Abstract
During the past few years, tissue-engineered skin constructs has offered great promise in the treatment of deep burns and various skin-related disorders. The overall impact of bioengineered skin research, as a multidisciplinary field, has also enhanced our understanding of the structure-function relations and the physiological processes of tissue regeneration within these constructed three-dimensional skin models. Despite of the fact that currently available bioengineered skin products have a range of problems such as patient safety, clinical efficacy and convenience of use, they are still one of the most advanced strategies because of their wide use and potential development in the biomedical field. Here, the challenges and developments in tissue-engineered skin research are discussed. Central to the discussion is the extensive application and future prospects of these bioengineered skin constructs.
Keywords: tissue-engineered skin, biomedicine, biomaterial, cell, wound healing
Introduction
The need is great for safe and effective therapeutic options for wound coverage and tissue repair in diverse clinical settings, such as in acute skin wounds, burns and chronic skin ulcers, which represent a major burden on world health care costs. Autografting with split-thickness skin is the preferred treatment for coverage of excised burn wounds, but donor sites for autografting are limited in patients with very large burns. In these patients, wound coverage requires repeated harvesting of available donor sites, which is associated with pain and scarring at the donor site and lengthy hospital stays. Moreover, in the developed world, life expectancy and affluence have increased so markedly that chronic wound associated with aging and diabetes has become significant. The traditional treatment of relatively small chronic wounds has included the use of topical agents and grafting of split- or full-thickness skin. Skin grafts can provide timely wound coverage but may lead to painful donor sites, which are slow to heal and may be unsuccessful because of underlying deficiencies in wound healing [1]. As well, although congenital skin conditions and diseases affect a relatively small fraction of the population, they represent significant challenges for wound coverage [2].
Because of the limited use of autografts and allografts and the large medical need for wound coverage in these diverse patient populations, bioengineered skin has been developed to provide new alternatives for clinicians to restore various skin defects. Over the last 30 years, bioengineered skin research, as one of the most active fields in biological substitutes, has extended the prospects for improving outcomes for patients with acute and chronic skin wounds. The first-documented bioengineered skin product, cultured epithelial autografts, appeared 30 years ago. Rheinwald and Green isolated and cultured keratinocytes from skin, which was further developed into small sheets of cells, two or three cell-layers thick [3]. Evidence suggests that cultured skin cells returned to a patient retain the capacity to self-renew for a lifetime [4]. However, delivering keratinocytes as small sheets is problematic. The sheets are fragile, and many of the keratinocytes are already differentiated beyond the point to which they can take any active part in providing a new epithelium. Hence, the company Genzyme further created living epithelial sheets with this technology and has marketed them since 1987 as EpiCel®[5]. The sheets are designed for temporary use for deep partial-thickness and full-thickness burns. Because cultured keratinocytes alone are of limited value in treating full-thickness defects without a well-vascularized dermal wound bed, advanced tissue sciences were used to develop a scaffold seeded with dermal fibroblasts and covered with a layer of keratinocytes, like Apligraf®and Dermagraft® [6,7]. Some new ideas were the acellular dermal substitute designed for the management of major burns. Integra®is a representative dermal replacement product composed of bovine tendon collagen with a glycosaminoglycan (chondroitin-6-sulphate) added, onto which a silicone membrane is sealed to the upper surface to act as a temporary epidermal substitute layer [8]. These products above can act as temporary wound covers or permanent skin replacements, depending on their design and composition.
Currently, bioengineered skin products usually possess a similar structure that imitates the natural skin but lacks several important structures and functions. No models of bioengineered skin can completely replicate the anatomy, physiology, biological stability or aesthetic nature of uninjured skin and no manufactured skin substitute has provided an outcome consistently comparable to an autograft [9]. Even with the drawbacks, bioengineered skin is still a promising viable approach to providing a protective barrier for skin replacement therapy. Bioengineered skin substitutes should have essential characteristics such as appropriate physical and mechanical properties; controlled degradation; sterility, non-toxicity and non-antigenicity; minimal inflammatory reactivity and provision of a vital barrier function. Additionally, three factors are crucial in the development of bioengineered skin [10]. First, for any cultured cells, scaffold materials or cell-material constructs may carry the risk of transmitting viral or bacterial infection and may also entail risk of disease, so it should have indispensable proven safety and benefit to patients. As well, bioengineered skin should heal well and facilitate angiogenesis. To achieve this objective, bioengineered skin substitutes must attach well to the wound bed, be supported by new vasculature, not be rejected by the immune system and be capable of self-repair. Also, the skin appendages such as sweat glands, sebaceous glands and hair follicles should be considered the main structures in bioengineered skin design and construction. Finally, bioengineered skin substitutes should have clear evidence of convenience of clinical use.
Clinical status
The technology of bioengineering skin is feasible and some products are already on the market, including cultured epithelial autografts (Epicel®, acellular dermal substitutes (Integra® Alloderm®, cell-populated dressings (TransCyte®, cellular dermal substitutes (Dermagraft®, and bilayered skin substitutes (Apligraf® Orcel®. Admittedly, these products bring a new development to therapeutic medicine and offer great promise in the treatment of burns, donor sites, chronic skin ulcers (e.g., venous, pressure and diabetic foot ulcers), and various other dermatological conditions. Current available bioengineered skin substitutes can be categorized by function into three groups: some replace the epidermal layer only, some provide a dermal substitute, and a small number provide both. The categories of main products that are available on the market, along with their characteristics and clinical uses are summarized in Table 1. In some clinical conditions (such as non-healing skin ulcers and superficial burns), simply transferring laboratory-expanded cells can benefit patients, but the treatment of major full-thickness burns requires the replacement of both dermis and epidermis. These skin-substitute products can act as temporary wound covers (dressing) or permanent skin replacements and provide an environment that stimulates their repair, depending on the design and composition.
Table 1.
Commercially available bioengineered skin products
| Category | Commercial name | Advantages | Disadvantages | Clinical uses |
|---|---|---|---|---|
| Epidermal cover | Epicel | large area of permanent wound coverage with little risk of rejection | longer time required to produce fragile confluent sheets | permanent closure in greater burn wound |
| EpiDex | cells with higher proliferative capacity; can be cryopreserved for repeat applications | longer time required from harvesting to produce; fragile | smaller chronic leg ulcers | |
| Dermal replacement | Integra | encourages ingrowth of fibroblasts and epithelial cells; epidermal layer removed when donor sites available for autografting | bovine collagen presents antigenicity and disease risk; longer time required to expand the dermal autograft | large burns and limited autograft donor sites |
| Alloderm | preserves matrix similar to normal human; Not rejected; no viral transmission | potential problems of graft rejection and disease transfer | burns and other wounds; repair of soft tissue defects | |
| TransCyte | dermal fibroblasts secrete ECM components and growth factors to aid wound healing; direct visualization of the wound bed | nylon mesh not biodegradable; rejection and disease risk from fibroblasts | excised burns awaiting placement of autograft | |
| Dermagraft | neonatal fibroblast rapidly proliferate to produce ECM components and growth factors to aid wound healing; facilitating observation of the underlying wound | potential risk of rejection and disease risk from fibroblasts | full-thickness foot ulcers | |
| Composite substitutes | Apligraf (Graftskin) | graft take comparable to autografts with good cosmetic results; improves granulation tissue deposition and no rejection observed | risk of chronic graft rejection and disease from allogeneic keratinocytes and fibroblasts | venous leg ulcers and diabetic foot ulcers |
| OrCel | provides a favourable environment for host cell migration and a source of cytokines and growth factors | only a biological dressing; risk of rejection and disease from bovine collagen and other cells | split-thickness donor sites in patients with burn |
The most basic and primary function of bioengineered skin products is to act as a barrier to fluid loss and microbial contamination. For example, Integra is designed for temporary use only, and its most important function is to serve as a template to generate a neodermis. Integra is a widely used option for covering excised burn wounds and is particularly valuable for patients with large burns and limited autograft donor sites [11]. Thirty-one patients who underwent Integra grafting for reconstructive surgery in 39 operational sites were observed in the clinic, with the length of follow-up ranging from 0.5 to 4 years; for 29 patients and 37 sites completing the study, the results were considered good in 28 cases, average in 6 and poor in just 3 [8]. The disadvantages of using Integra in reconstructive surgery are the necessity of two operations and the risks of infection under the silicone layer, the silicone becoming detached and recurrence of contraction. However, the advantages of Integra include its immediate availability, the availability of large quantities, the simplicity and reliability of the technique, and the pliability and cosmetic appearance of the resulting cover.
Alloderm is similar to Integra in that it is intended to provide a matrix for dermal tissue remodeling. It is composed of human allograft skin that has been screened for the absence of transmissible pathogens and then processed to remove epidermal components and all dermal cells. The ability of an acellular allograft dermal matrix to function as a permanent dermal transplant in full-thickness and deep partial-thickness burns was evaluated. This study consisted of a pilot phase (24 patients) to identify the optimal protocol and a study phase (43 patients) to evaluate graft performance. After 14-day take periods, the dermal matrix was statistically equivalent to the control autografts. Histology of the dermal matrix showed neovascularization and neoepithelialization without evidence of rejection. Assessment of wounds showed that thin split-thickness autografts plus allograft dermal matrix were equivalent to thicker split-thickness autografts [12]. This product might be useful for repair of soft-tissue defects, as in abdominal wall reconstruction [13].
TransCyte is used as a temporary covering for excised burns awaiting placement of autografts [14]. It has the advantage of being transparent for direct visualization of the wound bed, and the cells are destroyed to reduce the risk of immune response before grafting. Like TransCyte, Dermagraft is prepared with human neonatal fibroblasts, but in this skin substitute the fibroblasts are cryopreserved to maintain cell viability, and the matrix is made from a bioabsorbable polyglactin mesh [15]. Dermagraft is indicated for use in the treatment of full-thickness foot ulcers. It functions by providing a dermal matrix that facilitates re-epithelialization by the patient’s own keratinocytes. A study undertaken at 35 centers throughout the U.S. enrolled 314 patients to evaluate complete wound closure by 12 weeks. Patients were randomized to received either the Dermagraft or a control (conventional therapy). In terms of complete wound closure by week 12, 30.0% (39 of 130) of patients with wounds treated by Dermagraft healed as compared with 18.3% (21 of 115) of control patients (P = 0.023). The overall incidence of adverse events was similar for both the Dermagraft and control groups, but the Dermagraft group experienced significantly fewer ulcer-related adverse events [16].
Apligraf is a bi-layered living-skin construct consisting of a dermis and a well-differentiated epidermis. It has been useful for the treatment of venous leg and diabetic foot ulcers and has increased the proportion of wounds healed and decreased the time required for wound closure [17]. A trial comparing Apligraf and standardized wound care to standardized wound care alone in the treatment of diabetes-related neuropathic foot ulcers that were difficult to heal showed Apligraf as a valuable treatment adjunct in patients. Five of 9 patients (56%) receiving Apligraf therapy and standardized wound care showed complete healing, as compared with 3 of 8 control patients (37%) [18]. OrCel is similar in composition to Apligraf and it is designed for grafting to partial-thickness wounds to provide a favorable matrix for host cell migration and has been indicated for use in the treatment of donor sites in patients with burns. OrCel can be considered a biologic dressing because the components are intended for temporary wound coverage [19].
For larger wounds, such as giant nevi or burn wounds, autologous cells are generally required for permanent wound closure. The benefit of autologous skin substitutes is that once they have engrafted, permanent wound closure is accomplished and physiologic stability restored. Currently, Epicel is the only autologous cultured skin product commercially available in the United States; cultured epidermal autografts are sheets of autologous keratinocytes attached to a supportive petrolatum gauze backing that is removed approximately 1 week after grafting. Epicel has been extremely valuable for patients with very large (>60% TBSA) burns in whom the availability and/or quality of donor sites is poor [20]. EpiDex is an innovative grafting product composed of autologous keratinocytes cultured from the outer root sheath of anagen hair follicles transplanted with a supportive silicone membrane [21]. This product has improved the healing of chronic leg ulcers that are relatively small [22]. The common disadvantage of epithelial autografts is mechanical fragility, which results from the absence of an integrated dermal component at the time of grafting.
The variety of bioengineered skin products currently available has contributed to improved treatment of burns, chronic skin wounds, and congenital skin disorders. Although the skin substitutes are improved in quality and will become more similar to native skin autografts, they are not yet delivering significant progress in terms of clinical outcomes and have yet to replace the current “gold standard” of an autologous skin graft. Recent studies are addressing increasing homology to native human skin and improving functional outcomes of bioengineered skin. For example, to enhance the performance of bioengineered skin, genetic modification of cells has been used [23]. After being transplanted to full-thickness skin wounds on athymic mice, genetically modified skin containing FGF-7-expressing keratinocytes seeded onto acellular human dermis could secrete significant levels of FGF-7 and form a thicker and hyperproliferative epidermis with about four times the number of cells per square centimeter. Revascularization is more obvious because bioengineered skin expressing FGF-7 also produces more VEGF. Additionally, incorporation of other cell types may improve the effectiveness of bioengineered skin. Endothelial cells have been currently incorporated in bioengineered skin to initiate angiogenesis in vitro [24,25]. The pigmented skin models resulting from incorporation of melanocytes will benefit from a more thorough understanding of melanocyte function and factors that regulate skin pigmentation [26,27]. The combinatorial use of different skin cell types seems to contribute to the next generation of more functional and physiologically bioengineered skin products.
Clinically based research models
Despite the clinical success, bioengineered skin products have suffered commercial setbacks in recent years. However, the economic failure of bioengineered skin products should not be an obstacle in their being representative equivalents of human skin for research purposes. Currently, the in vitro-reconstructed 3-D skin models are widely used as promising tools in the laboratory to study all major principles in skin biology. The available products are composed of the epidermal layer only or both the epidermal and dermal layers. Within each layer, various types of cells can be incorporated, including keratinocytes, melanocytes and Langerhans cells in the epidermal layer and fibroblasts and endothelial cells in the dermal layer. Bioengineered skin products as an alternative to animal experimentation offer a way to not only concede to demands of regulatory authorities, animal welfare organizations, consumers and scientists but also provide a means to improve and extend our knowledge of biological processes in the skin. Although the models cannot be used as a complete substitute for in vivo animal or human studies because of their lacking any functioning vasculature or immune system, they have a significant advantage in their capability for adding or deleting different cell types to assess their relevance to the aspect of skin biology under investigation. This process cannot be readily done in vivo. These skin equivalents offer well-characterized models for studies of many areas, such as investigation of cell-cell and cell-ECM interactions, wound healing, angiogenesis, regulation of pigmentation, skin contraction and investigation of skin diseases such as melanoma invasion, psoriasis and skin blistering disorders, and the therapeutic strategies.
Test models to evaluate skin irritation in vitro
One of the most popular applications of bioengineered skin is to provide an examination platform for skin irritation caused by pharmaco-toxicological chemicals in vitro, if, ideally, dermal irritation can be evaluated in healthy volunteers in clinical trials (patch tests) under medical surveillance [28]. Nevertheless, this evaluation is not always possible because of ethical, safety and economic reasons. Animal models have been established as an alternative method for skin irritation testing for decades. However, animal models may not be appropriate testing platforms because of differences between animal and human physiology, in that the response does not always match the human response [29]. Testing for skin irritation in animals potentially causes them pain and discomfort, and the results are not always predictive for those found in humans. Additionally, pressure is increased for ethical laws on animal experimentation. Equipped with a normalized epidermal barrier function, bioengineered skin models could replace the traditional animal or human tests for determining the dermal irritation potential of a raw material or a finished product. Although such models cannot completely remove the need for some animal experimentation for the lack of immune and circulatory responses, they can massively reduce the number of animal dermatotoxicity tests in the cosmetology and dermatology fields.
Currently, the available skin products for testing skin irritation in vitro include EpiDerm, Episkin, Apligraf and Skinethic. The keratinocytes of all these products develop an organized stratum corneum, which resembles a functional barrier. Keratinocytes have been shown to be important in initiating, modulating and regulating skin irritation [30,31]. Certainly, bioengineered skin products differ from normal human skin in some characteristics. Compared to normal skin, bioengineered equivalents vary in the penetration rate of substances through the stratum corneum, with an approximately 10-30-fold higher permeability. This probably results in an over-prediction of irritants because of the higher penetration rate of applied substances and the higher availability of the substances in the living keratinocytes [32]. The quality of bioengineered human skin equivalents has now reached suitability for skin toxicity testing. The models are valuable for short-term toxicity assays to achieve a first impression of the toxic potential of a test compound. For the assessment of early stages of irritation and long-term exposure to mild irritants, specific protocols have still to be developed.
Wound healing study models
Skin wound healing encompasses a series of orchestrated cellular and molecular processes that act to repair the damaged tissue and reestablish the barrier function [33]. To fully understand the complexity of wound healing and the differences between regeneration and the normal outcome of tissue repair, namely, fibrosis and scarring, model systems that mimic the processes of normal wound repair and scarring must be developed. Animal wound models have established the necessary foundations for understanding reepithelialization, keratinocyte differentiation patterns, angiogenesis, inflammation, and scar formation. In particular, pig is an excellent model because of the anatomic and physiologic similarities of pig and human skin, but the difficulty of handling large animals and the high cost have restricted its wide use [34]. In contrast, small-animal models may not be appropriate testing platforms because of differences between animal and human physiology [35]. Consequently, in vitro wound models involving human cells in 3-D environments have been useful in obtaining quantitative data and delineating the complexity of wound healing.
Mimicking the skin structure, in vitro-constructed skin models consisting of epidermal keratinocytes growing on dermal equivalents represent a realistic biological tool that affords the opportunity to study the molecular mechanisms of cellular repair in vivo [36]. The scaffold-based skin models exhibit a superior epidermal architecture, with renormalized differentiation achieving and maintaining many traits of tissue homeostasis [37]. Thus, the regulation of keratinocyte growth and differentiation, the dynamics of basement membrane (BM) formation and the role of epithelial-mesenchymal interactions could be assessed. Furthermore, new insights could be gained into wound healing processes such as reepithelialisation and epidermal barrier development. As well, the in vitro skin models are of reliable robustness to perform long-term growth and differentiation of cells, thus enabling studies of later phases of skin regeneration and tissue stabilization. In addition, advances in the preparation of bioengineered skins demonstrate the ease of adding to the dermal component different cell types such as capillary-forming endothelial cells and even hair, which suggests that improvements to this model may afford the opportunity to study the effects of cell-cell interactions in wound healing. Advances in understanding the complex process of wound healing and the development of novel therapies would benefit from models that closely mimic the physiology of human wounds. Despite these benefits, bioengineered skin lacks the complexity of the tissue in vivo and cannot be used to study important aspects of healing such as vascularization or regeneration of nerve endings.
Human skin disease models and therapeutic systems
The stratum corneum is an important permeability barrier for the skin. The disorganization of the skin protective barrier characterizes some skin diseases, and psoriasis is a common example. Psoriasis does not exist as a spontaneous disease in the skin of lower animals, but some features of psoriasis have been induced in murine skin by genetic or immune manipulations. Even so, the structure of murine skin imposes serious limitations on resulting cellular alterations and, so far, psoriasis has not been faithfully reproduced by manipulation of native skin in any lower species [38]. Better model systems are needed to dissect the interactions of many complex molecular pathways or networks in the skin and for testing possible therapeutic targets for psoriasis and other inflammatory diseases. Because most therapeutic systems are directed toward human care, the ideal model system is one that represents certain aspects of human physiology but does not require human volunteers for experiments. Hence, vitroin and in vivo models based on bioengineered skin have been created with cells from patients to replicate aspects of the disease and for research into the disease and the subsequent development of therapeutic strategies.
For example, psoriatic skin is known to be more permeable than normal human skin. An in vitro human psoriatic model has been created by engineering the pathological substitutes with cells isolated from psoriatic biopsies and the auto-assembly method, which could be used to investigate the mechanisms of abnormal keratinocyte growth and to study cell-cell interactions [39]. The substitutes produced with psoriatic keratinocytes show a higher rate of proliferation and an under/overexpression of certain differentiation markers in comparison to healthy substitutes. This observation indicates that even if the in vitro cellular proliferation is accelerated, some differences between healthy and psoriatic cells are maintained in vitro, which suggests that this model can be used to study hyperproliferative or abnormal differentiation diseases such as psoriasis. This model could be used to better understand the mechanisms implicated in psoriasis and could be an excellent tool for the study of accelerated cellular differentiation of psoriatic keratinocytes. Although the clinical and histological characteristics of psoriasis are maintained in the pathological skin substitutes for a sufficient period of time to test pharmacological drugs, the models should be further developed to include elements of the immune system that may help determine the roles of different T-cell subsets and cytokines in psoriasis [40].
Bioengineered skin models for contraction studies
Skin graft contraction is a common and intractable problem. Wound contracture and scarring might be also considered more by clinicians and research scientists because for the patient, these issues are as important as successful restoration of barrier function and more must be done to improve the quality of the final result. Moreover, wound contraction leads to distortion of the surrounding tissues with associated cosmetic deformity and limitation of joint mobility; scars in the skin are less resistant to ultraviolet radiation, and sweat glands and hair follicles do not grow back within scar tissue. Despite 50 years of research in this area, the treatment and prevention of graft contraction has progressed very little and understanding the underlying mechanism remains poor. To identify the cellular activity underlying contraction, several research groups have developed bioengineered skin models for in vitro and in vivo use and have studied the contraction of these models. Bioengineered skin models for contraction studies offer an interesting perspective on what may be happening in vivo following skin grafting.
Although fibroblasts and myofibroblasts are very effective in contracting collagen gels, they appear ineffective at contracting more complex dermal architecture in these in vitro bioengineered skin models. However, keratinocytes are effective in contracting both collagen gels and the complex dermal architecture of bioengineered skin both in vitro and in vivo [41,42]. The knowledge that keratinocyte differentiation seems to be inextricably bound with contraction of bioengineered skin (regardless of any role that the fibroblast may or may not play) offers an easily accessible target for topical therapy to reduce skin graft contraction. This model is also applied to identify new pharmacological targets to block contraction. Recent evidence shows that the combination of early mechanical restraint and hydrogel delivery of topical agents to delay keratinocyte differentiation or prevent crosslink formation should be explored in the hope that this may significantly reduce graft contraction [43].
Summary and future prospects
The launch of early bioengineered skin products was heralded by much excitement in the medical, scientific, and financial communities, but time has tempered the enthusiasm for these products owing to their high cost and narrow clinical utility. More significantly, these high-cost products to some extent have not been considerably more effective than standard treatments. Skin bioengineering seemed to suffer from the pain of the developmental bottleneck at the beginning of this century. Currently, 30 years of research and experience has resulted in a relatively mature understanding the bioengineered skin and a relatively clear comprehension of the challenges facing skin bioengineering. Despite the pitfalls experienced in light of unrealistic expectations both clinically and commercially, conclusions of little room for improvement in this field are premature. The first-approved products, Dermagraft, Apligraf, and Epicel, are still being marketed and new ones, such as Or-Cel and Epidex, continue to be developed [44]. With hope, new strategies will be developed to conquer at least some of the problems identified in this review.
From cell biology and wound healing knowledge, increasing technological advances have been made in the last few years, with significant opportunities for multi-disciplinary groups to overcome the existing challenges. Eliminating or reducing the risk of disease transmission remains a major problem for bioengineered skin application. For this reason, synthetic collagen-mimetic materials or recombinant-produced collagen and reliable cells are needed to improve the safety. Next, the conventional bioengineered skin products are most likely to cause graft contraction and scarring at the margins. Understanding the biology of wound healing and in vitro models of bioengineered skin are offering insight into how to prevent these issues, and the new bioengineered skin products that may overcome the drawbacks involved in current products are now being developed as more effective therapies for patients.
Although the challenge of building a completely functional skin is ostensibly insurmountable, multi-disciplinary researchers, including biologists, clinicians, engineers, and biologists, continue to work together to meet this challenge. Recent and ongoing studies are addressing the preparation of bioengineered skin containing additional cell types to increase homology to native human skin and improve functional outcome. Figure 1 summarizes these promising strategies that offer the complete restoration of functional bioengineered skin. For example, incorporation of endothelial cells has been studied to initiate angiogenesis in bioengineered skin grafts in vitro [45]. Human umbilical-vein endothelial cells are readily available but can only be used in allogeneic skin substitutes. For clinical application of autologous endothelialized skin substitutes, use of dermal endothelial cells is optimal, and these cells should be isolated from the same skin biopsy used for preparation of fibroblast and keratinocyte cultures. Another potential cell type is melanocytes, which could have an important role in correcting absent or irregular pigmentation. Bioengineered skin will benefit from a more thorough understanding of melanocyte function and factors that regulate skin pigmentation. An exciting possibility for development of complete skin substitutes involves the addition of hair-follicle stem cells. Multipotent skin stem cells are known to reside in the hair-follicle bulge and have inherent capacity for regeneration. The regenerative role of bulge cells from the skin is multiple: they not only contribute to the production of epidermis and hair follicles but also are key to the formation of sebaceous glands. Induced pluripotent stem cells (iPS) is another source for seed cells in skin engineering, and as a research tool, they provide unprecedented opportunities to investigate reprogramming mechanisms and regenerative medicine. An exciting prospect is to make possible the bioengineering of other appendages, such as sweat glands within skin substitutes, and the initial results are positive.
Figure 1.
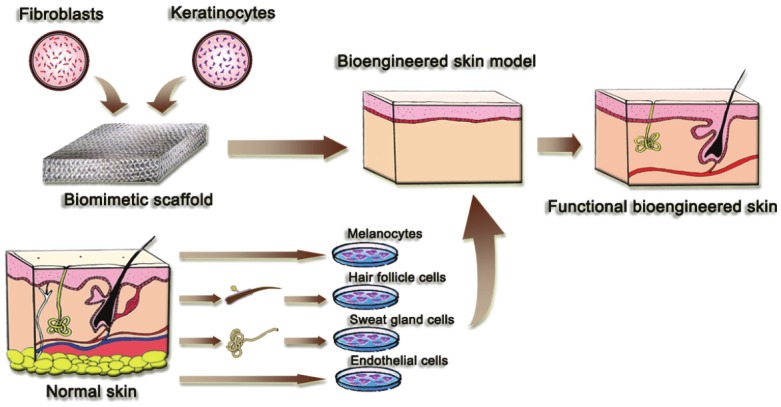
Promising strategies for complete restoration of functional bioengineered skin
Such integrated bioengineered skin should fulfill many normal functions and move more quickly from laboratory to clinical use with advances in research and development technologies.
Acknowledgements
This study was supported in part by the National Basic Science and Development Program (973 Program 2012CB518105) and the National Natural Science Foundation (30672176, 30770966) of China.
Competing interest statement
The authors declare that they have no competing financial interests.
References
- 1.Phillips TJ, Gilchrest BA. Cultured epidermal grafts in the treatment of leg ulcers. Adv Dermatol. 1990;5:33–48. [PubMed] [Google Scholar]
- 2.Uitto J, Pulkkinen L. Molecular genetics of heritable blistering disorders. Arch Dermatol. 2001;137:1458–61. doi: 10.1001/archderm.137.11.1458. [DOI] [PubMed] [Google Scholar]
- 3.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinising colonies from single cells. Cell. 1975;6:331–43. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 4.Rheinwald JG, Green H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature. 1977;265:421–4. doi: 10.1038/265421a0. [DOI] [PubMed] [Google Scholar]
- 5.Wright KA, Nadire KB, Busto P, Tubo R, McPherson JM, Wentworth BM. Alternative delivery of keratinocytes using a polyurethane membrane and the implications for its use in the treatment of full-thickness burn injury. Burn. 1998;24:7–17. doi: 10.1016/s0305-4179(97)00075-2. [DOI] [PubMed] [Google Scholar]
- 6.Bello YM, Falabella AF. The role of Graftskin Apligraf®in difficult-to-heal venous leg ulcers. JWound Care. 2003;11:182–3. doi: 10.12968/jowc.2002.11.5.26402. [DOI] [PubMed] [Google Scholar]
- 7.Marston WA, Hanft J, Norwood P, Pollak R. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26:1701–5. doi: 10.2337/diacare.26.6.1701. [DOI] [PubMed] [Google Scholar]
- 8.Dantzer E, Braye FM. Reconstructive surgery using an artificial dermis Integra: results with 39 grafts. Br J Plast Surg. 2001;54:659–64. doi: 10.1054/bjps.2001.3684. [DOI] [PubMed] [Google Scholar]
- 9.Metcalfe AD, Ferguson MW. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J R Soc Interface. 2007;4:413–37. doi: 10.1098/rsif.2006.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacNeil S. Progress and opportunities for tissue-engineered skin. Nature. 2007;445:870–80. doi: 10.1038/nature05664. [DOI] [PubMed] [Google Scholar]
- 11.Wisser D, Steffes J. Skin replacement with a collagen based dermal substitute, autologous keratinocytes and fibroblasts in burn trauma. Burns. 2003;29:375–80. doi: 10.1016/s0305-4179(03)00013-5. [DOI] [PubMed] [Google Scholar]
- 12.Wainwright D, Madden M, Luterman A, Hunt J, Monafo W, Heimbach D, Kagan R, Sittig K, Dimick A, Herndon D. Clinical evaluation of an acellular allograft dermal matrix in full-thickness burns. J Burn Care Rehabil. 1996;17:124–36. doi: 10.1097/00004630-199603000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Menon NG, Rodriguez ED, Byrnes CK, Girotto JA, Goldberg NH, Silverman RP. Revascularization of human acellular dermis in full-thickness abdominal wall reconstruction in the rabbit model. Ann Plast Surg. 2003;50:523–7. doi: 10.1097/01.SAP.0000044252.76804.6B. [DOI] [PubMed] [Google Scholar]
- 14.Lorenz RR, Dean RL, Hurley DB, Chuang J, Citardi MJ. Endoscopic reconstruction of anterior and middle cranial fossa defects using acellular dermal allograft. Laryngoscope. 2003;113:496–501. doi: 10.1097/00005537-200303000-00019. [DOI] [PubMed] [Google Scholar]
- 15.Kumar RJ, Kimble RM, Boots R, Pegg SP. Treatment of partial-thickness burns: a prospective randomized trial using Transcyte®. Aust N Z J Surg. 2004;74:622. doi: 10.1111/j.1445-1433.2004.03106.x. [DOI] [PubMed] [Google Scholar]
- 16.Marston WA, Hanft J, Norwood P, Pollak R. The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: results of a prospective randomized trial. Diabetes Care. 2003;26:1701–5. doi: 10.2337/diacare.26.6.1701. [DOI] [PubMed] [Google Scholar]
- 17.Eaglstein WH, Iriondo M, Laszlo K. A composite skin substitute (Graftskin) for surgical wounds: a clinical experience. Dermatol Surg. 1995;21:839–43. doi: 10.1111/j.1524-4725.1995.tb00709.x. [DOI] [PubMed] [Google Scholar]
- 18.Sams HH, Chen J, King LE. Graftskin treatment of difficult to heal diabetic foot ulcers: one center’s experience. Dermatol Surg. 2002;28:698–703. doi: 10.1046/j.1524-4725.2002.02019.x. [DOI] [PubMed] [Google Scholar]
- 19.Stephens R, Wilson K, Silverstein P. A premature infant with skin injury successfully treated with bilayered cell matrix. Ostomy/Wound Manage. 2002;48:34–8. [PubMed] [Google Scholar]
- 20.Carsin H, Ainaud P, Le Bever H, Rives J, Lakhel A, Stephanazzi J, Lambert F, Perrot J. Cultured epithelial autografts in extensive burn coverage of severely traumatized patients: a five year single-center experience with 30 patients. Burns. 2000;26:379–87. doi: 10.1016/s0305-4179(99)00143-6. [DOI] [PubMed] [Google Scholar]
- 21.Yang JS, Lavker RM, Sun TT. Upper human hair follicle contains a subpopulation of keratinocytes with superior in vitro proliferative potential. J Invest Dermatol. 1993;101:652–9. doi: 10.1111/1523-1747.ep12371671. [DOI] [PubMed] [Google Scholar]
- 22.Limat A, Mauri D, Hunziker T. Successful treatment of chronic leg ulcers with epidermal equivalents generated from cultured autologous outer root sheath cells. J Invest Dermatol. 1996;107:128–35. doi: 10.1111/1523-1747.ep12298415. [DOI] [PubMed] [Google Scholar]
- 23.Erdag G, Medalie DA, Rakhorst H, Krueger GG, Morgan JR. FGF-7 expression enhances the performance of bioengineered skin. Mol Ther. 2004;10:76–85. doi: 10.1016/j.ymthe.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 24.Sahota PS, Burn JL, Heaton M, Freedlander E, Suvarna SK, Brown NJ, Mac Neil S. Development of a reconstructed human skin model for angiogenesis. Wound Repair Regen. 2003;11:275–84. doi: 10.1046/j.1524-475x.2003.11407.x. [DOI] [PubMed] [Google Scholar]
- 25.Pollman MJ, Naumovski L, Gibbons GH. Endothelial cell apoptosis in capillary network remodeling. J Cell Physiol. 1999;178:359–70. doi: 10.1002/(SICI)1097-4652(199903)178:3<359::AID-JCP10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 26.Abdel-Malek ZA. Endocrine factors as effectors of integumental pigmentation. Dermatol Clin. 1988;6:175–83. [PubMed] [Google Scholar]
- 27.Nordlund JJ, Abdel-Malek ZA, Boissy RE, Rheins LA. Pigment cell biology: an historical review. J Invest Dermatol. 1989;92:53S–60S. doi: 10.1111/1523-1747.ep13074988. [DOI] [PubMed] [Google Scholar]
- 28.York M, Griffiths HA, Whittle E, Basketter DA. Evaluation of a human patch test for the identification and classification of skin irritation potential. Contact Dermatitis. 1996;34:204–12. doi: 10.1111/j.1600-0536.1996.tb02175.x. [DOI] [PubMed] [Google Scholar]
- 29.Fentem JH, Briggs D, Chesne C, Elliott GR, Harbell JW, Heylings JR, Portes P, Roguet R, van de Sandt JJ, Botha PA. Aprevalidation study on in vitro tests for acute skin irritation. Results and evaluation by the Management Team. Toxicol In Vitro. 2001;15:57–93. doi: 10.1016/s0887-2333(01)00002-9. [DOI] [PubMed] [Google Scholar]
- 30.Poumay Y, Coquette A. Modelling the human epidermis in vitro: tools for basic and applied research. Arch Dermatol Res. 2007;298:361–9. doi: 10.1007/s00403-006-0709-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boelsma E, Gibbs S, Faller C, Ponec M. Characterization and comparison of reconstructed skin models:morphological and immunohistochemical evaluation. Acta Dermato Venereol. 2000;80:82–8. [PubMed] [Google Scholar]
- 32.Perkins MA, Osborne R, Rana FR, Ghassemi A, Robinson MK. Comparison of in vitro and in vivo human skin response to consumer products and ingredients with a range of irritancy potential. Toxicol Sci. 1999;48:218–29. doi: 10.1093/toxsci/48.2.218. [DOI] [PubMed] [Google Scholar]
- 33.Laplante AF, Germain L, Auger FA, Moulin V. Mechanisms of wound reepithelialization: Hints from a tissue-engineered reconstructed skin to long-standing questions. FASEB J. 2001;15:2377–89. doi: 10.1096/fj.01-0250com. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen. 2001;9:66–76. doi: 10.1046/j.1524-475x.2001.00066.x. [DOI] [PubMed] [Google Scholar]
- 35.Garlick JA, Taichman LB. Fate of human keratinocytes during reepithelialization in an organotypic culture model. Lab Invest. 1994;70:916–24. [PubMed] [Google Scholar]
- 36.Geer DJ, Swartz DD, Andreadis ST. Fibrin promotes migration in a three-dimensional in vitro model of wound regeneration. Tissue Eng. 2002;8:787–98. doi: 10.1089/10763270260424141. [DOI] [PubMed] [Google Scholar]
- 37.Bell E, Ehrlich HP, Buttle DJ, Nakatsuji T. Living tissue formed in vitro and accepted as skin-equivalent tissue of full thickness. Science. 1981;211:1052–4. doi: 10.1126/science.7008197. [DOI] [PubMed] [Google Scholar]
- 38.Lebwohl M. Psoriasis. Lancet. 2003;361:1197–204. doi: 10.1016/S0140-6736(03)12954-6. [DOI] [PubMed] [Google Scholar]
- 39.Bernard G, Auger M, Soucy J, Pouliot R. Physical characterization of the stratum corneum of an in vitro psoriatic skin model by ATR-FTIR and Raman spectroscopies. Biochim Biophys Acta. 2007;1770:1317–23. doi: 10.1016/j.bbagen.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 40.Barker CL, McHale MT, Gillies AK, Waller J, Pearce DM, Osborne J, Hutchinson PE, Smith GM, Pringle JH. The development and characterization of an in vitro model of psoriasis. J Invest Dermatol. 2004;123:892–901. doi: 10.1111/j.0022-202X.2004.23435.x. [DOI] [PubMed] [Google Scholar]
- 41.Ralston DR, Layton C, Dalley AJ, Boyce SG, Freedlander E, MacNeil S. Keratinocytes contract human dermal extracellular matrix and reduce soluble fibronectin production by fibroblasts in a skin composite model. Br J Plast Surg. 1997;50:408–15. doi: 10.1016/s0007-1226(97)90327-1. [DOI] [PubMed] [Google Scholar]
- 42.Chakrabarty KH, Heaton M, Dalley AJ, Dawson RA, Freedlander E, Khaw PT, Mac Neil S. Keratinocyte-driven contraction of reconstructed human skin. Wound Repair Regen. 2001;9:95–106. doi: 10.1046/j.1524-475x.2001.00095.x. [DOI] [PubMed] [Google Scholar]
- 43.Harrison CA, Gossiel F, Layton CM, Bullock AJ, Johnson T, Blumsohn A, MacNeil S. Use of an in vitro model of tissue engineered skin to investigate the mechanism of skin graft contraction. Tissue Eng. 2006;12:3119–33. doi: 10.1089/ten.2006.12.3119. [DOI] [PubMed] [Google Scholar]
- 44.Ehrenreich M, Ruszczak Z. Update on tissue-engineered biological dressings. Tissue Eng. 2006;12:2407–24. doi: 10.1089/ten.2006.12.2407. [DOI] [PubMed] [Google Scholar]
- 45.Supp DM, Wilson-Landy K, Boyce ST. Human dermal microvascular endothelial cells form vascular analogs in cultured skin substitutes after grafting to athymic mice. FASEB J. 2002;16:797–804. doi: 10.1096/fj.01-0868com. [DOI] [PMC free article] [PubMed] [Google Scholar]


