Abstract
Background. We performed a prospective study to determine the disease burden of respiratory syncytial virus (RSV) and human metapneumovirus (HMPV) in older adults in comparison with influenza virus.
Methods. During 3 consecutive winters, we enrolled Davidson County (Nashville, TN) residents aged ≥50 years admitted to 1 of 4 hospitals with acute respiratory illness (ARI). Nasal/throat swabs were tested for influenza, RSV, and HMPV with reverse-transcriptase polymerase chain reaction. Hospitalization rates were calculated.
Results. Of 1042 eligible patients, 508 consented to testing. Respiratory syncytial virus was detected in 31 participants (6.1%); HMPV was detected in 23 (4.5%) patients; and influenza was detected in 33 (6.5%) patients. Of those subjects aged ≥65 years, 78% received influenza vaccination. Compared with patients with confirmed influenza, patients with RSV were older and more immunocompromised; patients with HMPV were older, had more cardiovascular disease, were more likely to have received the influenza vaccination, and were less likely to report fever than those with influenza. Over 3 years, average annual rates of hospitalization were 15.01, 9.82, and 11.81 per 10 000 county residents due to RSV, HMPV, and influenza, respectively.
Conclusions. In adults aged ≥50 years, hospitalization rates for RSV and HMPV were similar to those associated with influenza.
Although rates of viral respiratory infections in children are well established, fewer reports exist on the role of viral respiratory infections in respiratory disease in older adults. Respiratory syncytial virus (RSV) is a common virus in childhood, but it also has been reported as a cause of significant morbidity and mortality in the older adult population [1–4]. Rates of adult hospitalization for RSV previously have been estimated using modeling and not based on prospective, population-based, laboratory-confirmed data using sensitive molecular detection methods [2, 5–8]. Human metapneumovirus (HMPV), like RSV, is a paramyxovirus commonly detected in children but also identified in older adults [9, 10]. Little is known about rates of adult hospitalizations due to HMPV. Respiratory syncytial virus and HMPV are challenging to differentiate from influenza in older adults because of the similarity in the clinical presentation of these viruses, their seasonal overlap [10–12], and the difficulty in diagnosis. Rapid antigen tests for RSV and influenza virus have poor sensitivity in older adults, and rapid assays for HMPV are not widely available [13–18]. In addition, RSV and HMPV are difficult to culture [19–21]. Molecular methods offer high sensitivity in adults [19, 22–27] and can accurately detect these viruses as well as influenza virus in older populations [28, 29].
Defining the rates of illness due to RSV and HMPV may support vaccine development for the prevention of disease and drug development for treatment of RSV and HMPV infection. This study was designed to establish rates of hospitalizations associated with RSV and HMPV in older adults using sensitive molecular techniques and to compare these rates to the rates of influenza-associated hospitalizations in the setting of high influenza immunization rates [28, 30].
Methods
Study Design
During 3 successive influenza seasons—2006–2007, 2007–2008, and 2008–2009—individuals aged ≥50 years hospitalized with respiratory symptoms or nonlocalizing fever were prospectively enrolled into an ongoing study of influenza vaccine effectiveness in older adults [30]. After informed consent, adult residents of Davidson County (Nashville), Tennessee, were enrolled, as described previously. During the 2006–2007 season, enrollment occurred at 1 academic and 1 community hospital; during the 2007–2008 season, enrollment occurred at 1 academic hospital; and during the 2008–2009 season, enrollment occurred at 1 academic and 3 community hospitals. The number of participating hospitals each year was based on availability of research staff and funding. Recruitment occurred yearly from November through April, based on the circulation of influenza virus. In November, patients were enrolled 2 days a week, and this was increased to 4–5 days per week when influenza virus was identified for2 consecutive weeks in the Vanderbilt University Clinical Laboratory. Davidson County residents aged ≥50 years who were admitted during a 24-hour surveillance period for each enrollment day were eligible if they had any respiratory symptoms (ie, cough, nasal congestion, coryza, dyspnea, or wheezing) or nonlocalizing fever. At the time of consent, nose and throat swabs were obtained and subjects were asked to consent to have specimens stored for future diagnostic studies.
Demographic and Clinical Information
Subjects completed questionnaires, and medical record review captured age, sex, race, medical comorbidities, smoking (self-reported within the past 6 months), use of specific medications (home oxygen, corticosteroids, and immunosuppressants), influenza vaccination status, clinical symptoms, admission to an intensive care unit, endotracheal intubation, length of hospitalization, and status at discharge.
Laboratory Methods
Influenza testing was performed as previously described [30]. Using real-time reverse-transcriptase polymerase chain reaction (RT-PCR), frozen specimens previously evaluated for influenza were tested for RSV using methods published by the Centers for Disease Control and Prevention (CDC) [31] and for HMPV using primers and probes modified from Maertzdorf et al [32 and unpublished data], if the subjects had agreed to additional testing of samples. To insure the quality of the specimens collected, samples were also tested for β-actin (Applied Biosystems) during the 2006–2008 seasons and for RNase P during the 2008–2009 season. If either of the controls were negative in 3 consecutive tests, the RT-PCR results for that specimen were categorized as indeterminate and excluded from the analysis.
Analyses
Only specimens from subjects who gave permission for future testing were included in these analyses. Descriptive analyses were performed using the Fisher exact test for categorical values and logistical regression for continuous variables, using STATA version 9. The numerator for rate calculations was the weighted number of virus-specific hospitalizations. Numbers were weighted to account for number of days of enrollment, proportion of eligible patients enrolled, and the proportion of county respiratory hospitalizations at surveillance hospitals. To determine the proportion of Davidson County residents hospitalized at surveillance hospitals for acute respiratory illness during the surveillance periods, the Hospital Discharge Data System, which receives information from all inpatient discharges from Tennessee hospitals, was used. Denominators for rate calculations were age-specific Davidson County population numbers from the census annual July estimate. We calculated 95% confidence intervals (CIs) for all rates using 1000 bootstrap samples.
RESULTS
Characterization of Enrolled Patients
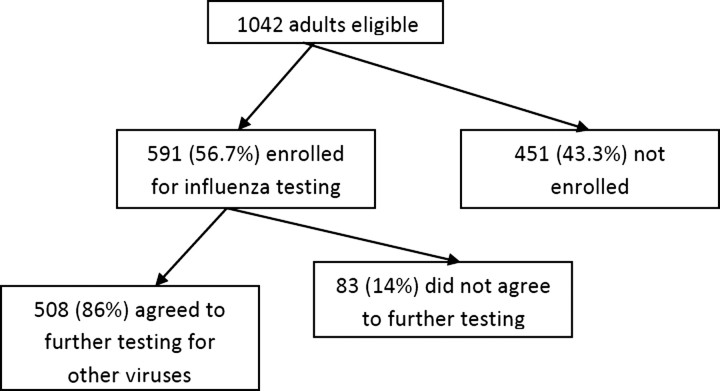
During the 3-year study period, 1042 Davidson County residents aged ≥50 years and hospitalized with acute respiratory symptoms were identified as eligible for enrollment on surveillance days. Of these, 926 (89%) were approached, and 591 (64%) of these were enrolled (Figure 1). Patients who were not approached included 21% who were discharged or missed, and 2% who were non-English speaking. Of those approached, reasons for nonenrollment included: patient refusal (26%), surrogate decision maker refusal (4%), physician refusal (<1%), and lack of legal guardian or surrogate decision maker (12%). Those who refused enrollment were significantly older than those enrolled (median age, 73 vs 68; P = .0001). Of those enrolled, 508 of 591 patients consented to additional testing of samples. There were no exclusions due to inadequate samples. The 83 subjects who refused further sample testing were older (median age, 71.4 vs 66; P = .01) and more likely to be white (87.8% vs 63.6%; P < .0001). Enrolled subjects had a median age of 68 years, most lived independently or with family (90.2%), and had >1 chronic illness (64.5%) (Table 1). Approximately 71% of subjects had received influenza vaccination for that influenza season, including 78% of those aged >65 years.
Figure 1.
Eligibility and enrollment: 1042 were approached for enrollment, 591 agreed to testing of specimens for influenza alone, and of those 591, 508 agreed to further testing for other viruses.
Table 1.
Characteristics of Enrolled, Tested, and Infected Subjects
| Characteristic | Enrolled (n = 591) | Samples Tested for RSV, HMPV, and Influenza (n = 508) | RSV Infection (n = 31/508; 6.1%) | HMPV Infection (n = 23/508; 4.5%) | Influenza Infection (n = 33/508; 6.5%) |
|---|---|---|---|---|---|
| Age in years, median (IQR) | 68 (58–78.4) | 66 (58–78) | 68 (56–78) | 76.2 (66–83.2)a | 60 (55–70) |
| Age group, no. (%)b | |||||
| 50–64 years | 254 (43) | 226 (44.5) | 12 (38.7) | 4 (17.4) | 23 (69.7) |
| ≥65 years | 337 (57) | 282 (55.5) | 19 (61.3) | 19 (82.6) | 10 (30.3) |
| Duration of symptoms in days, median (IQR) | 5 (3–8) | 5 (3–8) | 6.5 (4–7) | 5 (4–8) | 5 (3–9) |
| Sex, no. (%) | |||||
| Male | 239 (40.4) | 203 (40) | 14 (45.2) | 8 (34.8) | 9 (27.3) |
| Female | 352 (59. 6) | 305 (60) | 17 (54.8) | 15 (65.2) | 24 (72.7) |
| Race/ethnicity, no. (%) | |||||
| White | 395 (66.8) | 323 (63.6) | 22 (71) | 14 (60.9) | 19 (57.6) |
| Black | 185 (31.3) | 175 (34.5) | 8 (25.8) | 8 (34.8) | 14 (42.4) |
| Living situation, no. (%) | |||||
| Independent | 154 (26.1) | 127 (25) | 8 (25.8) | 4 (17.4) | 4 (12.1) |
| With family | 379 (64.1) | 330 (65) | 18 (58.1) | 16 (69.6) | 26 (78.8) |
| In nursing facility | 52 (8.8) | 46 (9.1) | 4 (12.9) | 3 (13) | 3 (9.1) |
| Chronic illnesses, no. (%) | |||||
| Cardiovascular disease | 351 (59.4) | 303 (59.7) | 15 (48.4) | 18 (78.3)a | 17 (51.5) |
| Pulmonary disease | 381 (64.5) | 322 (63.4) | 21 (67.7) | 14 (60.9) | 23 (69.7) |
| Diabetes mellitus | 234 (39.6) | 207 (40.8) | 12 (38.7) | 7 (30.4) | 13 (39.4) |
| Immunodeficiencyc | 288 (48.7) | 241 (47.4) | 19 (61.3)a | 9 (39.1) | 11 (33.3) |
| Smoker (within last 6 months), no. (%) | 138 (23.4) | 120 (23.6) | 8 (25.8) | 1 (4.4)a | 14 (42.4) |
| Flu vaccination, no. (%) | 424 (71.7) | 366 (72.1) | 20 (64.5) | 20 (87)a | 17 (51.5) |
Abbreviations: HMPV, human metapneumovirus; IQR, interquartile range; RSV, respiratory syncytial virus.
a Differences were significant (P < .05) when compared with influenza virus.
b Differences between both RSV and influenza and HMPV and influenza were significant (P < .05).
c Transplant, cancer, splenectomy, HIV/AIDS, steroid use, chemotherapy, immunosuppression
Clinical Presentation of Specific Viral Infection
The RT-PCR of nasal swab specimens identified 31 (6.1%) of 508 patients with RSV, 23 (4.5%) with HMPV, and 33 (6.5%) with influenza; there were no coinfections. Compared with patients with influenza, patients with RSV were more likely to be aged ≥65 years (P = .01) or to be immunocompromised (organ transplant, cancer, splenectomy, human immunodeficiency virus/AIDS, steroids, chemotherapy, immunosuppressive therapy) (P = .03). Compared with patients with influenza, patients with HMPV were older (P = .003), had more cardiovascular disease (P = .04), were less likely to be smokers (P = .002), were more likely to have received the influenza vaccination (P = .006), and were less likely to report fever (P = .004). Gender, race, and living situation (independent or with family) were not associated with specific viral infections. Nearly all patients (78 of 87; 89.7%) infected with 1 of the 3 study viruses had >1 medical comorbidity (Table 1).
The median duration of symptoms prior to presentation was 6.5 days with RSV, 5 days with HMPV, and 5 days with influenza. Overall hospital length of stay, admission to and length of stay in the intensive care unit, need for mechanical ventilation, and death were not different among those infected with any of the 3 viruses (Table 2).
Table 2.
Symptoms at Admission and Clinical Outcomes for Hospitalized Patients with Respiratory Syncytial Virus (RSV), Human Metapneumovirus (HMPV), or Influenza
| Characteristic | RSV (n = 31) | HMPV (n = 23) | Influenza Virus (n = 33) |
|---|---|---|---|
| Duration of symptoms in days, median (IQR) | 6.5 (4–7) | 5 (4–8) | 5 (3–9) |
| Symptoms/signs, no. (%) | |||
| Congestion/ rhinorrhea | 25 (80.65) | 20 (86.96) | 25 (75.76) |
| Sore throat | 19 (61.29) | 7 (30.43) | 13 (39.39) |
| Cough | 30 (96.77) | 23 (100) | 32 (96.97) |
| Dyspnea | 27 (87.1) | 20 (86.96) | 25 (75.76) |
| Wheezing | 25 (80.65) | 19 (82.61) | 27 (81.82) |
| Earache | 10 (32.26) | 3 (13.04) | 4 (12.12) |
| Fever | 22 (70.97) | 12 (52.17)a | 29 (87.88) |
| Nausea/vomiting/ diarrhea | 12 (38.71) | 10 (43.48) | 17 (51.52) |
| Decreased appetite | 19 (61.29) | 10 (43.48) | 21 (63.64) |
| Myalgias | 12 (38.71)a | 7 (30.43)a | 21 (63.64) |
| Headache | 20 (64.52) | 9 (39.13) | 18 (54.55) |
| Fatigue | 28 (90.32) | 19 (82.61) | 30 (90.91) |
| Altered mental statusb | 8/20 (40) | 5/10 (50) | 6/19 (31.58) |
| Clinical outcomes | |||
| Length of stay in days, median (IQR) | 3 (2–6) | 3 (1–4) | 4 (2–5) |
| ICU admission, no. (%) | 3 (9.68) | 3 (13.04) | 2 (6.06) |
| Length of stay in ICU in days, median (IQR) | 5 (3–12) | 4 (3–5) | 2.5 (1–4) |
| Mechanical ventilation, no. (%) | 1 (3.23) | 1 (4.35) | 0 (0) |
| In-hospital death, no. (%) | 2 (6.45) | 2 (8.7) | 0 (0) |
Abbreviations: ICU, intensive care unit; IQR, interquartile range.
a Differences were significant (P < .05) when compared with influenza virus.
b Not recorded in year one.
Viral-Specific Hospitalization Rates
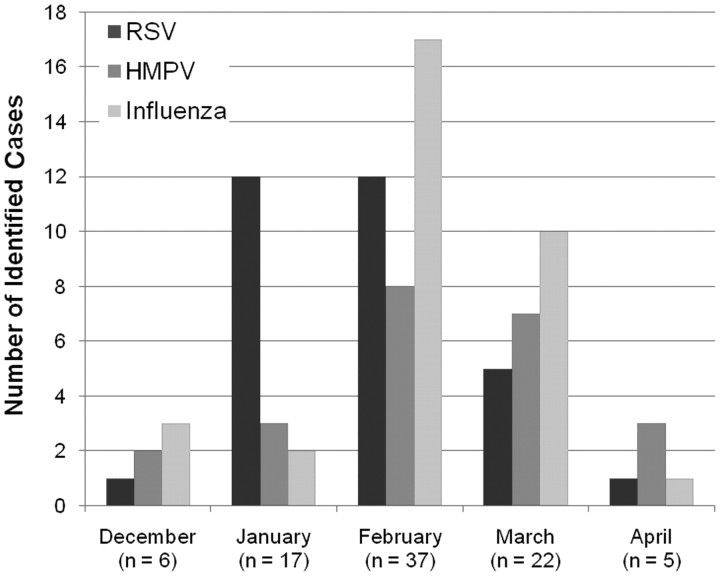
All 3 viruses circulated during the surveillance period from December through April, with no detection of any of the 3 viruses in November in any of the 3 study seasons (Figure 2). Respiratory syncytial virus peaked in January and February, whereas influenza virus and HMPV peaked in February.
Figure 2.
Seasonal variation of virus illness.The timeline of documented infections associated with each of the 3 viruses tested is illustrated. Data from all 3 years are included in this graph.
There were no coinfections detected in our cohort. Over the 3-year period studied, average annual rates of hospitalization in adults aged ≥50 years were 15.01, 9.82, and 11.81 per 10 000 residents due to RSV, HMPV, and influenza, respectively (Table 3). For those subjects aged ≥50 years, hospitalization rates for both RSV and HMPV were similar to those for influenza. Rates of hospitalizations for adults aged ≥65 years were higher for HMPV (P = .05) than influenza but not statistically different for RSV (P = .07) (Table 3).
Table 3.
Weighted Rates of Hospitalization, Adjusting for Number of Days of Enrollment, Proportion of Eligible Patients Enrolled, and the Proportion of County Respiratory Hospitalizations at Surveillance Hospitals
| RSV |
HMPV |
Influenza Virus |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age Group | Population | No. of Cases | Weight | Rate/10 000 (95% CI) | No. of Cases | Weight | Rate/10 000 (95% CI) | No. of Cases | Weight | Rate/10 000 (95% CI) |
| 50–64 years | 308 329 | 12 | 254 | 8.2 (3.3–12.3) | 4 | 57 | 1.8 (.3–4.0) | 23 | 356 | 11.5 (6.8–16.2) |
| ≥65 years | 200 711 | 19 | 510 | 25.4 (13.1–38.0) | 19 | 443 | 22.1 (12.1–33.7) | 10 | 247 | 12.3 (5.3–21.4) |
| Overall | 509 040 | 31 | 764 | 15.0 (8.6–19.8) | 23 | 500 | 9.8 (5.8–14.4) | 33 | 603 | 11.8 (7.6–16.2) |
Abbreviations: CI, confidence interval; HMPV, human metapneumovirus; RSV, respiratory syncytial virus.
DISCUSSION
Respiratory syncytial virus and HMPV are well-known pathogens in the pediatric population and are increasingly recognized as significant pathogens in older adults [2, 4, 8, 11, 34–37]. The burden of illness due to RSV is substantial [4]. In 1 prospective study involving 1471 patients hospitalized due to respiratory illness with an average age of 75 years, 9.7% of hospitalizations were due to RSV, and 15% of these required intensive care [4]. In a cohort of adults with both private and government insurance, RSV was estimated to cause 23.4 hospitalizations for pneumonia and influenza per 10 000 person-years in high-risk adults aged ≥65 years [8]. A study of Tennessee nursing home residents enrolled in Medicaid, a government-funded healthcare program for low-income families, estimated that the illness burden of RSV was similar to influenza and accounted for 150 acute respiratory hospitalizations, 760 courses of antibiotics, and 170 deaths per 10 000 nursing home patients annually [2]. In a 9-year study of death certificate data performed by Thompson et al, the mortality rate attributed to RSV-associated respiratory and circulatory illness in persons aged >65 years was estimated to be 2.7 per 10 000 person-years [38]. Similar published data are not available for HPMV.
Using prospectively collected data and specimens over 3 years, we determined that RSV and HMPV accounted for 6.1% and 4.5% of hospitalizations for acute respiratory illness during the winter viral respiratory season, respectively. The percentage of hospitalized adults with these 2 respiratory viruses was similar to that which has been previously reported [4, 9, 10, 39].
Because our studies were prospective and population-based rather than based on modeling, we were able to establish laboratory-confirmed hospitalization rates for RSV, HMPV, and influenza. Over the 3-year study period, we found aggregate annual rates of hospitalization to be 15.01, 9.82, and 11.81 per 10 000 residents due to RSV, HMPV, and influenza, respectively. Thus, both RSV and HMPV, as well as influenza, cause a substantial burden of illness in older adults. Our RSV hospitalization rate for adults aged >65 years was 25.4 per 10 000. This rate for low- and high-risk patients combined falls between rates reported by Mullooly et al, who used modeling to estimate RSV hospitalization rates to be 10.6 per 10 000 for low-risk patients aged >65 years and 44.4 per 10 000 for high-risk patients aged ≥65 years [8]. We found that those individuals infected with influenza were younger than those with either RSV or HMPV and were less likely to be vaccinated. In addition, in those aged ≥65 years, the rate of hospitalizations associated with RSV (rate ratio, 2.06; 95% CI, .8–5.0) and HMPV (rate ratio, 1.79; 95% CI, .8–4.8) was almost double that of hospitalization rates for influenza (P = .05, P = .07, respectively). This may be due in part to the effectiveness of the influenza vaccine and the relatively high vaccination rates in those aged >65 years. In our population, 71% of the enrolled subjects had received influenza vaccination, including 78% of those aged >65 years. Our previous work in this population estimated that influenza vaccine was 61% effective in preventing hospitalizations in older adults [30].
Our study has several limitations. We may have underrepresented rates of RSV and HMPV due to the surveillance period being tailored to the circulation of influenza. For example, RSV cases may have been missed due to low surveillance in November, and HMPV may have been missed due to no surveillance into May. Another limitation was our small sample size, making it difficult to draw comparisons of the clinical presentations seen with each virus. A final limitation was that the study only included 1 geographic location, middle Tennessee.
Because both RSV and HMPV can be devastating to the immunocompromised host, the identification of these illnesses in the hospitalized patient could contribute to a reduction of nosocomial spread. Outbreaks in long-term care facilities and assisted living facilities [40–44] suggest that patients with these viral illnesses should be isolated. In a study by Falsey et al, 17% of healthy older adults and close to half of high-risk adults with symptomatic RSV had contact with their healthcare provider (including office visits, emergency room visits, and hospital admissions), and 16% of them were hospitalized [4], providing potentially dangerous exposures to older patients, especially those with weakened immune systems. Currently, the CDC recommends contact precautions in addition to standard precautions when RSV or HMPV are highly suspected or confirmed [45].
Respiratory syncytial virus and HMPV both cause a significant number of hospitalizations in adults aged ≥50 years, especially among those aged ≥65 years. Because their clinical presentation mimics that of influenza, clinical suspicion and sensitive molecular diagnostic methods are needed to detect these infections. In addition, hospitalization rates of RSV and MPV are higher than hospitalization rates of influenza in adults aged ≥65 years, likely due to successful vaccination of the older population in the United States. Antiviral therapy and vaccines for the prevention and treatment of RSV and HMPV should be pursued to reduce the impact of these important viral agents in older adults.
Notes
Financial support. This work was supported by multiple sources: surveillance for the first 2 years was funded by the National Institute of Health Vaccine and Treatment Evaluation Unit (N01 AI25462) (Kathryn M. Edwards MD, site PI) and during the third year by the Centers for Disease Control and Prevention (1U181P000184-01) (Marie Griffin MD, site PI). K. W. was funded by The Agency for Healthcare Research and Quality T32 HS013833. H. K. T. received salary support and career development from the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) (1K23AI074863-01). J. V. W. received support from the NIH/NIAID (AI085062-01). The study was also supported in part by Vanderbilt CTSA grant 1 UL1 RR024975 from the National Center for Research Resources, NIH. The funders did not participate in the design or conduct of the study; collection, management, analysis, or interpretation of the data; nor preparation, review, or approval of the manuscript.
Potential conflicts of interest. M. R. G. has received research funds from the CDC. K. M. E. has received funding from the NIH and the CDC to evaluate the impact of influenza vaccines and study new influenza vaccines. J. V. W. serves on the scientific advisory board of Quidel. H. K. T. has received research funds from sanofi pasteur. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Dowell SF, Anderson LJ, Gary HE, Jr, et al. Respiratory syncytial virus is an important cause of community-acquired lower respiratory infection among hospitalized adults. J Infect Dis. 1996;174:456–62. doi: 10.1093/infdis/174.3.456. [DOI] [PubMed] [Google Scholar]
- 2.Ellis SE, Coffey CS, Mitchel EF, Jr, Dittus RS, Griffin MR. Influenza- and respiratory syncytial virus–associated morbidity and mortality in the nursing home population. J Am Geriatr Soc. 2003;51:761–7. doi: 10.1046/j.1365-2389.2003.51254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falsey AR, Walsh EE. Respiratory syncytial virus infection in adults. Clin Microbiol Rev. 2000;13:371–84. doi: 10.1128/cmr.13.3.371-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–59. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 5.Griffin MR, Coffey CS, Neuzil KM, Mitchel EF, Jr, Wright PF, Edwards KM. Winter viruses: influenza- and respiratory syncytial virus–related morbidity in chronic lung disease. Arch Intern Med. 2002;162:1229–36. doi: 10.1001/archinte.162.11.1229. [DOI] [PubMed] [Google Scholar]
- 6.Han LL, Alexander JP, Anderson LJ. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J Infect Dis. 1999;179:25–30. doi: 10.1086/314567. [DOI] [PubMed] [Google Scholar]
- 7.Jansen AG, Sanders EA, Hoes AW, van Loon AM, Hak E. Influenza- and respiratory syncytial virus–associated mortality and hospitalisations. Eur Respir J. 2007;30:1158–66. doi: 10.1183/09031936.00034407. [DOI] [PubMed] [Google Scholar]
- 8.Mullooly JP, Bridges CB, Thompson WW, et al. Influenza- and RSV-associated hospitalizations among adults. Vaccine. 2007;25:846–55. doi: 10.1016/j.vaccine.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 9.Walsh EE, Peterson DR, Falsey AR. Human metapneumovirus infections in adults: another piece of the puzzle. Arch Intern Med. 2008;168:2489–96. doi: 10.1001/archinte.168.22.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnstone J, Majumdar SR, Fox JD, Marrie TJ. Human metapneumovirus pneumonia in adults: results of a prospective study. Clin Infect Dis. 2008;46:571–4. doi: 10.1086/526776. [DOI] [PubMed] [Google Scholar]
- 11.Falsey AR, Cunningham CK, Barker WH, et al. Respiratory syncytial virus and influenza A infections in the hospitalized elderly. J Infect Dis. 1995;172:389–94. doi: 10.1093/infdis/172.2.389. [DOI] [PubMed] [Google Scholar]
- 12.Osterhaus A, Fouchier R. Human metapneumovirus in the community. Lancet. 2003;361:890–1. doi: 10.1016/S0140-6736(03)12785-7. [DOI] [PubMed] [Google Scholar]
- 13.Falsey AR, McCann RM, Hall WJ, Criddle MM. Evaluation of four methods for the diagnosis of respiratory syncytial virus infection in older adults. J Am Geriatr Soc. 1996;44:71–3. doi: 10.1111/j.1532-5415.1996.tb05641.x. [DOI] [PubMed] [Google Scholar]
- 14.Walsh EE, Falsey AR. A simple and reproducible method for collecting nasal secretions in frail elderly adults, for measurement of virus-specific IgA. J Infect Dis. 1999;179:1268–73. doi: 10.1086/314726. [DOI] [PubMed] [Google Scholar]
- 15.Boivin G, Abed Y, Pelletier G, et al. Virological features and clinical manifestations associated with human metapneumovirus: a new paramyxovirus responsible for acute respiratory-tract infections in all age groups. J Infect Dis. 2002;186:1330–4. doi: 10.1086/344319. [DOI] [PubMed] [Google Scholar]
- 16.Gerna G, Sarasini A, Percivalle E, Genini E, Campanini G, Grazia Revello M. Simultaneous detection and typing of human metapneumovirus strains in nasopharyngeal secretions and cell cultures by monoclonal antibodies. J Clin Virol. 2006;35:113–6. doi: 10.1016/j.jcv.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casiano-Colon AE, Hulbert BB, Mayer TK, Walsh EE, Falsey AR. Lack of sensitivity of rapid antigen tests for the diagnosis of respiratory syncytial virus infection in adults. J Clin Virol. 2003;28:169–74. doi: 10.1016/s1386-6532(03)00002-7. [DOI] [PubMed] [Google Scholar]
- 18.Percivalle E, Sarasini A, Visai L, Revello MG, Gerna G. Rapid detection of human metapneumovirus strains in nasopharyngeal aspirates and shell vial cultures by monoclonal antibodies. J Clin Microbiol. 2005;43:3443–6. doi: 10.1128/JCM.43.7.3443-3446.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falsey AR, Formica MA, Walsh EE. Diagnosis of respiratory syncytial virus infection: comparison of reverse transcription-PCR to viral culture and serology in adults with respiratory illness. J Clin Microbiol. 2002;40:817–20. doi: 10.1128/JCM.40.3.817-820.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendoza J, Rojas A, Navarro JM, Plata C, de la Rosa M. Evaluation of three rapid enzyme immunoassays and cell culture for detection of respiratory syncytial virus. Eur J Clin Microbiol Infect Dis. 1992;11:452–4. doi: 10.1007/BF01961862. [DOI] [PubMed] [Google Scholar]
- 21.van den Hoogen BG, de Jong JC, Groen J, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–24. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falsey AR, Criddle MC, Walsh EE. Detection of respiratory syncytial virus and human metapneumovirus by reverse transcription polymerase chain reaction in adults with and without respiratory illness. J Clin Virol. 2006;35:46–50. doi: 10.1016/j.jcv.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Falsey AR, Formica MA, Hennessey PA, Criddle MM, Sullender WM, Walsh EE. Detection of respiratory syncytial virus in adults with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:639–43. doi: 10.1164/rccm.200510-1681OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falsey AR, Formica MA, Treanor JJ, Walsh EE. Comparison of quantitative reverse transcription-PCR to viral culture for assessment of respiratory syncytial virus shedding. J Clin Microbiol. 2003;41:4160–5. doi: 10.1128/JCM.41.9.4160-4165.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaye M, Skidmore S, Osman H, Weinbren M, Warren R. Surveillance of respiratory virus infections in adult hospital admissions using rapid methods. Epidemiol Infect. 2006;134:792–8. doi: 10.1017/S0950268805005364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pabbaraju K, Wong S, McMillan T, Lee BE, Fox JD. Diagnosis and epidemiological studies of human metapneumovirus using real-time PCR. J Clin Virol. 2007;40:186–92. doi: 10.1016/j.jcv.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Talbot HK, Falsey AR. The diagnosis of viral respiratory disease in older adults. Clin Infect Dis. 50:747–51. doi: 10.1086/650486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poehling KA, Talbot HK, Williams JV, et al. Impact of a school-based influenza immunization program on disease burden: comparison of two Tennessee counties. Vaccine. 2009;27:2695–700. doi: 10.1016/j.vaccine.2009.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talbot HK, Poehling KA, Williams JV, et al. Influenza in older adults: impact of vaccination of school children. Vaccine. 2009;27:1923–7. doi: 10.1016/j.vaccine.2009.01.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talbot HK, Griffin MR, Chen Q, Zhu Y, Williams JV, Edwards KM. Effectiveness of seasonal vaccine in preventing confirmed influenza-associated hospitalizations in community dwelling older adults. J Infect Dis. 2011;203:500–8. doi: 10.1093/infdis/jiq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–98. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maertzdorf J, Wang CK, Brown JB, et al. Real-time reverse transcriptase PCR assay for detection of human metapneumoviruses from all known genetic lineages. J Clin Microbiol. 2004;42:981–6. doi: 10.1128/JCM.42.3.981-986.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klemenc JAaJVW. Nashville, TN: Vanderbilt University School of Medicine; 2010. Unpublished data. [Google Scholar]
- 34.Falsey AR, Dallal GE, Formica MA, et al. Long-term care facilities: a cornucopia of viral pathogens. J Am Geriatr Soc. 2008;56:1281–5. doi: 10.1111/j.1532-5415.2008.01775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falsey AR, Treanor JJ, Betts RF, Walsh EE. Viral respiratory infections in the institutionalized elderly: clinical and epidemiologic findings. J Am Geriatr Soc. 1992;40:115–9. doi: 10.1111/j.1532-5415.1992.tb01929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Treanor J, Falsey A. Respiratory viral infections in the elderly. Antiviral Res. 1999;44:79–102. doi: 10.1016/S0166-3542(99)00062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walsh EE, Peterson DR, Falsey AR. Is clinical recognition of respiratory syncytial virus infection in hospitalized elderly and high-risk adults possible? J Infect Dis. 2007;195:1046–51. doi: 10.1086/511986. [DOI] [PubMed] [Google Scholar]
- 38.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 39.Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187:785–90. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- 40.Boivin G, De Serres G, Hamelin ME, et al. An outbreak of severe respiratory tract infection due to human metapneumovirus in a long-term care facility. Clin Infect Dis. 2007;44:1152–8. doi: 10.1086/513204. [DOI] [PubMed] [Google Scholar]
- 41.Honda H, Iwahashi J, Kashiwagi T, et al. Outbreak of human metapneumovirus infection in elderly inpatients in Japan. J Am Geriatr Soc. 2006;54:177–80. doi: 10.1111/j.1532-5415.2005.00575_10.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Louie JK, Schnurr DP, Pan CY, et al. A summer outbreak of human metapneumovirus infection in a long-term-care facility. J Infect Dis. 2007;196:705–8. doi: 10.1086/519846. [DOI] [PubMed] [Google Scholar]
- 43.Sorvillo FJ, Huie SF, Strassburg MA, Butsumyo A, Shandera WX, Fannin SL. An outbreak of respiratory syncytial virus pneumonia in a nursing home for the elderly. J Infect. 1984;9:252–6. doi: 10.1016/s0163-4453(84)90530-9. [DOI] [PubMed] [Google Scholar]
- 44.Agius G, Dindinaud G, Biggar RJ, et al. An epidemic of respiratory syncytial virus in elderly people: clinical and serological findings. J Med Virol. 1990;30:117–27. doi: 10.1002/jmv.1890300208. [DOI] [PubMed] [Google Scholar]
- 45.Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35:S65–164. doi: 10.1016/j.ajic.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]