Abstract
It is estimated worldwide that over 6 million people per annum experience a burn injury. Despite advances in management and improved survival rates, the incidence of hypertrophic scarring remains high. These scars are particularly common after burns and are often raised, red, hard and may cause abnormal sensations. Such pathological scarring can lead to severe functional impairment, psychological morbidity, and costly long term healthcare. Wound healing is an inherent process which restores the integrity of the skin after injury and although scarring is a frequent by-product, the scarless wound healing observed in early human gestational fetuses suggests that it is not an essential component of the response. This has lead to a large body of research attempting to understand the mechanisms behind scarring and in turn prevent it. One of the main focuses of recent research has been the role played by the growth factor TGF-β in the process of both wound healing and scar formation. The three isoforms (TGF-β1, TGF-β2 and TGF-β3) appear to have overlapping functions and predominantly mediate their effects through the intracellular SMAD pathway. Initial research suggested that TGF-β1 was responsible for the fibrotic scarring response whereas the scarless wound healing seen in fetal wounds was due to increased levels of TGF-β3. However, the reality appears to be far more complex and it is unlikely that simply altering the ratio of TGF-β isoforms will lead to scarless wound healing. Other aspects of the TGF-β system that appear promising include the downstream mediator CTGF, the proteoglycan decorin and the binding protein p311. Other putative mechanisms which may underlie the pathogenesis of hypertrophic scars include excessive inflammation, excessive angiogenesis, altered levels of matrix metalloproteinases, growth factors, and delayed apoptosis of fibrotic myofibroblasts either due to p53 genetic alterations or tensile forces across the wound. If an effective treatment for hypertrophic scars following burns injury is to be developed then further work must be carried out to understand the basic mechanisms of pathological scarring.
Keywords: Burn scarring, hypertrophic scarring, pathological scarring, regeneration, TGF-β, wound healing
Introduction
It has been estimated that over 6.6 million people world-wide suffer a burns injury annually [1]. Though many burn injuries require no medical intervention, a number require medical treatment and the American Burn Association estimate that 500,000 patients seek medical attention a year [2]. Survival following extensive burns has improved over recent years with advancement in fluid resuscitation, new antibiotics, skin replacements and specialised burns centres. However, the incidence, treatment and prevention of scarring, particularly hypertrophic scarring has not improved with survival [3]. Patients following a burn injury may require long term medical intervention for their scars and their related issues. Patients suffering pathological scarring such as hypertrophic scars can suffer from disfigurement, disability, stigmatization, disruption of daily activities, as well as psychological issues [4-7].
Hypertrophic scars are often raised, red, hard, and usually have abnormal sensations, which can include pain and tenderness [8,9]. The incidence of hypertrophic scarring has varied in studies between 32-67% but rises to 75% in children, young adults and those with pigmented skin [10-14]. Some hypertrophic scars particularly those associated with thermal injuries are associated with contractures [15,16], which are not only disfiguring but when occurring over a joint can result in loss of functionality and disability [17].
Scarring
Wound healing is an inherent process, which restores the integrity of skin as quickly as possible. Restoration of the skin is essential, due to the skins importance in survival through the prevention of infection, fluid loss and other vital functions. Some have suggested that wound healing evolved for speed, to allow the wound to heal quickly reducing the risk of infection [18]. Wound healing is a dynamic process with at one end of the spectrum an over exuberance resulting in pathological scarring while at the other end a non-healing or chronic wound. However, it has been known since the 1970’s that scarring is not required for wound healing with early human gestational fetuses healing cutaneous wounds perfectly without the formation of scar tissue [19]. Variation in the outcome of wound healing is not just seen between racial groups, individuals, gender and age but can also vary within the same individual. Hypertrophic scarring has been shown to be more common following certain injuries such as burns, delayed epithelisation or wounds occurring in areas of high tension for example the deltoid and sternal regions or areas of movement [20-22]. Others, have suggested that the depth of the wound may be associated with hypertrophic scarring, with fibroblasts derived from the deep dermis resembling fibroblasts from hypertrophic scarring [23]. Singer and Clark suggest that hypertrophic scarring is caused by an aberrant form of wound healing demonstrated by a constitutively active proliferative phase [24]. Though other theories have been proposed and will be discussed later.
Hypertrophic scars are raised, abnormally pigmented and can cause itching or abnormal sensations. Unlike keloids, hypertrophic scars remain within the boundary of the original injury and hypertrophic scars can regress with time. Hypertrophic scars have been shown to have a preponderance of collagen type III fibres orientated parallel to the epidermal surface. They are often composed of nodules containing myofibroblasts, differentiated fibroblasts expressing alpha smooth muscle actin, collagen filaments and other extracellular matrices [25].
TGF-β and hypertrophic scars
TGF-β is a family of growth factors involved in a number of essential cellular functions. The three isoforms of TGF-β (TGF-β1, -β2, -β3) are secreted as inactive latent precursors that require activation prior to binding to the TGF-β receptors [26]. The three isoforms, share 60-80% homology and are encoded by different genes. However, the isoforms are believed to activate the same intracellular signalling pathways and appear in vitro to have overlapping biological functions, though with differing in vitro expression [27]. However, knockout mice have shown that the three isoforms appear to play different roles in both development and homeostasis [28-31]. All three isoforms appear to be present in wound healing, and even wounds from early human foetuses, which repair cutaneous wounds perfectly, have been shown to contain all three TGF-β isoforms [32]. However, these isoforms in fetal wounds not only differ in levels of expression, duration in the wound, but also in their biological activity [32-37]. TGF-β is involved in a number of processes in wound healing: inflammation, stimulating angiogenesis, fibroblast proliferation, collagen synthesis and deposition and remodelling of the new extracellular matrix [38,39]. Interestingly chronic, non-healing wounds often show a loss of TGF-β1 signalling [40,41].
All three isoforms are believed to bind and signal through the two TGF-β receptors (TβRI and TβRII) [26]. TβRII is constutively phosphorylated and on binding of the ligand, phosphorylates TβRI. Phosphorylation of the receptor complex activates the SMAD intracellular signalling pathway through the receptor Smads (Smad-2 and Smad-3) and co-Smad 4. The receptor SMADs and Smad-4 cross over the nuclear membrane where they regulate a number of genes [26,41]. TGF-β can also activate a number of non-Smad signalling pathways whose function in TGF-β remains to be elucidated [26].
Fibroblasts derived from hypertrophic scars have been shown, by a number of groups, to have an altered phenotype compared to fibroblasts derived from normal scars or uninjured dermis [43-45]. Wang and colleagues showed that hypertrophic derived fibroblasts and hypertrophic scar tissue produced more mRNA and protein for TGF-β1 than normal skin or fibroblasts derived from normal skin, suggesting a possible role for TGF-β1 in hypertrophic scar formation [45]. Not only have hypertrophic derived fibroblasts shown more TGF-β1 expression, but they have also been shown to have a prolonged expression of the TGF-β receptors compared to normal skin [46]. The group suggest that this expression of TGF-β is persistent compared to normal wound healing where receptor expression decreases during the remodelling phase [46]. The group suggest that this persistence of receptor expression may result in a feedback loop resulting in a fibrotic phenotype [46]. Others have suggested that the three isoforms of TGF-β have different temporal effects on wound healing and scarring, and any disruption in this expression pattern may result in hypertrophic scar formation [47].
Fibroblasts derived from hypertrophic scars have been shown to have an alteration in TGF-β signalling. Studies have indicated increased expression and phosphorylation of the receptor Smads-2 and/or 3 in hypertrophic scarring [48,49]. Xie and colleagues showed that the Smad inhibitor, Smad 7, showed no up regulation in response to TGF-β1 compared to fibroblasts derived from normal skin [48]. Kopp et al showed that over expressing Smad 7 prevented collagen contraction in both normal and hypertrophic scar derived fibroblasts [49]. Interestingly, animals lacking Smad-3 show improved wound-healing (increased rate of re-epithelization, reduced infiltration of monocytes). While in a bleomycin-lung fibrosis model, mice lacking Smad-3 showed suppression of type I procollagen mRNA expression and an attenuation of the fibrotic process [50,51; Figure 1].
Figure 1.
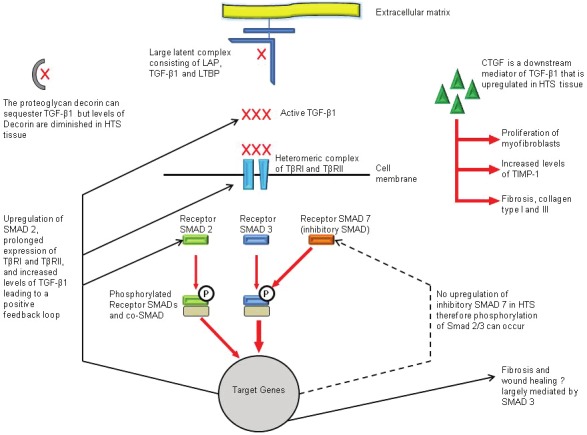
Summary of TGF-β signalling in hypertrophic scarring.
Rorison et al [52] showed that plasma levels of TGF-β maybe a predictive indicator in children who develop hypertrophic scars after a burn. They showed that children whose burns healed well and without hypertrophic scarring showed elevation of TGF-β in their plasma for two weeks post burn. However, those who developed hypertrophic scarring did not demonstrate this early increase in TGF-β levels [52]. Others have shown an increased frequency of CD4+/TGF-β producing T cells in blood from burns patients, and this was further identified in hypertrophic scar tissue [53]. Wang and colleagues suggested that these cells may regulate the functions of dermal fibroblasts resulting in hypertrophic scar formation. They showed that dermal fibroblasts showed increased proliferation, alpha smooth muscle expression and collagen gel contraction and collagen synthesis when treated with medium derived from CD4+ T lymphocytes from a burn patient compared to normal patients [53].
CTGF (CCN2) a downstream mediator of TGF-β1, though co-ordinately expressed, CTGF has been shown to be involved in the formation of fibrosis and TGF-β1’s pro-contractile activity is believed to mediated by CTGF [54,55; Figure 1]. CTGF has been shown to be elevated in fibroblasts derived from hypertrophic scars in both unstimulated hypertrophic scar fibroblasts and following stimulation with any of the three isoforms of TGF-β [56]. Sisco and colleagues showed in an animal model that inhibiting the action of CTGF through antisense oligonucleotides limited hypertrophic scar formation through the reduction of myofibroblasts, decreased TIPM-1 and collagens types I and III, but did not alter wound closure, therefore CTGF may be a potential future therapeutic target in the prevention of hypertrophic scarring [57].
Proteoglycans are specialised glycoproteins which also contain linear polysaccharides, glycosaminoglycans. Proteoglycans are known to play a role in cell signalling and can interact and modulate proteins found in the extracellular matrix. The proteoglycan decorin is known to bind to the three TGF-β isoforms and inhibits their activity by sequestering the isoforms to the extracellular matrix. Decorin also plays a role in regulating collagen fibrillogenesis, and has been shown to interact with other growth factors regulating their action, including CTGF [58]. Both fibromodulin and decorin have been shown to have lower levels or delayed expression in post-burn hypertrophic scars [59,60]. This low or reduced expression may explain the irregular collagen organisation and increased extracellular matrix production in pathological scarring [59]. The use of recombinant human decorin in in vitro studies has shown that decorin plays a role in reducing hypertrophic fibroblast proliferation, collagen synthesis and collagen contraction with decorin inhibiting both basal and TGF-β1 enhanced contraction in both normal and hypertrophic scar fibroblasts [61,62].
P311 a binding protein of the TGF-β1 latency associated protein, has been suggested to be involved in myofibroblast differentiation and fibrosis [63], though its biological function remains largely unknown. P311 has been found to be over expressed in hypertrophic scar tissue and other fibrotic lesions [64,65], and appears involved in wound healing as it is expressed by myofibroblasts, though is absent once the wound is healed [63]. The role that P311 has on TGF-β expression remains unclear, in human derived skin cells forced expression of p311 increases both TGF-β1 and collagen type 1 (COL1A1) mRNA expression [66 ], other human fibrotic tissues show elevation of both P311 and TGF-β1 suggesting a role for both in fibrosis [65]. However, in the mouse fibroblast cell lines, NIH 3T3 and C3H10, P311 inhibited TGF-β1 and TβRII expression with a subsequent decrease in collagen expression [63].
Other mechanisms in the development of hypertrophic scarring
Though the exact pathophysiology of hypertrophic scars is unknown a number of theories besides the role of TGF-β have been proposed (Figure 2). Table 1 shows examples of other mechanisms shown to be aberrant in hypertrophic scars. Understanding the mechanisms behind hypertrophic scars will help with therapeutic treatments to help prevent or reduce hypertrophic scarring.
Figure 2.
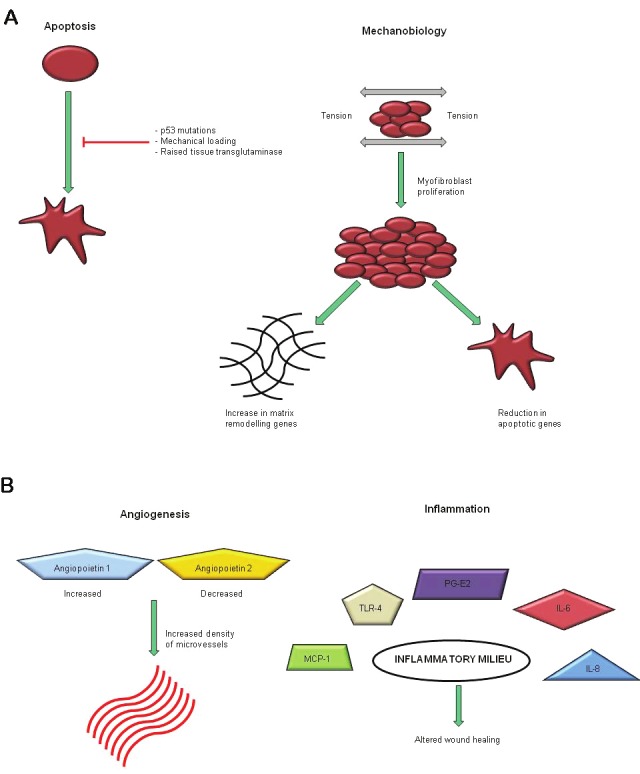
Summary of some of the mechanisms believed to be involved in the formation of hypertrophic scars.
Table 1.
Potential mechanisms involved in the development of hypertrophic scarring. Summarised in Figure 2
| Mechanism | Results | Reference |
|---|---|---|
| Apoptosis | -p53 alterations in hypertrophic scar derived cells | [44,67-71] |
| -Mechanical loading inhibits cellular apoptosis | ||
| -Bcl-2 increased in peripheral blood mononuclear cell fractions from burns patients with hypertrophic scars compared to burns patients who had normal healing | ||
| -failure to undergo apoptosis in hypertrophic scar fibroblasts due to over expression of tissue transglutaminase | ||
| -myofibroblasts from hypertrophic scars fail to undergo apoptosis in response to apoptotic inducers | ||
| -Decreased Fas expression in vivo and in vitro | ||
| Mechanobiology, tension | -Cyclical stretching influences expression of genes, growth factors etc | [72-74] |
| -Stretching causes number of myofibroblasts to increase | ||
| -mechanical strain upregulates matrix remodelling genes but down regulates cellular apoptosis genes | ||
| Angiogenesis and angiogenic factors | -Decrease in angiopoietin1/angiopoietin 2 ratio in hypertrophic scars (following surgery), microvessel density higher in hypertrophic scars | [75] |
| Inflammation | -Increased toll-like receptor-4,prostaglandin E2, IL-6, IL-8 and MCP-1 in hypertrophic derived fibroblasts (following burn) compared to normal matched dermal fibroblasts | [76-79] |
| -Increase in IL-6 in hypertrophic burn scar fibroblasts compared to normal control fibroblasts | ||
| -Increased IL-15 in hypertrophic scars compared to normal scars and skin | ||
| -HTS after a burn showed a polarized Th2 systemic reponse- increased T cells and Th2 fibrogenic cytokines | ||
| MMP | -Higher TIMP-1 and 2 mRNA in hypertrophic scars | [80,81] |
| Higher MMP2 mRNA | ||
| Higher TIMP-1 in sera than normal scar | ||
| -Higher MMP2 in hypertrophic compared to normal | ||
| Extra cellular matrix | -Distribution and organization of fibrillin-1 and elastin different between normal skin and pathological scars (hypertrophic and keloids) | [82] |
| Growth factors | -Increased expression TGF-β1, β2, β3, bFGF and VEGF in keratinocytes from burn scars at 1 month compared to matched normal. TGFβ3 elevated in hypertrophic scars than normal. | [83-85] |
| -IGF-1 – increased number of cells producing IGF1 in hypertrophic scar tissue compared to normal skin samples |
Treatment
To date, there remains no definitive treatment to either prevent or reduce any form of scarring. Peer reviewed data on the effectiveness of scar prevention treatments are few and far between. Clinical studies on current treatments are often inadequate due to small numbers of patients, lack of well designed controls and a lack of standardisation in scar outcome measurements [86]. A number of mechanisms associated with the development of hypertrophic scarring have been used to manipulate the scarring potential. Table 2 reviews a number of the current and potential therapies involved in different mechanisms associated with developing hypertrophic scarring.
Table 2.
Therapies and potential therapies for hypertrophic scarring
| Mechanism | Drug | Reference |
|---|---|---|
| Apoptosis/ reducing proliferation | Intralesional corticosteroids, | [87-92] |
| IFN-α2b | ||
| Combinations of treatment | ||
| Compression | ||
| Fibrostat | ||
| Extracellular matrix | Silicone gel (various mechanisms including remodelling of extra cellular matrix) | [93-95] |
| AZX-100 (Capstone Therapeutics; peptide analogue of HSP 20) | ||
| Minocycline | ||
| Inflammation | IFN-α2b | [88] |
| Growth factors | Receptor tyrosine kinase inhibitors (SU9518, SU11657, Imatinib/Gleevec) | [96] |
A number of groups are studying the manipulation of TGF-β in the prevention of both normal scarring and hypertrophic scarring. TGF-β neutralising antibodies have been shown to inhibit fibrosis in a number of animal models [97,98], however, reduction in TGF-β signalling has been linked with chronic or non-healing wounds [40,41]. Blocking TGF-β through a number of natural TGF-β inhibitors, such as decorin, biglycan, LAP, may block the fibrotic TGF-β response, but not affect the TGF-β immune response [99]. Other methods of manipulating TGF-β include blocking the TGF-β receptors with kinase inhibitors (for example SD-208), a dominant negative TGFβRII (TbetaRIIDeltacyt), which have been shown to prevent fibrosis in animal models by blocking profibrotic gene expression [100,101]. Ahn and colleagues in 2010 showed that betaglycan (the TGF-β III receptor) inhibited Smad signalling and Akt and ERK phosphoryation [102], and in vivo models suggest that soluble betaglycan may prevent fibrosis in animal models [103]. Few clinical studies have been performed on the manipulation of TGF-β1 and its isoforms to prevent dermal scarring. Ferguson and colleagues published three double-bind placebo controlled studies (phase I/II) using the administration of TGF-β3 prophylatically [104], however the Phase 3 trial appears to have been unsuccessful [105].
Problems
Though there has been a huge array of research conducted on hypertrophic scarring, however, our understanding of its pathophysiology, and potential therapeutic agents remain unclear. There are problems with current research with as yet no definitive animal model available, though studies have described a number of animal models including the rabbit and the red Duroc pig [106,107]. However it remains to be seen if these models are an adequate comparison compared to human scarring. Further, comparisons between research studies has been hampered due to a number of variations between studies for example the aetiology of the hypertrophic scarring (thermal injury v injury v surgery), maturity of the scar, any previous treatments, and the location of the hypertrophic scar which may all impact research findings. Research is almost always conducted after the fact; there have been few if any research conducted on the tissue prior or during the injury, and then following on after the injury. In human patients this may be difficult due to ethical permission, but this may indicate not only potentially susceptible individuals but also where wound healing goes awry.
Conclusion
Hypertrophic scarring is a common abnormality of wound healing often associated with thermal injuries. To date, the mechanism behind this form of scarring remains unclear with much research focussing on the pro-fibrotic growth factor TGF-β1. Further, as yet there is no definitive treatment to reduce or prevent any scarring though the market place is large with many patients finding their scarring unacceptable.
References
- 1.ReSurge International. http://www.org/about/gfx/fs09_burns%20factsheet.pdf (last accessed June 9th 2011) [Google Scholar]
- 2.American Burn Association Fact Sheet. 2011 Available at. http://www.ameriburn.org/resources_factsheet.php. accessed 4th may 2011. [Google Scholar]
- 3.Sheridan RL. Burn Care: Results of Technical and Organizational Progress. JAMA. 2003;290:719–722. doi: 10.1001/jama.290.6.719. [DOI] [PubMed] [Google Scholar]
- 4.Taal L, Faber AW. Posttraumatic stress and maladjustment among adult bun survivors 1 to 2 years post burn. Part III The interview data. Burns. 1998;24:399–405. doi: 10.1016/s0305-4179(98)00053-9. [DOI] [PubMed] [Google Scholar]
- 5.Robert R, Meyer W, Bishop S, Rosenberg L, Murphy L, Blakeney P. Disfiguring burn scars and adolescent self esteem. Burns. 1999;25:581–585. doi: 10.1016/s0305-4179(99)00065-0. [DOI] [PubMed] [Google Scholar]
- 6.Dorfmuller M. Psychological management and after-care of severely burned patients. Unfallchirurg. 1995;98:213–217. [PubMed] [Google Scholar]
- 7.Woo SH, Seul JH. Optimising the Correction of Severe Post burn Hand Deformities by using Aggressive Contracture Releases and fasciocutaneous free tissue transfers. Plast Reconst Surg. 2001;107:1–8. doi: 10.1097/00006534-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Ehrlich HP, Kelley SF. Hypertrophic Scar: An interruption in the remodelling of repair- a laser Doppler blood flow study. Plast Reconstr Surg. 1992;90:993–998. [PubMed] [Google Scholar]
- 9.Rudolph R. Wide spread scars, hypertrophic scars and keloids. Clin Plast Surg. 1987;14:253–260. [PubMed] [Google Scholar]
- 10.Deitch EA, Wheelaham TM, Rose MP, Clothier J, Cotter J. Hypertrophic burn scars: analysis of variables. J Trauma. 1983;23:895–898. [PubMed] [Google Scholar]
- 11.Dedovic Z, Koupilova I, Brychta P. Time trends in incidence of hypertrophic scarring in children treated for burns. Acta Chir Plast. 1999;41:87–90. [PubMed] [Google Scholar]
- 12.McDonald WS, Deitch EA. Hypertrophic skin grafts in burned patients: A prospective analysis of variables. J Trauma. 1987;27:147–150. doi: 10.1097/00005373-198702000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Spurr ED, Shakespeare PG. Incidence of hypertrophic scarring in burn-injured children. Burns. 1990;16:179–181. doi: 10.1016/0305-4179(90)90034-t. [DOI] [PubMed] [Google Scholar]
- 14.Bombaro KM, Engrav LH, Carrougher GJ, Wiechman SA, Faucher L, Costa BA, Heimbach DM, Rivara FP, Honari S. What is the prevalence of hypertrophic scarring following burns? Burns. 2003;29:299–302. doi: 10.1016/s0305-4179(03)00067-6. [DOI] [PubMed] [Google Scholar]
- 15.Gangemi EN, Gregori D, Berchialla P, Zingarelli E, Cairo M, Bollero D, Ganem J, Capocelli R, Cuccuru F, Cassano P, Risso D, Stella M. Epidemiology and risk factors for pathologic scarring after burn wounds. Arch Facial Plast Surg. 2008;10:93–102. doi: 10.1001/archfaci.10.2.93. [DOI] [PubMed] [Google Scholar]
- 16.Schneider JC, Holvanahalli R, Helm P, Goldstein R, Kowalske K. Contractures in burn injury: defining the problem. J Burn Care Res. 2006;27:508–514. doi: 10.1097/01.BCR.0000225994.75744.9D. [DOI] [PubMed] [Google Scholar]
- 17.Herndon DN, Lemaster J, Beard S, Bernstein N, Lewis SR, Rutan TC, Winkler JB, Cole M, Biarnason D, Gore D. The quality of life after a major thermal injury in children: an analysis of 12 survivors with greater than or equal to 80% total body, 70% third-degree burns. J Trauma. 1986;26:609–619. [PubMed] [Google Scholar]
- 18.Bayat A, McGrouther DA, Ferguson MWJ. Skin scarring. BMJ. 2003;326:88–92. doi: 10.1136/bmj.326.7380.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rowlatt U. Intrauterine wound healing in a 20 week human fetus. Virchows Arch A Pathol Anat Histol. 1979;381:353–361. doi: 10.1007/BF00432477. [DOI] [PubMed] [Google Scholar]
- 20.Mustoe TA, Cooter RD, Gold MH, Hobbs FD, Ramelet AA, Shakespeare PG, Stella M, Téot L, Wood FM, Ziegler UE International Advisory Panel on Scar Management. International clinical recommendations on scar management. Plast Reconstr Surg. 2002;110:560–571. doi: 10.1097/00006534-200208000-00031. [DOI] [PubMed] [Google Scholar]
- 21.Muir IF. On the nature of keloid and hypertrophic scars. Br J Plast Surg. 1990;43:61–69. doi: 10.1016/0007-1226(90)90046-3. [DOI] [PubMed] [Google Scholar]
- 22.Mutalik S. Treatment of keloids and hypertrophic scars. Indian J Dermatol Venereol Leprol. 2005;71:3–8. doi: 10.4103/0378-6323.13777. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Dodd C, Shankowsky HA, Sctt PG, Tredget EE. Deep dermal fibroblasts contribute to hypertrophic scarring. Lab Invest. 2008;88:1278–1290. doi: 10.1038/labinvest.2008.101. [DOI] [PubMed] [Google Scholar]
- 24.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 25.Slemp AE, Kirschner RE. Keloids and scars: a review of keloids and scars, their pathogenesis, risk factors, and management. Curr Opin Pediatr. 2006;18:396–402. doi: 10.1097/01.mop.0000236389.41462.ef. [DOI] [PubMed] [Google Scholar]
- 26.Klass BR, Grobbelaar AO, Rolfe KJ. Transforming growth factor beta 1 signalling, wound healing and epiair: a multifunctional cytokine with clinical implications for wound repair, a delicate balance. Postgrad Med J. 2009;85:9–14. doi: 10.1136/pgmj.2008.069831. [DOI] [PubMed] [Google Scholar]
- 27.Roberts AB, Sporn MB. Differential expression of the TGF-beta isoforms in embryogenesis suggests specific roles in developing and adult tissues. Mol Reprod Dev. 1992;32:91–98. doi: 10.1002/mrd.1080320203. [DOI] [PubMed] [Google Scholar]
- 28.Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFβ2 knockout mice have multiple developmental defects that are nonoverlapping with other TGFβ knockout phenotypes. Development. 1997;124:2659–2670. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diebold RJ, Eis MJ, Yin M, Ormsby I, Boivin GP, Darrow BJ, Saffitz JE, Doetschman T. Early onset multifocal inflammation in the transforming growth factor β1- null mouse is lymphocyte mediated. Proc Natl Acad Sci USA. 1995;92:12215–12219. doi: 10.1073/pnas.92.26.12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaartinen V, Voncken JW, Shuler C, Warbuton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development ad cleft palate in mice lacking TGF-β3 indicates defects of epithelial mesenchymal interaction. Nat Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- 31.Kukarni AB, Huh c-G Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor β1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cowin AJ, Holmes TM, Brosnan P, Ferguson MW. Expression of TGF-beta and its receptors in murine fetal and adult dermal wounds. Eur J Dermatol. 2001;11:424–431. [PubMed] [Google Scholar]
- 33.Rolfe KJ, Richardson J, Vigor C, Irvine LM, Grobbelaar AO, Linge C. A role for TGF-beta1-induced cellular responses during wound healing of the non-scarring early human fetus? J Invest Dermatol. 2007;127:2656–2667. doi: 10.1038/sj.jid.5700951. [DOI] [PubMed] [Google Scholar]
- 34.Rolfe KJ, Irvine LM, Grobbelaar AO, Linge C. Differential gene expression in response to transforming growth factor- beta 1 by fetal and postnatal dermal fibroblasts. Wound Repair Regen. 2007;15:897–906. doi: 10.1111/j.1524-475X.2007.00314.x. [DOI] [PubMed] [Google Scholar]
- 35.Chen W, Fu X, Ge S, Sun T, Zhou G, Jiang D, Sheng Z. Ontogeny of expression of transforming growth factor beta and its receptors and their possible relationship with scarless healing in human fetal skin. Wound Repair Regen. 2005;13:68–75. doi: 10.1111/j.1067-1927.2005.130109.x. [DOI] [PubMed] [Google Scholar]
- 36.Soo C, Beanes SR, Hu FY, Zhang X, Dang C, Chang G, Wang Y, Nishimura I, Freymiller E, Longaker MT, Lorenz HP, Ting K. Ontogenetic transition in fetal wound transforming growth-factor beta regulation correlates with collagen organization. Am J Pathol. 2003;163:2459–2476. doi: 10.1016/s0002-9440(10)63601-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin P, Dickson MC, Millan FA, Akhurst RJ. Rapid induction and clearance of TGFβ1 is an early response to wounding in the mouse embryo. Dev Genetics. 1993;14:225–238. doi: 10.1002/dvg.1020140309. [DOI] [PubMed] [Google Scholar]
- 38.Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH, Fauci AS. Transforming growth factor-β: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nall AV, Brownlee RE, Colvin CP, Schultz G, Fein D, Cassisi NJ, Nguyen T, Kalra A. Transforming growth factor beta 1 improves wound healing and random flap survival in normal and irradiated rats. Arch Otolaryngol. 1996;122:171–177. doi: 10.1001/archotol.1996.01890140057011. [DOI] [PubMed] [Google Scholar]
- 40.Pastar I, Stojadinovic O, Krzyzanowska A, Barrientos S, Stuelten C, Zimmerman K, Blumenberg M, Brem H, Tomic-Canic M. Attenuation of the transforming growth factor beta-signaling pathway in chronic venous ulcers. Mol Med. 2010;16:92–101. doi: 10.2119/molmed.2009.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim BC, Kim HT, Park SH, Cha JS, Yufit T, Kim SJ, Falanga V. Fibroblasts from chronic wounds show altered TGF-β signalling and decreased TGF-β Type II receptor expression. J Cell Physiol. 2003;195:331–336. doi: 10.1002/jcp.10301. [DOI] [PubMed] [Google Scholar]
- 42.Schiller M, Javelaud D, Mauviel A. TGF-beta-induced SMAD signalling and gene regulation: consequences for extracellular matrix remodelling and wound healing. J Dermatol Sci. 2004;35:83–92. doi: 10.1016/j.jdermsci.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Ali SS, Hajrah NH, Ayuob NN, Moshref SS, Abuzinadah OA. Morphological and morphometric study of cultured fibroblast from treated and untreated abnormal scar. Saudi Med J. 2010;30:874–881. [PubMed] [Google Scholar]
- 44.Linge C, Richardson J, Vigor C, Clayton E, Hardas B, Rolfe K. Hypertrophic scar cells fail to undergo a form of apoptosis specific to contractile collagen- the role of transglutaminase. J Invest Dermatol. 2005;12:72–82. doi: 10.1111/j.0022-202X.2005.23771.x. [DOI] [PubMed] [Google Scholar]
- 45.Wang R, Ghahary A, Shen Q, Scott PG, Roy K, Tredget EE. Hypertrophic scar tissues and fibroblasts produce more transforming growth factor -beta 1 mRNA and protein than normal skin and cells. Wound Repair Regen. 2000;8:128–137. doi: 10.1046/j.1524-475x.2000.00128.x. [DOI] [PubMed] [Google Scholar]
- 46.Schmid P, Itin P, Cherry G, Bi C, Cox DA. Enhanced expression of transforming growth factor -beta type 1 and type II receptors in wound granulation tissue and hypertrophic scar. Am J Pathol. 1998;152:485–493. [PMC free article] [PubMed] [Google Scholar]
- 47.Lu L, Saulis AS, Liu WR, Roy NK, Chao JD, Ledbetter S, Mustoe TA. The temporal effects of anti-TGF-beta1, 2, and 3 monoclonal antibody on wound healing and hypertrophic scar formation. J Am Coll Surg. 2005;201:391–397. doi: 10.1016/j.jamcollsurg.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 48.Xie JL, Qi SH, Pan S, Xu YB, Li TZ, Liu XS, Li P. Expression of Smad protein by normal skin fibroblasts and hypertrophic scar fibroblasts in response to transforming growth factor beta 1. Dermatol Surg. 2008;34:1216–1224. doi: 10.1111/j.1524-4725.2008.34261.x. [DOI] [PubMed] [Google Scholar]
- 49.Kopp J, Preis E, Said H, Hafemann B, Wickert L, Gressner AM, Pallua N, Dooley S. Abrogation of transforming growth factor β signalling by SMAD7 inhibits collagen gel contraction of human dermal fibroblasts. J Biol Chem. 2006;280:21570–21576. doi: 10.1074/jbc.M502071200. [DOI] [PubMed] [Google Scholar]
- 50.Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE, Anzano M, Greenwell-Wild T, Wahl SM, Deng C, Roberts AB. Mice lacking Smad 3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- 51.Zhao J, Shi W, Wang YL, Chen H, Bringas P, Dato MB, Frederick JP, Wang XF, Warburton D. Smad 3 deficiency attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol. 2002;282:L585–L593. doi: 10.1152/ajplung.00151.2001. [DOI] [PubMed] [Google Scholar]
- 52.Rorison P, Thomlinson A, Hassan Z, Roberts SA, Ferguson MW, Shah M. Longitudinal changes in plasma Transforming growth factor beta-1 and post-burn scarring. Burns. 2010;36:89–96. doi: 10.1016/j.burns.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Jiao H, Stewart TL, Shankowsky HA, Scott PG, Tredget EE. Increased TGF-beta producing CD4+ T lymphocytes in postburn patients and their potential interaction with dermal fibroblasts in hypertrophic scarring. Wound Repair Regen. 2007;15:530–539. doi: 10.1111/j.1524-475X.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- 54.Shi-Wen X, Leask A, Abraham D. Regulation and function of connective tissue growth factor/CCN2 in tissue repair, scarring and fibrosis. Cytokine Growth factor Rev. 2008;19:133–144. doi: 10.1016/j.cytogfr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Daniels JT, Schultz GS, Blalock TD, Garrett Q, Grotendorst GR, Dean NM, Khaw PT. Mediation of Transforming growth factor-β1- stimulated matrix contraction by fibroblasts. A role for connective tissue growth factor in contractile scarring. Am J Pathol. 2003;163:2043–2052. doi: 10.1016/s0002-9440(10)63562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colwell AS, Phan TT, Kong W, Longaker MT, Lorenz PH. Hypertrophic scar fibroblasts have increased connective tissue growth factor expression after transforming growth factor -beta stimulation. Plast Reconstr Surg. 2005;116:1387–1390. doi: 10.1097/01.prs.0000182343.99694.28. [DOI] [PubMed] [Google Scholar]
- 57.Sisco M, Kryger ZB, O’Shaughnessy KD, Kim PS, Schultz GS, Ding XZ, Roy NK, Dean NM, Mustoe TA. Antisense inhibit of connective tissue growth factor (CTGF/CCN2) mRNA limits hypertrophic scarring without affecting wounding in vivo. Wound Repair Regen. 2008;16:661–673. doi: 10.1111/j.1524-475X.2008.00416.x. [DOI] [PubMed] [Google Scholar]
- 58.Vial C, Guiterrez J, Santander C, Cabrera D, Brandan E. Decorin interacts with CTGF/CCN2 through LRR12 inhibiting its biological activity. J Biol Chem. 2011;286:24242–24252. doi: 10.1074/jbc.M110.189365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sayani K, Dodd CM, Nedelec B, Shen YJ, Ghahary A, Tredget EE, Scott PG. Delayed appearance of decorin in healing burn scars. Histopathol. 2000;36:262–272. doi: 10.1046/j.1365-2559.2000.00824.x. [DOI] [PubMed] [Google Scholar]
- 60.Scott PG, Dodd CM, Tredget EE, Ghahary A, Rahemtulla F. Immunohistochemical localization of the proteoglycans decorin, biglycan and versican and transforming growth factor beta in human post-burn hypertrophic and mature scars. Histopathol. 1995;26:423–431. doi: 10.1111/j.1365-2559.1995.tb00249.x. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Z, Li XJ, Liu Y, Zhang X, Li YY, Xu WS. Recombinant human decorin inhibits cell proliferation and down regulates TGF-beta 1 production of hypertrophic scar fibroblasts. Burns. 2007;33:634–641. doi: 10.1016/j.burns.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Z, Garron TM, Li XJ, Liu Y, Zhang X, Li YY, Xu WS. Recombinant human decorin inhibits TGF-beta 1 - induced contraction of collagen lattice by hypertrophic scar fibroblasts. Burns. 2009;35:527–537. doi: 10.1016/j.burns.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 63.Pan D, Zhe X, Jakkaraju S, Taylot GA, Schuger L. P311 induces a TGF-β-independent, nonfibrogenic myofibroblasts phenotype. J Clin Invest. 2002;110:1349–1358. doi: 10.1172/JCI15614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu J, Ma B, Yi S, Wang Z, He W, Luo G, Chen X, Wang X, Chen A, Barisoni D. Gene expression of early hypertrophic scar tissue screened by means of cDNA microarrays. J Trauma. 2004;57:1276–1286. doi: 10.1097/01.ta.0000108997.49513.dc. [DOI] [PubMed] [Google Scholar]
- 65.Wang F, Xie X, Fan J, Wang L, Guo D, Yang L, Ma X, Zhang L, Li Z. Expression of P311, a transforming growth factor beta latency-associated protein-binding protein, in human kidneys with IgA nephropathy. Int Urol Nephrol. 2010;42:811–819. doi: 10.1007/s11255-009-9681-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan J, Peng X, Luo G, Ma B, Cao C, He W, Yuan S, Li S, Wilkins JA, Wu J. Investigating the role of P311 in the hypertrophic scar. PLoS One. 2010;5:e9995. doi: 10.1371/journal.pone.0009995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Felice B, Garbi C, Santoriello M, Santillo A, Wilson RR. Differential apoptosis markers in human keloids and hypertrophic scar fibroblasts. Mol Cell Biochem. 2009;327:191–201. doi: 10.1007/s11010-009-0057-x. [DOI] [PubMed] [Google Scholar]
- 68.Aarabi S, Bhatt KA, Shi Y, Paterno J, Chang EI, Loh SA, Holmes JW, Longaker MT, Yee H, Gurtner GC. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007;21:3250–3261. doi: 10.1096/fj.07-8218com. [DOI] [PubMed] [Google Scholar]
- 69.Wassermann RJ, Polo M, Smith P, Wang X, Ko F, Robson MC. Differential production of apoptosis-modulating proteins in patients with hypertrophic burn scar. J Surg Res. 1998;75:74–80. doi: 10.1006/jsre.1998.5267. [DOI] [PubMed] [Google Scholar]
- 70.Moulin V, Larochelle S, Langlois C, Thibault I, Lopez-Vallé CA, Roy M. Normal skin wound and hypertrophic scar myofibroblasts have differential responses to apoptotic inducers. J Cell Physiol. 2004;198:350–358. doi: 10.1002/jcp.10415. [DOI] [PubMed] [Google Scholar]
- 71.Akasaka Y, Fujita K, Ishikawa Y, Asuwa N, Inuzuka K, Ito M, Masauda T, Akishima Y, Zhang L, Ito K, Ishii T. Detection of apoptosis in keloids and a comparative study on apoptosis between keloids, hypertrophic scars normal healed flat scars and dermatofibroma. Wound Repair Regen. 2001;9:501–506. doi: 10.1046/j.1524-475x.2001.00501.x. [DOI] [PubMed] [Google Scholar]
- 72.Ogawa R, Akaishi S, Huang C, Dohi T, Aoki M, Omori Y, Koike S, Kobe K, Akimoto M, Hyakusoku H. Clinical applications of basic research that shows reducing skin tension could prevent and treat abnormal scarring; the importance of fascial/subcutaneous tensile reduction sutures and flap surgery for keloid and hypertrophic scar reconstruction. J Nippon Med Sch. 2011;78:68–76. doi: 10.1272/jnms.78.68. [DOI] [PubMed] [Google Scholar]
- 73.Junker JP, Kratz C, Tollbäck A, Kratz G. Mechanical tension stimulates the transdifferentiation of fibroblast into myofibroblasts in human burn scars. Burns. 2008;34:942–946. doi: 10.1016/j.burns.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 74.Derderian CA, Bastidas N, Lerman OZ, Bhatt KA, Lin SE, Voss J, Holmes JW, Levine JP, Gurtner GC. Mechanical strain alters gene expression in an in vitro model of hypertrophic scarring. Ann Plast Surg. 2005;55:69–75. doi: 10.1097/01.sap.0000168160.86221.e9. [DOI] [PubMed] [Google Scholar]
- 75.Van der Veer WM, Niessen FB, Ferreira JA, Zwiers PJ, de Jong EH, Middelkoop E, Molema G. Time course of the angiogenic response during normotrophic and hypertrophic scar formation in humans. Wound Repair Regen. 2011;19:292–301. doi: 10.1111/j.1524-475X.2011.00692.x. [DOI] [PubMed] [Google Scholar]
- 76.Wang J, Hori K, Ding J, Huang Y, Kwan P, Ladak A, Tredget EE. Toll-like receptors expressed by dermal fibroblasts contributes to hypertrophic scarring. J Cell Physiol. 2011;226:1265–1273. doi: 10.1002/jcp.22454. [DOI] [PubMed] [Google Scholar]
- 77.Xue H, McCauley RL, Zhang W, Martini DK. Altered interleukin-6 expression in fibroblasts from hypertrophic burn scars. J Burn Care Rehabil. 2000;21:142–146. doi: 10.1097/00004630-200021020-00010. [DOI] [PubMed] [Google Scholar]
- 78.Castagnoli C, Trombotto C, Ariotti S, Milesimo M, Ravarino D, Magliacani G, Ponzi AN, Stella M, Teich-Alasia S, Novelli F, Musso T. Expression and role of IL-15 in post-burn hypertrophic scars. J Invest Dermatol. 1999;113:238–245. doi: 10.1046/j.1523-1747.1999.00647.x. [DOI] [PubMed] [Google Scholar]
- 79.Tredget EE, Yang L, Delehanty M, Shankowsky H, Scott PG. Polarized Th2 cytokine production in patients with hypertrophic scar following thermal injury . J Interferon Cytokine Res. 2006;26:179–189. doi: 10.1089/jir.2006.26.179. [DOI] [PubMed] [Google Scholar]
- 80.Ulrich D, Ulrich F, Unglaub F, Piatkowski A, Pallua N. Matrix metalloproteinases and tissue inhibitors of metalloprotienases in patients with different types of scars and keloids. J Plast Reconstr Aesthet Surg. 2010;63:1015–1021. doi: 10.1016/j.bjps.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 81.Tanriverdi-Akhisaroglu S, Menderes A, Oktay G. Matrix metalloproteinase -2 and -9 activities in human keloids, hypertrophic and atropic scars: a pilot study. Cell Biochem Funct. 2009;27:81–87. doi: 10.1002/cbf.1537. [DOI] [PubMed] [Google Scholar]
- 82.Amadeu TP, Braune AS, Porto LC, Desmouliére A, Costa AM. Fibrillin-1 and elastin are differentially expressed in hypertrophic scars and keloids. Wound Repair Regen. 2004;12:169–174. doi: 10.1111/j.1067-1927.2004.012209.x. [DOI] [PubMed] [Google Scholar]
- 83.Hakvoot T, Altun V, van Zuijlen PP, de Boer WI, van Schadewii WA, van der Kwast TH. Transforming growth factor beta (1)- beta (2) - beta (3), basic fibroblast growth factor and vascular endothelial growth factor expression in keratinocytes of burns scars. Eur Cytokine Netw. 2000;11:233–239. [PubMed] [Google Scholar]
- 84.Ghahary A, Shen YJ, Wang R, Scott PG, Tredget EE. Expression and localization of insulin-like growth factor-1 in normal and postburn hypertrophic scar tissue in human. Mol Cell Biochem. 1998;183:1–9. doi: 10.1023/a:1006890212478. [DOI] [PubMed] [Google Scholar]
- 85.Ghahary A, Shen YJ, Nedelec B, Scott PG, Tredget EE. Enhanced expression of mRNA for insulin-like growth factor-1 in post burn hypertrophic scar tissue and its fibrogenic role by dermal fibroblasts. Mole Cell Biochem. 1995;148:25–32. doi: 10.1007/BF00929499. [DOI] [PubMed] [Google Scholar]
- 86.Reish RG, Eriksson E. Scars: a review of emerging and currently available therapies. Plast Reconstr Surg. 2008;122:1068–1078. doi: 10.1097/PRS.0b013e318185d38f. [DOI] [PubMed] [Google Scholar]
- 87.Niessen F, Spauwen P, Schalkwijk J, Kon M. On the nature of hypertrophic scars and keloids: a review. Plast Reconstr Surg. 1999;104:1435–1458. doi: 10.1097/00006534-199910000-00031. [DOI] [PubMed] [Google Scholar]
- 88.Tredget E, Shankowsky H, Pannu R, Nedelec B, Iwashina T, Ghahary A, Taerum TV, Scott PG. Transforming growth beta in thermally injured patients with hypertrophic scars: effects of interferon alpha-2b. Plast Reconstr Surg. 1998;102:1317–1328. doi: 10.1097/00006534-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 89.Renò F, Sabbatini M, Lombardi F, Stella M, Pezzuto C, Magliacani G, Cannas M. In vitro mechanical compression induces apoptosis and regulates cytokines release in hypertrophic scars. Wound Repair Regen. 2003;11:331–336. doi: 10.1046/j.1524-475x.2003.11504.x. [DOI] [PubMed] [Google Scholar]
- 90.Haurani MJ, Foreman K, Yang JJ, Siddiqui A. 5-Fluorouracil treatment of problematic scars. Plast Reconstr Surg. 2009;123:139–148. doi: 10.1097/PRS.0b013e3181904d1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Daroughen A, Asilian A, Shariati F. Intralesional triamcinolon alone or in combination with 5-fluorouracil for the treatment of keloid and hypertrophic scars. Clin Exp Dermatol. 2009;34:219–223. doi: 10.1111/j.1365-2230.2007.02631.x. [DOI] [PubMed] [Google Scholar]
- 92.Dolynchuk K, Ziesmann M, Serletti J. Topical putrescine (Fibrostat) in treatment of hypertophic scars: phase II study. Plast Reconstr Surg. 1996;97:117–123. doi: 10.1097/00006534-199601000-00019. [DOI] [PubMed] [Google Scholar]
- 93.Borgognoni L. Biological effects of silicone gel sheeting. Wound Repair Regen. 2002;10:118–121. doi: 10.1046/j.1524-475x.2002.00205.x. [DOI] [PubMed] [Google Scholar]
- 94.Gilman T. Silicone sheet for the treatment and prevention of hypertrophic scar: a new proposal for the mechanism of efficacy. Wound Repair Regen. 2003;11:235. doi: 10.1046/j.1524-475x.2003.11313.x. [DOI] [PubMed] [Google Scholar]
- 95.Kuhn M, Moffit M, Smith P, Lyle WG, Ko F, Meltzer DD, Robson MC. Silicone sheeting decreases fibroblast activity and downregulates TGF-beta 2 in hypertrophic scar model. Internat J Surg Invest. 2001;2:467–474. [PubMed] [Google Scholar]
- 96.Abdollahi A, Li M, Ping G, Plathow C, Domhan S, Kiessling F, lee LB, McMahnon G, Gröne HJ, Lipson KE, Huber PE. Inhibition of platelet-derived growth factor signalling attenuates pulmonary fibrosis. J Experiment Med. 2005;201:925–935. doi: 10.1084/jem.20041393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shah M, Foreman D, Ferguson M. Control of scarring in adult wounds by neutralising antibody to transforming growth factor beta. Lancet. 1992;339:213–214. doi: 10.1016/0140-6736(92)90009-r. [DOI] [PubMed] [Google Scholar]
- 98.McCormick L, Zhang Y, Tootell E, Gilliam A. Anti-TGFbeta treatment prevents skin and lung fibrosis in murine sclerodermatous graft-versus host disease: a model for human scleroderma. J Immunol. 1999;163:5693–5699. [PubMed] [Google Scholar]
- 99.Zhang Y, McCormick L, Gilliam A. Latency associated peptide prevents skin fibrosis in murine sclerodermatous graft-versus host disease, a model for human scleroderma. J Invest Dermatol. 2003;121:713–719. doi: 10.1046/j.1523-1747.2003.12517.x. [DOI] [PubMed] [Google Scholar]
- 100.Bonniaud P, Margetts P, Kolb M, Schroeder JA, Kapoun AM, Damm D, Murphy A, Chakravarty S, Dugar S, Higgins L, Protter AA, Gauldie J. Progressive transforming growth factor-beta1 induced lung fibrosis is blocked by an orally active ALK5 kinase inhibitor. Am J Resp Cit Care Med. 2005;171:889–898. doi: 10.1164/rccm.200405-612OC. [DOI] [PubMed] [Google Scholar]
- 101.Marquez-Aquirre A, Sandoval-Rodriguz A, Gonzalez-Cuevas J, Bueno-Topete M, Navarro-Partida J, Arellano-Olivera I, Lucano-Laneros S, Armendariz-Borunda J. Adenoviral delivery of dominant negative transforming growth factor beta type II receptor up-regulates transcriptional repressor SKI-like oncogene, decreases matrix metalloproteinase 2 in hepatic stellate cell and prevents liver fibrosis in rats. J Gene Med. 2009;11:207–219. doi: 10.1002/jgm.1303. [DOI] [PubMed] [Google Scholar]
- 102.Ahn JY, Park S, Yun YS, Song JY. Inhibition of type III TGF-β- receptor aggravates lung fibrotic response. Biomed Pharmacother. 2010;64:472–476. doi: 10.1016/j.biopha.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 103.Juárez P, Vilchis-Landeros MM, Ponce-Coria J, Mendoza V, Hermández-Pando R, Bobadilla NA, López-Casillas F. Soluble betaglycan reduces renal damage progression in db/db mice. Am J Physiol Renal Physiol. 2007;292:F321–F329. doi: 10.1152/ajprenal.00264.2006. [DOI] [PubMed] [Google Scholar]
- 104.Ferguson MW, Duncan J, Bond J, Bush J, Durani P, So K, Taylor L, Chantrey J, Mason T, James G, Laverty H, Occleston NL, Sattar A, Ludlow A, O’Kane S. Prophylactic administration of avotermin for improvement of skin scarring: three double-blind, placebo controlled , phase I/II studies. Lancet. 2009;373:1264–1274. doi: 10.1016/S0140-6736(09)60322-6. [DOI] [PubMed] [Google Scholar]
- 105. http://www.renovo. com/en/products/juvista. [Google Scholar]
- 106.Wei YJ, Yan XQ, Ma L, Wu JG, Zhang H, Qin LP. Oleanolic acid inhibits hypertrophic scarring in the rabbit ear model. Clin Exp Dermatol. 2011;36:528–533. doi: 10.1111/j.1365-2230.2010.04012.x. [DOI] [PubMed] [Google Scholar]
- 107.Zhu KQ, Carrougher GJ, Gibran NS, Isik FF, Engrav LH. Review of the female Duroc/Yorkshire pig model of fibroproliferative scarring. Wound Repair Regen. 2007;15(Suppl 1):S32–S39. doi: 10.1111/j.1524-475X.2007.00223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


