Abstract
Many species approach, inspect and signal towards their predators. These behaviours are often interpreted as predator-deterrent signals—honest signals that indicate to a predator that continued hunting is likely to be futile. However, many of these putative predator-deterrent signals are given when no predator is present, and it remains unclear if and why such signals deter predators. We examined the effects of one such signal, the tail-flag display of California ground squirrels, which is frequently given both during and outside direct encounters with northern Pacific rattlesnakes. We video-recorded and quantified the ambush foraging responses of rattlesnakes to tail-flagging displays from ground squirrels. We found that tail-flagging deterred snakes from striking squirrels, most likely by advertising squirrel vigilance (i.e. readiness to dodge a snake strike). We also found that tail-flagging by adult squirrels increased the likelihood that snakes would leave their ambush site, apparently by elevating the vigilance of nearby squirrels which reduces the profitability of the ambush site. Our results provide some of the first empirical evidence of the mechanisms by which a prey display, although frequently given in the absence of a predator, may still deter predators during encounters.
Keywords: predator–prey, animal communication, predator-deterrent signal, rattlesnake, ground squirrel
1. Introduction
When encountering a predator, prey often exhibit conspicuous vocalizations or displays that are thought to deter predator attacks. These predator-deterrent signals can operate through many mechanisms, but they are usually categorized as either quality advertisement (signaller indicates its ability to escape a pursuing predator), predator detection (signaller indicates it has seen an ambush predator) or both [1,2]. Consequently, both predator and prey are thought to benefit from predator-deterrent signals because both parties avoid an energetically costly escalation that was unlikely to be successful. The majority of studies that test for a predator-deterrent function of prey displays use humans as surrogates for natural predators [3–6]. Such studies have yielded valuable insights into prey–predator communication from the perspective of the signaller. However, it is often unknown whether these signals actually influence predator behaviour, as the responses of free-ranging predators go unexamined. Determining whether predators respond to prey signals is critical for establishing a functional relationship between prey and predator behaviour.
The few studies that have examined predator responses to predator-deterrent signals have focused on signals that are displayed primarily during encounters with predators [7–10]. However, several researchers have noted that many putative predator-deterrent signals are also frequently given in the absence of predators [11–15] Although exhibiting conspicuous signals in the absence of a receiver may seem maladaptive, several plausible explanations for this pattern have been offered, including (i) dishonest predator detection [11]; (ii) deflecting attacks of undetected predators to non-vital body parts, such as a tail [14,15]; or (iii) deterring attacks from undetected predators by honestly advertising vigilance [12,13]. In the last case, predators are thought to be less likely to attack signallers because predators would only be willing to forgo crypsis if such an attack had a high probability of success. Thus, prey deter predators simply by advertising their vigilance towards the possibility of an attack, even if the predator remains undetected. Although past studies have found some support for all of these different functions by examining prey display behaviour towards surrogate predators, none have been able to examine the responses of free-ranging predators to prey signals. Thus, it has been difficult to definitively establish whether prey signals that are frequently displayed when no predator is present actually deter predators during encounters.
Here, we examined the effect of a putative predator-deterrent signal, the tail-flag display of the California ground squirrel (Otospermophilus beecheyi), on the ambush behaviour of free-ranging northern Pacific rattlesnakes (Crotalus oreganus oreganus). When encountering rattlesnakes, both pup (recently weaned) and adult ground squirrels approach, often within striking distance, and invariably tail-flag (wave tail side-to-side) [16–18]. Ground squirrels increase the temperature of their tail when tail-flagging towards infrared-sensitive rattlesnakes, but not when tail-flagging towards non-infrared-sensitive gopher snakes [19]. This suggests that tail-flagging serves a specific function for communicating with snake predators. One possible function of the infrared tail-flag signal may be to disrupt the image of squirrels (sensory confusion), making it more difficult for snakes to successfully target the squirrel's body during a strike. Tail-flagging is also sometimes paired with physical harassment (throwing substrate and biting the snake), and consequently may function to threaten snakes that the signaller will escalate to physical attacks unless they leave the area [16].
However, as is the case with putative predator-deterrent signals in other systems, squirrels also frequently tail-flag when no snake predator is present [20,21]. These non-snake tail-flags occur at times and places where squirrels are likely to encounter snakes, suggesting that tail-flagging is associated with squirrel vigilance towards undetected snakes. Additionally, tail-flagging by adults (but not pups) in this context increases the vigilance of nearby squirrels [20,21]. Tail-flagging may function similarly during snake encounters to advertise to nearby squirrels that a snake has been detected. If this were true, we would expect snakes to associate repeated, prolonged tail-flagging from adult squirrels with a reduced probability of encountering squirrels at an ambush site, and consequently leave ambush sites sooner than they normally would. Although dozens of studies have examined ground squirrel tail-flagging behaviour and its effects on conspecifics (reviewed in Owings & Coss [17]), the influence of tail-flagging on the foraging behaviour of free-ranging snakes has not been quantified.
We used natural encounters between ground squirrels and rattlesnakes to test two hypotheses about the function of tail-flagging when ground squirrels confront rattlesnakes. Specifically, we tested whether tail-flagging (i) deters rattlesnakes from striking, and/or (ii) causes rattlesnakes to abandon ambush sites sooner than they normally would. In addition, we explored potential mechanisms that may mediate rattlesnake responses to tail-flagging displays. For strike-deterrence, these mechanisms include: (i) sensory confusion, (ii) predator detection, and (iii) vigilance advertisement. For ambush site abandonment, potential mechanisms include: (i) harassment threat and (ii) reduced hunting success through advertising predator detection to conspecifics. Our study represents the first systematic analysis of how a putative predator-deterrent signal may still influence predator behaviour, even when frequently given in the absence of predators.
2. Material and methods
(a). Study sites
We conducted our study at two field sites, Camp Ohlone and Frog Pond, in Sunol and Ohlone Regional Wildernesses in Alameda County, CA, USA. Camp Ohlone (37°29′9.27″ N, 121°44′33.47″ W) is composed of riparian woodland interspersed with non-native fruit and nut orchards because of its location at a historic homestead. This field site has been used for several previous studies of snake–squirrel interactions [20–23]. Frog Pond (37°29′58.28″ N, 121°46′24.91″ W), located approximately 3 km west, consists of cattle grazed grassland and scattered oak woodland. We chose these sites because of their high densities of both northern Pacific rattlesnakes and California ground squirrels. We conducted our research between 18 April and 15 July 2009 and 2010, as this is the period during which recently weaned squirrel pups are most vulnerable to snake predation [17].
(b). Study animals
Using a combination of trap lines and active searching, we captured 22 adult rattlesnakes and surgically implanted temperature-sensitive radio transmitters (Holohil Systems, models AI-2T and SI-2T) coupled to iButton temperature loggers (Maxim, model DS1921G) coated in Plasti Dip (Plasti Dip International, Blaine, MN, USA) using the methods of Reinert & Cundall [24] (details in electronic supplementary material, section S1a). Once a snake appeared to recover from a surgery, as evidence by frequent tongue-flicking, we released the snake at its capture location (always within 24 h of surgery). We began regularly tracking the movements and body temperatures of radio-tagged individuals once they began moving from their capture/release location and adopting ambush foraging postures. For this study, we report data from only those individual snakes that foraged primarily in microhabitats with resident ground squirrels (11 males and four females; total length range: 78.7–106.5 cm; weight range: 410–980 g).
(c). Natural observations
Once a rattlesnake began hunting in microhabitats with resident ground squirrels, we monitored its hunting behaviour using radio telemetry and portable video surveillance units (hereafter, PVSUs; details in electronic supplementary material, section S1b). Upon relocating a snake, we positioned PVSUs 1–2 m from it or the burrow entrance from which its radio signal was centred. In addition to monitoring snakes with PVSUs, we opportunistically positioned observers with digital camcorders (Sony Handycam) 5–15 m away to document aspects of snake and squirrel behaviour that occurred outside the PVSU camera frame (e.g. snakes interacting with squirrels as they moved to a new ambush site; details in electronic supplementary material, section S1c).
We monitored snake movements by checking their position with radio telemetry approximately every 1–2 h, or by direct observation. When snakes moved to new ambush sites, we repositioned PVSUs and continued our manned observations. To maximize our snake observation time, we quickly estimated short-distance movements (less than 50 m) to within 1 m by calibrating our strides to 1 m. We used a handheld GPS (Garmin Geko, ±6 m accuracy) to determine long-distance movements (more than 50 m).
(d). Strike behaviour
To examine whether tail-flagging influences rattlesnake strike behaviour, we examined PVSU recordings of squirrels coming within strike range of rattlesnakes. Squirrels were within strike range if they came within 31 cm (furthest strike distance observed from our recordings) of the 180° arc, extending from either side of a snake's head to the front, while it was in ambush position. We classified snakes as being in an ambush position if they were coiled outside of a burrow or if their head was visible at a burrow entrance, as we observed predatory strikes from both of these positions. From these videos, we quantified: (i) squirrel age, (ii) whether the squirrel tail-flagged, (iii) the squirrel's distance to snake, and (iv) if the snake struck. If the snake struck, we recorded: (i) whether the squirrel attempted to dodge and (ii) the accuracy of the snake strike.
During our study period, adult squirrels are easily differentiated from recently weaned pups based on their body size, so we classified squirrel age as either adult or pup (details in electronic supplementary material, section S1d). We categorized squirrels as tail-flagging if they exhibited any side-to-side tail movement during an encounter [18]. We did not quantify tail-flagging more precisely because squirrels were often obscured by vegetation or their tail was partially outside the frame of the camera. We measured the distance to snake (±1 cm) as the tip of the snake's head to the closest point on the squirrel's body, either during the squirrel's closest approach when the snake did not strike, or the squirrel's distance immediately preceding a strike. We used the head of the snake as a reference for estimating distances [25] to the nearest centimetre in the program ImageJ [26] (details in electronic supplementary material, section S1e).
We categorized prey as dodging [27] if their post-strike trajectory of movement deviated from their pre-strike trajectory by greater than 45°. In other words, squirrels dodged if they made a sudden movement in a new direction after the snake started moving towards them during a strike, but before the head of the snake reached them.
We categorized snake strike accuracy as either accurate or not. To classify strike accuracy, we compared the vector of the strike movement to the space occupied by the head and body of the prey in the video frame immediately preceding strike initiation (i.e. the frame used to measure distance to snake, above). Strikes were classified as accurate if the strike trajectory passed through the space occupied by the prey upon strike initiation. This measurement enabled us to examine if tail-flagging is associated with decreased strike accuracy.
To test the hypothesis that tail-flagging deters snakes from striking, we used a logistic regression with penalized maximum-likelihood estimates in R (‘logistf’ package in R; v. 2.13.0) [28–30]. Specifically, we examined whether snake strike behaviour (binary response variable) is a function of whether squirrels tail-flagged (binary explanatory variable) and included distance to snake, both as a covariate and interaction term, since it is a known determinant of snake strike success [27]. We included squirrel age as a covariate in the model, but since it was not significant (n = 15 adults and 11 pups, likelihood ratio = 1.49, 95% CI = −1.14–13.45, χ2 = 1.12, p = 0.29), we eliminated this variable to preserve our sample size. This allowed us to retain three encounters in which we were unable to accurately classify the squirrel as a pup or adult. Although we recorded multiple strike events for some snakes (n = 10 snakes, median number per snake = 2, range = 1–7), we assumed that all strike events were independent samples because they all involved independent prey items under a unique set of circumstances (e.g. unique spatial and temporal location of prey). Finally, to explore the mechanisms by which tail-flagging influences snake strike behaviour, we used Fisher's exact tests to examine whether squirrel tail-flagging was associated with (i) squirrel dodging and (ii) snake accuracy.
(e). Ambush site behaviour
To examine the effect of tail-flagging on snake ambush behaviour, we used our PVSUs and manned observations to record the time until snakes abandoned an ambush site. We defined an ambush site as a 5 m radius extending from the snake's previous ambush position, which meant that snakes had to move continuously 5 m or more to abandon their ambush site. Five metres represent the maximum observed radius of a maternal female ground squirrel's core use area (i.e. the smallest area in which 50% of activity occurs) at our Camp Ohlone site (calculated from [31]). Thus, snakes moving 5 m or more were likely to enter a different squirrel's core use area. We only analysed ambush sites in which snakes had already established an ambush position prior to an observed squirrel encounter.
While hunting in squirrel microhabitats, snakes were active primarily during the day and spent the majority of nights underground in squirrel burrow systems. Within each ambush site, we recorded the time of each observed squirrel interaction. We defined an interaction as the squirrel repeatedly tail-flagging within 1 m of a visible snake. When possible, we identified the age of the interacting squirrel as either pup or adult. All documented interactions took place between 08.00 and 19.00 h; therefore, our calculation of time spent in ambush sites was limited to this snake–squirrel mutual activity period. We also recorded the time of each strike on any squirrel (both hits and misses).
We conducted a Cox proportional hazards regression (PHREG) analysis [32] to examine the effect of interactions on the time until snakes abandoned an ambush site. Cox PHREG is a survival analysis procedure that examines the effect of predictor variables on the time until events occur (details in electronic supplementary material, section S1f). We modelled the cumulative number of adult and pup interactions within an ambush site as time-dependent covariates. Additionally, since our data consist of multiple observations of the same snakes, we modelled snake ID as an indicator of correlated observations. We report the Wald test statistic which does not assume independence among correlated observations [33]. To examine the potential mechanisms influencing snake responses, we modelled the effect of these predictor variables on time until snakes struck squirrels within an ambush site. We also recorded whether any interactions escalated to physical harassment (i.e. throwing substrate or biting the snake) or could be classified as mobbing (more than one squirrel simultaneously displaying at the snake). We conducted all Cox PHREG analyses in R using the ‘survival’ package [33]. We tested the proportional hazards assumption of our Cox PHREG models and found that no variables significantly deviated from this assumption (all variables in both models: p > 0.05). All data for strike and ambush site behavioural analyses are available in the Dryad repository: http://dx.doi.org/10.5061/dryad.v21bb.
3. Results
(a). Strike behaviour
We recorded 29 instances (15 tail-flag and 14 no tail-flag events) of squirrels approaching within striking range of ambush foraging rattlesnakes. Sample video recordings from this study are publicly viewable at the Clark laboratory website (http://www.bio.sdsu.edu/pub/clark/Site_2/Videos_Home.html). The majority of tail-flagging events we recorded (10/15) consisted of squirrels that appeared to unknowingly move within strike range, and upon becoming vigilant of the snake, stared towards it and began tail-flagging. The other five occasions came from squirrels that were already vigilant of the snake and re-approached to a closer distance (mean = 21 cm, s.d. = 7.2).
We found that the effect of tail-flagging on snake strike behaviour was strongly dependent on distance to snake (10 snakes, n = 29, likelihood ratio test on 3 d.f. = 21.9, p < 0.001; figure 1). Specifically, the probability of snakes striking at tail-flagging squirrels decreases with distance (tail-flagging–distance interaction, likelihood ratio = −0.36, 95% CI = −3.43 to −0.03, χ2 = 4.69, p = 0.03). For example, at a distance of 13 cm from the snake, the probability of a snake striking a tail-flagging squirrel drops below 50 per cent. In contrast, the probability of snakes striking non-tail-flagging squirrels remains high within the snake's strike range (figure 1).
Figure 1.
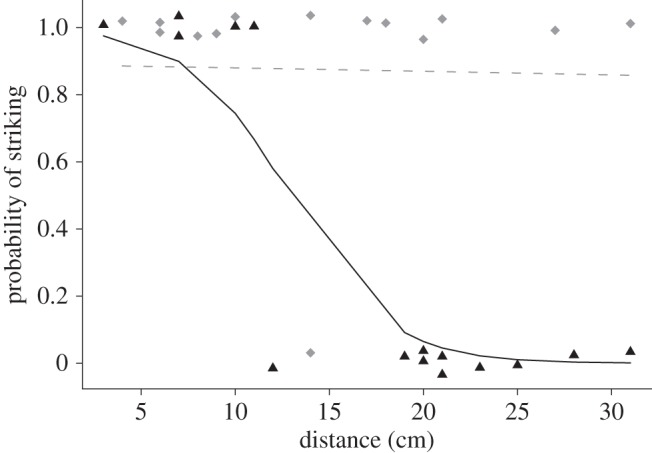
Rattlesnake responses (strike, 1; no strike, 0) to squirrels that either tail-flagged (black triangle) or not (grey diamond) at various distances within their observed strike range (31 cm). All data points were either 1 or 0; we jittered the location of data points around 1 and 0 to avoid overlapping values. The black solid line indicates our model's predicted probability of snakes striking at tail-flagging squirrels as a function of distance. The grey-dashed line indicates the predicted probability of snakes striking at non-tail-flagging squirrels as a function of distance.
The strike deterrent effect of tail-flagging may be explained by the significant association between squirrel tail-flagging prior to snake strikes and their attempts to dodge the strike (Fisher's exact test, p = 0.044; figure 2). Specifically, 100 per cent (5/5) of tail-flagging squirrels attempted to dodge snake strikes, whereas only 42 per cent (5/12) of non-tail-flagging squirrels dodged, supporting a vigilance advertisement function of tail-flagging (one non-tail-flagging squirrel was dropped from analysis owing to ambiguity of dodge movement). Snakes only struck at tail-flagging squirrels at short distances (less than 12 cm), yet these squirrels still successfully dodged 80 per cent (4/5) of strikes. In contrast, non-tail-flagging squirrels (all distances considered) only successfully dodged 54 per cent (7/13) of strikes; however, this difference was not statistically significant (Fisher's exact test, p = 0.596).
Figure 2.
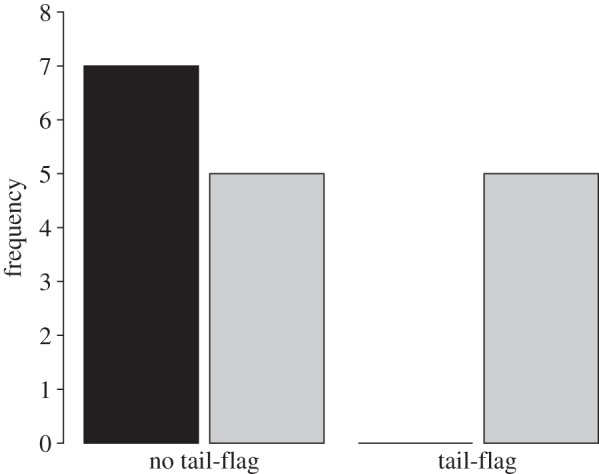
Frequency of tail-flagging and non-tail-flagging ground squirrels that attempted to dodge rattlesnake strikes. Black bars, no dodge; grey bars, dodge.
In contrast to expectations from the sensory confusion hypothesis, snake strikes were quite accurate; that is, they almost always passed through the area occupied by the squirrel's body upon strike initiation. Accuracy did not depend on whether the squirrel tail-flagged (5/5 = 100% accuracy) or not (12/13 = 92% accuracy; Fisher's exact test, p = 1.0).
(b). Ambush site behaviour
Our ambush site data come from 14 different snakes (11 males and three females) occupying 64 different ambush sites over 60.4 days (08.00–19.00 h) of near continuous observation (78% of 60.4 days). During this period, we documented 18 predatory strikes (10 hits and eight misses) and 45 interactions with ground squirrels (32 adult, 12 pup and one of unknown age interactions). The majority of our recorded interactions (30/45) consisted of squirrels approaching and tail-flagging towards sedentary, coiled snakes. During these interactions, all ambush foraging snakes remained virtually immobile (e.g. occasional head adjustments and tongue-flicking) until the squirrel left; ambush site abandonments never occurred in the presence of squirrels. Although squirrels invariably tail-flagged during interactions, less than 5 per cent (2/45) ever escalated to physical harassment. These two harassment interactions consisted of squirrels throwing substrate that contacted the snake; on neither of these two occasions did snakes exhibit an overt response (i.e. no hissing, defensive coiling, rattling or defensive striking). None of the observed interactions in this dataset consisted of more than one squirrel simultaneously confronting the snake.
The age of the signalling squirrel influenced snake decisions to abandon its ambush site (Wald test = 9.65, d.f. = 3, p = 0.022; figure 3a). Rattlesnakes were 1.6 times more likely to abandon ambush sites following each adult interaction (hazard ratio = 1.58, 95% CI = 1.00–2.48, z = 1.97, p = 0.049), whereas pup interactions had no statistically significant effect (hazard ratio = 2.40, 95% CI = 0.76–7.58, z = 1.49, p = 0.136). We found no interactive effect between pup and adult interactions on the probability of snake abandonment (hazard ratio = 0.81, 95% CI = 0.37–1.78, p = 0.594).
Figure 3.
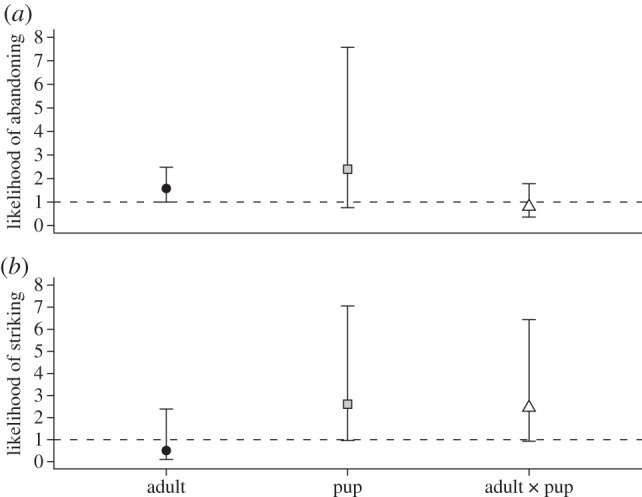
Plot of the estimated likelihood (hazard ratio ± 95% confidence intervals) of rattlesnakes (a) abandoning an ambush site and (b) striking a squirrel within an ambush site, after each tail-flagging display from an adult, pup or both. The dashed line with y-intercept = 1 indicates the likelihood under the null hypothesis.
The age of the signalling squirrel also had consequences on the likelihood of snakes striking subsequent squirrels in the area (Wald test = 30.4, d.f. = 3, p < 0.001; figure 3b). With every pup interaction, rattlesnakes tended to be more likely to strike a squirrel within the ambush site, but not significantly so (hazard ratio = 2.61, 95% CI = 0.96–7.06, z = 1.89, p = 0.059). In contrast, adult squirrel interactions tended to decrease the likelihood of striking a future squirrel, but this was not a significant decrease (hazard ratio = 0.51, 95% CI = 0.11–2.39, z = −0.85, p = 0.394). There was also a trend for the interaction between pup and adult signalling to increase the probability of future snakes strikes (hazard ratio = 2.45, 95% CI = 0.93–6.44, z = 1.82, p = 0.068), but this appears to be driven by the main effect of pup signalling.
4. Discussion
(a). Strike behaviour
Rattlesnakes alter their strike behaviour in response to tail-flagging displays of squirrels. We found that the probability of ambush foraging rattlesnakes striking at tail-flagging squirrels significantly decreased with increasing distance from the snake, yet remained high at all distances for non-tail-flagging squirrels (figure 1). We also found that tail-flagging was associated with squirrel readiness to dodge a snake strike (figure 2). This pattern suggests that tail-flagging deters snakes from striking because tail-flags honestly advertise squirrel vigilance and readiness to avoid attacks. Although vigilance advertisement has been suggested as a mechanism by which prey signalling might deter predators [12,13], our study is the first to demonstrate that a vigilance advertisement signal actually deters attack from a free-ranging predator.
Most studies of predator-deterrent signals involving displays towards ambush predators focus on predator detection signals, whereby the prey signal notifies the predator it has been detected. However, our results show that initial tail-flagging is not always, or even usually, correlated with detection of the snake, per se. Squirrels advertising their detection of a snake could do so while staying outside of the snake's limited strike range (31 cm). In our study, we frequently observed squirrels adopt alert postures and tail-flag within this effective strike range, where they are more vulnerable to predation [27], instead of immediately dodging away from the snake. This behaviour is more consistent with the hypothesis that these squirrels were unsure of the presence, identity or orientation of the snake, as snakes are usually well camouflaged either in burrow entrances or vegetation, with their body only partially visible. In this scenario, squirrels may tail-flag to advertise their vigilance to a potential strike, even if the snake remains undetected. We also recorded several instances of tail-flagging squirrels, which were already aware of the snake, approaching within strike range (but not typically closer than 21 cm). These closer approaches may enable squirrels to gain additional information regarding the species, size and exact posture of the snake (ambush or basking). Because venomous, non-venomous and non-predatory snakes of various sizes are common in their environment [16,34], such information would be useful in mounting a response appropriate to the nature of the threat. This information may be especially important for pups that are just learning to recognize snake predators [35].
In further support of a vigilance advertisement function, previous work in this system has found that over 90 per cent of tail flag displays occur when no snake is present [20,21]. When squirrels tail-flag outside the presence of an actual snake, they do so mostly while investigating microhabitats that snakes often use as ambush sites, such as thick vegetation and burrow entrances [20,21]. Given the frequency of these non-snake tail-flags, we think it is unlikely that this signal functions as a dishonest predator detection signal sensu Murphy [11]; theoretical models predict that signalling systems can support only a low frequency of dishonesty before becoming unstable [36].
Another potential function of predator-deterrent signals is to confuse the sensory systems of the predator [37], or to deflect an attack to a less-vulnerable area of the body, such as a tail [14,15]. Ground squirrels increase the temperature of their tail when tail-flagging towards infrared-sensitive rattlesnakes [19]. However, we found that tail-flagging did not influence the accuracy of snake strikes, suggesting that sensory confusion is an unlikely mechanism by which tail-flagging deters snake strikes. One caveat of our analysis is that snakes only struck at tail-flagging squirrels at close range. Because snake strikes are inherently more accurate at close distances (closer prey present larger targets), our data provide only a limited test of the sensory confusion hypothesis. A more explicit test of this hypothesis would quantify snake strike accuracy in response to different tail temperatures of a tail-flagging squirrel, which future work will attempt to do with a biorobotic California ground squirrel [38].
There are aspects of squirrel signalling behaviour other than the tail flag that could confound our conclusions. For example, tail-flagging squirrels were usually oriented towards snakes in a stereotyped, elongate posture. Therefore, it is unclear if the pattern of strike deterrence we observed would hold for tail-flagging squirrels that are not oriented towards an actual snake, but are tail-flagging only because they are in rattlesnake microhabitat. Examining the interaction between tail-flagging, approach distance and squirrel orientation on rattlesnake strike behaviour will give further insight to the efficacy of tail-flagging in buffering squirrels from attack by undetected snakes. Although it may be unrealistic to experimentally manipulate these behaviours in free-ranging squirrels, future studies will be able to separate correlated aspects of the signal using a biorobotic squirrel [38].
(b). Ambush site behaviour
Whether rattlesnakes abandoned ambush sites in response to tail-flagging displays depended on the age of the squirrel. After each adult display, the likelihood of a rattlesnake abandoning a site increased by a factor of 1.6 (figure 3a). We did not find a significant decrease in the probability of snakes ambushing a squirrel after each adult display (figure 3b), but this may be confounded by snakes being more likely to abandon ambush sites. In contrast, rattlesnakes were not more likely to abandon sites after being tail-flagged at by pups (figure 3a), and tended to experience an increase in their probability of ambushing squirrels if they remained at the site (figure 3b).
These different consequences of pup and adult signalling on ambush site selection behaviour of snakes are consistent with previous research in this system. Hersek & Owings [20] showed that even when no snake is present, adult squirrel tail-flagging elicits an increase in snake vigilance of nearby adults and pups. Furthermore, adult squirrels will return to check on snakes at previous interaction sites, and thus appear to be aware of the long durations and microhabitat features used by foraging rattlesnakes [17]. Combined with our results, snakes appear more likely to abandon ambush sites in response to prolonged adult tail-flagging because these displays may advertise to nearby squirrels that a snake has been detected, thereby reducing the profitability of the ambush site. In contrast, pup tail-flagging in the absence of rattlesnakes appears to have no effect on snake vigilance of nearby pups or adults, even though squirrels were more likely to remain in the general vicinity of a tail-flagging pup [21]. Given that squirrels pups are also a spatially clumped resource [39], this may explain why our snakes did not abandon ambush sites in response to pup signals (i.e. ambush site remains profitable). Although Hersek & Owings [20,21] focused on adult and pup signalling in the absence of rattlesnakes, our observations of snake behaviour match the predicted outcomes of their results in the context of direct encounters with snakes.
Physical harassment and mobbing of rattlesnakes by ground squirrels are commonly cited behaviours, but past studies typically elicit squirrel behaviours by staging encounters with caged or tethered rattlesnakes [16,17]. Our dataset on free-ranging rattlesnakes only contained two recordings of squirrels throwing substrate (dirt and rocks) that actually contacted snakes, and on neither occasion did snakes respond defensively or immediately leave its ambush site. Therefore, direct harassment and mobbing are not likely mechanisms influencing ambush site selection of free-ranging adult rattlesnakes. Anecdotally, we have observed squirrels escalate to more aggressive harassment of moving adult rattlesnakes (non-focal individuals) through direct physical attack and mobbing, but these events were rare (see also [22,23]).
The pattern we observed of a predator-deterrent signal influencing the ambush site selection behaviour of predators is not limited to this study [7,40,41]. However, in these studies [7,40,41], it is unclear whether predators abandon sites because the signalling prey has detected the predator, or because the signal is advertising the predator's presence to the nearby prey. To evaluate the relative contribution of these two functions, future research should explicitly examine whether the benefits to the signaller rely primarily on the increased vigilance of other potential prey in the vicinity, or simply on the information that the signaller perceives the predator.
5. Conclusion
Although predator-deterrent signals are purportedly given by a variety of taxa (e.g. fish, lizards, birds and mammals), the dearth of studies incorporating predator responses has hindered our progress in understanding the specific functions and evolutionary dynamics of prey–predator communication. Ground squirrel tail-flagging appears to operate as a predator-deterrent signal through two distinct mechanisms. First, during the initial approach and investigation of a snake or snake habitat, tail-flagging signals the vigilance of the squirrel, communicating to any nearby rattlesnakes that attempts to strike the signaller will likely be futile. Second, the prolonged inspection and repeated tail-flagging by adult squirrels reveals the presence and location of the rattlesnake to other squirrels in the vicinity [20,21], which increases the probability of the rattlesnake leaving its ambush site. Our study serves as a demonstration that a single anti-predator signal, even when displayed frequently in the absence of a predator, can still influence predator behaviour through multiple mechanisms. Much is to be gained by future research that focuses on the mechanisms by which prey signals affect predator behaviours, and the consequences such interactions may have on the evolutionary dynamics of predator and prey interactions.
Acknowledgements
All methods adhered to were approved by the Institutional Animal Care and Use Committee at San Diego State University (APF 10-09-025C).
We would like to dedicate this manuscript to the late Dr Donald H. Owings; we are forever in his debt for the guidance and insight he provided to us on the rattlesnake–ground squirrel system. The San Francisco Water District and East Bay Regional Park staff played a pivotal role in facilitating our research by providing housing and allowing us to conduct our study on their land. Karen Swaim lent us snake traps during the first year of this study, for which we are thankful. We thank our volunteer field assistants: Caesar Rahman, Jessica Fort, Michelle Goh, Zach Cavas, Elinor Israel, Emily Mastrelli, Sean Tangco, Emily Stulik, Cleo Grieve, Hossein Ayazi and Eric Schroeder. Without their help, this research would not have been possible. For help in reviewing snake videos, we thank: Rey Ayon, Alex Janes, Adriana Scurto, Cristina Lever, Sean Tangco, Maji Ghulam, Jessica Rinauro, Kenneth Huang and Tracy Grimes. For insightful comments on this manuscript, we thank Shannon Hoss, Frank Santana, Tara Luckau, Bree Putman, J. P. Montagne, Stephen Rice, Laura Williams, Rebecca Lewison, Barbara Bailey, Valerie Clark and Krystal Rypien. This research was funded by an Animal Behavior Society Student Research grant, American Museum of Natural History Theodore Roosevelt grant and San Diego State University's Mabel Myers Memorial Scholarship to M.A.B. as well as grants from the National Geographic Society (Waitts Grant W17-08) and the National Science Foundation (DBI-0951010) to R.W.C.
References
- 1.Bradbury J. W., Vehrencamp S. L. 2011. Principles of animal communication, 2nd edn. Sunderland, MA: Sinauer Associates [Google Scholar]
- 2.Caro T. 2005. Antipredator defenses in birds and mammals. Chicago, IL: University of Chicago Press [Google Scholar]
- 3.Cooper W. E., Jr, Pérez-Mellado V., Baird T. A., Caldwell J. P., Vitt L. J. 2004. Pursuit deterrent signalling by the bonaire whiptail lizard Cnemidophorus murinus. Behaviour 141, 297–311 10.1163/156853904322981860 (doi:10.1163/156853904322981860) [DOI] [Google Scholar]
- 4.Cooper W. E. 2011. Pursuit deterrence, predation risk, and escape in the lizard Callisaurus draconoides. Behav. Ecol. Sociobiol. 65, 1833–1841 10.1007/s00265-011-1191-5 (doi:10.1007/s00265-011-1191-5) [DOI] [Google Scholar]
- 5.Caro T. M. 1995. Pursuit-deterrence revisited. Trends Ecol. Evol. 10, 500–503 10.1016/S0169-5347(00)89207-1 (doi:10.1016/S0169-5347(00)89207-1) [DOI] [PubMed] [Google Scholar]
- 6.Woodland D. J., Jaafar Z., Knight M.-L. 1980. The ‘pursuit deterrent’ function of alarm signals. Am. Nat. 115, 748–753 10.1086/283596 (doi:10.1086/283596) [DOI] [Google Scholar]
- 7.Zuberbühler K., Jenny D., Bshary R. 1999. The predator deterrence function of primate alarm calls. Ethology 105, 477–490 10.1046/j.1439-0310.1999.00396.x (doi:10.1046/j.1439-0310.1999.00396.x) [DOI] [Google Scholar]
- 8.Cresswell W. 1994. Song as a pursuit-deterrent of skylark, and its occurrence relative to other anti-predation behaviours of skylark (Alauda arvensis) on attack by merlins (Falco columbarius). Behav. Ecol. Sociobiol. 34, 217–223 10.1007/BF00167747 (doi:10.1007/BF00167747) [DOI] [Google Scholar]
- 9.Caro T. M. 1986. The functions of stotting in Thomson's gazelles: some tests of the predictions. Anim. Behav. 34, 663–684 10.1016/S0003-3472(86)80052-5 (doi:10.1016/S0003-3472(86)80052-5) [DOI] [Google Scholar]
- 10.FitzGibbon C. D., Fanshawe J. H. 1988. Stotting in Thomson's gazelles: an honest signal of condition. Behav. Ecol. Sociobiol. 23, 69–74 10.1007/BF00299889 (doi:10.1007/BF00299889) [DOI] [Google Scholar]
- 11.Murphy T. G. 2007. Dishonest ‘preemptive’ pursuit-deterrent signal? Why the turquoise-browed motmot wags its tail before feeding nestlings. Anim. Behav. 73, 965–970 10.1016/j.anbehav.2006.10.020 (doi:10.1016/j.anbehav.2006.10.020) [DOI] [Google Scholar]
- 12.Randler C. 2006. Is tail wagging in white wagtails, Motacilla alba, an honest signal of vigilance? Anim. Behav. 71, 1089–1093 10.1016/j.anbehav.2005.07.026 (doi:10.1016/j.anbehav.2005.07.026) [DOI] [Google Scholar]
- 13.Carder M. L., Ritchison G. 2009. Tail pumping by Eastern Phoebes: an honest, persistent predator-deterrent signal? J. Field Ornithol. 80, 163–170 10.1111/j.1557-9263.2009.00218.x (doi:10.1111/j.1557-9263.2009.00218.x) [DOI] [Google Scholar]
- 14.Cooper W. E., Jr 2001. Multiple roles of tail display by the curly-tailed lizard Leiocephalus carinatus: pursuit deterrent and deflective roles of a social signal. Ethology 107, 1137–1149 10.1046/j.1439-0310.2001.00754.x (doi:10.1046/j.1439-0310.2001.00754.x) [DOI] [Google Scholar]
- 15.Cooper W. 1998. Conditions favoring anticipatory and reactive displays deflecting predatory attack. Behav. Ecol. 9, 598–604 10.1093/beheco/9.6.598 (doi:10.1093/beheco/9.6.598) [DOI] [Google Scholar]
- 16.Owings D. H., Coss R. G. 1977. Snake mobbing by California ground squirrels: adaptive variation and ontogeny. Behaviour 62, 50–69 10.1163/156853977X00045 (doi:10.1163/156853977X00045) [DOI] [Google Scholar]
- 17.Owings D. H., Coss R. G. 2008. Hunting California ground squirrels: constraints and opportunities for Northern Pacific rattlesnakes. In Biology of the rattlesnakes (eds Hayes W. K., Beaman K. R., Cardwell M. D., Bush S. P.). Loma Linda, CA: Loma Linda University Press [Google Scholar]
- 18.Hennessy D. F., Owings D. H., Rowe M. P., Coss R. G., Leger D. W. 1981. The information afforded by a variable signal: constraints on snake-elicited tail flagging by California ground squirrels. Behaviour 78, 188–226 10.1163/156853981X00329 (doi:10.1163/156853981X00329) [DOI] [Google Scholar]
- 19.Rundus A. S., Owings D. H., Joshi S. S., Chinn E., Giannini N. 2007. Ground squirrels use an infrared signal to deter rattlesnake predation. Proc. Natl Acad. Sci. USA 104, 14 372–14 376 10.1073/pnas.0702599104 (doi:10.1073/pnas.0702599104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hersek M. J., Owings D. H. 1993. Tail flagging by adult California ground squirrels—a tonic signal that serves different functions for males and females. Anim. Behav. 46, 129–138 10.1006/anbe.1993.1168 (doi:10.1006/anbe.1993.1168) [DOI] [Google Scholar]
- 21.Hersek M. J., Owings D. H. 1994. Tail flagging by young California ground squirrels, Spermophilus beecheyi: age-specific participation in a tonic communicative system. Anim. Behav. 48, 803–811 10.1006/anbe.1994.1304 (doi:10.1006/anbe.1994.1304) [DOI] [Google Scholar]
- 22.Hennessy D. F., Owings D. H. 1988. Rattlesnakes create a context for localizing their search for potential prey. Ethology 77, 317–329 10.1111/j.1439-0310.1988.tb00213.x (doi:10.1111/j.1439-0310.1988.tb00213.x) [DOI] [Google Scholar]
- 23.Swaisgood R. R., Owings D. H., Rowe M. P. 1999. Conflict and assessment in a predator–prey system: ground squirrels versus rattlesnakes. Anim. Behav. 57, 1033–1044 10.1006/anbe.1998.1069 (doi:10.1006/anbe.1998.1069) [DOI] [PubMed] [Google Scholar]
- 24.Reinert H. K., Cundall D. 1982. An improved surgical implantation method for radio-tracking snakes. Copeia 1982, 702–705 10.2307/1444674 (doi:10.2307/1444674) [DOI] [Google Scholar]
- 25.Klauber L. M. 1972. Rattlesnakes: their habits, life histories, and influence on mankind. Berkeley, CA: University of California Press [Google Scholar]
- 26.Abràmoff M. D., Hospitals I., Ram S. J. 2004. Image processing with ImageJ. Biophotonics Int. 11, 36–42 10.1117/1.3589100 (doi:10.1117/1.3589100) [DOI] [Google Scholar]
- 27.Clark R. W., Tangco S., Barbour M. A. 2012. Field recordings reveal factors influencing predator strike success of free-ranging rattlesnakes (Crotalus spp.). Anim. Behav. 84, 183–190 10.1016/j.anbehav.2012.04.029 (doi:10.1016/j.anbehav.2012.04.029) [DOI] [Google Scholar]
- 28.Firth D. 1993. Bias reduction of maximum likelihood estimates. Biometrika 80, 27–38 10.1093/biomet/80.1.27 (doi:10.1093/biomet/80.1.27) [DOI] [Google Scholar]
- 29.Ploner M., Dunkler D., Southworth H., Heinz G. 2010. logistf: Firth's bias reduction logistic regression. R package version 1.10. See http://CRAN.R-project.org/package=logistf
- 30.R Development Core Team 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 31.Boellstorff D. E., Owings D. H. 1995. Home range, population structure, and spatial organization of California ground squirrels. J. Mammal. 76, 551–561 10.2307/1382363 (doi:10.2307/1382363) [DOI] [Google Scholar]
- 32.Cox D. R. 1972. Regression models and life-tables. J. R. Stat. Soc. B Methodol. 34, 187–220 10.2307/2985181 (doi:10.2307/2985181) [DOI] [Google Scholar]
- 33.Therneau T. 2012. A package for survival analysis in S. R package version 2.36–14 See: http://cran.r-project.org/web/packages/survival/index.html)
- 34.Fitch H. S. 1949. Study of snake populations in central California. Am. Midland Nat. 41, 513–579 10.2307/2421774 (doi:10.2307/2421774) [DOI] [Google Scholar]
- 35.Coss R. G. 1991. Context and animal behavior III: the relationship between early development and evolutionary persistence of ground squirrel antisnake behavior. Ecol. Psychol. 3, 277–315 10.1207/s15326969eco0304_1 (doi:10.1207/s15326969eco0304_1) [DOI] [Google Scholar]
- 36.Searcy W. A., Nowicki S. 2005. The evolution of animal communication: reliability and deception in signaling systems. Princeton, NJ: Princeton University Press [Google Scholar]
- 37.Dugatkin L. A., Godin J.-G. J. 1992. Prey approaching predators: a cost–benefit perspective. Annal. Zool. Fennici 29, 233–252 [Google Scholar]
- 38.Joshi S. S., Johnson R., Rundus A. S., Clark R. W., Barbour M., Owings D. H. 2012. Robotic squirrel models for the study of squirrel–rattlesnake interaction in laboratory and natural settings. IEEE Robot. Autom. Mag. 18, 59–68 10.1109/MRA.2011.942121 (doi:10.1109/MRA.2011.942121) [DOI] [Google Scholar]
- 39.Boellstorff D. E., Owings D. H., Penedo M. C. T., Hersek M. J. 1994. Reproductive behaviour and multiple paternity of California ground squirrels. Anim. Behav. 47, 1057–1064 10.1006/anbe.1994.1144 (doi:10.1006/anbe.1994.1144) [DOI] [Google Scholar]
- 40.Clark R. W. 2005. Pursuit-deterrent communication between prey animals and timber rattlesnakes (Crotalus horridus): the response of snakes to harassment displays. Behav. Ecol. Sociobiol. 59, 258–261 10.1007/s00265-005-0032-9 (doi:10.1007/s00265-005-0032-9) [DOI] [Google Scholar]
- 41.FitzGibbon C. D. 1994. The costs and benefits of predator inspection behaviour in Thomson's gazelles. Behav. Ecol. Sociobiol. 34, 139–148 10.1007/BF00164184 (doi:10.1007/BF00164184) [DOI] [Google Scholar]


