Abstract
Phosphorylation of G protein–coupled receptors (GPCRs, which are also known as seven-transmembrane spanning receptors) by GPCR kinases (GRKs) plays essential roles in the regulation of receptor function by promoting interactions of the receptors with β-arrestins. These multifunctional adaptor proteins desensitize GPCRs, by reducing receptor coupling to G proteins and facilitating receptor internalization, and mediate GPCR signaling through β-arrestin–specific pathways. Detailed mapping of the phosphorylation sites on GPCRs targeted by individual GRKs and an understanding of how these sites regulate the specific functional consequences of β-arrestin engagement may aid in the discovery of therapeutic agents targeting individual β-arrestin functions. The β2-adrenergic receptor (β2AR) has many serine and threonine residues in the carboxyl-terminal tail and the intracellular loops, which are potential sites of phosphorylation. We monitored the phosphorylation of the β2AR at specific sites upon stimulation with an agonist that promotes signaling by both G protein–mediated and β-arrestin–mediated pathways or with a biased ligand that promotes signaling only through β-arrestin–mediated events in the presence of the full complement of GRKs or when either GRK2 or GRK6 was depleted. We correlated the specific and distinct patterns of receptor phosphorylation by individual GRKs with the functions of β-arrestins and propose that the distinct phosphorylation patterns established by different GRKs establish a “barcode” that imparts distinct conformations to the recruited β-arrestin, thus regulating its functional activities.
INTRODUCTION
G protein (heterotrimeric GTP-binding protein)–coupled receptors (GPCRs), which are also known as seven-transmembrane spanning receptors (7TMRs), regulate most physiological processes in humans and are one of the most important target classes of therapeutic agents (1). Classically, GPCR signaling is mediated through coupling to heterotrimeric G proteins, subsequently triggering a series of intracellular signaling cascades and ultimately leading to changes in cellular physiology. After their activation, various GPCRs are phosphorylated by GPCR kinases (GRKs) and subsequently recruit one or both of the two isoforms of cytosolic β-arrestins (β-arrestin1 and β-arrestin2). β-Arrestin binding uncouples the receptor from the G protein, thus terminating or attenuating G protein–mediated signaling (desensitization), and facilitates clathrin-mediated endocytosis (internalization) of the receptor (2). In addition to their role in the termination of G protein–mediated signaling, β-arrestins also serve as multi-functional adaptors and signal transducers, linking GPCRs to a growing list of signaling molecules, including mitogen-activated protein kinase (MAPK), the tyrosine kinase c-Src, and the Ser-Thr kinase Akt (3). Whereas classical agonists stimulate both G protein–mediated and β-arrestin–mediated signaling mechanisms, “biased ligands” can selectively activate G protein or β-arrestin functions and thus elicit distinct biological effects (4). For example, carvedilol (Coreg), which was considered a β antagonist or “β blocker” because it did not trigger G protein–mediated signaling by the β2-adrenergic receptor (β2AR), selectively stimulates β-arrestin–mediated signaling (5, 6).
Phosphorylation of GPCRs on their C termini and intracellular loops by GRKs is generally required for β-arrestin binding. In contrast to the plethora of GPCRs, there are only seven members in the GRK family, and of those, only GRKs 2, 3, 5, and 6 are ubiquitously expressed. Studies with “loss-of-function” techniques such as small interfering RNA (siRNA) to delete individual GRKs or combinations of GRKs have suggested that distinct GRKs may contribute differently to the processes of receptor desensitization, endocytosis, and signaling (7–9). These findings raise the question of how receptor phosphorylation by different GRKs might enable distinct functions of β-arrestins.
We hypothesized that the different GRKs might phosphorylate distinct sets of sites on the C terminus and internal loops of the receptor, thereby establishing a “barcode” that would instruct or determine the conformation assumed by the β-arrestin, which would, in turn, determine its functional capabilities. We determined the functional capability of β-arrestin bound to the β2AR phophorylated by different GRKs and the sites of agonist-induced phosphorylation of the receptor by different GRKs. We also evaluated whether a β-arrestin–biased ligand, such as carvedilol, induced phosphorylation events distinct from those induced by an unbiased agonist, such as isoproterenol. Although protein kinase A (PKA) also phosphorylates β2ARs, we did not explore its contributions in these assays because inhibition of PKA activity affects neither the rate nor the amounts of β-arrestin recruitment to β2ARs (10).
MATERIALS AND METHODS
HEK293 β2AR stable and transiently transfected cell lines
HEK293 cells were obtained from the American Type Culture Collection and maintained in designated culture media at 37°C in a humidified 5% CO2 incubator. Briefly, early-passage HEK293 cells were transiently transfected with 5 µg of FLAG-tagged receptor plasmid. β2AR protein was verified by radioligand binding. HEK293 cells stably transfected with FLAG-tagged β2ARs have been previously described (13, 36).
siRNA transfection
The sequences of the control, GRK2, and GRK6 siRNAs were published previously (7, 8). HEK293 cells stably transfected with FLAG-tagged β2AR were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma) for SILAC analysis or in minimal essential medium (MEM) (Sigma) for cell-based assays supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% penicillin/streptomycin. Cells were grown to ~40% confluence in 150-mm dishes and were transfected with 50 µg (90 µl of 20 µM) of siRNA with the GeneSilencer transfection reagent (Genlantis) as described (37). Seventy-two hours after transfection, cells were serum-starved for at least 4 hours before ligand treatment.
β2AR desensitization in GloSensor cells
To maintain consistency during desensitization assays, we generated a stable cell line with a cAMP reporter. Early-passage HEK293 cells were transfected with 2.5 µg of GloSensor receptor plasmid (Promega), and positive clones were selected with hygromycin B (100 µg/ml). GloSensor transfection and transcription were confirmed by stimulation of endogenous β2ARs with isoproterenol in 12 different clonal lines. The clonal line with the highest sensitivity to isoproterenol was then further characterized with forskolin treatment.
HEK293 cells stably transfected with Promega’s GloSensor enzyme were seeded on 96-well plates 48 hours after GRK siRNA treatment. The next day (72 hours after transfection), the cells were preequilibrated with 2% of the GloSensor cAMP reagent in MEM (supplemented with 10% FBS) for 2 hours at 37°C. Endogenous β2ARs were then stimulated with either a vehicle (DMSO) or 100 nM isoproterenol for 5 min and subsequently washed two times with 150 µl of 37°C MEM (supplemented with 10% FBS) for 5 min. After addition of 100 µl of fresh MEM to the cells, β2ARs were rechallenged with isoproterenol in a dose-dependent manner. GloSensor luciferase activities were then measured in duplicate or triplicate for at least n = 6 with the NOVOstar microplate reader (BMG Labtech) in a luminescence mode, and the dose-response data were plotted and fit with GraphPad Prism. An Emax analysis was performed on these dose-response data. The maximal response for each fit was calculated and then normalized within each data group, and the treated maximal response (100 nM isoproterenol pretreatment) was subtracted from the nontreated maximal response (DMSO pretreatment) to determine the change in Emax.
Internalization assay
Receptor internalization was measured by CGP-12177 radioligand binding on monolayers of cells plated on poly-d-lysine–coated 12-well dishes (Biocoat) in MEM buffered with 10 mM Hepes (pH 7.5) and supplemented with 0.1% bovine serum albumin (w/v) (serum-starving medium). Binding was performed in triplicate with 10 nM CGP-12177 in the presence or absence of 20 µM ICI1 18551 (to define nonspecific binding). After incubation at 4°C for 90 min, the cells were placed on ice and washed several times with phosphate-buffered saline (PBS) containing calcium and magnesium. Cells were solubilized in 0.1 N NaOH and 0.1% SDS and counted for 3H. Agonist-induced internalization was defined as loss of cell surface receptors as previously described (32). GraphPad Prism software was used for data analyses.
Phosphorylated ERK assay
HEK293 cells stably transfected with the β2AR were grown at low confluence and split to 6- or 12-well plates. Cells were incubated in serum-free media at least 4 hours before stimulation with either 10 µM isoproterenol or 10 µM carvedilol. After stimulation, cells were solubilized directly into 2× SDS sample buffer followed by sonication with a microtip for 15 s. For each experimental condition, an equal portion of cells was used for protein determination (Bradford). Equal micrograms of cell lysate were separated on 4 to 20% tris-glycine polyacrylamide gels and transferred to nitrocellulose filters for immunoblotting. GraphPad Prism software was used for data analyses.
BRET assay
BRET assays were performed as previously described (15). Briefly, 48 hours after transient cotransfection with β2AR, the BRET β-arrestin biosensor, and siRNAs, cells were plated on fibronectin-coated, 96-well microplates (white wall, clear bottom) in serum-containing media. Before the assay, cells were washed twice with 100 µl of PBS at 37°C. The transparent bottom of the plate was then covered with a white-backed tape adhesive and cells were then incubated for 10 min at 37°C with coelenterazine h, an intracellular luminophore (5 µM final concentration). Cells were then stimulated with isoproterenol for 15 min and light emission was detected as previously described (15). The BRET ratio was determined as the ratio of light emitted by YFP and the light emitted by RLuc. The values were corrected by subtracting the background signals before isoproterenol stimulation.
Generation of antibodies specific for phosphorylated residues of β2AR
Antibodies that recognize the β2AR phosphorylated on specific sites were generated with a standard phosphorylation-specific polyclonal antibody production protocol (rabbit) (GenScript USA Inc.). The amino acid sequences for the synthesized phosphopeptides used to immunize rabbits are as follows: anti–β2AR-pS246: QNL{pSer}QVEQDGRC; anti–β2AR-pS261/pS262: GLRR{pSER}{pSER}KFCLKEHK; anti–β2AR-pS396: FVGHQGTVP{pSER}DNID; and anti–β2AR-pS407/pS411:QGRNC{pSER}TND{pSER}LL.
Briefly, a cysteine was added to the C termini of the phosphopeptides and conjugated to KLH (keyhole limpet hemocyanin). The conjugates were mixed at a 1:1 ratio with Freund’s adjuvant and injected into rabbits for the production of anti–β2AR-pS246, anti–β2AR-pS261/pS262, anti–β2AR-pS396, and anti–β2AR-pS407/pS411. Following a standard immunization and boosting schedule, serum was obtained from multiple production bleeds and purified using the phosphopeptide and cross-adsorbed with the nonphosphorylated peptide. The specificity of the antisera was tested with ELISA (enzyme-linked immunosorbent assay) and dot blot analyses.
Western blot analysis
HEK293 cells stably transfected with FLAG-tagged β2AR were used for immunoprecipitations unless otherwise noted. Cells were lysed in glycerol lysis buffer [10 mM Hepes (pH 7.4), 150 mM NaCl, 10% glycerol, and 0.1% NP-40] and adjusted to equal protein concentration by protein assay before immunoprecipitation with the M2 beads recognizing the FLAG epitope (Sigma). In some experiments, the β2ARs were purified from the cells with an alprenolol-agarose purification protocol. Equal amounts of protein were separated on tris-glycine polyacrylamide gels (Invitrogen) and transferred to polyvinylidine fluoride membranes for immunoblotting. GRKs were detected with isoform-specific antibodies from Santa Cruz Biotechnology Inc. according to the manufacturer’s protocol. β2AR phosphorylation at the pair of residues Ser345 and Ser346 and the pair Ser355 and Ser356 was detected by immunoblotting with the commercially available phosphorylation site–specific antibodies anti–β2AR-pS345/pS346 and anti–β2AR-pS355/pS356 (Santa Cruz), respectively. β2AR phosphorylation at Ser246, the pair Ser261 and Ser262, S396, and the pair Ser407 and Ser411 was detected by immunoblotting with newly generated phosphorylation site–specific antibodies anti–β2AR-pS246, anti–β2AR-pS261/pS262, anti–β2ARpS396, and anti–β2AR-pS407/pS411, respectively. Phosphorylated ERK1/2 and total ERK1/2 were detected by immunoblotting with an antibody that recognizes phosphorylated ERK1/2 (Cell Signaling) and an antibody that recognizes ERK1/2 (Upstate Biotechnology), respectively. Chemiluminescence detection was performed with horseradish peroxidase–coupled secondary antibody (Amersham Biosciences) and SuperSignal West Pico reagent (Pierce) or Femto reagent (Pierce). Chemiluminescence was quantified by a charge-coupled device camera (Syngene ChemiGenius2).
SILAC and siRNA depletion of GRK2 and GRK6
The HEK293 line stably transfected with FLAG-tagged β2AR was generated in our lab as previously described (13). The cells were maintained in SILAC “light” and “heavy” media. The SILAC medium was prepared from custom-ordered DMEM powder without arginine, lysine, and leucine (Gibco, formula # 03-5080EB) (Gibco/Invitrogen). [13C6, 15N2]Lysine (50 mg/liter) and [13C6, 15N]leucine (50 mg/liter) (Cambridge Isotope Laboratories) were added to the heavy medium, whereas equal concentrations of conventional lysine and leucine were added to the light medium; both heavy and light media were supplemented with l-arginine (84 mg/liter), 10% dialyzed FBS (Hyclone) (Thermo Scientific), 1% penicillin/streptomycin, and G418 (150 µg/ml). In some experiments, [13C6, 15N2]lysine (50 mg/liter) and [13C6, 15N4]arginine (25 mg/liter) (Cambridge Isotope Laboratories) were added to the heavy medium, whereas equal concentrations of conventional arginine and lysine were added to the light medium, and both heavy and light media were supplemented with l-leucine (104 mg/liter), l-proline (10 mg/liter), 10% dialyzed FBS (Hyclone) (Thermo Scientific), 1% penicillin/streptomycin, and G418 (150 µg/ml). When the cells reached ~80% confluence, they were serum-starved for 24 hours. To map the phosphorylation sites on the β2AR induced by isoproterenol or carvedilol, we treated the light labeled cells with 10 µM isoproterenol or carvedilol for 5 min before harvesting, and the heavy labeled cells served as control without any treatment, or vice versa.
To map the sites phosphorylated by GRK2 or GRK6, we treated the light labeled cells with CTL siRNA and the heavy labeled cells with GRK2 or GRK6 siRNA, or vice versa. After siRNA treatment, both light and heavy cells were stimulated with 10 µM isoproterenol for 5 min before harvesting. Equal numbers of light and heavy cells (generally thirty 150-mm culture dishes for each) were mixed, flash-frozen in liquid nitrogen, and stored at −100°C. The SILAC experiments were repeated at least three times. Silencing was quantified by immunoblotting. Only experiments with verified silencing were used.
β2AR purification and digestion
The β2ARs were purified with an alprenolol-agarose affinity purification procedure as previously described (38). Briefly, crude membrane fractions were prepared from the HEK293 line stably transfected with FLAG-tagged β2AR (2 pmol/mg), and receptors were extracted with 1× buffer [20 mM tris-HCl (pH 8.0), 100 mM NaCl, and 2 mM EDTA] containing 1% DDM (n-dodecyl β-d-maltoside). Alprenolol-agarose affinity beads were used to isolate the β2ARs from the extracts. Purified receptors were eluted with 150 µl of 5 mM alprenolol in 1× buffer with 0.01% DDM. Protease and phosphatase inhibitors were added to all the buffers as described previously (39).
Purified β2ARs were reduced with 5 mM dithiothreitol (DTT) at 65°C for 20 min and alkylated with 10 mM iodoacetamide in the dark at room temperature for 20 min. In-solution digestion was performed with trypsin (5 to 10 ng/µl; modified, sequencing grade, Promega) or chymotrypsin (15 ng/µl; Sigma) in 50 mM NH4HCO3 (pH 8.0) for 18 hours at 37°C. An equal volume of 100% acetonitrile (CH3CN) was added to the digested samples and then they were dried under vacuum on a SpeedVac evaporator. The peptide samples were desalted with handmade Stage Tips (40) and lyophilized with a SpeedVac before either MS analyses or phosphopeptide enrichment.
Immobilized metal ion affinity chromatography
For phosphopeptide enrichment, the dried peptides were resuspended in 150 µl of IMAC wash/equilibration buffer (25 mM formic acid, 40% acetonitrile) and added to 25 µl of a 1:1 slurry of precharged IMAC resin [Fe(III)-loaded IMAC slurry] (PHOS-Select Iron Affinity Gel, Sigma-Aldrich). The IMAC resin was prewashed three times in 1 ml of wash/equilibration buffer. Samples were agitated for 90 min at room temperature and washed three times with 150 µl of wash/equilibration buffer. Bound peptides were eluted twicewith 45 µl of 50 mM KH2PO4/NH3 (pH 10.0) and acidified with 45 µl of 5% formic acid and 5% acetonitrile. All samples were resuspended in 40 µl of 5% formic acid and desalted on C18 resin, using handmade Stage Tips (40). Peptides were eluted with 5% formic acid and 50% acetonitrile, lyophilized with a SpeedVac, reconstituted in 5% formic acid and 5% acetonitrile (with Agilent 1100 series binary pump) or 0.1% trifluoroacetic acid, 2% acetonitrile, and 25 mM citrate [Waters nanoACQUITY Ultra Performance LC (UPLC) System], and subjected to LC-MS/MS analysis.
MS and data analyses
LC-MS/MS analyses were performed on a Thermo Scientific LTQ Orbitrap XL or LTQ-FT mass spectrometer (Thermo Fisher) with a Finnigan Nanospray II electrospray ionization source as described previously (41). Enriched phosphopeptides were loaded by a Famos autosampler onto a 125 µm (inside diameter) by 15 cm fused-silica microcapillary column packed in-house with C18 reversed-phase resin (Magic C18AQ; 5-µm particles; 200-Å pore; Michrom Bioresources Inc.) and separated with an Agilent 1100 series binary pump. In some experiments, peptides were injected onto a 75 µm by 150 mm BEH C18 column (particle size, 1.7 µm; Waters) and separated with a Waters nanoACQUITY UPLC System.
Instrument control and primary data processing were done with the Xcalibur software package. The LTQ Orbitrap XL or LTQ-FT was operated in data-dependent mode using a TOP10 strategy (42). MS/MS spectra were searched with the SEQUEST algorithm against a composite database containing the human β2AR sequence or β2AR with its interacting proteins, as well as their reverse sequences. Search parameters allowed for three missed tryptic cleavages, a mass tolerance of ±80 parts per million (ppm), a static modification of 57.02146 daltons (carboxyamidomethylation) on cysteine, and up to six total dynamic modifications: 79.96633 daltons (phosphorylation) on serine, threonine, and tyrosine; 15.99491 daltons (oxidation) on methionine; 7.01764 daltons on leucine or 10.00827 daltons on arginine; and 8.01420 daltons on lysine. Search results were filtered to include <1% matches to reverse sequences by restricting the mass tolerance window, and setting thresholds for Xcorr and dCn′ (defined as the normalized difference between Xcorr values of the top-ranked candidate peptide and the next candidate with a different amino acid sequence). Matches for phosphopeptides were validated manually with special consideration of intense fragment ions formed through cleavage N-terminal to proline residues and neutral losses of phosphoric acid.
The probability of correct phosphorylation site localization for each phosphorylation site was measured with an Ascore algorithm as described previously (43). Sites with Ascore ≥13 (P ≤ 0.05) were considered confidently localized, and those with Ascore ≥19 (P ≤ 0.01) were considered to be localized with near certainty. Peptide quantification was performed with the Vista program (44) as well as by manual calculation with Qual Browser (version 2.0.7). In brief, the theoretical mass of both heavy and light variants of each peptide was calculated and used to identify ion peaks in the high mass accuracy precursor scans for each. The intensity of the peaks was used to construct ion chromatograms. Candidate peaks were required to fall within a tolerance window of ±10 ppm from the calculated mass and were filtered to require the predicted isotopic distribution. For each isotopic variant, the background-subtracted area under the curve was determined as a function of elution time and used to calculate the heavy-to-light abundance ratio.
RESULTS
Silencing of either GRK2 or GRK6 impairs β2AR desensitization and internalization
We wanted to determine which GRKs enable which β-arrestin functions. Evidence from the literature suggests that GPCRs use specific GRKs, or subsets thereof, and that this specificity is dictated not only by receptor type but also by cell type (10). For these studies, we used human embryonic kidney (HEK) 293 cells, which endogenously express β2AR and in which, previous studies have shown, only GRK2 and GRK6 regulate β-arrestin2 recruitment to the β2AR (the preferred β-arrestin isoform for this receptor) (10). The absolute amounts of GRK2 and GRK6 in HEK293 cells were 4.5 ± 1.2 and 3.0 ± 0.6 fmol/mg, respectively, as determined by Western blot analysis with purified proteins (fig. S1).
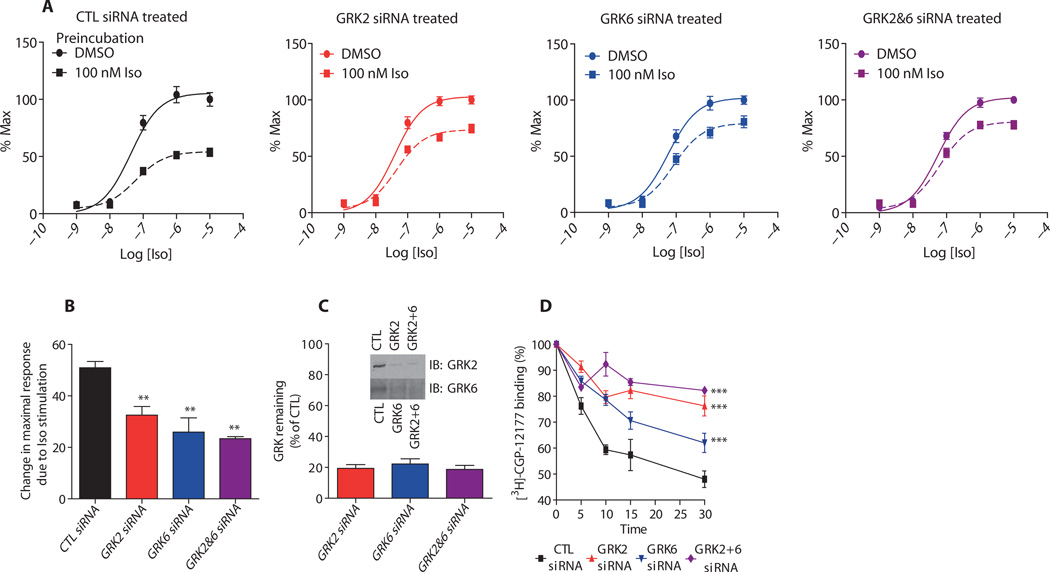
The β2AR is a Gs-coupled receptor that when activated with an agonist, such as isoproterenol, stimulates the production of adenosine 3′,5′-monophosphate (cAMP), which we detected with the live-cell cAMP biosensor, GloSensor (11). We first tested the effect of altering the receptor phosphorylation pattern by siRNA knockdown of GRKs on Gs-dependent cAMP generation by the β2AR (Fig. 1). Endogenous β2ARs in HEK293 cells stably transfected with GloSensor were prestimulated with either vehicle [dimethyl sulfoxide (DMSO)] or 100 nM isoproterenol for 5 min, then washed and rechallenged with isoproterenol in a dose-dependent manner. (We performed these experiments with HEK293 cells that had not been transfected to overexpress the β2AR, because in cells stably transfected with β2AR, the GloSensor signal did not return to a basal amount after prestimulation and subsequent washes, making an analysis of desensitization suboptimal in those cells. However, cells stably transfected or transiently transfected with the β2AR were used in the other cellular assays to increase the sensitivity of the responses.) In cells transfected with control (CTL) siRNA, pretreatment with isoproterenol induced a 50% loss of the maximal cAMP signal when rechallenged (Fig. 1A). Cells transfected with siRNA targeting GRK2, GRK6, or both GRK2 and GRK6 showed impairment of this desensitization (Fig. 1A). This effect of GRK knockdown was evident in the significant reduction in the change in the maximal cAMP response (Emax) after isoproterenol pretreatment (Fig. 1B). Thus, knocking down either GRK2 or GRK6 alone was almost as effective as knocking down both. Knockdown efficiency was comparable in all three cases (Fig. 1C).
Fig. 1.
The effect of knockdown of GRK2, GRK6, or both GRK2 and GRK6 on desensitization and internalization of the β2AR. (A) HEK293 cells stably transfected with the GloSensor reporter and the indicated siRNAs were pretreated with 100 nM isoproterenol for 5 min, washed two to three times over 10 min, and then assayed for cAMP accumulation after restimulation with isoproterenol at the indicated concentrations (10−9 to 10−4 M). The fits of the data are sigmoidal, and the amounts of cAMP were normalized within each group and plotted as percent of the maximal response achieved. (B) The data from (A) are shown here as the baseline-corrected changes in maximal response upon pretreatment with isoproterenol. All data shown are the means ± SEM on dose-response data performed in duplicate or triplicate for at least n = 6. Statistical significance was assessed by one-way analysis of variance (ANOVA) with Bonferroni post-test to compare each group versus CTL siRNA–treated cells (**P < 0.01). (C) Representative immunoblot (IB) for silencing of GRK2, GRK6, or both demonstrates the siRNA transfection efficiency. Quantification (SE ± SEM) of at least six independent siRNA transfections used for various experiment types is shown. (D) Effect of the indicated siRNAs on the internalization of the β2AR. Internalization was determined with 3H-CGP-12177 and ICI-118551 to detect nonspecific binding as described in Materials and Methods. The percent receptor internalized is shown as a loss of 3H-CGP-12177 binding, and the values represent the means ± SEM from at least three independent experiments. Statistical comparison of the curves was determined with two-way ANOVA between GRK siRNA–transfected cells and control cells (***P < 0.001).
β-Arrestins scaffold elements of the endocytic machinery, such as the adaptor protein AP-2 and the coat protein clathrin, which target agonist-activated 7TMRs to clathrin-coated pits for internalization (12). Therefore, we analyzed the rate and extent of internalization of the receptor after depletion of either GRK2 or GRK6 alone or in combination in HEK293 cells stably transfected with β2AR (Fig. 1D). In CTL siRNA–transfected cells, stimulation with 10 µM isoproterenol led to rapid internalization with a maximum of 50% of the receptor internalized in 30 min. Depletion of GRK2 slowed the initial rate of internalization and reduced the maximum internalization to 20%. GRK6 siRNA treatment also slowed the initial rate of β2AR internalization and significantly reduced the maximum internalization (to 35%), but this effect was less than what we observed for GRK2 depletion (Fig. 1D).Knockdown of bothGRK2 and GRK6 together almost completely blocked receptor internalization. Thus, these data demonstrate that GRK2 and GRK6 differentially regulate internalization.
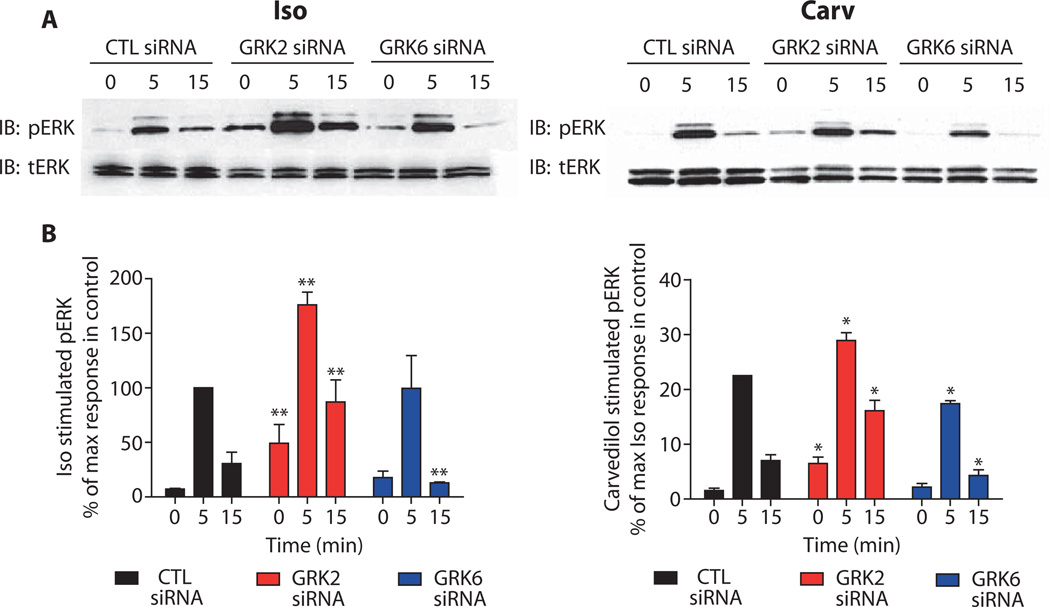
Knockdown of specific GRKs alters β2AR-stimulated phosphorylation of ERK
The β2AR can signal to extracellular signal–regulated kinase (ERK) through a β-arrestin–dependent pathway in response to either isoproterenol or carvedilol stimulation (6, 13).To test whether GRK2 or GRK6 contributed to ERK phosphorylation activated by the β2AR, we stimulated HEK293 cells stably transfected with the β2AR with either isoproterenol or a weak β-arrestin–biased agonist, carvedilol, after they had been treated with GRK-specific siRNAs (Fig. 2). We analyzed ERK1 and ERK2 (ERK1/2) phosphorylation at two times after stimulation, 5 and 15 min. Previous studies with this cell line have shown that at 5 min after isoproterenol stimulation, ERK1/2 activation peaks and contains both a G protein–mediated and a β-arrestin–mediated component, with the G protein component dominating (13). In contrast, at later time points, such as 15 min after stimulation, β-arrestin–dependent ERK activation predominates (13). We found that in isoproterenol-stimulated, CTL siRNA–transfected cells, ERK1/2 activation was robust at the 5-min time point, whereas the 15-min time point showed a lesser amount of ERK1/2 activation (Fig. 2, A, left panel, and B, black bars, left panel). Carvedilol stimulation in CTL siRNA–transfected cells produced a smaller response than did isoproterenol in terms of ERK1/2 phosphorylation because of the lack of G protein coupling in response to this agonist and because of its relatively weak ability to stimulate β-arrestin signaling (Fig. 2, A, right panel, and B, black bars, right panel) (note the different y-axis scale in the left and right graphs) (6).
Fig. 2.
The roles of GRK2 and GRK6 in β2AR-mediated ERK activation. (A) siRNA-transfected cells stably carrying the β2AR were serum-starved and then stimulated with either 10 µM isoproterenol (left panel) or 10 µM carvedilol (right panel) at 37°C for the indicated time points. Both phosphorylated ERK (pERK) and total ERK (tERK) were visualized by Western blotting. (B) Signals from (A) were quantified by densitometry and expressed as the percent of phosphorylated ERK1/2 obtained at the 5-min stimulation in control (CTL) siRNA–transfected cells. Data represent the means ± SEM from at least three independent experiments. Statistical significance was determined with a one-way ANOVA with Bonferroni post-test to correct for multiple comparisons between each GRK siRNA–transfected and control-transfected cells (*P < 0.05; **P < 0.01).
GRK2 depletion tended to increase agonist-stimulated ERK1/2 phosphorylation by either isoproterenol or carvedilol at both time points tested (Fig. 2B, red bars). However, it should be noted that there was an increase in basal ERK activation upon GRK2 knockdown possibly due to the increase in constitutive activation of the receptor. In contrast, GRK6 siRNA–transfected cells stimulated with isoproterenol showed no change in ERK1/2 phosphorylation at 5 min, but significantly less ERK1/2 phosphorylation at the 15-min time point when compared with CTL siRNA–transfected cells (Fig. 2B, blue bars). GRK6 depletion also led to significant decreases in carvedilol-stimulated ERK1/2 activation at both time points (Fig. 2B, blue bars). These results demonstrate that GRK6-mediated phosphorylation of the β2AR is important for β-arrestin–dependent ERK1/2 activation, whereas GRK2 phosphorylation of the receptor may inhibit β2AR signaling to ERK1/2.
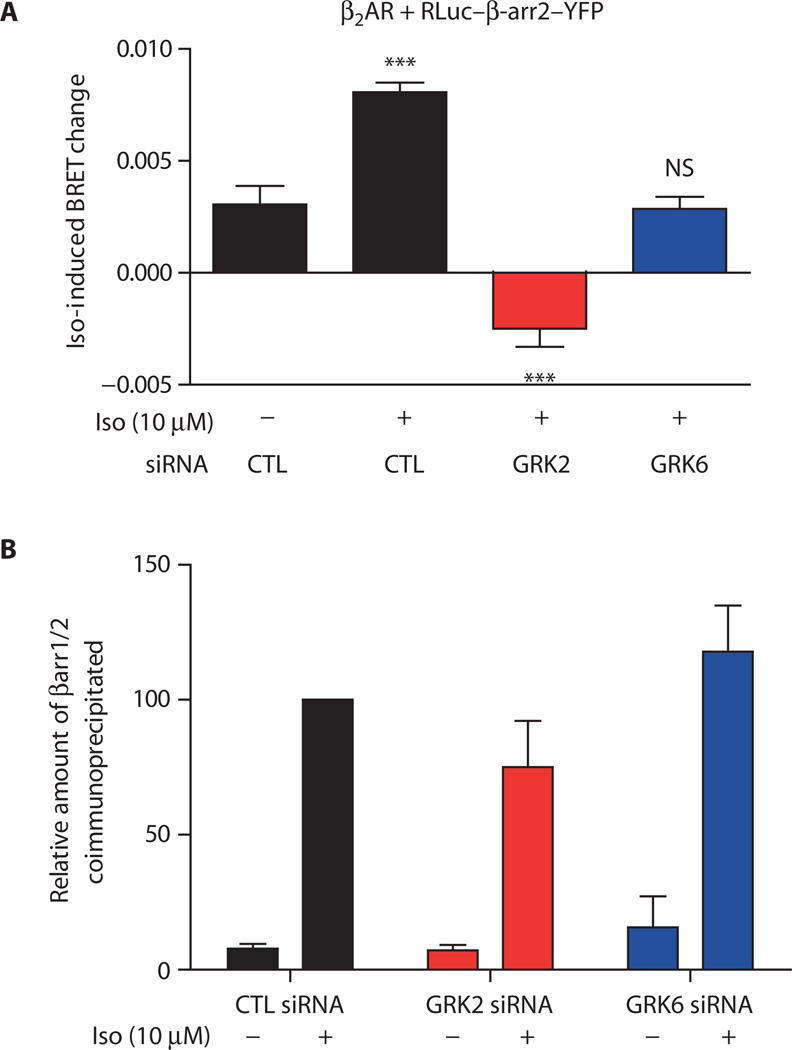
GRK2 and GRK6 phosphorylation dictate β-arrestin2 conformation
To test the hypothesis that phosphorylation of the β2AR, mediated by either GRK2 or GRK6, elicits distinct conformations of β-arrestin, we used a bioluminescence resonance energy transfer (BRET)–based biosensor for β-arrestin2 (14, 15). In this biosensor, RLuc–β-arr-YFP, the N terminus of β-arrestin2 is fused to bioluminescent Renilla luciferase (RLuc) and the C terminus is fused to yellow fluorescent protein (YFP). This biosensor displays quantitatively distinct responses depending on whether β-arrestins are selectively activated: Wild-type receptors stimulated by full agonists display directionally opposite changes in BRET signals compared to either wild-type receptors stimulated by β-arrestin–biased agonists or β-arrestin–biased mutant receptors stimulated by any agonist (15). HEK293 cells transiently transfected with the β2AR and the RLuc–β-arr2–YFP biosensor were stimulated with 10 µM isoproterenol after treatment with GRK-specific siRNAs (Fig. 3A). Isoproterenol stimulation of CTL siRNA–transfected cells resulted in an increase in the intramolecular BRET, which is consistent with previous reports (15) and is indicative of a conformational change in β-arrestin2 upon recruitment to the β2AR (Fig. 3A; CTL siRNA). Isoproterenol stimulation of β2AR after GRK2 siRNA treatment decreased the intramolecular BRET (Fig. 3A, red bar), indicating a different conformation of β-arrestin2 in the GRK2 siRNA–transfected cells compared with that in the CTL siRNA–transfected cells. The GRK6 siRNA–transfected cells showed no significant change in the BRET signal (Fig. 3A, blue bar).
Fig. 3.
β2AR phosphorylation dictates β-arrestin conformation without affecting recruitment. (A) HEK293 cells transiently cotransfected with β2AR and the β-arrestin2 BRET biosensor (RLuc–β-arr-YFP) were transfected with either control (CTL), GRK2, or GRK6 siRNAs. Changes in intramolecular BRET upon stimulation of β2AR by isoproterenol (10 µM for 15 min) were measured. Data are the means ± SEM of six independent experiments, each performed in quadruplicate. ***P < 0.001; NS, not significant between basal and stimulated conditions as determined by one-way ANOVA with Bonferroni post-test. (B) Immunoprecipitation of stably transfected FLAG-β2AR followed by Western blot analysis shows that agonist-induced β-arrestin association after 15-min stimulation with 10 µM isoproterenol was unaffected by knockdown of GRK2 or GRK6. Data shown are the means ± SEM from three independent experiments. One-way ANOVA and Bonferroni post-tests indicated no significant difference between the groups of data compared to control (CTL). The antibody used for the Western analysis detects both β-arrestin1 and β-arrestin2 (βarr1/2).
Previous studies indicated that silencing of individual GRKs in HEK293 cells reduces only the rate, but not the extent, of β-arrestin2 recruitment as assessed by fluorescence resonance energy transfer (FRET) (9). Therefore, we tested β-arrestin2 recruitment by measuring the amount that was chemically cross-linked to the receptor 15 min after stimulation with 10 µM isoproterenol (Fig. 3B) and found that β-arrestin2 recruitment to β2AR at 15 min was comparable in control-transfected cells and those in which GRK2 or GRK6 had been knocked down. Moreover, because HEK293 cells contain two endogenous β-arrestin isoforms (β-arrestin1 and β-arrestin2), we tested whether transient transfection of β-arrestin2 under the conditions used here precluded recruitment of endogenous β-arrestin1 as would be expected. Only β-arrestin2 recruitment was detectable after chemical cross-linking and immunoprecipitation of FLAG-tagged β2ARs from cells also transfected with β-arrestin2 (fig. S2). Because the extent of β-arrestin2 recruitment was not significantly affected by GRK6 knockdown, the lack of change in BRET signal suggests that β-arrestin2 adopts a unique conformation after GRK6 knock-down and that this β-arrestin2 conformation is different from that in either CTL or GRK2 siRNA–transfected cells. Thus, the BRET data and the recruitment data (Fig. 3) suggest that β-arrestin2 can adopt three distinct conformations: Under control conditions, the increase in BRET reflects one conformation, the decrease in BRET when GRK2 is knocked down reflects a second conformation, and the lack of a change in BRET signal when GRK6 is knocked down reflects a third conformation. These data suggest that distinct phosphorylation patterns on the β2AR produced by the two different GRKs result in distinct β-arrestin2 conformations.
Mass spectrometry identifies 13 phosphorylation sites on the β2AR
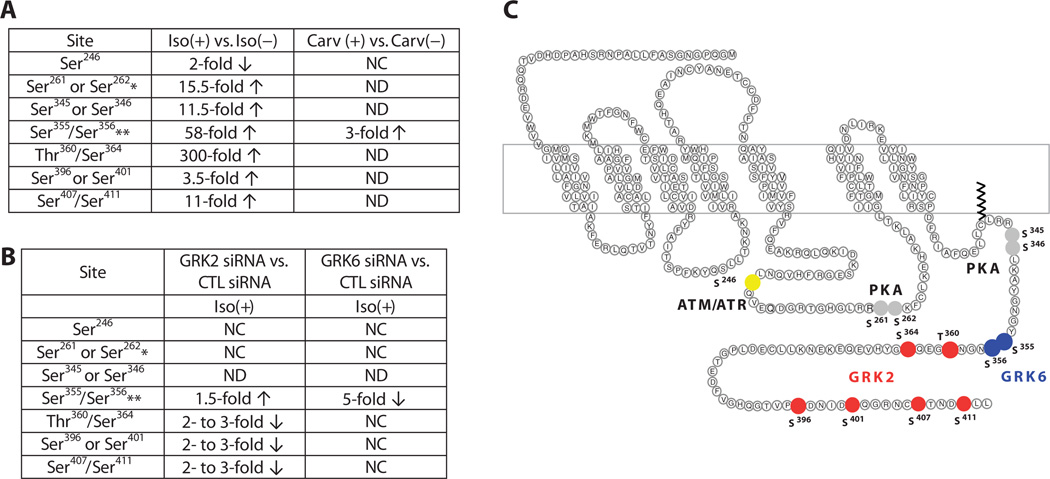
To determine whether GRK2 and GRK6 target distinct sites, we characterized the phosphorylation sites on the β2AR with a mass spectrometry (MS)–based proteomic approach (see Materials and Methods and fig. S3 for details). We used SILAC (stable isotope labeling with amino acids in cell culture) (16) to detect the difference in the relative amounts of phosphorylation of each site before and after agonist stimulation. Receptors were isolated from HEK293 cells stably transfected with human β2AR tagged with the FLAG epitope and the purified β2ARs were digested, and phosphopeptides, which had been enriched by IMAC (immobilized metal ion affinity chromatography), were analyzed by online liquid chromatography–tandem mass spectrometry (LC-MS/MS).
We identified 13 phosphorylation sites (Ser246, Ser261, Ser262, Ser345, Ser346, Ser355, Ser356, Thr360, Ser364, Ser396, Ser401, Ser407, and Ser411) on the β2AR. Upon stimulation with 10 µM isoproterenol for 5 min, these sites exhibited a wide range in the change in phosphorylation (Fig. 4A). Several of the sites identified are phosphorylated by kinases other than GRKs. For example, the pair Ser261 and Ser262 and the pair Ser345 and Ser346 are consensus sites for PKA, whereas Ser246 is a consensus site for ATM (ataxia telangiectasia mutated) phosphorylation (Fig. 4, A and C), and all of these have been previously reported as phosphorylated by the respective kinases (17, 18).
Fig. 4.
Quantitative analysis of β2AR phosphorylation. (A) Summary of phosphorylation changes in response to agonist stimulation as determined by quantitative LC-MS/MS. HEK293 cells stably transfected with the β2AR were treated with 10 µM isoproterenol (Iso) or carvedilol (Carv) for 5 min before harvesting. Fold changes in phosphorylation were calculated relative to samples without agonist treatment. *, singly phosphorylated peptides were detected when either one of the listed sites was phosphorylated; **, the listed two sites were identified as a cluster when both sites were phosphorylated; CTL, control; NC, no change; ND, not detected. (B) Summary of phosphorylation changes in response to GRK2 or GRK6 knock-down as determined by quantitative LC-MS/MS. GRK2 siRNA or GRK6 siRNA was used to knock down the individual kinases in HEK293 cells stably transfected with the β2AR. Fold changes were calculated relative to samples with CTL siRNA treatment. Both GRK siRNA– and CTL siRNA–treated cells were stimulated with 10 µM isoproterenol for 5 min before harvesting. (C) Mapping GRK2 and GRK6 phosphorylation sites on the β2AR. GRKs responsible for phosphorylation of individual residues were determined by siRNA treatment combined with quantitative MS. Significant fold changes of the phosphorylated residue clusters are shown and indicated by filled circles. Residues that exhibited a decrease in the extent of phosphorylation upon GRK2 knockdown are indicated in red and those that exhibited a decreasewithGRK6 knockdown are shown in blue. PKA consensus sites are shown in gray and the site phosphorylated by ATM is shown in yellow.
The consensus site for ATM phosphorylation, Ser246, was the only site that exhibited a decrease in phosphorylation (50%) upon isoproterenol stimulation (Fig. 4A and fig. S4). We confirmed this dephosphorylation event by Western blotting with site-specific antibodies that recognize the β2AR when it is phosphorylated or dephosphorylated at Ser246 (fig. S4, right panels in A and B).
Six and two phosphorylation sites on the β2AR map to targets of GRK2 and GRK6, respectively
To determine the phosphorylation sites on the β2AR for which GRK2 and GRK6 are responsible, we knocked down each of these kinases individually from cells and then used SILAC to quantitatively measure the extent of phosphorylation of each site in cells stimulated with isoproterenol. Depletion of GRK6 specifically reduced isoproterenol-promoted phosphorylation of Ser355 and Ser356 by fivefold compared to the extent of phosphorylation stimulated by isoproterenol in the presence of CTL siRNA (Fig. 4B and fig. S5). Depletion of GRK2 reduced isoproterenol-promoted phosphorylation of Thr360, Ser364, Ser396, Ser401, Ser407, and Ser411 by two to threefold (Fig. 4B). Thus, both GRK2 and GRK6 phosphorylate the receptors in response to the full agonist isoproterenol, but target unique regions and sites (Fig. 4C). Depletion of GRK2 increased isoproterenol-promoted phosphorylation of Ser355 and Ser356 by 1.5-fold (Fig. 4B and fig. S5, B and C), suggesting that GRK2 inhibited GRK6-mediated phosphorylation of these sites.
In contrast to isoproterenol, which triggered a change in the phosphorylation status of all 13 sites, 10 µM carvedilol stimulation for 5 min only induced an increase in phosphorylation of Ser355 and Ser356 (Fig. 4A), the sites phosphorylated by GRK6 (Fig. 4C), which was confirmed by Western blot analysis of purified, FLAG-tagged β2AR with an antibody specific for the pSer355 and pSer356 form of the receptor (fig. S6). This selective phosphorylation profile is consistent with the β-arrestin–biased signaling induced by this ligand.
Antibodies against phosphorylation-specific residues confirm the presence of a β2AR phosphorylation barcode
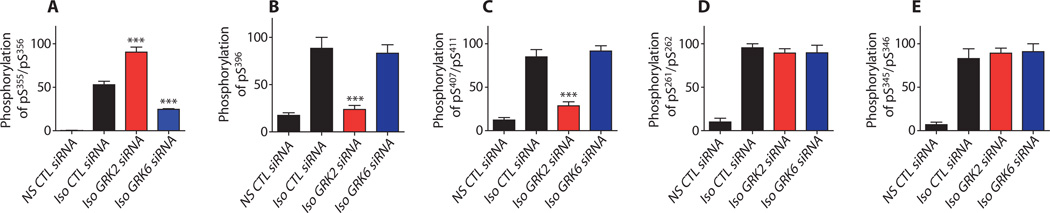
On the basis of the phosphorylation sites identified on the β2AR by MS, we generated several antibodies that specifically recognize the receptor phosphorylated at specific sites (anti–β2AR-pS261/pS262, anti–β2AR-pS396, and anti–β2AR-pS407/pS411) (see Materials and Methods for details) to complement the commercially available phosphorylation site–specific antibodies (anti–β2AR-pS345/pS346 and anti–β2AR-pS355/pS356). With this panel of phosphorylation site–specific antibodies, we performed Western blot analyses by probing the purified, FLAG-tagged β2AR from stably transfected HEK293 cells treated with various siRNAs. This analysis confirmed that GRK2 and GRK6 target distinct sites (Fig. 5 and fig. S7), with GRK6 phosphorylating Ser355 and Ser356 (Fig. 5A) and GRK2 phosphorylating Ser396, Ser407, and Ser411 (Fig. 5, B and C, and fig. S7, A and B). Western blot analysis also confirmed that GRK2 and GRK6 had reciprocal effects on phosphorylation of Ser355 and Ser356 (Fig. 5A). In addition, the extents of phosphorylation of the PKA sites (Ser261Ser262, Ser345, and Ser346) were not affected by either GRK siRNA treatment (Fig. 5, D and E, and fig. S7, C and D). Thus, these Western blot data confirm the MS results and provide further support for a signaling-specific phosphorylation barcode for the β2AR.
Fig. 5.
Confirmation of site-specific β2AR phosphorylation by GRK2 and GRK6 with phosphorylation site–specific antibodies. β2ARs were purified from HEK293 cells stably transfected with the β2AR that were transfected with CTL, GRK2, or GRK6 siRNA. Cells were stimulated with or without 10 µM isoproterenol for 5 min before the receptors were pulled down using an alprenolol-agarose affinity purification procedure. Western blot analyses of the purified β2ARs were then performed with phosphorylation site–specific antibodies. At least three independent experiments were quantified in (A) to (E) and data shown are the means ± SEM. Statistical significance was assessed by one-way ANOVA with Bonferroni post-test comparing CTL siRNA–treated cells versus either GRK2 or GRK6 siRNA–treated cells (***P < 0.001). (A to E) β2AR phosphorylation (A) at pSer355 and pSer356, (B) at pSer396, C) at pSer407 and pSer411, D) at the PKA consensus sites pSer261 and pSer262, and (E) at the PKA consensus sites pSer345 and pSer346. Representative Western blots for the data shown in (A) are shown in fig. S4C, and the data quantified are nonstimulated (0 min) and the 5-min data points. Representative Western blots for the remaining panels are shown in fig. S5.
DISCUSSION
Here, we used MS-based quantitative proteomic approaches to map phosphorylation sites on the β2AR, determined the GRKs responsible for phosphorylation of the sites, and delineated conformation-specific β-arrestin capabilities imparted by these specific phosphorylation events. We found that in HEK293 cells, GRK2 sites were primarily responsible for β2AR internalization, GRK6 sites contributed to β-arrestin–mediated ERK activation, and both GRKs contributed to desensitization. We also demonstrated that a β-arrestin–biased ligand, carvedilol, induced a phosphorylation pattern distinct from that of an unbiased, full agonist, isoproterenol. Finally, our data are consistent with the model that different phosphorylation patterns on the β2AR elicited by either GRK2 or GRK6 can induce distinct β-arrestin conformations. These data are consistent with the receptor phosphorylation barcode hypothesis, whereby the distinct pattern of phosphorylation triggers specific downstream signaling. Our data further support the idea that distinct patterns of multisite phosphorylation on a receptor by different GRKs differentially enable β-arrestin functions by inducing distinct β-arrestin conformations.
The present study found roles for both GRK2 and GRK6, whereas Violin et al. had previously reported that in HEK293 cells, GRK6 was the primary GRK responsible for mediating β2AR desensitization as assessed by a quantitative analysis of real-time cAMP dynamics (10). However, the experimental approach used in the present work differs in a number of respects. Violin et al. monitored changes in cAMP abundance over a 12-min time period after stimulation with 1 µM isoproterenol to study the kinetics of cAMP accumulation and degradation during this constant exposure to isoproterenol. In contrast, here we prestimulated the cells for 5 min with 100 nM isoproterenol, then washed the cells and rechallenged them with varying concentrations of isoproterenol (1 nM to 10 µM) 15 min after the initial stimulation. We measured cAMP within 5 min, representing a single ~20-min time point after the initial stimulation. Thus, in Violin et al. (10), the experiment ended with the desensitization that had occurred within 12 min, whereas here we examined desensitization at a single later time point, 20 min. It is possible that there are temporal differences in the mechanisms of desensitization with GRK6 important at early times and both GRK2 and GRK6 important later. In addition, there may be differences in the contributions of the GRKs in conditions of continuous versus transient exposure to ligand. Moreover, Violin et al. used the PKA antagonist H89 to block phosphodiesterase (PDE)–mediated cAMP degradation, a process activated by PKA. PKA promotes the translocation of GRK2, but not GRK6, to the membrane and thereby enhances its activity toward the receptor (19), and the use of H89 may have obscured a contribution of GRK2 in the previous study.
Distinct signaling in response to specific GRK phosphorylation is supported by studies of several other GPCRs, including the vasopressin 2 receptor (V2R), the chemokine receptor CXCR4, and the angiotensin 1A receptor (AT1AR), for which GRK6 or GRK5 promotes β-arrestin–mediated ERK activation, whereas GRK2 or GRK3 opposes it (7, 8, 20). In the case of the CCR7 chemokine receptor overexpressed in HEK293 cells, although both endogenous ligands CCL19 and CCL21 induce G protein activation, calcium mobilization, and G protein–dependent and β-arrestin2–dependent ERK activation with equal potency, only activation by CCL19 promotes robust desensitization (21). CCL19 leads to robust CCR7 phosphorylation and β-arrestin2 recruitment catalyzed by both GRK3 and GRK6, whereas CCL21 activates GRK6 alone and leads to weaker β-arrestin2 recruitment (22). Although these data suggested a correlation between specific CCR7 phosphorylation and β-arrestin2–dependent activities, the relevant sites phosphorylated by different GRKs were not determined.
A study of the chemokine receptor CXCR4, with MS in conjunction with antibodies that recognized site-specific phosphorylation, mapped phosphorylation sites upon stromal cell–derived factor 1 stimulation in HEK293 cells stably transfected with the CXCR4 (20).Of the 18 potential serine and threonine phosphorylation sites on the C terminus of CXCR4, 3 sites were identified as phosphorylated by MS, and an additional 4 were identified with phosphorylation site–specific antibodies. GRK6 accounted for most of the phosphorylation sites identified. Although no GRK2 or GRK3 sites were found, multiple GRKs regulate CXCR4 signaling, including GRK2. Silencing of either GRK2 or GRK6 by siRNA increased calcium mobilization, whereas knockdown of GRK3 or GRK6 decreased ERK1/2 activation. GRK2 knockdown enhanced ERK1/2 activation, suggesting coordination among the GRKs in terms of signaling, although no mechanistic explanation could be deduced in the absence of identified GRK2 phosphorylation sites.
Synthetic phosphopeptides corresponding to the C-terminal sequences of two GPCRs bind to and induce conformational changes in β-arrestins (23, 24). Moreover, phosphopeptides derived from the sequence of the V2R tail (a member of the “class B” receptors that bind β-arrestins tightly) induce conformational changes in β-arrestin distinct from those observed with phosphopeptides from β2AR (a member of the “class A” receptors that bind β-arrestin much less tightly) (12). β-Arrestins in these distinct conformations also interact differently with E3 ubiquitin ligases and deubiquitinases, and this may explain the differences in trafficking of class A receptors (rapidly recycle after dissociation from β-arrestins) versus class B receptors (recycle slowly and remain tightly bound to β-arrestins). Furthermore, the trafficking behavior of β2AR could be converted from that of a class A receptor to that of a class B receptor by transfection of GRK5 or GRK6, but not by GRK2, which suggests that the sites phosphorylated by GRK6 promote a more stable interaction between β-arrestin and the β2AR (13).
Biased ligands of GPCRs that activate β-arrestin signaling in the absence of G protein activation induce conformations of β-arrestin2 that are distinct from those induced by unbiased ligands, as assessed by an intramolecular β-arrestin2 BRET biosensor (15). That the biased ligand carvedilol triggered a pattern of receptor site phosphorylation distinct from that obtained with the unbiased agonist isoproterenol is consistent with these findings. Carvedilol is a β blocker that is effective in the treatment of heart failure and selectively stimulates β-arrestin–mediated signaling (5, 6). This signaling may contribute to the unique clinical efficacy of carvedilol in the treatment of heart failure. Therefore, carvedilol may serve as a prototype for a new generation of therapeutic β2AR ligands. One hypothesis to explain the selectivity of carvedilol for β-arrestin–mediated β2AR signaling is that it induces a specific conformation of the receptor, leading to receptor phosphorylation by specific GRK subtypes. We determined that stimulation of the β2AR with carvedilol induced phosphorylation only of Ser355 and Ser356 by GRK6, which contrasts with the change in phosphorylation at all 13 identified sites, including those for GRK2 and GRK6, in response to the full agonist isoproterenol. This result suggests that, whereas isoproterenol stimulation recruits both GRK2 and GRK6 to the receptor, carvedilol stimulation recruits only GRK6. This fits the notion that membrane association and activation of GRK2 occur through its interaction with Gβγ subunits (25, 26). Without activation of G proteins during carvedilol stimulation, it is likely that GRK2 is not targeted to the membrane. These data further suggest that biased ligands, by inducing distinct receptor conformations and G protein coupling, are able to recruit distinct GRKs.
We tested the hypothesis that distinct receptor phosphorylation patterns established by the different GRKs induce structurally and functionally distinct conformations of the bound β-arrestins. Using an intramolecular β-arrestin2 BRET biosensor, we found that GRK2 siRNA treatment (resulting in GRK-mediated phosphorylation by GRK6 on Ser355 and Ser356, the phosphorylation pattern of carvedilol) produced the same directionally negative change in the BRET ratio as we previously demonstrated with several β-arrestin–biased ligands in multiple GPCR systems (15). In addition, GRK6-siRNA treatment led to no change in the BRET signal despite robust recruitment of β-arrestin, which suggests yet a third distinct β-arrestin conformation. These data indicate that distinct phosphorylation patterns on a 7TMR result in unique β-arrestin conformations.
The barcode mechanism of specific signaling is not supported by all studies. Mutants of the AT1AR in which all potential C-terminal phosphorylation sites are removed by truncation or substitution with alanine still recruit β-arrestins, albeit in a weaker class A pattern than its wild type counterpart that induces a strong class B pattern (27). The mutant receptors activate ERK to the same extent as do wild-type AT1AR. However, in the absence of receptor phosphorylation, β-arrestin–mediated desensitization and endocytosis of AT1ARs are largely abrogated. In contrast, when all of the phosphorylation sites on the β2AR are mutated to alanine, the receptor neither binds β-arrestin nor stimulates ERK in a β-arrestin–dependent manner (27). Thus, for the β2AR, phosphorylation appears to be a prerequisite for β-arrestin recruitment and β-arrestin–mediated signaling, whereas this does not appear to be the case for the AT1AR.
A limited number of studies reporting the mapping of specific phosphorylation sites on 7TMRs are available. Trester-Zedlitz et al. reported that a peptide (residues 339 to 369) of the human β2AR purified and subsequently trypsin-digested from HEK293 cells contained multiple sites and showed that the net phosphorylation of this peptide increased with agonist stimulation (28). However, they were unable to assign specific phosphorylation sites or to detect any of the distal C-terminal sites. Fredericks et al. used purified, recombinant human β2AR in conjunction with GRK2 or GRK5 to delineate overlapping patterns of phosphorylation sites with these two GRKs in vitro (29). However, the physiological relevance of these studies is somewhat uncertain because of the high concentrations of receptors and GRKs used in these in vitro experiments. Mutation of all serine and threonine residues to alanines or glycines in the C terminus of the β2AR prevents agonist-stimulated phosphorylation (30), the interaction between the receptor and β-arrestin (31), and β-arrestin–mediated processes of desensitization (32), internalization (31), and ERK activation (27).
A number of studies targeting various combinations of four phosphorylation sites (Ser355, Ser356, Thr360, and Ser364) on the β2AR have shown that this region is important for β-arrestin binding and that loss of these sites impairs, to varying extents, receptor desensitization and internalization (31–35). Krasel et al. showed that although phosphorylation of these four sites (two of which we assigned as GRK6 sites here) can promote β-arrestin2 interaction with the β2AR, it is phosphorylation distal to residue 381 (assigned as GRK2 sites here) that is required for a high-affinity interaction between the receptor and β-arrestin2 (31). However, at least for β2AR trafficking, those four sites are not the only contributors. A Leu381 β2AR truncation mutant demonstrated strong interaction with β-arrestin2 but failed to internalize, suggesting that β-arrestin binding in and of itself is not sufficient for receptor internalization (31). Deletion of only the last eight residues of the β2AR C terminus (ΔASN405) also results in the failure of receptor internalization (31). These data suggest that, whereas both the distal and the proximal phosphorylation residues of the β2AR are important for β-arrestin binding, it is the distal residues (assigned as GRK2 sites here) that confer high-affinity binding and also coordinate protein-protein interactions that facilitate internalization. These data are also consistent with our finding that silencing of GRK2 leads to more marked impairment of β2AR internalization than does silencing of GRK6.
In summary, we have quantitatively mapped sites on the β2AR phosphorylated in response to stimulation with an unbiased agonist, isoproterenol, and a β-arrestin–biased ligand, carvedilol. We demonstrate that of the 13 sites phosphorylated in response to isoproterenol, only 2 (S355 and S356) are phosphorylated in response to carvedilol. Moreover, these correspond to the only sites for which phosphorylation is mediated by GRK6. Phosphorylation of the different sets of sites by the two GRKs engenders the distinct functionality of β-arrestin by inducing different conformations of the receptor-bound β-arrestin. These findings are consistent with a model where the patterning of receptor phosphorylation sites by different GRKs establishes a barcode that determines the conformation of the bound β-arrestins and, subsequently, its functional capabilities. Understanding such barcodes for various receptors may be useful in screening for therapeutic agents.
Supplementary Material
Acknowledgments
We gratefully acknowledge K. Kubota, J. Villen, W. Haas, B. Zhai, and X. Li of the Gygi laboratory for valuable assistance with the MS experiments. We are also grateful to E. Whalen, J. Violin, and J. Sun for stimulating ideas and helpful discussions. We also thank X. Jiang, D. Capel, and L. Langevin for excellent technical assistance and Q. Lennon and D. Addison for excellent secretarial assistance.
Funding: R.J.L. is an investigator with the Howard Hughes Medical Institute. This work was supported in part by grants from the U.S. NIH (HL16037 and HL70631) to R.J.L.
Footnotes
SUPPLEMENTARY MATERIALS
www.sciencesignaling.org/cgi/content/full/4/185/ra51/DC1
Fig. S1. Endogenous GRK2 and 6 in HEK293 cells.
Fig. S2. Specific β-arrestin2 recruitment to the β2AR.
Fig. S3. Overview of β2AR phosphorylation analysis by MS-based quantitative proteomics.
Fig. S4. Dephosphorylation of β2AR Ser246 by isoproterenol stimulation.
Fig. S5. Phosphorylation of Ser355/Ser356 on β2AR as detected by LC-MS/MS and Western blotting.
Fig. S6. Western blot analysis of phosphorylation of β2AR on Ser355/Ser356 in response to isoproterenol and carvedilol stimulation.
Fig. S7. Western blot analysis of β2AR phosphorylation at Ser396, Ser407/Ser411, Ser261/Ser262, and Ser345/Ser346.
Author contributions: K.N.N. and K.X. participated in experimental design, performed the experiments, analyzed the data, generated all the figures, and contributed to the writing of the manuscript; S.A., C.M.L., and S.R. participated in the desensitization and ERK phosphorylation experiments; S.K.S. participated in the internalization experiment, some data analysis, and presentation; A.K.S. participated in the BRET experiment; R.T.S. and M.R.H. participated in antibody generation; T.-Y.H. participated in cell culture and receptor purification; E.A.B. generated the GloSensor stable cell line; S.P.G. participated in MS experimental design and data analysis; and R.J.L. designed the experiments, analyzed the results, and wrote the manuscript.
Competing interests: The authors declare that they have no competing interests.
Data availability: The MS data can be viewed at http://lefkolab.org/RAW-data.287.0.html.
References
- 1.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 2.Shenoy SK, Lefkowitz RJ. Seven-transmembrane receptor signaling through β-arrestin. Sci. STKE. 2005;2005:cm10. doi: 10.1126/stke.2005/308/cm10. [DOI] [PubMed] [Google Scholar]
- 3.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 4.Violin JD, Lefkowitz RJ. β-Arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol. Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Kim IM, Tilley DG, Chen J, Salazar NC, Whalen EJ, Violin JD, Rockman HA. β-Blockers alprenolol and carvedilol stimulate β-arrestin-mediated EGFR transactivation. Proc. Natl. Acad. Sci. U.S.A. 2008;105:14555–14560. doi: 10.1073/pnas.0804745105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ. A unique mechanism of β-blocker action: Carvedilol stimulates β-arrestin signaling. Proc. Natl. Acad. Sci. U.S.A. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ren XR, Reiter E, Ahn S, Kim J, Chen W, Lefkowitz RJ. Different G protein-coupled receptor kinases govern G protein and β-arrestin-mediated signaling of V2 vasopressin receptor. Proc. Natl. Acad. Sci. U.S.A. 2005;102:1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J, Ahn S, Ren XR, Whalen EJ, Reiter E, Wei H, Lefkowitz RJ. Functional antagonism of different G protein-coupled receptor kinases for β-arrestin-mediated angiotensin II receptor signaling. Proc. Natl. Acad. Sci. U.S.A. 2005;102:1442–1447. doi: 10.1073/pnas.0409532102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butcher AJ, Prihandoko R, Kong KC, McWilliams P, Edwards JM, Bottrill A, Mistry S, Tobin AB. Differential G-protein-coupled receptor phosphorylation provides evidence for a signaling bar code. J. Biol. Chem. 2011;286:11506–11518. doi: 10.1074/jbc.M110.154526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Violin JD, Ren XR, Lefkowitz RJ. G-protein-coupled receptor kinase specificity for β-arrestin recruitment to the β2-adrenergic receptor revealed by fluorescence resonance energy transfer. J. Biol. Chem. 2006;281:20577–20588. doi: 10.1074/jbc.M513605200. [DOI] [PubMed] [Google Scholar]
- 11.Binkowski B, Fan F, Wood K. Engineered luciferases for molecular sensing in living cells. Curr. Opin. Biotechnol. 2009;20:14–18. doi: 10.1016/j.copbio.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Shenoy SK, Modi AS, Shukla AK, Xiao K, Berthouze M, Ahn S, Wilkinson KD, Miller WE, Lefkowitz RJ. β-Arrestin-dependent signaling and trafficking of 7-transmembrane receptors is reciprocally regulated by the deubiquitinase USP33 and the E3 ligase Mdm2. Proc. Natl. Acad. Sci. U.S.A. 2009;106:6650–6655. doi: 10.1073/pnas.0901083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, Reiter E, Premont RT, Lichtarge O, Lefkowitz RJ. β-Arrestin-dependent, G protein-independent ERK1/2 activation by the β2 adrenergic receptor. J. Biol. Chem. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- 14.Charest PG, Terrillon S, Bouvier M. Monitoring agonist-promoted conformational changes of β-arrestin in living cells by intramolecular BRET. EMBO Rep. 2005;6:334–340. doi: 10.1038/sj.embor.7400373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shukla AK, Violin JD, Whalen EJ, Gesty-Palmer D, Shenoy SK, Lefkowitz RJ. Distinct conformational changes in β-arrestin report biased agonism at seven-transmembrane receptors. Proc. Natl. Acad. Sci. U.S.A. 2008;105:9988–9993. doi: 10.1073/pnas.0804246105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteomics. 2002;1:376–386. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 17.Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, III, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, Shiloh Y, Gygi SP, Elledge SJ. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 18.Clark RB, Friedman J, Dixon RA, Strader CD. Identification of a specific site required for rapid heterologous desensitization of the β-adrenergic receptor by cAMP-dependent protein kinase. Mol. Pharmacol. 1989;36:343–348. [PubMed] [Google Scholar]
- 19.Cong M, Perry SJ, Lin FT, Fraser ID, Hu LA, Chen W, Pitcher JA, Scott JD, Lefkowitz RJ. Regulation of membrane targeting of the G protein-coupled receptor kinase 2 by protein kinase A and its anchoring protein AKAP79. J. Biol. Chem. 2001;276:15192–15199. doi: 10.1074/jbc.M009130200. [DOI] [PubMed] [Google Scholar]
- 20.Busillo JM, Armando S, Sengupta R, Meucci O, Bouvier M, Benovic JL. Site-specific phosphorylation of CXCR4 is dynamically regulated by multiple kinases and results in differential modulation of CXCR4 signaling. J. Biol. Chem. 2010;285:7805–7817. doi: 10.1074/jbc.M109.091173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohout TA, Nicholas SL, Perry SJ, Reinhart G, Junger S, Struthers RS. Differential desensitization, receptor phosphorylation, β-arrestin recruitment, and ERK1/2 activation by the two endogenous ligands for the CC chemokine receptor 7. J. Biol. Chem. 2004;279:23214–23222. doi: 10.1074/jbc.M402125200. [DOI] [PubMed] [Google Scholar]
- 22.Zidar DA, Violin JD, Whalen EJ, Lefkowitz RJ. Selective engagement of G protein coupled receptor kinases (GRKs) encodes distinct functions of biased ligands. Proc. Natl. Acad. Sci. U.S.A. 2009;106:9649–9654. doi: 10.1073/pnas.0904361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nobles KN, Guan Z, Xiao K, Oas TG, Lefkowitz RJ. The active conformation of β-arrestin1: Direct evidence for the phosphate sensor in the N-domain and conformational differences in the active states of β-arrestins1 and-2. J. Biol. Chem. 2007;282:21370–21381. doi: 10.1074/jbc.M611483200. [DOI] [PubMed] [Google Scholar]
- 24.Xiao K, Shenoy SK, Nobles K, Lefkowitz RJ. Activation-dependent conformational changes in β-arrestin 2. J. Biol. Chem. 2004;279:55744–55753. doi: 10.1074/jbc.M409785200. [DOI] [PubMed] [Google Scholar]
- 25.Lefkowitz RJ, Inglese J, Koch WJ, Pitcher J, Attramadal H, Caron MG. G-protein-coupled receptors: Regulatory role of receptor kinases and arrestin proteins. Cold Spring Harb. Symp. Quant. Biol. 1992;57:127–133. doi: 10.1101/sqb.1992.057.01.016. [DOI] [PubMed] [Google Scholar]
- 26.Pitcher JA, Inglese J, Higgins JB, Arriza JL, Casey PJ, Kim C, Benovic JL, Kwatra MM, Caron MG, Lefkowitz RJ. Role of βγ subunits of G proteins in targeting the β-adrenergic receptor kinase to membrane-bound receptors. Science. 1992;257:1264–1267. doi: 10.1126/science.1325672. [DOI] [PubMed] [Google Scholar]
- 27.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. β-Arrestins and cell signaling. Annu. Rev. Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 28.Trester-Zedlitz M, Burlingame A, Kobilka B, von Zastrow M. Mass spectrometric analysis of agonist effects on posttranslational modifications of the β-2 adrenoceptor in mammalian cells. Biochemistry. 2005;44:6133–6143. doi: 10.1021/bi0475469. [DOI] [PubMed] [Google Scholar]
- 29.Fredericks ZL, Pitcher JA, Lefkowitz RJ. Identification of the G protein-coupled receptor kinase phosphorylation sites in the human β2-adrenergic receptor. J. Biol. Chem. 1996;271:13796–13803. doi: 10.1074/jbc.271.23.13796. [DOI] [PubMed] [Google Scholar]
- 30.Bouvier M, Hausdorff WP, De Blasi A, O’Dowd BF, Kobilka BK, Caron MG, Lefkowitz RJ. Removal of phosphorylation sites from the β2-adrenergic receptor delays onset of agonist-promoted desensitization. Nature. 1988;333:370–373. doi: 10.1038/333370a0. [DOI] [PubMed] [Google Scholar]
- 31.Krasel C, Zabel U, Lorenz K, Reiner S, Al-Sabah S, Lohse MJ. Dual role of the β2-adrenergic receptor C terminus for the binding of β-arrestin and receptor internalization. J. Biol. Chem. 2008;283:31840–31848. doi: 10.1074/jbc.M806086200. [DOI] [PubMed] [Google Scholar]
- 32.Hausdorff WP, Bouvier M, O’Dowd BF, Irons GP, Caron MG, Lefkowitz RJ. Phosphorylation sites on two domains of the β2-adrenergic receptor are involved in distinct pathways of receptor desensitization. J. Biol. Chem. 1989;264:12657–12665. [PubMed] [Google Scholar]
- 33.Seibold A, January BG, Friedman J, Hipkin RW, Clark RB. Desensitization of β2-adrenergic receptors with mutations of the proposed G protein-coupled receptor kinase phosphorylation sites. J. Biol. Chem. 1998;273:7637–7642. doi: 10.1074/jbc.273.13.7637. [DOI] [PubMed] [Google Scholar]
- 34.Seibold A, Williams B, Huang ZF, Friedman J, Moore RH, Knoll BJ, Clark RB. Localization of the sites mediating desensitization of the β2-adrenergic receptor by the GRK pathway. Mol. Pharmacol. 2000;58:1162–1173. doi: 10.1124/mol.58.5.1162. [DOI] [PubMed] [Google Scholar]
- 35.Vaughan DJ, Millman EE, Godines V, Friedman J, Tran TM, Dai W, Knoll BJ, Clark RB, Moore RH. Role of the G protein-coupled receptor kinase site serine cluster in β2-adrenergic receptor internalization, desensitization, and β-arrestin translocation. J. Biol. Chem. 2006;281:7684–7692. doi: 10.1074/jbc.M500328200. [DOI] [PubMed] [Google Scholar]
- 36.Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated β2-adrenergic receptor and β-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- 37.Ahn S, Nelson CD, Garrison TR, Miller WE, Lefkowitz RJ. Desensitization, internalization, and signaling functions of β-arrestins demonstrated by RNA interference. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1740–1744. doi: 10.1073/pnas.262789099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caron MG, Srinivasan Y, Pitha J, Kociolek K, Lefkowitz RJ. Affinity chromatography of the β-adrenergic receptor. J. Biol. Chem. 1979;254:2923–2927. [PubMed] [Google Scholar]
- 39.Xiao K, McClatchy DB, Shukla AK, Zhao Y, Chen M, Shenoy SK, Yates JR, III, Lefkowitz RJ. Functional specialization of β -arrestin interactions revealed by proteomic analysis. Proc. Natl. Acad. Sci. U.S.A. 2007;104:12011–12016. doi: 10.1073/pnas.0704849104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rappsilber J, Ishihama Y, Mann M. Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal. Chem. 2003;75:663–670. doi: 10.1021/ac026117i. [DOI] [PubMed] [Google Scholar]
- 41.Xiao K, Sun J, Kim J, Rajagopal S, Zhai B, Villen J, Haas W, Kovacs JJ, Shukla AK, Hara MR, Hernandez M, Lachmann A, Zhao S, Lin Y, Cheng Y, Mizuno K, Ma’ayan A, Gygi SP, Lefkowitz RJ. Global phosphorylation analysis of β-arrestin-mediated signaling downstream of a seven transmembrane receptor (7TMR) Proc. Natl. Acad. Sci. U.S.A. 2010;107:15299–15304. doi: 10.1073/pnas.1008461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haas W, Faherty BK, Gerber SA, Elias JE, Beausoleil SA, Bakalarski CE, Li X, Villen J, Gygi SP. Optimization and use of peptide mass measurement accuracy in shotgun proteomics. Mol. Cell. Proteomics. 2006;5:1326–1337. doi: 10.1074/mcp.M500339-MCP200. [DOI] [PubMed] [Google Scholar]
- 43.Beausoleil SA, Villen J, Gerber SA, Rush J, Gygi SP. A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat. Biotechnol. 2006;24:1285–1292. doi: 10.1038/nbt1240. [DOI] [PubMed] [Google Scholar]
- 44.Bakalarski CE, Elias JE, Villen J, Haas W, Gerber SA, Everley PA, Gygi SP. The impact of peptide abundance and dynamic range on stable-isotope-based quantitative proteomic analyses. J. Proteome Res. 2008;7:4756–4765. doi: 10.1021/pr800333e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.