:ANSOCO]2.0.CO;2) [DOI] [Google Scholar]
- 27.Tereshchenko V. S. 1996. A reconstruction of the locomotion of Protoceratops. Paleontol. J. 30, 232–245 [Google Scholar]
- 28.Senter P. 2007. Analysis of forelimb function in basal ceratopsians. J. Zool. 273, 305–314 10.1111/j.1469-7998.2007.00329.x (doi:10.1111/j.1469-7998.2007.00329.x) [DOI] [Google Scholar]
- 29.Hutchinson J. R. 2001. The evolution of pelvic osteology and soft tissues on the line to extant birds (Neornithes). Zool. J. Linn. Soc. Lond. 131, 123–168 10.1111/j.1096-3642.2001.tb01313.x (doi:10.1111/j.1096-3642.2001.tb01313.x) [DOI] [Google Scholar]
- 30.Bryant H. N., Seymour K. L. 1990. Observations and comments on the reliability of muscle reconstruction in fossil vertebrates. J. Morphol. 206, 109–117 10.1002/jmor.1052060111 (doi:10.1002/jmor.1052060111) [DOI] [PubMed] [Google Scholar]
- 31.Boyd C. A., Brown C. M., Scheetz R. D., Clark J. A. 2009. Taxonomic revision of the basal neornithischian taxa Thescelosaurus and Bugenasaura. J. Vertebr. Paleontol. 29, 758–770 10.1671/039.029.0328 (doi:10.1671/039.029.0328) [DOI] [Google Scholar]
- 32.Kirkland J. I., Deblieux D. D. 2010. New basal centrosaurine ceratopsian skulls from the Wahweap Formation (Middle Campanian), Grand Staircase–Escalante Nation Monument, southern Utah. In New perspectives on horned dinosaurs: the Royal Tyrrell Museum Ceratopsian Symposium (eds Ryan M. J., Chinnery-Allgeier B. J., Eberth D. A.), pp. 117–140 Bloomington, IN: Indiana University Press [Google Scholar]
- 33.Maidment S. C. R. 2010. Stegosauria: a historical review of the body fossil record and phylogenetic relationships. Swiss J. Geosci. 103, 199–210 10.1007/s00015-010-0023-3 (doi:10.1007/s00015-010-0023-3) [DOI] [Google Scholar]
- 34.McDonald A. T., Horner J. R. 2010. New material of ‘Styracosaurus’ ovatus from the Two Medicine Formation of Montana. In New perspectives on horned dinosaurs: the Royal Tyrrell Museum Ceratopsian Symposium (eds Ryan M. J., Chinnery-Allgeier B. J., Eberth D. A.), pp. 156–168 Bloomington, IN: Indiana University Press [Google Scholar]
- 35.McDonald A. T., Barrett P. M., Chapman S. D. 2010. A new basal iguanodont (Dinosauria: Ornithischia) from the Wealden (Lower Cretaceous) of England. Zootaxa 2569, 1–43 [Google Scholar]
- 36.Prieto-Marquez A. 2010. Global phylogeny of hadrosauidae (Dinosauria: Ornithopoda) using parsimony and Baysian methods. Zool. J. Linn. Soc. Lond. 159, 435–503 10.1111/j.1096-3642.2009.00617.x (doi:10.1111/j.1096-3642.2009.00617.x) [DOI] [Google Scholar]
- 37.Sampson S. D., Loewen M. A., Farke A. A., Roberts E. M., Forster C. A., Smith J. A., Titus A. L. 2010. New horned dinosaurs from Utah provide evidence for intracontinental dinosaur endemism. PLoS ONE 5, 1–12 10.1371/journal.pone.0012292 (doi:10.1371/journal.pone.0012292) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu X., Wang K., Zhao X., Sullivan C., Chen S. 2010. A new leptoceratopsid (Ornithischia: Ceratopsia) from the Upper Cretaceous of Shandong, China and its implications for neoceratopsian evolution. PLoS ONE 5, 1–14 10.1371/journal.pone.0013835 (doi:10.1371/journal.pone.0013835) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson R. S., Parish J. C., Maidment S. C. R., Barrett P. M. 2011. Phylogeny of the ankylosaurian dinosaurs (Ornithischia: Thyreophora). J. Syst. Palaeontol. 10, 301–312 10.1080/14772019.2011.569091 (doi:10.1080/14772019.2011.569091) [DOI] [Google Scholar]
- 40.Maddison D. R., Maddison W. P. 2003. MacClade 4: analysis of phylogeny and character evolution, version 4.06. Sunderland, MA: Sinauer Associates [Google Scholar]
- 41.Hutchinson J. R., Gatesy S. M. 2006. Beyond the bones. Nature 440, 292–294 10.1038/440292a (doi:10.1038/440292a) [DOI] [PubMed] [Google Scholar]
- 42.Paul G. S. 1997. Dinosaur models: the good, the bad, and using them to estimate the mass of dinosaurs. In DinoFest Int. Proc. (eds Wolberg D. L., Stump E., Rosenberg G. D.), pp. 129–154 Philadelphia, PA: The Academy of Natural Sciences [Google Scholar]
- 43.McCrea R. T., Lockley M. G., Meyer C. A. 2001. Global distribution of purported ankylosaur track occurrences. In The armored dinosaurs (ed. Carpenter K.), pp. 413–454 Bloomington, IN: Indiana University Press [Google Scholar]
- 44.Carpenter K. 1982. Skeletal and dermal armor reconstruction of Euoplocephalus tutus (Ornithischia: Ankylosauridae) from the Late Cretaceous Oldman Formation of Alberta. Can. J. Earth Sci. 19, 689–697 10.1139/e82-058 (doi:10.1139/e82-058) [DOI] [Google Scholar]
- 45.Lockley M. G., Wright J. L. 2001. Trackways of large quadrupedal ornithopods from the Cretaceous: a review. In Mesozoic vertebrate life (eds Tanke D. H., Carpenter K.), pp. 428–442 Bloomington, IN: Indiana University Press [Google Scholar]
- 46.Kemp T. S. 2007. The origin of higher taxa: macroevolutionary processes, and the case of the mammals. Acta Zool. Stockholm 88, 3–22 10.1111/j.1463-6395.2007.00248.x (doi:10.1111/j.1463-6395.2007.00248.x) [DOI] [Google Scholar]