Abstract
We reported earlier that during chronic toxoplasmosis CD8+ T cells become functionally exhausted with concomitant PD-1 upregulation, leading to eventual host mortality. However, how immune exhaustion specifically mediates attrition of CD8 polyfunctionality, a hallmark of potent T-cell response, during persistent infections has not been addressed. In this study, we demonstrate that PD-1 is preferentially expressed on polyfunctional memory CD8+ T cells, which renders them susceptible to apoptosis. In vitro blockade of the PD-1–PD-L1 pathway dramatically reduces apoptosis of polyfunctional and interferon γ+/granzyme B− memory but not effector CD8+ T cells. In summary, the present report underscores the critical role of the PD-1–PD-L1 pathway in mediating attrition of this important CD8+ T-cell subset and addresses the mechanistic basis of how αPD-L1 therapy reinvigorates polyfunctional CD8 response during chronic infections. The conclusions of this study can have profound immunotherapeutic implications in combating recrudescent toxoplasmosis as well other chronic infections.
Effective CD8+ T-cell response is critical for control of both acute and chronic Toxoplasma infection [1, 2]. Mice lacking CD8+ T cells exhibit inability to clear infection [3], and depletion of CD8+ rather than CD4+ T cells had a greater effect on mortality of chronically infected animals [4]. Moreover, chronically infected susceptible mice strains show reduced number of intracerebral CD8+ T cells with concomitant toxoplasmic encephalitis (TE) development [5]. Combined, these studies underline the important role of this T-cell subset in controlling Toxoplasma infection during the chronic phase.
Interferon γ (IFN-γ) is critical for mediating protective immunity to Toxoplasma, and during chronic infection CD8+ T cells are a major source of this cytokine, which is essential for controlling parasite reactivation [6, 7]. CD8+ T cells can also provide effector function via production of other cytokines, including tumor necrosis factor α [3, 8]. However, macrophages but not CD8+ T cells are the major producers of this cytokine during chronic toxoplasmosis [9]. Similarly, multiple studies using replication-competent strains of Toxoplasma have demonstrated that T cells produce minimal interleukin 2 during both acute and chronic Toxoplasma infection [10]. Apart from cytokines, CD8-mediated cytotoxicity most likely plays an important role in controlling chronic toxoplasmosis [10]. A recent study has demonstrated that CD8+ T cells are capable of eradicating cysts in sulfadiazine-treated immunodeficient mice by a perforin-dependent mechanism [11]. Based on these findings, IFN-γ and cytotoxicity appear to be the critical effector mechanisms for CD8-mediated control of this protozoan. Pardoxically, robust CD8+ T-cell response during the acute phase of infection does not ensure long-term survival of susceptible mice [12].
Recent studies from our laboratory [13] demonstrated that CD8+ T cells from chronically infected mice exhibit progressive functional exhaustion, concomitant with increased expression of PD-1, an inhibitory receptor on their surface. This dysfunction results in reactivation of latent infection, which eventually leads to death of the infected animals. In vivo blockade of PD-1 interaction with its receptor PD-L1, reinvigorated polyfunctional CD8+ T-cell response (ie, the capacity for a single T cell to exhibit multiple functions) and prevented death of infected animals. Incidentally, the observation that human immunodeficiency virus (HIV)–infected nonprogressors are superior at maintaining virus-specific polyfunctional CD8+ T cells compared with HIV- infected progressors [14] suggests that this polyfunctional population constitutes an important part of protective immune response against intracellular pathogens. However, how immune exhaustion causes attrition of polyfunctional CD8+ T cells during chronic infections has not been addressed in parasitic or even in viral models of persistent infection. Considering the prevalence of TE in Toxoplasma-seropositive AIDS patients [15], approaches that boost CD8 polyfunctionality can have profound implications against both HIV and Toxoplasma gondii infections. Despite wide Toxoplasma seropositivity in the global population, current initiatives for vaccine development against this parasite are prophylactic in nature [16]. Because the majority of the mortality caused by Toxoplasma is due to parasite reactivation in immunocompromised patients [17], there is an urgent need for development of immunotherapeutic vaccination approaches to combat this infection. In this study, we report for the first time that PD-1 is preferentially expressed on polyfunctional memory phenotype CD8+ T cells, which renders them susceptible to apoptosis. In vitro blockade of this pathway reduced active caspase 3 expression on both polyfunctional and IFN-γ+/granzyme B− memory phenotype CD8+ T cells. Thus the current study highlights a critical and arguably novel mechanism of how the PD-1–PD-L1 pathway promotes the attrition of polyfunctional CD8 response. More important, from a therapeutic perspective, this creates a strong basis for incorporating anti–PD-L1 as an adjuvant in immunotherapeutic vaccination approaches against chronic toxoplasmosis.
MATERIALS AND METHODS
Mice, Parasites, and Toxoplasma Lysate Antigen Preparation
Female C57BL/6 mice (National Cancer Institute) aged 6–8 weeks were infected with 10 ME49 cysts via oral route. Animal studies were carried out in agreement with Institutional Animal Care and Use Committee–approved guidelines. Toxoplasma lysate antigen (TLA) was prepared from the RH strain of the parasite, as previously described [18].
Lymphocyte Isolation, Cell Surface Staining, and Intracellular Staining
Single-cell suspension was prepared from spleen and brain using standard protocol. For direct ex vivo assessment of apoptosis, BD AnnexinV Kit was used as per manufacturer's protocol. Intracellular staining was performed after surface staining using Cytofix/Cytoperm Kit (BD Biosciences) as per manufacturer's protocol. For cytokine detection, restimulation was carried out for 16 hours with 30 μg/mL of TLA in supplemented media. For some samples, αPD-L1 (MIH5) antibody or isotype control antibody was added throughout the incubation period at a final concentration of 30 μg/mL.
Statistical Analysis
Statistical analysis was performed using Student t test with P < .05 considered statistically significant.
Additional details are available in the Supplementary Methods.
RESULTS
Majority of Polyfunctional CD8+ T Cells During Chronic Toxoplasmosis Exhibit a Memory Phenotype
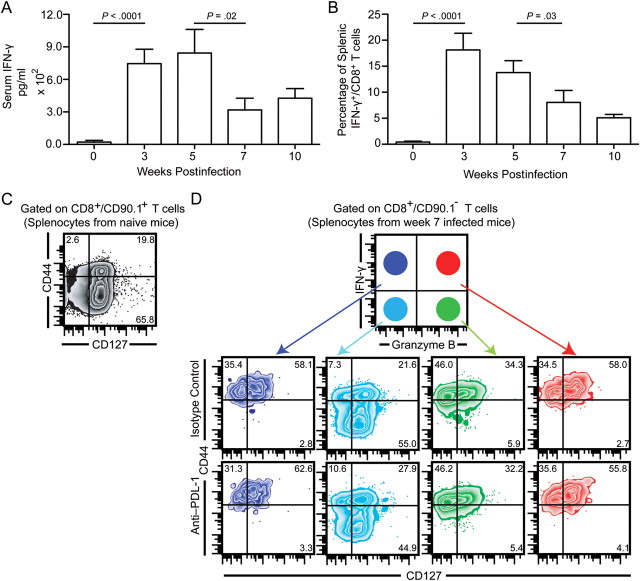
Toxoplasma infection induces a robust CD8+ T-cell response, which has been reported to be critical for protection against host infection [3]. However, during the late chronic phase, the parasite undergoes reactivation (ie, conversion from chronic stage–associated bradyzoite [slowly replicating, primarily encysted, relatively quiescent] to acute stage–associated tachyzoite [rapidly replicating]) [19]. Because IFN-γ has been shown to be critical for control of Toxoplasma [20], we measured serum levels of IFN-γ as well as production of this cytokine by splenic CD8+ T cells. Concomitant with parasite reactivation, decreased serum IFN-γ levels (Figure 1A) and reduced IFN-γ production by CD8+ T cells (Figure 1B) were noted during the late chronic phase. This is in agreement with our previously published data, which showed a decrease in polyfunctional (IFN-γ+/granzyme B+) CD8+ T-cell response in spleen and brain at week 7 postinfection [13]. Because recent studies in viral models have established polyfunctionality as 1 of the most critical correlates of viral clearance or vaccine efficacy [21, 22], we next investigated whether the polyfunctional CD8+ T cells during the chronic phase of infection belonged to the effector or memory subset. To address this, we performed a 5-color flow cytometry on restimulated splenocytes, using well-established naive/effector/memory markers CD44 and CD127 [23] to discriminate various CD8 subsets. Irrespective of the time point examined, during chronic phase, the majority of CD8+/IFN-γ+/granzyme B− and CD8+/IFN-γ+/granzyme B+ T cells exhibited a memory phenotype (CD44hi/CD127hi), whereas CD8+/IFN-γ−/granzyme B+ T cells showed an effector subset phenotype (Figure 1C and 1D). In contrast, the overwhelming majority of CD8+/IFN-γ−/granzyme B− T cells were CD44lo/CD127hi, akin to the phenotype of naive splenocytes (Figure 1C and 1D).
Figure 1.
Majority of polyfunctional CD8+ T cells during chronic toxoplasmosis exhibit a memory phenotype. A, Mice infected with 10 ME49 cycts orally were evaluated for serum interferon γ (IFN-γ) by enzyme-linked immunosorbent assay at weeks 3, 5, 7, and 10 postinfection. B, Splenic CD8+ T cells at the above time points were assessed for IFN-γ production by restimulation of splenocytes with Toxoplasma lysate antigen. C and D, The IFN-γ+/granzyme B−, IFN-γ+/granzyme B+, IFN-γ−/granzyme B+, and IFN-γ−/granzyme B− splenic CD8+ T-cell subsets from chronically infected (week 7) mice were evaluated for expression of CD44 and CD127. Due to low number of IFN-γ– or granzyme B–expressing CD8+ T cells in naive spleens, the total CD8+ T-cell population was assessed for CD44 and CD127 expression. The data represent 2 experiments with at least 4 mice per group. Error bars represent standard deviation throughout.
Normal CD8+ T-Cell Development During T. gondii Infection
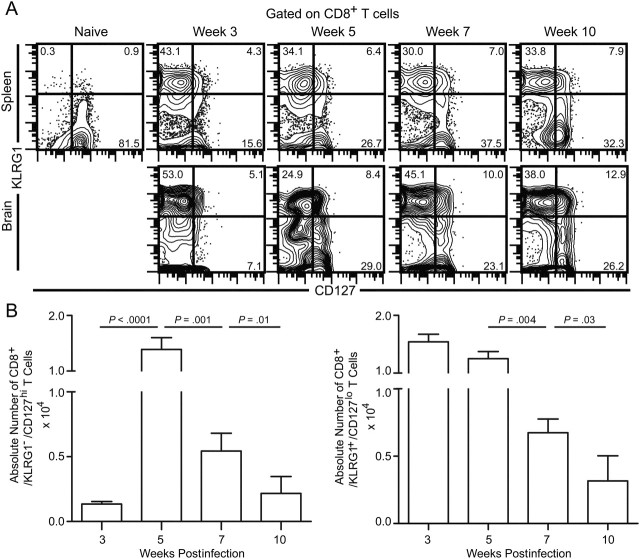
Because the majority of polyfunctional (IFN-γ+/granzyme B+) and IFN-γ+/granzyme B− CD8+ T cells exhibit a memory phenotype and there is a loss of polyfunctional CD8+ T-cell response during late chronic toxoplasmosis, we hypothesized a poor development or maintenance of memory CD8+ T cells during later phases of chronic toxoplasmosis. To address this, we evaluated the expression of KLRG1 and CD127 on CD8+ T cells. Previous studies have shown that effector cells express KLRG1 but minimal CD127, whereas the reverse is true for memory CD8+ T cells [24]. Because naive nonspecific CD8+ T cells also express CD127, we examined the phenotype in brain, a site that specifically recruits CD8+ T cells in a strictly antigen-specific manner [25]. Contrary to our expectations based on the degree of attrition of polyfunctionality, we only noted moderate differences in the frequency of CD127hi cells (Figure 2A). Although dramatic changes in the frequency of CD8+/CD127hi T cells were not noted in the brain, the absolute numbers of both effector and memory phenotype CD8+ T cells were reduced in the brain during the later phases of infection (Figure 2B).
Figure 2.
Frequency of memory phenotype CD8+ T cells is not dramatically altered at the effector site. A, KLRG1 and CD127 expression on CD8+ T cells from the spleen and brain was assessed at weeks 3, 5, 7, and 10 postinfection. B, The absolute number of CD8+/KLRG1−/CD127hi (left panel) and CD8+/KLRG1+/CD127lo (right panel) T cells in the brain at weeks 3, 5, 7, and 10 postinfection. The data represent 3 experiments with at least 4 mice per group.
Preferential Expression of PD-1 on Memory CD8+ T Cells
In a recent study, we reported that PD-1hi/CD8+ T cells were preferentially apoptotic as evaluated by elevated active caspase 3 expression [13]. Based on this report and the finding that the frequency of memory phenotype CD8+ T cells were only moderately affected at the effector site during late chronic toxoplasmosis, we hypothesized that PD-1 was expressed both on memory and effector CD8+ T cells. Hence, next we assessed the expression of the PD-1 molecule on the effector or memory CD8+ T-cell population during T. gondii infection. Because a previous study using the acute infection−causing Lymphocytic choriomeningitis virus (LCMV) Armstrong strain had shown that CD43 (activation-associated isoform) expression can be used to discriminate effector (CD43hi) from memory (CD43lo) CD8+ T cells, we examined the expression of this molecule on CD8+ T cells in the spleen and brain [26]. Polychromatic flow cytometry revealed that the majority of PD-1hi cells in both tissues coexpressed CD43 and that increase in PD-1 expression correlated with increase in CD43 expression (Figure 3A). To further verify if PD-1hi/CD43+, PD-1hi/CD43−, PD-1int/lo/CD43hi, and PD-1int/lo/CD43− CD8+ T cells belonged to memory or effector subsets, we examined them for expression of canonical memory markers CD44 and interleukin 7Rα (CD127) [23]. Surprisingly, despite elevated CD43 expression, the majority of PD-1hi/CD43hi cells in both tissues coexpressed high levels of CD44 and CD127 (Figure 3B and 3C). In contrast, most cells in the other 3 subsets, irrespective of CD43 expression, exhibited lower frequency of CD127hi cells (Figure 3C). To further verify memory/effector characteristics of PD-1–expressing CD8T cells, we evaluated various CD8 subsets for presence of Bcl-2, an antiapoptotic molecule that is expressed at high levels in memory CD8+ T cells [23, 27]. Although PD-1hi/CD44int/hi/CD127hi CD8 T cells expressed lower levels of this protein than PD-1int/lo/CD44int/hi/CD127hi cells (conventional memory), irrespective of PD-1 expression, CD44int/hi/CD127hi CD8+ T cells had much higher levels of this molecule than naive or effector phenotype CD8+ T cells (Figure 3D). These findings suggest that although the PD-1hi CD8+ T cells exhibit memory characteristics, they represent a phenotype distinct from both conventional memory and effector CD8+ T cells generated in acute infection models [26].
Figure 3.
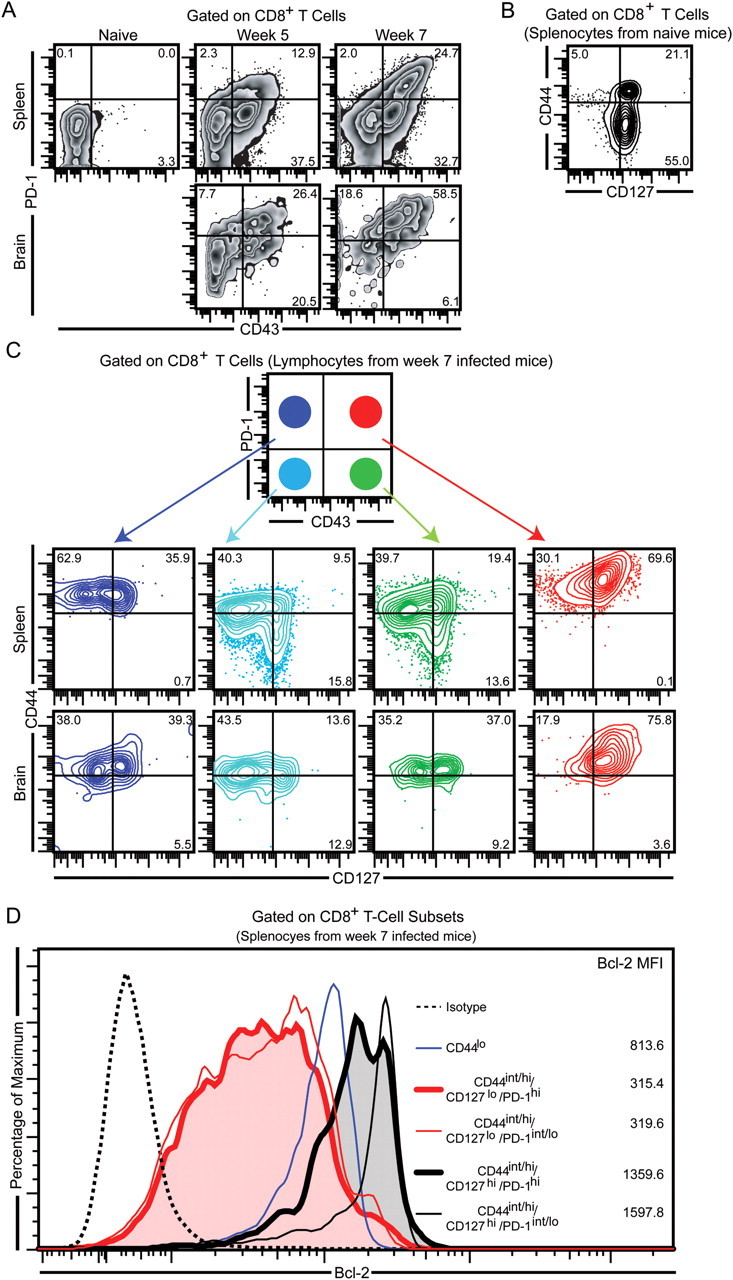
PD-1 is preferentially upregulated on CD43-expressing memory phenotype CD8+ T cells. A, Splenic and brain CD8+ T cells were evaluated for PD-1 and CD43 expression at week 5 and week 7 postinfection. (B and C), PD-1hi/CD43+, PD-1hi/CD43−, PD-1int/lo/CD43+, and PD-1int/lo/CD43−/CD8+ T-cell subsets from the spleen and brain of chronically infected mice (week 7) were further evaluated for CD44 and CD127 expression. Due to low numbers of PD-1hi– or CD43-expressing CD8+ T cells in naive spleens, the total CD8+ T-cell population was assessed for CD44 and CD127 expression. D, Splenic CD8+ T-cell subsets were assessed for Bcl-2 expression by intracellular staining. Numbers in the histogram denote mean fluorescence intensity. Data represent 3 experiments with at least 4 mice per group.
Increased Expression of PD-1 Is Associated With Apoptosis of Polyfunctional Memory CD8+ T Cells
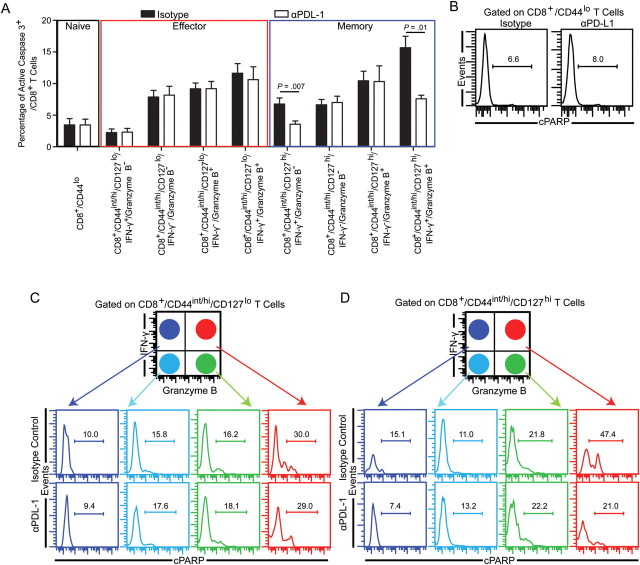
As mentioned previously, PD-1hi/CD8+ T cells during chronic toxoplasmosis express high levels of active caspase 3 [13]. Because a previous study demonstrated that despite elevated active caspase 3 expression anergic T cells do not undergo apoptosis [28], we wanted to revalidate our earlier findings by assessing the distribution of membrane phosphatidly serine using fluorochome-conjugated annexin V, which is considered a hallmark of apoptosis [29]. As shown in Figure 4A, the majority of annexin V+/CD8+ T cells expressed PD-1. To further verify our results, we assessed for the presence of cleaved poly(ADP ribose) polymerase (cPARP). Cleavage of PARP by caspase-dependent and independent mechanisms plays a critical role in mediating apoptosis [30, 31]. As expected the majority of cPARP+/CD8+ T cells were PD-1+ (Figure 4B). This suggests that active caspase 3 expression in PD-1hi/CD8+ T cells indeed correlates with their apoptotic potential. Because PD-1 is preferentially expressed on memory phenotype CD8+ T cells and polyfunctional CD8+ T cells primarily exhibited a memory phenotype, we hypothesized the PD-1–PD-L1 pathway causes preferential apoptosis of PD-1–expressing polyfunctional memory CD8+ T cells. To address this, we measured active caspase 3 expression on the relevant subsets in restimulated splenocytes. As expected, naive phenotype (CD8+/CD44lo) T cells exhibited minimal IFN-γ and granzyme B production and low levels of PD-1 and active caspase3 expression (Figure 4C). Next we analyzed polyfunctional populations in the effector CD8+ T-cell subset (CD8+/CD44int/hi/CD127lo) for apoptotic potential (Figure 4D). No dramatic difference in frequency of PD-1+/active caspase 3+–expressing cells was noted between CD8+/CD44int/hi/CD127lo/IFN-γ+/granzymeB−, CD8+/CD44int/hi/CD127lo/IFN-γ+/granzyme B+ and CD8+/CD44int/hi/CD127lo/IFN-γ−/granzyme B+ subsets. However PD-1− polyfunctional effector CD8+ T cells were moderately more apoptotic than the other subsets. Finally, we examined the memory subset by gating on CD8+/CD44int/hi/CD127hi cells (Figure 4E). In agreement with our previous observations with direct ex vivo staining, memory phenotype CD8+ T cells had a substantially higher frequency of PD-1–expressing CD8 T cells than naive phenotype or effector phenotype CD8+ T cells. In concurrence with our hypothesis, the highest levels of PD1+/active caspase+ memory CD8+ T cells was noted in the polyfunctional memory population. Combined, our data strongly suggest a strong correlation between PD-1 expression and apoptosis of polyfunctional memory CD8+ T cells.
Figure 4.
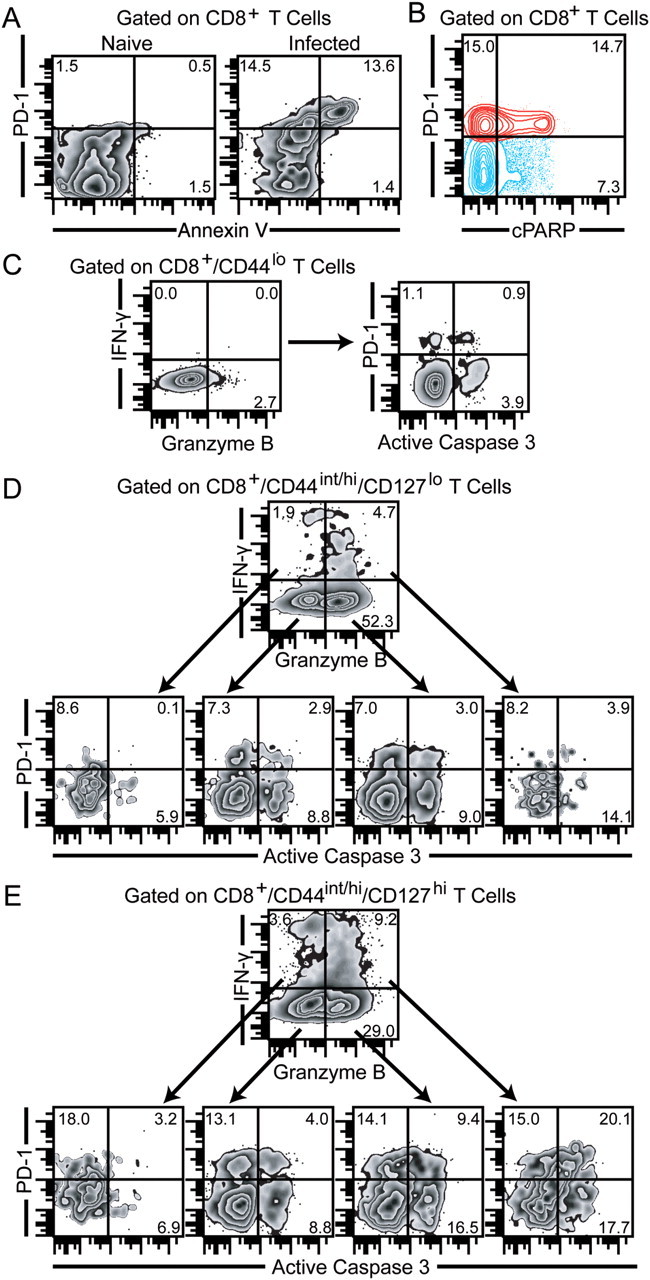
PD-1 expression correlates with apoptosis of polyfunctional memory phenotype CD8+ T cells. A, Splenic CD8+ T cells from chronically infected (week 7) mice were evaluated for PD-1 expression and annexin V reactivity. B, Cleavage of poly(ADP ribose) polymerase (cPARP) was measured in splenic CD8+ T cells from chronically infected (week 7) mice via intracellular staining. C, D, and E, PD-1 and active caspase 3 expression were assessed in interferon γ (IFN-γ)+/granzyme B−, IFN-γ−/granzyme B−, IFN-γ+/granzyme B+, and IFN-γ−/granzyme B+ naive/effector/memory phenotype CD8+ T cells by 7-color flow cytometry. The data represent 2 experiments with at least 4 mice per group.
PD-1–PD-L1 Blockade Reduces Apoptosis of Polyfunctional and IFN-γ+/CD8+ T Cells
To directly link the role of increased PD-1 expression on polyfunctional memory CD8+ T cells with their apoptosis, we performed in vitro treatment of splenocytes with isotype control or αPD-L1 antibody (MIH5, blocking but nondepleting) [32]. The rationale for performing in vitro studies is that apoptotic cells get rapidly eliminated in vivo, and thus an accurate assessment of apoptosis cannot be made. In vitro incubation of TLA-stimulated splenocytes for 16 hours with αPD-L1 did not alter CD8+ T-cell functionality or proliferation, irrespective of the subset examined (data not shown). It is possible that with longer multiday incubation we may observe increase in polyfunctionality in αPD-L1 treated cells, akin to the in vivo scenario [33]. However, with longer incubations, CD8+ T cells in αPD-L1 treated groups may undergo rapid expansion and, as a result, it will not be possible to discriminate the differential effects of apoptosis and expansion on the various CD8 subsets [33]. As expected, αPD-L1 treatment had no effect on apoptosis of naive phenotype CD8+ T cells (Figure 5A). Surprisingly, blockade of PD-1–PD-L1 pathway failed to reduce apoptosis of effector CD8+ T cells, irrespective of IFN-γ or granzyme B production (Figure 5A). Similar to its effect on naive and effector phenotype CD8+ T cells, αPD-L1 treatment did not affect the apoptosis of memory phenotype CD8+/CD44int/hi/CD127hi/IFN-γ−/granzyme B− cells or CD8+/CD44int/hi/CD127hi/IFN-γ−/granzyme B+ cells (Figure 5A). However, αPD-L1 treatment was equally efficacious in reducing the apoptosis of the both polyfunctional and CD8+/CD44int/hi/CD127hi/IFN-γ+/granzyme B− memory phenotype cells. Because annexin V staining is incompatible with intracellular staining, we further verified αPD-L1–mediated reduction in polyfunctional memory CD8+ T-cell apoptosis by assaying CD8+ T-cell subsets for cPARP. As shown in Figure 5B–D, αPD-L1 treatment reduced cleavage of PARP in polyfunctional and CD8+/CD44int/hi/CD127hi/IFN-γ+/granzyme B− memory phenotype cells but not in naive or effector phenotype CD8+ T cells. Considering that IFN-γ is considered to be critical in mediating protection against Toxoplasma as well other pathogens [20], the mechanistic insight offered by the current study can have important ramifications in incorporating αPD-L1 antibody in immunotherapeutic applications
Figure 5.
In vitro αPD-L1 treatment prevents apoptosis of polyfunctional memory phenotype CD8+ T cells. Splenocytes from chronically infected (week 7) mice were treated with αPD-L1 and stimulated overnight with Toxoplasma lysate antigen. A, Active caspase 3 expression in various CD8 subsets in isotype and αPD-L1–treated splenocytes. B, Cleavaged poly(ADP ribose) polymerase (cPARP) expression in isotype and αPD-L1–treated naive phenotype CD8+ T cells. C, cPARP expression in interferon γ (IFN-γ)+/granzyme B−, IFN-γ−/granzyme B−, IFN-γ+/granzyme B+, and IFN-γ−/granzyme B+ effector phenotype CD8+ T cells treated with αPD-L1 or control antibody. D, IFN-γ– or granzyme B–producing memory phenotype CD8+ T cells in isotype or αPD-L1–treated splenocytes were evaluated for cPARP expression by polychromatic flow cytometry. The data represent 2 experiments with at least 4 mice per group.
DISCUSSION
Recent studies, primarily in viral models, have established T-cell polyfunctionality as a critical predictor of efficacious antiviral immune response and vaccine potency [34]. However, the role of polyfunctional CD8+ T cells in parasitic or bacterial infection models has not been thoroughly investigated. Unlike chronic viral models, in mycobacterial infection, disease progression is not associated with contraction of polyfunctional T cells [35]. This makes a pathogen-specific study of polyfunctionality and host morbidity and mortality critical for determining whether gain or loss of polyfunctionality is linked to pathogen clearance. In contrast, in the Toxoplasma model, as we have previously demonstrated [13, 36] and further reiterated in the current study, loss of polyfunctional CD8+ T cells is strongly associated with PD-1–mediated CD8+ exhaustion and recrudescence of disease. Moreover αPD-L1 treatment not only upregulated polyfunctional CD8+ T-cell response but also prevented parasite reactivation. Combined, this creates a strong basis for investigating how PD-1 mediates attrition of polyfunctional CD8+ T cells during chronic toxoplasmosis.
In this study, we demonstrate for the first time that during chronic toxoplasmosis, the majority of the polyfunctional CD8+ T cells exhibit a memory phenotype. Although PD-1–expressing memory phenotype polyfunctional CD8+ T cells were preferentially apoptotic, paradoxically, the proportion of memory CD8+ T cells at the effector site (brain) was not dramatically altered. Incidentally, in chronic infection models, there is a continuous recruitment of naive T cells, which contributes to the pathogen-specific T-cell response [37]. Based on our observation in the T. gondii model that the frequency of memory CD8+ T cells is not dramatically affected but they are preferentially apoptotic, it is tempting speculate that naive T cells recruited during the chronic phase preferentially differentiate to memory precursor effector cells. This hypothesis is further supported by the observation that inflammatory cytokines are reduced during chronic toxoplasmosis. Incidentally, reduced inflammation as shown by the Kaech lab [24] results in preferential differentiation to memory precursor effector CD8+ T cells. Hence, in future studies it will be important to address this issue using adoptive T-cell transfer approaches and partial bone marrow chimeras.
However, the above hypothesis does not explain why the absolute numbers of both effector and memory CD8+ T cells are drastically reduced during chronic toxoplasmosis. One explanation could be reduced thymic output during chronic toxoplasmosis. Although thymic atrophy has been reported in the Toxoplasma model, its role on CD8+ T-cell response development and disease pathogenesis has not been extensively studied [38]. Apart from thymic atrophy, reduced effector or memory T-cell number could be a consequence of attenuated naive T-cell priming. As reported by our laboratory earlier, PD-L1, the receptor of PD-1, is also highly expressed during late chronic toxoplasmosis [13]. Incidentally, PD-L1 is expressed on both nonhematopoietic cells and hematopoietic cells, including antigen-presenting cells (APC) [39]. How PD-L1 expression on APC modulates their priming ability (costimulation, cytokines) has not been addressed in the Toxoplasma model. Apart from aforementioned effect on recent thymic emigrants, the possibility of antiapoptotic cytokines like interleukin 15 (primarily produced by APC) [40, 41] directly affecting baseline survival of preexisting memory and effector cells independent of CD8-intrinsic PD-1 expression needs to be considered in future studies
One of the hallmarks of robust CD8+ T-cell immunity is the capacity to mount a potent recall response [42]. As demonstrated by our laboratory previously, CD8+ T cells during the later stages of chronic infection are deficient in this regard [13]. Interestingly, CD43hi memory CD8+ T cells, which constitute the dominant memory phenotype of the CD8+ T-cell population during late chronic toxoplasmosis, have been shown to elicit poor recall response in the Sendai virus model [43]. Incidentally, CD43 has been shown to decrease expression of Bcl-2, an antiapoptotic molecule, on CD8+ T cells during LCMV infection [44]. During chronic toxoplasmosis, because the majority of PD-1–expressing memory phenotype CD8+ T cells coexpress high levels of CD43, the contribution of this molecule toward apoptosis of exhausted CD8+ T cells needs to be addressed in future investigations.
Because PD-1 was preferentially expressed on memory phenotype CD8+ T cells during chronic toxoplasmosis, we next investigated if PD-1–expressing cells in the memory subset were polyfunctional or monofunctional. Although PD-1 expression on memory CD8+ T cells did not have a 1 to 1 correlation with levels of IFN-γ and granzyme B expression, PD-1–expressing memory CD8+ T cells were indeed more apoptotic. Although high levels of active caspase 3 were also noted for polyfunctional effector CD8+ T cells, this subset exhibited minimal PD-1 expression. This suggests that apart from PD-1, other PD-1–independent, apoptosis-inducing mechanisms are at play. Considering that exhaustion in viral models has been associated with upregulation of multiple inhibitory receptors, the role of molecules such as Tim-3, 2B4, CTLA-4, Lag3, CD160, and others need to be investigated for their role in inducing apoptosis of polyfunctional CD8+ T cells [45]. Although CD8+/CD44int/hi/CD127hi/IFN-γ+/granzyme B− memory phenotype cells exhibited comparatively lower proportions of PD-1+/active caspase 3+ cells, in vitro αPD-L1 treatment resulted in decrease of apoptosis in this subset, similar to polyfunctional memory CD8+ T cells. Paradoxically, despite considerable expression of both PD-1 and active caspase 3, the CD8+/CD44int/hi/CD127hi/IFN-γ−/granzyme B+ subset remained refractory to the antiapoptotic effects of in vitro αPD-L1 treatment. As mentioned earlier, this may be due to coexpression of other inhibitory receptors, which may dampen the ameliorative effects of αPD-L1. Alternatively, this subset may require prolonged αPD-L1 treatment for reducing its apoptotic potential. These possibilities may not be mutually exclusive.
Reinvigoration of CD8+ T-cell response via αPD-L1 administration has profound significance for treatment of tumors and chronic infections such as hepatitis B, hepatitis C, and HIV [46, 47]. Development of a robust polyfunctional T-cell response is considered 1 of the hallmarks of potent antipathogen immunity. However, few reports have addressed the mechanistic basis for the development of polyfunctional response. A recent study demonstrated that strong antigen stimulation results in development of polyfunctional T-cell response [48]. However, in chronic infections where not only high antigen burden is present but also elevated levels of CD8+ T cell–expressed PD-1, polyfunctionality is lost. How the PD-1–PD-L1 pathway causes attrition of this polyfunctional response in chronic infections has not been investigated before. The current study strongly suggests that, not withstanding the per cell decrease in function, PD-1 expression on polyfunctional memory CD8+ T cells strongly predisposes them to apoptosis. Significantly, transient in vitro blockade of the PD-1–PD-L1 pathway is potent enough to reduce apoptosis of this important subset. Despite the reduction in apoptosis in αPD-L1–treated splenocytes, no dramatic change in Bcl-2 levels was noted in CD8+ T cells (data not shown). Although this suggests that blockade of the PD-1–PD-L1 pathway does not affect Bcl-2 expression, the role of specific antiapoptotic molecules, such as Bcl-xl, and pro-apoptotic molecules, such as Bak, Bax, Bid, Bim, and Bad, need to be considered in future studies [49]. In summary, the conclusions of this present report have profound implications for immunotherapeutic regimens targeting polyfunctional CD8 T-cell response against chronic infections.
Supplementary Data
Supplementary materials are available at the Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank M. Azuma for providing MIH5 hybridoma.
Financial support. This work was supported by the National Institutes of Health (grant AI-33325 to I. A. K.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Denkers EY, Sher A, Gazzinelli RT. CD8+ T-cell interactions with Toxoplasma gondii: implications for processing of antigen for class-I-restricted recognition. Res Immunol. 1993;144:51–7. doi: 10.1016/s0923-2494(05)80099-9. [DOI] [PubMed] [Google Scholar]
- 2.Gigley JP, Bhadra R, Khan IA. CD8 T cells and Toxoplasma gondii: a new paradigm. J Parasitol Res. 2011;2011:243796. doi: 10.1155/2011/243796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denkers EY, Yap G, Scharton-Kersten T, et al. Perforin-mediated cytolysis plays a limited role in host resistance to Toxoplasma gondii. J Immunol. 1997;159:1903–8. [PubMed] [Google Scholar]
- 4.Gazzinelli R, Xu Y, Hieny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992;149:175–80. [PubMed] [Google Scholar]
- 5.Deckert-Schluter M, Schluter D, Schmidt D, Schwendemann G, Wiestler OD, Hof H. Toxoplasma encephalitis in congenic B10 and BALB mice: impact of genetic factors on the immune response. Infect Immun. 1994;62:221–8. doi: 10.1128/iai.62.1.221-228.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan IA, Ely KH, Kasper LH. Antigen-specific CD8+ T cell clone protects against acute Toxoplasma gondii infection in mice. J Immunol. 1994;152:1856–60. [PubMed] [Google Scholar]
- 7.Suzuki Y. Host resistance in the brain against Toxoplasma gondii. J Infect Dis. 2002;185(Suppl 1):S58–65. doi: 10.1086/337999. [DOI] [PubMed] [Google Scholar]
- 8.Gazzinelli RT, Amichay D, Sharton-Kersten T, Grunwald E, Farber JM, Sher A. Role of macrophage-derived cytokines in the induction and regulation of cell-mediated immunity to Toxoplasma gondii. Curr Top Microbiol Immunol. 1996;219:127–39. doi: 10.1007/978-3-642-51014-4_12. [DOI] [PubMed] [Google Scholar]
- 9.Gazzinelli RT, Eltoum I, Wynn TA, Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-alpha and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol. 1993;151:3672–81. [PubMed] [Google Scholar]
- 10.Bhadra R, Gigley JP, Khan IA. The CD8 T-cell road to immunotherapy of toxoplasmosis. Immunotherapy. 2011;3:789–801. doi: 10.2217/imt.11.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki Y, Wang X, Jortner BS, et al. Removal of Toxoplasma gondii cysts from the brain by perforin-mediated activity of CD8+ T cells. Am J Pathol. 2010;176:1607–13. doi: 10.2353/ajpath.2010.090825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scharton-Kersten TM, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1261–73. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhadra R, Gigley JP, Weiss LM, Khan IA. Control of Toxoplasma reactivation by rescue of dysfunctional CD8+ T-cell response via PD-1–PDL-1 blockade. Proc Natl Acad Sci USA. 2011;108:9196–201. doi: 10.1073/pnas.1015298108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Betts MR, Nason MC, West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–9. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nissapatorn V. Toxoplasmosis in HIV/AIDS: a living legacy. Southeast Asian J Trop Med Public Health. 2009;40:1158–78. [PubMed] [Google Scholar]
- 16.Kur J, Holec-Gasior L, Hiszczynska-Sawicka E. Current status of toxoplasmosis vaccine development. Expert Rev Vaccines. 2009;8:791–808. doi: 10.1586/erv.09.27. [DOI] [PubMed] [Google Scholar]
- 17.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–76. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 18.Khan IA, Kasper LH. IL-15 augments CD8+ T cell-mediated immunity against Toxoplasma gondii infection in mice. J Immunol. 1996;157:2103–8. [PubMed] [Google Scholar]
- 19.Lyons RE, McLeod R, Roberts CW. Toxoplasma gondii tachyzoite-bradyzoite interconversion. Trends Parasitol. 2002;18:198–201. doi: 10.1016/s1471-4922(02)02248-1. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–8. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 21.Meythaler M, Wang Z, Martinot A, et al. Early induction of polyfunctional simian immunodeficiency virus (SIV)-specific T lymphocytes and rapid disappearance of SIV from lymph nodes of sooty mangabeys during primary infection. J Immunol. 2011;186:5151–61. doi: 10.4049/jimmunol.1004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makedonas G, Betts MR. Polyfunctional analysis of human T cell responses: importance in vaccine immunogenicity and natural infection. Springer Semin Immunopathol. 2006;28:209–19. doi: 10.1007/s00281-006-0025-4. [DOI] [PubMed] [Google Scholar]
- 23.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–8. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 24.Joshi NS, Cui W, Chandele A, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–95. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galea I, Bernardes-Silva M, Forse PA, van Rooijen N, Liblau RS, Perry VH. An antigen-specific pathway for CD8 T cells across the blood-brain barrier. J Exp Med. 2007;204:2023–30. doi: 10.1084/jem.20070064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrington LE, Galvan M, Baum LG, Altman JD, Ahmed R. Differentiating between memory and effector CD8 T cells by altered expression of cell surface O-glycans. J Exp Med. 2000;191:1241–6. doi: 10.1084/jem.191.7.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J Immunol. 2000;164:3950–4. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- 28.Puga I, Rao A, Macian F. Targeted cleavage of signaling proteins by caspase 3 inhibits T cell receptor signaling in anergic T cells. Immunity. 2008;29:193–204. doi: 10.1016/j.immuni.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kietselaer BL, Hofstra L, Dumont EA, Reutelingsperger CP, Heidendal GA. The role of labeled annexin A5 in imaging of programmed cell death. From animal to clinical imaging. Q J Nucl Med. 2003;47:349–61. [PubMed] [Google Scholar]
- 30.Assefa Z, Vantieghem A, Garmyn M, et al. p38 Mitogen-activated protein kinase regulates a novel, caspase-independent pathway for the mitochondrial cytochrome c release in ultraviolet B radiation-induced apoptosis. J Biol Chem. 2000;275:21416–21. doi: 10.1074/jbc.M002634200. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y, Zhao S, Song J. Caspase-dependent apoptosis and -independent poly(ADP-ribose) polymerase cleavage induced by transforming growth factor beta1. Int J Biochem Cell Biol. 2004;36:223–34. doi: 10.1016/s1357-2725(03)00215-2. [DOI] [PubMed] [Google Scholar]
- 32.Dias P, Giannoni F, Lee LN, et al. CD4 T-cell help programs a change in CD8 T-cell function enabling effective long-term control of murine gammaherpesvirus 68: role of PD-1–PD-L1 interactions. J Virol. 2010;84:8241–9. doi: 10.1128/JVI.00784-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrovas C, Casazza JP, Brenchley JM, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–92. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almeida JR, Price DA, Papagno L, et al. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med. 2007;204:2473–85. doi: 10.1084/jem.20070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young JM, Adetifa IM, Ota MO, Sutherland JS. Expanded polyfunctional T cell response to mycobacterial antigens in TB disease and contraction post-treatment. PLoS One. 2010;5:e11237. doi: 10.1371/journal.pone.0011237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhadra R, Gigley JP, Khan IA. Cutting edge: CD40-CD40 ligand pathway plays a critical CD8-intrinsic and -extrinsic role during rescue of exhausted CD8 T cells. J Immunol. 2011;187:4421–5. doi: 10.4049/jimmunol.1102319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vezys V, Masopust D, Kemball CC, et al. Continuous recruitment of naive T cells contributes to heterogeneity of antiviral CD8 T cells during persistent infection. J Exp Med. 2006;203:2263–9. doi: 10.1084/jem.20060995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLeod R, Estes RG, Mack DG, Cohen H. Immune response of mice to ingested Toxoplasma gondii: a model of toxoplasma infection acquired by ingestion. J Infect Dis. 1984;149:234–44. doi: 10.1093/infdis/149.2.234. [DOI] [PubMed] [Google Scholar]
- 39.Mueller SN, Vanguri VK, Ha SJ, et al. PD-L1 has distinct functions in hematopoietic and nonhematopoietic cells in regulating T cell responses during chronic infection in mice. J Clin Invest. 2010;120:2508–15. doi: 10.1172/JCI40040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohteki T, Tada H, Ishida K, et al. Essential roles of DC-derived IL-15 as a mediator of inflammatory responses in vivo. J Exp Med. 2006;203:2329–38. doi: 10.1084/jem.20061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhadra R, Guan H, Khan IA. Absence of both IL-7 and IL-15 severely impairs the development of CD8 T cell response against Toxoplasma gondii. PLoS One. 2010;5:e10842. doi: 10.1371/journal.pone.0010842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zammit DJ, Cauley LS, Pham QM, Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22:561–70. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med. 2007;204:1625–36. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Onami TM, Harrington LE, Williams MA, et al. Dynamic regulation of T cell immunity by CD43. J Immunol. 2002;168:6022–31. doi: 10.4049/jimmunol.168.12.6022. [DOI] [PubMed] [Google Scholar]
- 45.Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1–PD-1 ligand blockade. J Exp Med. 2006;203:2223–7. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lukens JR, Cruise MW, Lassen MG, Hahn YS. Blockade of PD-1/B7-H1 interaction restores effector CD8+ T cell responses in a hepatitis C virus core murine model. J Immunol. 2008;180:4875–84. doi: 10.4049/jimmunol.180.7.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beuneu H, Lemaitre F, Deguine J, et al. Visualizing the functional diversification of CD8+ T cell responses in lymph nodes. Immunity. 2010;33:412–23. doi: 10.1016/j.immuni.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 49.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]