Abstract
Background. Human monocytic ehrlichiosis is one of the most prevalent tick-borne zoonoses caused by infection with Ehrlichia chaffeensis. Although E. chaffeensis lacks entire lipopolysaccharide and most peptidoglycan biosynthesis genes, it induces inflammatory cytokines and chemokines. Ehrlichia chaffeensis components that induce inflammation and the responsive host cell pathway are not known.
Methods. Expression of penicillin-binding protein (PBP) in E. chaffeensis was analyzed by reverse-transcription polymerase chain reaction and Bocillin FL binding assay. Next, recombinant PBP, which was high-pressure liquid chromatography purified, and native PBP of E. chaffeensis were investigated for their ability to induce proinflammatory cytokines in the human monocytic leukemia cell line THP-1 and bone marrow–derived macrophages (BMDMs) from wild-type and MyD88 knockout mice.
Results. Expression of PBP by E. chaffeensis was upregulated during its intracellular life cycle. PBP induced interleukin 8 or CXCL2, tumor necrosis factor α, interleukin 1β, and interleukin 10 in THP-1 cells and BMDMs. Cytokine induction by PBP was MyD88-dependent. Removal of PBP from E. chaffeensis lysate using penicillin affinity column and a complementation assay confirmed cytokine-inducing activity of native PBP.
Conclusions. The cytokine-inducing activity by E. chaffeensis PBP provides novel insights into pathogen-associated molecular patterns and pathogenesis of E. chaffeensis infection.
Human monocytic ehrlichiosis (HME), one of the most prevalent and potentially fatal tick-borne zoonoses, is caused by infection with Ehrlichia chaffeensis, a monocytotropic ehrlichia [1]. HME was identified in 1986 [2] and designated as a nationally notifiable disease in 1998 [3]. This disease is characterized by fever, headache, myalgia, anorexia, and chills, and is frequently accompanied by leukopenia, thrombocytopenia, anemia, and elevations in serum hepatic aminotransferase [4]. The only proven treatment is the broad-spectrum antibiotic doxycycline, and the only preventive measure available is tick repellent. Although E. chaffeensis lacks endotoxin or exotoxins, it induces proinflammatory cytokines and chemokines in vitro and in vivo [5, 6], which likely modulate HME disease processes and immunity [7].
Recognition of pathogen-associated molecular patterns (PAMPs) by the pattern recognition receptors of eukaryotic cells is a major pathways for cytokine induction [7]. Ehrlichia chaffeensis is an obligate intracellular gram-negative bacterial pathogen that belongs to the order Rickettsiales in the class Alphaproteobacteria. Ehrlichia chaffeensis, however, lacks 3 major PAMPs—lipopolysaccharide (LPS), peptidoglycan, and flagella [8, 9]. This suggests that cytokine induction by E. chaffeensis is dependent on other PAMPs or mechanisms. In fact, E. chaffeensis can induce inflammatory responses through MyD88-dependent NF-κB and ERK pathways, without the involvement of TRIF or Toll-like receptors (TLRs) [10]. Despite the loss of most genes for peptidoglycan biosynthesis [9], our multiple sequence alignment analysis by ClustalW version 2.1 software revealed that Ech_1067 encoding a low-molecular-weight penicillin-binding protein (PBP) homolog (34% amino acid identity with Escherichia coli PBP6 precursor P08506, dacC) is one of the rare genes of peptidoglycan synthesis conserved in E. chaffeensis and other sequenced members of the family Anaplasmataceae (except the genus Neorickettsia). Our aim was to determine whether E. chaffeensis Ech_1067 binds penicillin, is expressed during E. chaffeensis intracellular growth in human monocytes, and has any role in cytokine/chemokine induction.
METHODS
Ehrlichia chaffeensis
Ehrlichia chaffeensis was cultured and bacteria were isolated from THP-1 or DH82 cells as previously described [5, 6]. In brief, the 107 heavily infected cells were sonicated by a W-380 ultrasonic processor (Heat Systems) at level 2, input 50%, 30 cycles, and centrifuged at 1000g for 5 minutes. The supernatant was then filtered through a 2.7-µm filter (Whatman) to remove debris and centrifuged at 10 000g for 10 minutes, and the pellet containing freshly isolated host cell–free bacteria was used for synchronous infection as described [11]. Samples were collected at 0, 24, 48, and 72 hours after infection by centrifugation and total RNA was isolated from each sample. For cytokine assay, E. chaffeensis was isolated as above and kept at −80°C until use. The uninfected host cell lysate prepared in the same manner was used as control.
Recombinant Proteins
The DNA fragments encoding the full-length Ech_1067 without signal peptide (1–30 aa) and the full-length diaminopimelate aminotransferase (DPA, Ech_0886) were amplified by polymerase chain reaction (PCR) using E. chaffeensis chromosomal DNA as a template. The amplified fragments were digested with restriction enzymes and ligated into restriction enzyme–digested pET-33b(+) (Novagen). Escherichia coli Novablue (Novagen) was transformed and the plasmids were extracted. The cloned fragments were confirmed by DNA sequencing. Escherichia coli BL21 (DE3) (Novagen) cells were transformed with the plasmid and induced to express the recombinant proteins with isopropyl-thio-ß-d-galactoside (Gold Biotechnology). Recombinant proteins (rEch_1067 and rDPA) were purified from the soluble fraction with Ni-agarose (Sigma–Aldrich).
High-Pressure Liquid Chromatography
To further purify recombinant protein, high-pressure liquid chromatography (HPLC) was performed as previously described [12]. In brief, Ni-affinity-purified rEch_1067 was loaded onto a TSK G3000 SWXL gel filtration column (Tosho) placed in an ÄKTA purifier (GE Healthcare) to separate any E. coli protein contamination from the target recombinant protein. Proteins were eluted at a flow rate of 1 mL/min. The fractions were concentrated by evaporation and tested by GelCode Blue (Pierce) staining. Protein amounts were determined by bicinchoninic acid protein assay (Pierce).
Endotoxin Removal and Assay
To remove any endotoxin contamination from the protein solution, endotoxin removal kit (EndoClean, BioVintage) was used. Removal of endotoxin from the protein solution was confirmed by Toxinsensor gel clot endotoxin (Limulus amebocyte lysate [LAL]) assay kit (GenScript). In addition, polymyxin B sulfate at 20 µg/mL was used with the recombinant protein preparation.
Penicillin Affinity Separation
Penicillin affinity sepharose was prepared by coupling 6-aminopenicillanic acid (6-APA; Sigma–Aldrich) with HiTrap NHS-activated High Performance Sepharose (GE Healthcare). To remove PBPs from E. chaffeensis lysate, the bacterial lysate was centrifuged at 14 000g at 4°C for 5 minutes to remove debris and the supernatant was incubated with the penicillin affinity sepharose in the column at 37°C for 30 minutes, and the eluent was collected. Removal of PBPs from the eluent was confirmed by Bocillin FL (Invitrogen) detection.
Bocillin FL Detection of PBPs
Purified rEch_1067 and E. chaffeensis [11] were incubated with 50 µM Bocillin FL at 37°C for 30 minutes, and subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) as described [13]. The labeled protein was visualized with a Typhoon 9410 Imager (Amersham Biosciences). For fluorescence imaging, bacteria were cytocentrifuged onto glass slides. Bacteria were fixed with methanol for 5 minutes at −20°C and then incubated with dog serum against E. chaffeensis for 1 hour at 37°C. After being washed twice with phosphate-buffered saline (PBS; 137 mM NaCl, 4.7 mM KCl, 9.32 mM Na2HPO4, and 0.68 mM NaH2PO4, pH 7.4), the cells were incubated with the secondary antibody (Texas Red–conjugated goat antidog immunoglobulin G; Rockland) and 50 µM Bocillin FL at 37°C for 1 hour. Both antisera used were preabsorbed with uninfected THP-1 cells and at a 1:200 dilution in PBS.
Mouse Bone Marrow–Derived Macrophages
Six- to 12-week-old male MyD88−/− mice, originally developed by Adachi et al [14], and age- and sex-matched wild-type C57BL/6 mice were used in this study. All animal experiments were performed under the animal protocol approved by The Ohio State University Institutional Animal Care and Use Committee. Mouse bone marrow–derived macrophages (BMDMs) were prepared as previously described [10].
Stimulation of THP-1 cells and BMDMs
Stimulation of THP-1 cells was performed as previously described [5]. In brief, THP-1 cells at 3 × 105 cells per well or mouse BMDMs at 5 × 105 cells per well were plated in 24- or 48-well plates, respectively, and host cell–free E. chaffeensis [6], bacterial lysate (before and after affinity depletion of PBPs) or corresponding uninfected DH82 cell extract, or rEch_1067 or rDPA were added. After incubation at 37°C for 2 hours, the cells were harvested and the total RNA was extracted with RNeasy Mini Kit (Qiagen) for reverse-transcription (RT) PCR analysis.
Quantitative RT-PCR
Ehrlichia chaffeensis Ech_1067 was determined by quantitative RT-PCR. Complementary DNA (cDNA) was prepared from total RNA (1 μg) using Maxima reverse transcriptase (Fermentas) and random hexamers. The level of Ech_1067 at each time point was normalized against bacterial 16S ribosomal DNA (rDNA) as described [11]. The expression of human or mouse interleukin 8 (IL-8), tumor necrosis factor (TNF)-α, interleukin 10 (IL-10), interleukin 1β (IL-1β), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were quantified by RT-PCR using gene specific primers and normalized against that of human or mouse GAPDH, respectively [6, 10].
Statistical Analysis
Student t test and analysis of variance were applied to determine the differences among cDNA and DNA levels. A P value of <.05 was considered significant.
RESULTS
Expression of Ech_1067 by E. chaffeensis
We examined Ech_1067 expression during E. chaffeensis intracellular growth stages in THP-1 cells. Based on quantitative PCR of E. chaffeensis 16S rDNA gene, after 24 hours of the lag phase, E. chaffeensis began to replicate, with exponential growth occurring between 48 and 72 hours after infection (Figure 1A). Relative Ech_1067 messenger RNA (mRNA) expression normalized against 16S rDNA was upregulated at the start of growth stage and somewhat downregulated at mid to late exponential growth stage (Figure 1A).
Figure 1.
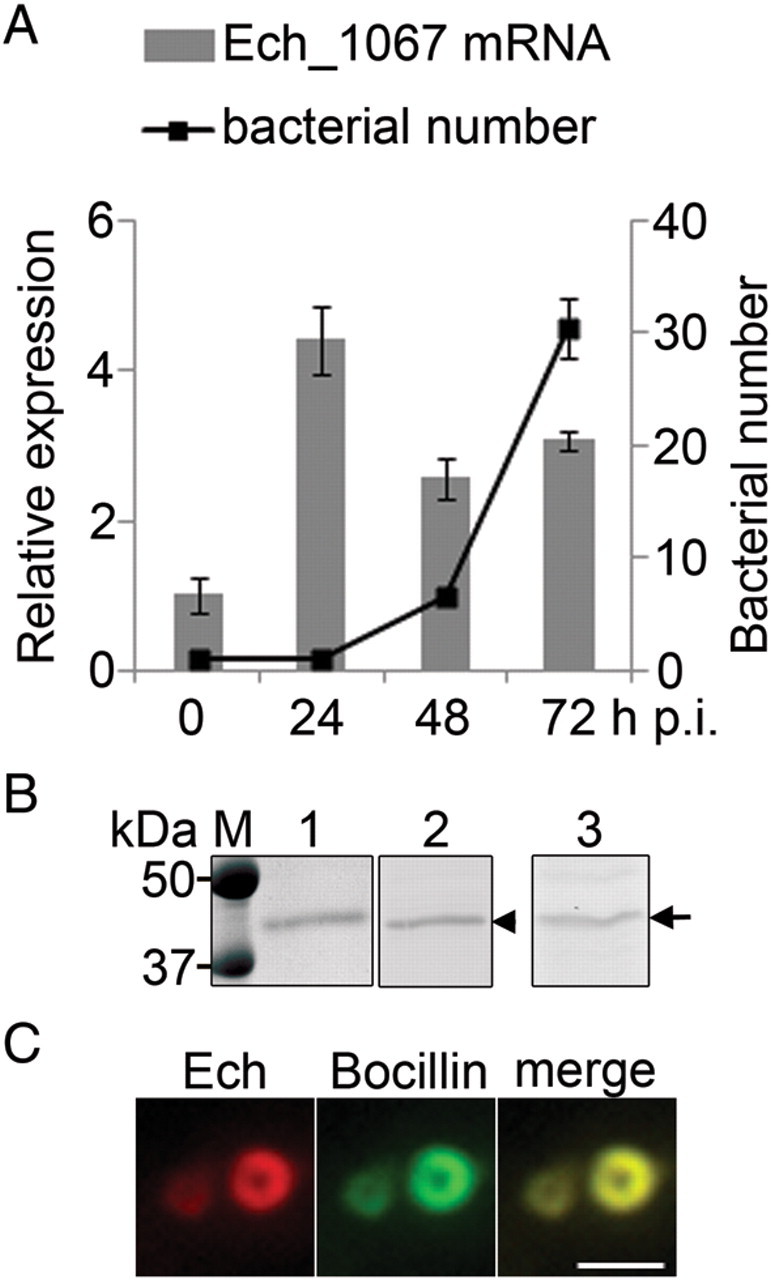
Ech_1067 temporal expression and Bocillin binding. A, DNA and mRNA prepared from synchronously infected THP-1 cells at different time points were subjected to quantitative 16S rDNA polymerase chain reaction (PCR) and Ech_1067 reverse-transcription PCR. Ech_1067 mRNA levels were normalized against 16S rDNA levels corresponding to bacterial number. The relative values to the amount of 0 h after infection are shown. Data are expressed as mean ± SD (n = 3) and are representative of 2 independent experiments with similar results. B, Purified rEch_1067 (arrowhead) and native Ech_1067 (arrow) bind Bocillin FL. Purified rEch_1067 (0.5 μg) was subjected to 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis followed by GelCode Blue staining (lane 1) and Bocillin FL staining (lane 2). The lysate prepared from infected THP-1 cells (4 × 105) was subjected to 12% SDS-PAGE analysis followed by Bocillin FL staining (lane 3). C, Immunofluorescence images of Bocillin binding to individual bacteria. Host cell–free bacteria were labeled with dog anti–Ehrlichia chaffeensis serum (Ech, red) and Bocillin (green). The right panel is a merged image viewed with green and red filters. Scale bar = 1 µm.
Ech_1067 is a PBP
Ehrlichia chaffeensis Ech_1067 penicillin-binding activity was determined by a fluorescent penicillin analog, Bocillin FL, that specifically binds to E. coli PBPs [13], and also was used to detect PBPs in other bacteria [13]. After incubation with Bocillin, both purified rEch_1067 and the native E. chaffeensis protein (predicted mature protein molecular mass, 37 kDa) showed strong penicillin-binding activity (Figure 1B). Ni-affinity purified recombinant E. chaffeensis SurE (Ech_0791) or uninfected THP-1 cell lysate was also incubated with Bocillin as a negative control, and no band was detected (data not shown). Therefore, we call Ech_1067 a PBP. To determine the native PBP localization, purified E. chaffeensis was incubated with Bocillin. PBP was predicted to be a cytoplasmic membrane protein by PSORTb version 3.0.2 (http://psort.org/psortb/index.html) analysis. By immunofluorescence labeling, dog immune serum against E. chaffeensis, which preferentially reacts with the surface of individual bacteria, revealed a ringlike labeling pattern. Bocillin bound to the native PBP also showed a ringlike labeling pattern, in agreement with its prediction on the bacterial membrane (Figure 1C).
Cytokine Induction by PBP of E. chaffeensis
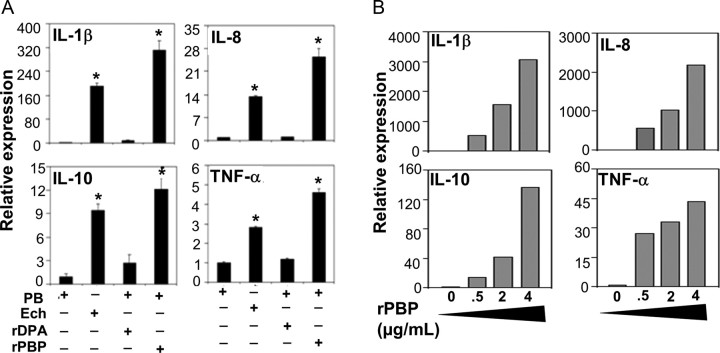
Recombinant PBP (rPBP) of E. chaffeensis that represents >99% purity after Ni-affinity chromatography followed by HPLC (Supplementary Figure 1) as estimated from the peak area, and SDS-PAGE and GelCode Blue staining, were used to determine its ability to induce cytokines in THP-1 cells. Any endotoxin contamination was removed from the HPLC-purified specimen using the endotoxin removal kit, as LAL test for rPBP revealed that endotoxin activity was below the 0.25 endotoxin units/mL detection limit. For further precaution, we also used polymyxin B sulfate, which binds and blocks LPS action [15]. The mRNA expression of the cytokines IL-8, TNF-α, IL-10, and IL-1β was highly induced in THP-1 cells by rPBP, but not by rDPA, another recombinant E. chaffeensis protein related to peptidoglycan biosynthesis, used as control (Figure 2A). The levels of expression of cytokine mRNAs in THP-1 cells in response to rPBP were dose-dependent above 0.5 µg/mL (Figure 2B).
Figure 2.
Cytokine induction by penicillin-binding protein (PBP) of Ehrlichia chaffeensis. A, Cytokine mRNA levels determined by quantitative real-time reverse-transcription polymerase chain reaction. THP-1 cells were incubated at 37°C for 2 h with E. chaffeensis (Ech, 25 µg/mL), polymyxin B sulfate (PB, 20 µg/mL) for lipopolysaccharide inhibition, or the combination of PB and recombinant E. chaffeensis diaminopimelate aminotransferase (rDPA, 0.5 µg/mL) or E. chaffeensis recombinant PBP (rPBP, 0.5 µg/mL). All data are normalized against human GAPDH mRNA and cytokine transcript amounts are shown relative to THP-1 cells treated with PB alone. Values are means ± SD of 3 independent samples. *P < .05 by Student t test compared with PB-alone control. B, Dose-dependent effect of rPBP (0.5–4 µg/mL). Abbreviations: IL, interleukin; TNF, tumor necrosis factor.
Cytokine Induction by PBP is MyD88-Dependent
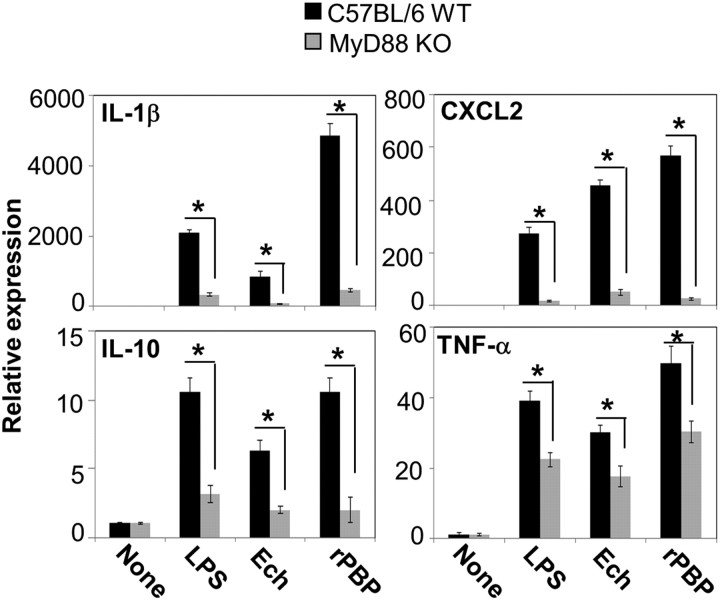
It has been recently reported that cytokine induction by E. chaffeensis in mouse BMDMs is MyD88-dependent [10]. To determine whether cytokine induction by E. chaffeensis PBP is also MyD88 dependent, we used MyD88−/− BMDMs for cytokine induction assay. CXCL2 and IL-1β induction by E. chaffeensis lysate, rPBP, and E. coli LPS was almost completely abolished in MyD88−/− BMDMs, whereas IL-10 and TNF-α expression was mostly and only partially abrogated (Figure 3).
Figure 3.
MyD88-dependent cytokine/chemokine induction by recombinant penicillin-binding protein (rPBP). Relative cytokine/chemokine mRNA levels from bone marrow–derived macrophages of wild-type (WT) or MyD88 knockout (MyD88 KO) mice incubated with Escherichia coli lipopolysaccharide (LPS) (10 ng/mL), Ehrlichia chaffeensis (Ech, 25 μg/mL), or rPBP (0.5 μg/mL) at 37°C for 2 h. Values are normalized against mouse GAPDH mRNA levels. Values shown are means ± SD of 3 independent samples. *P < .05, by analysis of variance. Abbreviations: IL, interleukin; TNF, tumor necrosis factor.
Cytokine-Inducing Activity of PBP-Depleted E. chaffeensis Was Complemented With rPBP
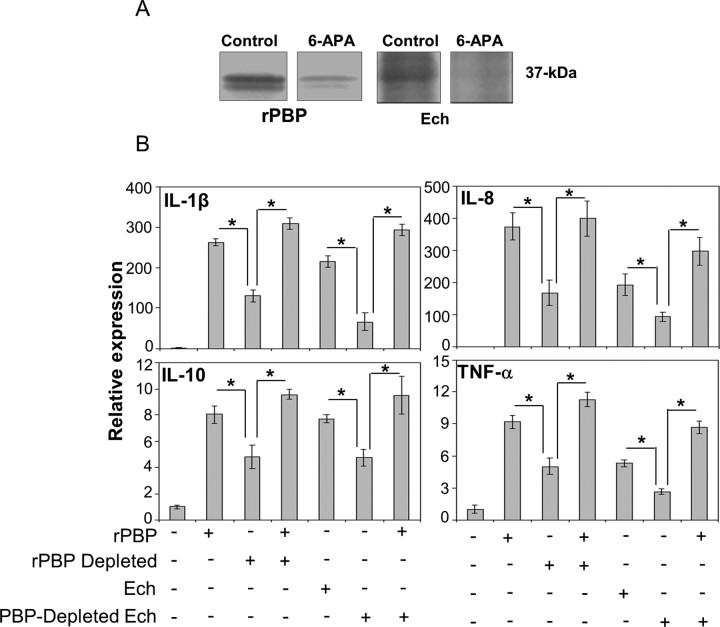
To determine whether PBP from E. chaffeensis is a significant factor in cytokine induction by whole E. chaffeensis, depletion and complementation assay of this protein was performed in THP-1 cells. PBP was removed from the rPBP and E. chaffeensis lysate using 6-APA–sepharose by covalent bond formation between PBP and 6-APA [16]. This procedure reduced approximately 70% of rPBP and 90% of native PBP as shown by Bocillin labeling (Figure 4A). Specific depletion of PBP from the E. chaffeensis lysate significantly reduced the bacterial cytokine-inducing activity compared with sham-treated control lysate, suggesting that native PBP is a significant factor for cytokine induction (Figure 4B). This reduced activity was restored when complemented with rPBP (Figure 4B), indicating that PBP is significantly responsible for E. chaffeensis cytokine and chemokine induction in THP-1 cells.
Figure 4.
Depletion and complementation of penicillin-binding protein (PBP) in Ehrlichia chaffeensis. A, Depletion of PBP from recombinant PBP (rPBP) (control, 0.5 µg/mL) and PBP from E. chaffeensis (Ech control, 25 µg/mL) by 6-aminopenicillanic acid (6-APA) (10 mg/mL) affinity chromatography as confirmed by Bocillin FL labeling. B, Cytokine induction in THP-1 cells by rPBP (0.5 µg/mL), PBP-partially depleted rPBP, PBP-partially depleted rPBP reconstituted with rPBP, E. chaffeensis (Ech, 25 µg/mL), PBP-depleted E. chaffeensis, and PBP-depleted E. chaffeensis reconstituted with rPBP. All data are normalized against human GAPDH mRNA. Values are means ± SD of 3 independent samples. *P < .05, by analysis of variance. Abbreviations: IL, interleukin; TNF, tumor necrosis factor.
DISCUSSION
Our results show that E. chaffeensis Ech_1067, which has C-terminal 110 aa, 26% identical to E. coli PBP5 and PBP6, indeed had penicillin-binding activity. Among 12 known E. coli PBPs, 5 are high-molecular-weight PBPs involved in new peptidoglycan polymerization and incorporation into the existing sacculus, and 7 are low-molecular-weight PBPs of less-known function [17]. Of low-molecular-weight PBPs, PBP5 has d-ala-d-ala-carboxypeptidase enzyme activity involved in peptidoglycan synthesis, but the function of PBP6 remains unknown [18]. PBP5 is upregulated in exponential growth stage and plays a major role in determining cell diameter, contour and morphology of E. coli, and expression of ampicillin resistance and peptidoglycan structure [19, 20]. PBP6 is upregulated at stationary phase and has a biological role in the stabilization of the peptidoglycan during stationary phase E. coli [18, 21]. Although E. chaffeensis PBP was highly expressed and localized to the bacterial membrane, as the majority of other genes involved in peptidoglycan synthesis are missing, the function of E. chaffeensis PBP is unlikely in peptidoglycan biosynthesis. Upregulation of this gene before the onset of exponential growth stage and throughout the growth suggests an important role of PBP for establishment of bacterial infection and growth.
The present study suggests that E. chaffeensis PBP is a PAMP released from live and/or lysed bacteria, inducing cytokines by THP-1 and mouse BMDM cells, and this effect is mediated through MyD88. To the best of our knowledge, PBP has not been reported to induce cytokines in any other bacteria, although immunogenicity of meningococcal PBP2 and protective activity of anti-PBP2 antibodies against meningococcal bacteremia in mice have been reported [22]. Therefore, whether this is a unique property of E. chaffeensis PBP or the first example of PBP as PAMP remains to be investigated. In addition, which domain or part of PBP has cytokine-inducing activity remains to be defined. Although affinity removal of PBP reduced cytokine-inducing activity of E. chaffeensis, the addition of ampicillin to rPBP did not reduce its cytokine-inducing activity (data not shown), suggesting that C-terminal penicillin-binding domain is not critical for cytokine induction. This also suggests penicillin treatment would not ameliorate cytokine-induced HME clinical signs. This is in agreement with the ineffectiveness of β-lactams for HME treatment [4].
The adaptor molecule MyD88 plays an important role along with TLR signaling. Although the E. chaffeensis–related bacterium Anaplasma phagocytophilum activates NF-κB through TLR2, but not TLR4 [23], a recent study from our lab [10] showed that E. chaffeensis induces cytokines and chemokines independent of TLR2/4, endosome acidification, and interleukin 1R1/18R1. TLR-independent cytokine induction was also reported with bacteria such as Burkholderia pseudomallei [24] and Vibrio parahaemolyticus [25]. But for E. chaffeensis, an adaptor molecule, MyD88, is involved in the signaling pathway [10]. Koh et al [26] reported that Ehrlichia muris induces interleukin 12, TNF-α, and interleukin 6 by mouse dendritic cells in a MyD88-dependent manner. None of the aforementioned studies identified bacterial components responsible for cytokine induction. While immune responses to E. chaffeensis lipoproteins [27], and other molecules such as glycolipid [28] and ehrlichial ankyrin repeat-containing protein p200, might contribute to cytokine and chemokine induction by Ehrlichia [29], our results showed that PBP is a significant E. chaffeensis factor activating MyD88-dependent inflammatory signaling pathway. The fact that both mouse BMDM and human leukemia cell line THP-1 respond to PBP indicate that human and mouse monocytes share a common PBP receptor and signaling pathways. Taken together, we can extrapolate from our results that E. chaffeensis PBP has an important role in HME pathogenesis and immune responses. This is the first report describing E. chaffeensis PBP and represents an inflammatory role recognized in a MyD88-dependent manner, which may hint at the potential application of this information to identify an HME therapy adjunct to doxycycline.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://www.oxfordjournals.org/our_journals/jid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank Dr Xin Li for the stock of C57BL/6 and MyD88−/− mice.
Financial support. This work was funded by the National Institutes of Health (grant number R01AI47885).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Dawson JE, Anderson BE, Fishbein DB, et al. Isolation and characterization of an Ehrlichia sp. from a patient diagnosed with human ehrlichiosis. J Clin Microbiol. 1991;29:2741–5. doi: 10.1128/jcm.29.12.2741-2745.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maeda K, Markowitz N, Hawley RC, Ristic M, Cox D, McDade JE. Human infection with Ehrlichia canis, a leukocytic rickettsia. N Engl J Med. 1987;316:853–6. doi: 10.1056/NEJM198704023161406. [DOI] [PubMed] [Google Scholar]
- 3.McQuiston JH, Paddock CD, Holman RC, Childs JE. The human ehrlichioses in the United States. Emerg Infect Dis. 1999;5:635–42. doi: 10.3201/eid0505.990504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paddock CD, Childs JE. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin Microbiol Rev. 2003;16:37–64. doi: 10.1128/CMR.16.1.37-64.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee EH, Rikihisa Y. Absence of tumor necrosis factor alpha, interleukin-6 (IL-6), and granulocyte-macrophage colony-stimulating factor expression but presence of IL-1beta, IL-8, and IL-10 expression in human monocytes exposed to viable or killed Ehrlichia chaffeensis. Infect Immun. 1996;64:4211–9. doi: 10.1128/iai.64.10.4211-4219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miura K, Rikihisa Y. Liver transcriptome profiles associated with strain-specific Ehrlichia chaffeensis-induced hepatitis in SCID mice. Infect Immun. 2009;77:245–54. doi: 10.1128/IAI.00979-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tizard I. Sickness behavior, its mechanisms and significance. Anim Health Res Rev. 2008;9:87–99. doi: 10.1017/S1466252308001448. [DOI] [PubMed] [Google Scholar]
- 8.Hotopp JC, Lin M, Madupu R, et al. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet. 2006;2:e21. doi: 10.1371/journal.pgen.0020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin M, Rikihisa Y. Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infect Immun. 2003;71:5324–31. doi: 10.1128/IAI.71.9.5324-5331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miura K, Matsuo J, Rahman MA, Kumagai Y, Li X, Rikihisa Y. Ehrlichia chaffeensis induces monocyte inflammatory responses through MyD88, ERK, and NF-kappaB but not through TRIF, interleukin-1 receptor 1 (IL-1R1)/IL-18R1, or Toll-like receptors. Infect Immun. 2011;79:4947–56. doi: 10.1128/IAI.05640-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng Z, Wang X, Rikihisa Y. Regulation of type IV secretion apparatus genes during Ehrlichia chaffeensis intracellular development by a previously unidentified protein. J Bacteriol. 2008;190:2096–105. doi: 10.1128/JB.01813-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumagai Y, Matsuo J, Hayakawa Y, Rikihisa Y. Cyclic di-GMP signaling regulates invasion by Ehrlichia chaffeensis of human monocytes. J Bacteriol. 2010;192:4122–33. doi: 10.1128/JB.00132-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao G, Meier TI, Kahl SD, Gee KR, Blaszczak LC. BOCILLIN FL, a sensitive and commercially available reagent for detection of penicillin-binding proteins. Antimicrob Agents Chemother. 1999;43:1124–8. doi: 10.1128/aac.43.5.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adachi O, Kawai T, Takeda K, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–50. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 15.Morrison DC, Jacobs DM. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976;13:813–8. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- 16.Amanuma H, Strominger JL. Purification and properties of penicillin-binding proteins 5 and 6 from Escherichia coli membranes. J Biol Chem. 1980;255:11173–80. [PubMed] [Google Scholar]
- 17.Spratt BG. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975;72:2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos JM, Lobo M, Matos AP, De Pedro MA, Arraiano CM. The gene bolA regulates dacA (PBP5), dacC (PBP6) and ampC (AmpC), promoting normal morphology in Escherichia coli. Mol Microbiol. 2002;45:1729–40. doi: 10.1046/j.1365-2958.2002.03131.x. [DOI] [PubMed] [Google Scholar]
- 19.Nelson DE, Young KD. Penicillin binding protein 5 affects cell diameter, contour, and morphology of Escherichia coli. J Bacteriol. 2000;182:1714–21. doi: 10.1128/jb.182.6.1714-1721.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sifaoui F, Arthur M, Rice L, Gutmann L. Role of penicillin-binding protein 5 in expression of ampicillin resistance and peptidoglycan structure in Enterococcus faecium. Antimicrob Agents Chemother. 2001;45:2594–7. doi: 10.1128/AAC.45.9.2594-2597.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Linden MP, de Haan L, Hoyer MA, Keck W. Possible role of Escherichia coli penicillin-binding protein 6 in stabilization of stationary-phase peptidoglycan. J Bacteriol. 1992;174:7572–8. doi: 10.1128/jb.174.23.7572-7578.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zarantonelli ML, Antignac A, Lancellotti M, Guiyoule A, Alonso JM, Taha MK. Immunogenicity of meningococcal PBP2 during natural infection and protective activity of anti-PBP2 antibodies against meningococcal bacteraemia in mice. J Antimicrob Chemother. 2006;57:924–30. doi: 10.1093/jac/dkl066. [DOI] [PubMed] [Google Scholar]
- 23.Choi KS, Scorpio DG, Dumler JS. Anaplasma phagocytophilum ligation to toll-like receptor (TLR) 2, but not to TLR4, activates macrophages for nuclear factor-kappa B nuclear translocation. J Infect Dis. 2004;189:1921–5. doi: 10.1086/386284. [DOI] [PubMed] [Google Scholar]
- 24.Hii CS, Sun GW, Goh JW, Lu J, Stevens MP, Gan YH. Interleukin-8 induction by Burkholderia pseudomallei can occur without Toll-like receptor signaling but requires a functional type III secretion system. J Infect Dis. 2008;197:1537–47. doi: 10.1086/587905. [DOI] [PubMed] [Google Scholar]
- 25.Shimohata T, Nakano M, Lian X, et al. Vibrio parahaemolyticus infection induces modulation of IL-8 secretion through dual pathway via VP1680 in Caco-2 cells. J Infect Dis. 2011;203:537–44. doi: 10.1093/infdis/jiq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koh YS, Koo JE, Biswas A, Kobayashi KS. MyD88-dependent signaling contributes to host defense against ehrlichial infection. PLoS One. 2010;5:e11758. doi: 10.1371/journal.pone.0011758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang H, Lin M, Wang X, et al. Proteomic analysis of and immune responses to Ehrlichia chaffeensis lipoproteins. Infect Immun. 2008;76:3405–14. doi: 10.1128/IAI.00056-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattner J, Debord KL, Ismail N, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–9. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 29.Zhu B, Nethery KA, Kuriakose JA, Wakeel A, Zhang X, McBride JW. Nuclear translocated Ehrlichia chaffeensis ankyrin protein interacts with a specific adenine-rich motif of host promoter and intronic Alu elements. Infect Immun. 2009;77:4243–55. doi: 10.1128/IAI.00376-09. [DOI] [PMC free article] [PubMed] [Google Scholar]