Abstract
Osteoporosis is common in human immunodeficiency virus (HIV)–infected persons. The etiology of osteoporosis in HIV-infected patients is likely multifactorial, involving traditional risk factors such as low body weight, hypogonadism, and smoking, as well as direct effects of chronic HIV infection and antiretroviral therapy. Emerging evidence suggests that the increasing prevalence of osteoporosis in HIV-infected persons translates into a higher risk of fracture, likely leading to excess morbidity and mortality as the HIV-infected population ages. This review addresses the epidemiology of osteoporosis, discusses the causes of low bone mineral density in HIV-infected persons, including the impact of specific antiretroviral therapies, and offers recommendations on screening and treating vitamin D deficiency and osteoporosis.
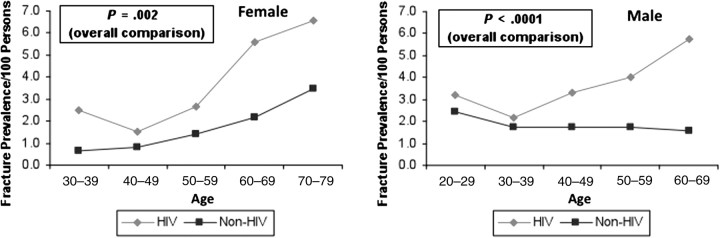
Osteoporosis, which affects >10 million Americans, is characterized by low bone mass, deterioration of bone tissue, disruption of bone architecture, and compromised bone strength, resulting in an increased risk of fracture [1, 2]. Osteoporosis is common in human immunodeficiency virus (HIV)–infected populations and is likely to become an important cause of morbidity and mortality as the HIV-infected population ages [3]. Data are emerging that suggest the increased risk of osteoporosis translates into a higher risk of osteoporosis-related fracture. In a population-based study at a large US healthcare system, the period prevalence of fractures of the spine, hip, and wrist, sites commonly associated with osteoporosis, was 60% higher in HIV-infected men and women compared with HIV-uninfected persons [4] (Figure 1). Similar results have been found in the Veterans Aging Cohort Study [5] and HIV Outpatient Study [6]. Early detection of osteoporosis, prior to the clinical presentation of fracture, and institution of appropriate treatment can decrease the burden of osteoporosis-associated fractures in HIV-infected persons. HIV-infected patients are also at increased risk for osteonecrosis of the hip and other bones, although a full discussion of osteonecrosis is beyond the scope of this review [7].
Figure 1.
Comparison of fracture prevalence in human immunodeficiency virus (HIV)–infected vs non-HIV-infected patients according to gender and age group. From Triant et al [4]. Copyright Endocrine Society 2008.
OSTEOPOROSIS: DEFINITION
In postmenopausal women and men aged ≥50 years, osteoporosis is defined as a dual-energy, x-ray absorptiometry (DXA)–derived bone mineral density (BMD) measurement at the hip or spine ≤2.5 standard deviations (SDs) below the mean BMD for a healthy, young, sex-matched population (T score) [2, 8]. The diagnosis of osteoporosis can also be made when a hip or spine fragility fracture is present, regardless of BMD [8]. A fragility fracture is generally defined as a fracture resulting from trauma equivalent to or less than a fall from a standing position. Osteopenia is defined as a T score between −1.0 and −2.5 [2]. In older populations, the risk of fracture increases by 2- to 3-fold for each SD decrease in BMD below the young normal mean [9]. For premenopausal women and men aged <50 years, a z score (SD below sex- and ethnicity-matched population of the same age) ≤−2.0 is considered abnormal [2]. In this patient population, an abnormal BMD should be interpreted within the context of the person’s risk for fracture, including prior fragility fracture, and the coexistence of diseases, conditions, or medications that may increase the risk of fracture.
ETIOLOGIES OF LOW BMD IN HIV INFECTION
Among HIV-infected persons, the etiology of osteoporosis is likely multifactorial. Traditional risk factors such as hypogonadism, smoking, alcohol use, opiate use, physical inactivity, low body weight, and vitamin D deficiency contribute to the increased risk, and the direct effects of antiretroviral therapy (ART) and chronic immune activation by HIV infection likely also play an important role [10–12]. Typically, bone remodeling involves the tightly coupled processes of bone resorption and bone formation. In untreated HIV, through direct viral effects and inflammatory effects, bone resorption and bone formation are uncoupled. In particular, in vitro studies have shown that HIV viral proteins Vpr and gp120 stimulate osteoclast activity [13, 14] and p55-gag suppresses osteoblast activity and increases osteoblast apoptosis [15]. In addition, inflammatory cytokines, such as tumor necrosis factor α in in vitro and in vivo studies [16] and interleukin 6 in in vitro studies [17, 18], promote osteoclastogenesis and bone resorption. High concentrations of HIV RNA have been associated with elevated levels of receptor activator of nuclear factor κB ligand (RANKL), an osteoblast-secreted cytokine that promotes osteoclast formation [19].
Specific components of antiretroviral therapy have also been implicated in the pathogenesis of reduced BMD in HIV-infected persons. Randomized controlled trials comparing BMD in protease inhibitor (PI) vs non-PI regimens have shown mixed results, with some studies revealing that PI-containing regimens led to decreased spine BMD and others showing no difference in total body or hip BMD between treatment groups [12, 20, 21]. The reason for the differential response by site deserves further study. Despite the mixed effects on BMD, cumulative exposure to boosted PI was found to be associated with an increased risk of fracture (hazard ratio [HR], 1.11; 95% confidence interval [CI], 1.05–1.18; P < .001) in a study of the Veterans Affairs Clinical Case Registry [22]. In particular, treatment with lopinavir/ritonavir led to a 17% increased risk of incident hip, vertebral, or wrist fracture [22].
Randomized trials comparing BMD in tenofovir vs nontenofovir regimens have consistently found that tenofovir is associated with significantly decreased BMD at the hip and spine [10, 23, 24]. The clinical relevance of these findings requires further study. A study of the Veteran Affairs Clinical Case Registry specifically aimed to define the impact of cumulative therapy with tenofovir vs other ART on the risk of osteoporotic fractures in HIV-infected patients during the pre–highly active ART (HAART) (1988–1995) and HAART (1996–2009) eras [22]. During the HAART era, cumulative exposure to tenofovir was associated with an increased risk of incident fracture (HR, 1.16; 95% CI, 1.08–1.24; P < .0001) [22].
The mechanisms underlying tenofovir’s effect on bone are not clear. Experimental models have shown that tenofovir impairs bone mineralization [25] through its effects on renal phosphate handling, resulting in increased bone turnover and osteomalacia [26]. These effects may be worsened with concomitant vitamin D deficiency [27].
Multiple studies have assessed the impact of ART initiation on bone mineral density and have generally shown a 2%–6% loss of BMD after 48–96 weeks of therapy, regardless of the type of ART initiated [20]. This degree of bone loss is larger than what would be expected by aging alone [28] and is comparable to the bone loss seen in women aged 50–59 over 2 years [29]. Importantly, lower CD4 cell count prior to ART initiation is associated with greater decreases in BMD, implying that earlier initiation of ART would attenuate the bone loss related to ART initiation [10].
Bone mineral density loss with ART initiation is likely explained by a rapid increase in bone turnover, as studies have shown significant increases in osteocalcin, a marker of bone formation, and c-telopeptide, a marker of bone resorption, following initiation of ART [24]. Markers of bone resorption are already elevated with untreated HIV infection and increase earlier and to a greater extent than markers of bone formation, creating a “catabolic window” during the first 6 months after ART initiation [30]. Although ART initiation is associated with significant bone loss, several longitudinal studies have shown that with continued ART use, BMD stabilizes over time [31, 32].
SCREENING FOR BONE DISEASE IN HIV
The National Osteoporosis Foundation recommends osteoporosis screening with DXA BMD testing for all women aged ≥65 years and men aged ≥70 years, regardless of clinical risk factors, and for adults aged >50 years who sustain a fracture [2]. Younger postmenopausal women and men aged 50–69 years should also undergo BMD testing if there is concern for osteoporosis based on their clinical risk factor profile [2]. Given the substantial evidence of the association between HIV infection and low BMD, McComsey et al, in their review of bone disease in HIV infection [33], recommended a screening DXA scan for all HIV-infected postmenopausal women and men aged ≥50. The cost-effectiveness of this screening approach has not yet been determined.
DIAGNOSIS AND WORK-UP OF SECONDARY CAUSES OF LOW BONE MINERAL DENSITY
Once a diagnosis of osteoporosis is made, then an investigation into secondary causes (disease processes and medications causing or exacerbating osteoporosis) should be undertaken. In HIV-infected persons, low BMD has been associated with low body weight, testosterone or estrogen deficiency, malabsorption, duration of HIV infection, glucocorticoids, lipodystrophy, insulin resistance, and hyperlactatemia [33]. Additionally, endocrine, renal, gastrointestinal, and hematologic disorders, such as vitamin D deficiency, hyperparathyroidism, subclinical hyperthyroidism, Cushing syndrome, exogenous corticosteroid use, phosphate wasting, idiopathic hypercalciuria, celiac sprue, multiple myeloma, and mastocytosis, are associated with low bone mineral density in both the general population and HIV-infected persons. Secondary causes can be suspected on the basis of a thorough history and physical examination, and laboratory testing can be used to confirm the diagnosis (Table 1).
Table 1.
Causes of Osteoporosis and Work-up
| Osteoporosis-Associated Condition | Laboratory Evaluation |
| Endocrine disorders | |
| Vitamin D deficiency | 25-hydroxyvitamin D |
| Hyperparathyroidism | iPTH, total calcium, phosphate, albumin, creatinine |
| Subclinical hyperthyroidism | TSH, free T4 |
| Hypogonadism | Males: free testosteroneFemales: estradiol, follicle-stimulating hormone, prolactin |
| Renal disorders | |
| Phosphate wasting | Simultaneous serum phosphate and creatinine and spot urine phosphate and creatinine to calculate fractional excretion of phosphate |
| Idiopathic hypercalciuria | 24-hour urinary calcium |
| Gastrointestinal disorders | |
| Celiac sprue | IgG and IgA antitissue transglutaminase |
| Hematologic disorders | |
| Multiple myeloma | Complete blood count, serum protein electrophoresis |
| Mastocytosis | Serum tryptase |
Abbreviations: IgA, immunoglobulin A; IgG, immunoglobulin G; iPTH, intact parathyroid hormone; TSH, thyroid stimulating hormone; T4, thyroxine.
Because tenofovir use can be associated with proximal tubule dysfunction, a high degree of suspicion for phosphate wasting is prudent for tenofovir-treated patients. Specifically, the authors recommend that patients with low trauma or atraumatic fractures or low BMD (T score <−1.0) on tenofovir undergo assessment for renal phosphate wasting, using simultaneous measurements of serum and urine phosphate and creatinine to calculate the fractional excretion of phosphate. If hyperphosphaturia is present (ie, if the fractional excretion of phosphate is >20%–30%), transition to a nontenofovir-containing ART regimen should be considered. In a patient with a fragility fracture or very low bone mineral density (T score <−3), it would be reasonable to avoid tenofovir if other antiretroviral options are available. Further work is needed to determine what clinical, biochemical, and genetic factors can identify those who will experience clinically significant loss of BMD and fracture with tenofovir therapy.
TREATMENT
After secondary causes of low BMD have been diagnosed and treated, pharmacologic treatment of osteoporosis should be considered for postmenopausal women and men aged ≥50 years with hip or vertebral fractures, a BMD T score ≤−2.5 at the femoral neck or spine, or low bone mass, meaning a BMD T score between −1.0 and −2.5 at the femoral neck or spine and a 10-year probability of hip fracture ≥3% or a 10-year probability of a major osteoporosis-related fracture ≥20% based on the US FRAX model [2]. The US FRAX model is a World Health Organization fracture risk assessment tool, useful in patients with low BMD and calibrated to US fracture and mortality rates. Notably, FRAX has not been validated in HIV-infected persons and may underestimate the 10-year risk of fracture in these patients [33].
The National Osteoporosis Foundation supports several strategies to reduce fracture risk, including adequate intake of calcium and vitamin D. The Institute of Medicine (IOM) recently recommended calcium 1000 mg/day for men and women aged 31–50 years, 1000 mg/day for men aged 51–70 years, and 1200 mg/day for women aged 51–70 years [34]. The safety of calcium supplementation has been questioned given recent studies showing an increased risk of cardiovascular events, but definitive conclusions have not been made [35]. Calcium intake requirements are best achieved through dietary sources, but calcium supplementation remains a good option for those who cannot achieve adequate calcium intake through diet alone.
Lifelong participation in 30 minutes of weight-bearing (jogging, dancing, walking) and muscle-strengthening exercise (ie, weight training) at least 3 days a week is also recommended, as such exercise may increase bone density [36]. Smoking cessation and limitation of alcohol intake are suggested [2]. Strategies to assess and reduce fall risk, such as checking and correcting vision and hearing, evaluating neurological problems, and referring patients to physical and occupational therapy, are also recommended.
VITAMIN D DEFICIENCY
Low vitamin D levels have been implicated in the pathogenesis of osteoporosis in the general population and in HIV-infected persons. Among 672 HIV-infected participants in a prospective, observational cohort study, 70.3% (95% CI, 68.1%–74.9%) were vitamin D insufficient or deficient (25-hydroxyvitamin D [25(OH)D] <20 ng/mL), compared with 79.1% (95% CI, 76.7%–81.3%) of US adults [37]. African American race, Hispanic ethnicity, and exposure to efavirenz were associated with an increased risk of vitamin D deficiency in this cohort [37]. Efavirenz may induce the catabolism of 25(OH)D through the induction of CYP24A, the enzyme that converts 25(OH)D to its inactive metabolite 24,25-dihydroxyvitamin D [38], and multiple studies have documented an independent, though modest, efavirenz effect on 25(OH)D [39–41].
The approach to screening for vitamin D deficiency is controversial. The IOM did not specify recommendations for screening in its recent report on dietary goals for vitamin D intake [34]. Conversely, the European AIDS Clinical Society recommends screening all HIV-infected persons at the time of diagnosis and at 2-year intervals thereafter [42]. The Endocrine Society recommends against universal screening for vitamin D deficiency and endorses screening in patients at high risk for deficiency, which includes those taking AIDS medication [43].
Arguments against the widespread screening for vitamin D deficiency in HIV-infected patients include the unclear benefit of vitamin D replacement for nonmusculoskeletal outcomes, the cost of laboratory testing, and the potential adverse effects of some replacement approaches. For example, high yearly loading doses (500 000 IU of vitamin D3) were associated with increased falls and fractures in a recent randomized controlled trial of postmenopausal women [44]. Although the dosing regimen used in this trial differed greatly from the regimens generally used in clinical practice, this unexpected result highlights that the optimal vitamin D replacement regimen is not known and some approaches may be associated with harm, despite achieving 25(OH)D levels in the target range of 30–50 ng/mL.
For these reasons, for HIV-infected individuals with normal BMD and no history of fractures or falls, we recommend against the routine testing of 25(OH)D. However, vitamin D deficiency should be investigated in HIV-infected persons with osteopenia or osteoporosis as part of the work-up of secondary causes of osteoporosis.
Vitamin D can be replaced by vitamin D2 (ergocalciferol) or the more potent and bioavailable vitamin D3 (cholecalciferol) [43]. Recently the IOM recommended 600 IU/day for men and women aged 31–70 years and 800 IU/day for men and women >70 years [34]. These recommendations are similar to those endorsed by the Endocrine Society, which suggest that adults aged 19–70 years and >70 years receive at least 600 and 800 IU/day, respectively, of vitamin D to maximize bone health and muscle function [43]. Among adults aged >65 years, the Endocrine Society recommends 800 IU/day of vitamin D for the prevention of falls and fractures [43]. Importantly, the guidelines note that 1500–2000 IU/day of vitamin D may be required to raise the blood level of 25(OH)D consistently above 30 ng/mL [43]. Our recommendations for dietary intake of vitamin D and the treatment of vitamin D insufficiency and deficiency are detailed in Table 2. Because of the effect of efavirenz on 25(OH)D concentrations, efavirenz-treated patients may require higher doses of vitamin D to achieve or maintain target concentrations (eg, approximately 2000 IU D3 per day in a patient not known to be vitamin D deficient).
Table 2.
Recommended Daily Intake of Vitamin D and Treatment Regimens for Vitamin D Deficiency in HIV-Infected Adults
| Vitamin D Level | Supplementation Regimen |
| >30 ng/mL | 1000 IU/day vitamin D3 |
| 21–29 ng/mL (insufficiency) | 2000 IU/day vitamin D3 |
| 15–19 ng/mL (deficiency) | Replacement: ergocalciferol 50 000 IU/week × 8 weeks (or equivalent of 6000 IU/day vitamin D3)a |
| Maintenance: vitamin D3 2000 IU/dayb | |
| <15 ng/mL (severe deficiency) | Replacement: Ergocalciferol 50 000 IU once or twice a week × 8–12 weeks (or equivalent of 6000 IU/day vitamin D3)a |
| Maintenance: vitamin D3 2000 IU/dayb |
Abbreviation: HIV, human immunodeficiency virus.
Consider a more aggressive replacement strategy if patient has secondary hyperparathyroidism, osteomalacia, malabsorption syndrome, or obesity or is taking medications that affect vitamin D metabolism.
Recheck 25-hydroxyvitamin D level after course of ergocalciferol, goal >30 ng/mL. Consider monitoring urinary calcium in patients with a history of nephrolithiasis and concurrent calcium supplemenation use.
BISPHOSPHONATES
Bisphosphonates are generally considered first-line therapy for persons with a history of fragility fracture and/or osteoporosis by DXA. Bisphosphonates bind to bone, decrease osteoclast activity, and decrease bone resorption. Of the available preparations, alendronate, ibandronate, risedronate, and zoledronic acid all decrease vertebral fracture risk by about 50% [45–48]. In addition, alendronate and zoledronic acid reduce the risk of hip fractures and nonvertebral fractures by 25%–50% in non-HIV-infected patients [45, 46]. Alendronate is given orally weekly, whereas ibandronate and risedronate are given orally monthly. Ibandronate and zoledronic acid can be given intravenously every 3 months or 1 year, respectively. Alendronate and zoledronic acid were shown to significantly improved BMD at the lumbar spine, total hip, and trochanter in HIV-infected patients treated for 48 weeks in randomized controlled trials [49–51]. Data on the effects of bisphosphonates on the risk of fracture in HIV-infected patients are not available.
Side effects (Supplementary Table) are similar for each of the oral bisphosphonates and include difficulty swallowing, esophageal inflammation, dyspepsia, and gastric ulcer. Osteonecrosis of the jaw has been reported, typically in cancer patients requiring multiple doses of intravenous bisphosphonate therapy, but its occurrence with oral bisphosphonates in the management of osteoporosis is rare, with an estimated incidence of <1 case per 100 000 person-years of exposure [52]. Acute phase reactions, characterized by arthralgias, myalgias, headache, fever, and bone pain, have been reported most commonly with intravenous ibandronate, and zoledronic acid and may be prevented with analgesic administration (ie, acetaminophen) in the days following dosing [53]. Bisphosphonate use has been linked with atrial fibrillation and esophageal cancer, but the available evidence is insufficient to establish a causal relationship [46, 54]. The occurrence of subtrochanteric fractures, or atypical femoral shaft fractures, is another rare event associated with bisphosphonate use, and such fractures are thought to occur due to drug-induced oversuppression of bone turnover [55]. These fractures occur after minimal or no trauma and may be preceded by leg pain; imaging reveals a sclerotic appearance to the subtrochanteric region [55].
The long-term safety of bisphosphonate use is unknown. Given the increasing recognition of rare adverse effects associated with long-term use and the lack of clear efficacy data with usage >5 years compared with a 5-year course, a Food and Drug Administration expert panel recently recommended the consideration of bisphosphonate cessation or a drug interruption after the 5 years of use. The optimal duration of a scheduled drug interruption (ie, “drug holiday”) has not been established.
SECOND-LINE THERAPY
Estrogen
Estrogen replacement therapy can be considered for osteoporosis in postmenopausal women as the Women’s Health Initiative found that hormone therapy reduced the risk of clinical vertebral fractures and hip fractures by 34% and other osteoporotic fractures by 23% among non-HIV-infected patients [2, 56]. However, the increased risk of myocardial infarction, stroke, invasive breast cancer, and venous thromboembolism associated with this therapy limits its use [56]. Oral and transdermal low-dose estrogen-only preparations (0.3 mg/day oral conjugated equine or esterified estrogens; 0.5 mg/day oral micronized estradiol; 0.025 mg/day transdermal 17β estradiol) have also been shown to prevent bone loss, but their role in fracture prevention has not been established [57]. Given the risks of estrogen-only replacement therapy, its use for osteoporosis prevention and treatment is limited. No data on the effects of estrogen on BMD or fractures are available specifically in HIV-infected populations.
Estrogen Agonist/Antagonist
Raloxifene is FDA approved for the treatment of postmenopausal osteoporosis [47]. Raloxifene reduces the risk of vertebral fracture and increases hip and spine BMD, although nonvertebral or hip fracture efficacy has not been demonstrated [47]. No data are available in HIV-infected populations.
Teriparatide
Teriparatide, recombinant human parathyroid hormone (1–34), stimulates bone formation and has been shown to reduce the risk of vertebral and nonvertebral fractures in men and postmenopausal women with osteoporosis [58]. It may be used in patients with very high fracture risk or patients in whom bisphosphonate therapy has failed. Therapy is typically for 2 years and once stopped, BMD declines quickly over the following year [59]. For this reason, antiresorptive therapy, generally with a bisphosphonate, is recommended after finishing a teriparatide course. Data regarding the safety and efficacy of teriparatide in HIV-infected patients is lacking, and this area deserves further investigation.
Denosumab
Denosumab, a human monoclonal antibody against RANKL that leads to reduced activation of RANK and decreased osteoclastogenesis, may be used in those persons with a history of osteoporotic fracture or multiple risk factors for fracture or those who have failed or are intolerant to other osteoporosis therapies [60]. In addition to its importance in bone metabolism, the RANKL/RANK interaction plays a role in immune regulation, by inducing proinflammatory cytokine and chemokine release from immune cells and enhancing dendritic cell–T cell interaction [61]. Therefore, blocking RANKL/RANK interaction may decrease monocyte and dendritic cell function and survival and theoretically may result in infection, tumors, and hematologic or immune dysfunction [61, 62]. This risk is of particular concern in HIV-infected persons, who are already at increased risk for infection. Further studies regarding the impact of denosumab on immune function are needed before its use can be recommended in HIV-infected persons. Reported adverse effects of denosumab include osteonecrosis of the jaw, serious skin infections, dermatitis, rashes, and eczema [63]. In addition, there was a slightly increased rate of malignancy in osteoporotic women treated with denosumab compared with placebo (4.0% vs 3.3.%) in preregistration randomized controlled trials [53]. Because the drug was only recently approved for use, data on long-term continued denosumab use will be necessary to clearly define the risks.
MONITORING THERAPY
Once osteoporosis therapy is started, DXA should be repeated in 1–2 years to determine treatment effect and stability of BMD [64]. If BMD is stable or improved, then less frequent DXA scans can be considered [64]. Treatment success is also based on the absence of fractures and the presence of stable or increasing BMD.
CONCLUSION
Aging-related comorbidities, such as osteoporosis, are increasing in importance for HIV-infected persons in the era of HAART, and fractures are likely to be a major source of morbidity. The etiology of osteoporosis in HIV-infected persons is multifactorial, including HIV disease– and treatment-specific risks and traditional risk factors, such as hypogonadism, smoking, and low body weight. We recommend screening all HIV-infected postmenopausal women and men aged ≥50 years, particularly those with additional risk factors for osteoporosis, with a DXA scan. Treatment guidelines should follow those established for the general population.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://www.oxfordjournals.org/our_journals/jid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support.
This work was supported by the National Insitute of Allergy and Infectious Diseases, National Insitutes of Health (grant number HRO1 AI093520 to T. T. B.). This supplement was supported by the Harvard Center for AIDS Research and an Educational Grant from Bristol Myers Squibb.
Potential conflicts of interest.
T. T. B. has received consulting fees from Gilead, ViiV Healthcare, and EMD-Serono; has received lecture fees from BMS, ViiV Healthcare, and Tibotec; has developed educational presentations for ViiV Healthcare, Gilead, and Tibotec; and has received research support from Merck and GSK. V. W. H. reports no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med. 1993;94:646–50. doi: 10.1016/0002-9343(93)90218-e. [DOI] [PubMed] [Google Scholar]
- 2.In: Foundation NO, ed. Washington, DC: National Osteoporosis Foundation; 2010. National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. [Google Scholar]
- 3.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS. 2006;20:2165–74. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 4.Triant VA, Brown TT, Lee H, Grinspoon SK. Fracture prevalence among human immunodeficiency virus (HIV)–infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab. 2008;93:3499–504. doi: 10.1210/jc.2008-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Womack JA, Goulet JL, Gibert C, et al. Increased risk of fragility fractures among HIV-infected compared to uninfected male veterans. PLoS One. 2011;6:e17217. doi: 10.1371/journal.pone.0017217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young B, Dao CN, Buchacz K, Baker R, Brooks JT. Increased rates of bone fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared with the US general population, 2000–2006. Clin Infect Dis. 2011;52:1061–8. doi: 10.1093/cid/ciq242. [DOI] [PubMed] [Google Scholar]
- 7.Morse CG, Mican JM, Jones EC, et al. The incidence and natural history of osteonecrosis in HIV-infected adults. Clin Infect Dis. 2007;44:739–48. doi: 10.1086/511683. [DOI] [PubMed] [Google Scholar]
- 8.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO study group. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 9.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–9. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallant JE, Staszewski S, Pozniak AL, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004;292:191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 11.Tebas P, Powderly WG, Claxton S, et al. Accelerated bone mineral loss in HIV-infected patients receiving potent antiretroviral therapy. AIDS. 2000;14:F63–7. doi: 10.1097/00002030-200003100-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tebas P, Umbleja T, Dube M, et al. In: Program and abstracts of the 14th Conference on Retroviruses and Opportunistic Infections (Los Angeles, CA) Alexandria, VA: CROI: 2007. Initiation of ART is associated with bone loss independent of the specific ART regimen: results of ACTG A5005s [abstract] [Google Scholar]
- 13.Fakruddin JM, Laurence J. HIV envelope gp120-mediated regulation of osteoclastogenesis via receptor activator of nuclear factor kappa B ligand (RANKL) secretion and its modulation by certain HIV protease inhibitors through interferon-gamma/RANKL cross-talk. J Biol Chem. 2003;278:48251–8. doi: 10.1074/jbc.M304676200. [DOI] [PubMed] [Google Scholar]
- 14.Fakruddin JM, Laurence J. HIV-1 Vpr enhances production of receptor of activated NF-kappaB ligand (RANKL) via potentiation of glucocorticoid receptor activity. Arch Virol. 2005;150:67–78. doi: 10.1007/s00705-004-0395-7. [DOI] [PubMed] [Google Scholar]
- 15.Cotter EJ, Malizia AP, Chew N, Powderly WG, Doran PP. HIV proteins regulate bone marker secretion and transcription factor activity in cultured human osteoblasts with consequent potential implications for osteoblast function and development. AIDS Res Hum Retroviruses. 2007;23:1521–30. doi: 10.1089/aid.2007.0112. [DOI] [PubMed] [Google Scholar]
- 16.Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–8. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashizume M, Hayakawa N, Mihara M. IL-6 trans-signalling directly induces RANKL on fibroblast-like synovial cells and is involved in RANKL induction by TNF-alpha and IL-17. Rheumatology (Oxford) 2008;47:1635–40. doi: 10.1093/rheumatology/ken363. [DOI] [PubMed] [Google Scholar]
- 18.Ishimi Y, Miyaura C, Jin CH, et al. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990;145:3297–303. [PubMed] [Google Scholar]
- 19.Gibellini D, Borderi M, De Crignis E, et al. RANKL/OPG/TRAIL plasma levels and bone mass loss evaluation in antiretroviral naive HIV-1-positive men. J Med Virol. 2007;79:1446–54. doi: 10.1002/jmv.20938. [DOI] [PubMed] [Google Scholar]
- 20.Brown TT, McComsey GA, King MS, Qaqish RB, Bernstein BM, da Silva BA. Loss of bone mineral density after antiretroviral therapy initiation, independent of antiretroviral regimen. J Acquir Immune Defic Syndr. 2009;51:554–61. doi: 10.1097/QAI.0b013e3181adce44. [DOI] [PubMed] [Google Scholar]
- 21.Duvivier C, Kolta S, Assoumou L, et al. Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV-1 infected naive patients. AIDS. 2009;23:817–24. doi: 10.1097/QAD.0b013e328328f789. [DOI] [PubMed] [Google Scholar]
- 22.Bedimo R, Zhang S, Dreschler H, Tebas P, Mallouf N. In: Program and abstracts of the 13th International Workshop on Adverse Drug Reactions and Comorbidities (Rome) London: International Medical Press: 2011. Risk of osteoporotic fractures associated with cumulative exposure to tenofovir and other antiretroviral agents. [Google Scholar]
- 23.McComsey GA, Kitch D, Daar ES, et al. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: AIDS Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis. 2011;203:1791–801. doi: 10.1093/infdis/jir188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stellbrink HJ, Orkin C, Arribas JR, et al. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis. 2010;51:963–72. doi: 10.1086/656417. [DOI] [PubMed] [Google Scholar]
- 25.Verhelst D, Monge M, Meynard JL, et al. Fanconi syndrome and renal failure induced by tenofovir: a first case report. Am J Kidney Dis. 2002;40:1331–3. doi: 10.1053/ajkd.2002.36924. [DOI] [PubMed] [Google Scholar]
- 26.Karras A, Lafaurie M, Furco A, et al. Tenofovir-related nephrotoxicity in human immunodeficiency virus-infected patients: three cases of renal failure, Fanconi syndrome, and nephrogenic diabetes insipidus. Clin Infect Dis. 2003;36:1070–3. doi: 10.1086/368314. [DOI] [PubMed] [Google Scholar]
- 27.Childs KE, Fishman SL, Constable C, et al. Short communication: inadequate vitamin D exacerbates parathyroid hormone elevations in tenofovir users. AIDS Res Hum Retroviruses. 2010;26:855–9. doi: 10.1089/aid.2009.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knoke JD, Barrett-Connor E. Weight loss: a determinant of hip bone loss in older men and women. The Rancho Bernardo Study. Am J Epidemiol. 2003;158:1132–8. doi: 10.1093/aje/kwg265. [DOI] [PubMed] [Google Scholar]
- 29.Warming L, Hassager C, Christiansen C. Changes in bone mineral density with age in men and women: a longitudinal study. Osteoporos Int. 2002;13:105–12. doi: 10.1007/s001980200001. [DOI] [PubMed] [Google Scholar]
- 30.Aukrust P, Haug CJ, Ueland T, et al. Decreased bone formative and enhanced resorptive markers in human immunodeficiency virus infection: indication of normalization of the bone-remodeling process during highly active antiretroviral therapy. J Clin Endocrinol Metab. 1999;84:145–50. doi: 10.1210/jcem.84.1.5417. [DOI] [PubMed] [Google Scholar]
- 31.Bolland MJ, Grey AB, Horne AM, et al. Bone mineral density remains stable in HAART-treated HIV-infected men over 2 years. Clin Endocrinol (Oxf) 2007;67:270–5. doi: 10.1111/j.1365-2265.2007.02875.x. [DOI] [PubMed] [Google Scholar]
- 32.Dolan SE, Kanter JR, Grinspoon S. Longitudinal analysis of bone density in human immunodeficiency virus-infected women. J Clin Endocrinol Metab. 2006;91:2938–45. doi: 10.1210/jc.2006-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McComsey GA, Tebas P, Shane E, et al. Bone disease in HIV infection: a practical review and recommendations for HIV care providers. Clin Infect Dis. 2010;51:937–46. doi: 10.1086/656412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dietary Reference Intakes for Calcium and Vitamin D. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Ross AC, Taylor CL, Yaktine AL, et al., eds. Washington, DC: National Academies Press. 2011. [PubMed] [Google Scholar]
- 35.Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women’s Health Initiative limited access dataset and meta-analysis. BMJ. 2011;342:d2040. doi: 10.1136/bmj.d2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howe TE, Shea B, Dawson LJ, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011;7:CD000333. doi: 10.1002/14651858.CD000333.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dao CN, Patel P, Overton ET, et al. Low vitamin D among HIV-infected adults: prevalence of and risk factors for low vitamin D levels in a cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clin Infect Dis. 2011;52:396–405. doi: 10.1093/cid/ciq158. [DOI] [PubMed] [Google Scholar]
- 38.Landriscina M, Altamura SA, Roca L, et al. Reverse transcriptase inhibitors induce cell differentiation and enhance the immunogenic phenotype in human renal clear-cell carcinoma. Int J Cancer. 2008;122:2842–50. doi: 10.1002/ijc.23197. [DOI] [PubMed] [Google Scholar]
- 39.Wohl D, Doroana M, Orkin C, et al. Program and abstracts of the 18th Conference on Retroviruses and Opportunistic Infections (Boston, MA) Alexandria, VA: CROI: 2011. Change in vitamin D levels smaller and risk of development of severe vitamin D deficiency lower among HIV-1-infected, treatment-naive adults receiving TMC278 compared with EFV: 48-week results from the phase III ECHO trial. [Google Scholar]
- 40.Brown TT, McComsey GA. Association between initiation of antiretroviral therapy with efavirenz and decreases in 25-hydroxyvitamin D. Antivir Ther. 2010;15:425–9. doi: 10.3851/IMP1502. [DOI] [PubMed] [Google Scholar]
- 41.Welz T, Childs K, Ibrahim F, et al. Efavirenz is associated with severe vitamin D deficiency and increased alkaline phosphatase. AIDS. 2010;24:1923–8. doi: 10.1097/QAD.0b013e32833c3281. [DOI] [PubMed] [Google Scholar]
- 42. Lundgren J. European AIDS clinical society guidelines: prevention and management of non-infectious co-morbidities in HIV. France 2011; Vol 5-4.
- 43.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 44.Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303:1815–22. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 45.Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–41. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 46.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–22. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 47.Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282:637–45. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 48.Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282:1344–52. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 49.Bolland MJ, Grey AB, Horne AM, et al. Annual zoledronate increases bone density in highly active antiretroviral therapy-treated human immunodeficiency virus–infected men: a randomized controlled trial. J Clin Endocrinol Metab. 2007;92:1283–8. doi: 10.1210/jc.2006-2216. [DOI] [PubMed] [Google Scholar]
- 50.McComsey GA, Kendall MA, Tebas P, et al. Alendronate with calcium and vitamin D supplementation is safe and effective for the treatment of decreased bone mineral density in HIV. AIDS. 2007;21:2473–82. doi: 10.1097/QAD.0b013e3282ef961d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.